Abstract
Background
Chronic inflammation might play a major role in the pathogenesis linking diabetes mellitus (DM) to cognition. In addition, DM might be the main driver of dementia risk. The purpose of the present study was to evaluate whether inflammation, glycation, or both are associated with the risk of developing all-cause dementia (ACD).
Methods
A nationwide population-based cohort study was conducted with 4113 participants. The data were obtained from the Taiwanese Survey on Prevalence of Hypertension, Hyperglycemia, and Hyperlipidemia (TwSHHH) in 2007, which was linked with the Taiwan National Health Insurance Research Database (NHIRD). The markers of inflammation, expressed as hs-CRP, and glycation, presented as HbA1c, were measured. High levels of hs-CRP and HbA1c were defined as values greater than or equal to the 66th percentile. Developed ACD was identified based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes.
Results
During 32,926.90 person-years, 106 individuals developed ACD in up to 8 years of follow-up. The study participants were separated into four categories by the top tertiles of hs-CRP and HbA1c based on the 66th percentile: high levels of both hs-CRP and HbA1c, only high levels of hs-CRP, only high levels of HbA1c, and non-high levels of hs-CRP nor HbA1c. Those who with a high level of only hs-CRP had the higher hazard for developing ACD (adjusted HR = 2.58; 95% CI = 1.29 ~ 5.17; P = 0.007), followed by the group with a high level of only HbA1c (adjusted HR = 2.52; 95% CI = 1.34 ~ 4.74; P = 0.004) and the group with high levels of both hs-CRP and HbA1c (adjusted HR = 2.36; 95% CI = 1.20 ~ 4.62; P = 0.012). Among those aged less than 65 years, hs-CRP was the only significant predictor of ACD risk (P = 0.046), whereas it did not yield any significant result in the elderly.
Conclusions
A higher risk of developing ACD was found not only in patients with high levels of inflammation but also high levels of glycated hemoglobin. Future studies should focus on the clinical implementation of hs-CRP or HbA1c to monitor cognitive deficits.
Similar content being viewed by others
Background
The etiology of neurodegenerative diseases is multifactorial and is attributable to several major risk factors, [1] including hypertension, diabetes mellitus (DM), high cholesterol, obesity, physical inactivity, smoking, and depression [2,3,4]. These predictors contributing to the development of dementia could be modified through healthy lifestyle behaviors [5]. Therefore, it is crucial to discover potential biomarkers for the early development and progression of cognitive impairment [6].
Chronic inflammation might play a major role in the pathogenesis of type 2 DM to Alzheimer’s disease (AD) [7]. The co-morbid conditions related to diabetes, such as obesity, high cholesterol, and hypertension, were negatively associated with brain function [8]. In addition, the relationship between increased systemic inflammation and cognitive dysfunction is thought to result from physical inactivity and cigarette smoking [9, 10]. In addition, anti-inflammatory treatments could reduce dementia risk among people with depressive disorder [11]. Furthermore, chronic low-grade inflammation, measured using levels of high-sensitivity C-reactive protein (hs-CRP), was associated with early stage β-amyloid accumulation, resulting in neuroinflammation in brain regions [12].
Diabetes mellitus was found to be the main driver of cardiovascular risk factors and the risk of dementia due to increased insulin resistance (IR), which is influenced by diabetes [13]. The marker of glycated hemoglobin (HbA1c) might be clinically useful as a surrogate for identifying the presence of both insulin resistance and dysglycemia, [14] for which the possible biological pathogenesis might be that chronic hyperglycemia plays a key role in linking diabetes and memory decline, likely through microvascular injury [15]. Nevertheless, although HbA1c is a surrogate biomarker for detecting insulin resistance, it is suggested to be used in combination with other biomarkers [16].
The association between hyperglycemia and AD via tau hyperphosphorylation has been demonstrated [17]. In addition, inflammation is regarded as a major driver of IR in AD, impairing the blood–brain barrier [18]. However, the damaging consequences of IR have different pathomechanisms in DM and AD [19]. Moreover, previous studies have shown the joint effect of inflammation and glycation on cardiovascular diseases, such as coronary artery diseases, [20] cardiovascular risks, [21] and advanced subclinical carotid atherosclerosis progression, [22] which share similar pathogenic features that contribute to cerebral white matter hyperintensities, atherogenesis, and focal dysregulation in cerebrovascular flow in the hippocampus, leading to cognitive decline [23, 24]. However, there is still no evidence that illustrates the impact of the combination of inflammation and glycation on the risk of developing dementia.
Therefore, the purpose of the present study was to determine whether there is a possible impact from inflammation, glycation, or both on the risk of developing all-cause dementia (ACD) during eight years of follow-up in a nationwide population-based study sample.
Methods
Study design and data sources
This study was a cohort study. Data were obtained from the Taiwanese Survey on Prevalence of Hypertension, Hyperglycemia, and Hyperlipidemia (TwSHHH) in 2002. A second follow-up of the TwSHHH was carried out in 2007, encompassing a longitudinal study with a nationally representative sample. It was then linked with the Taiwan National Health Insurance Research Database (NHIRD) between 2001 and 2015 to identify information on ACD diagnoses. Moreover, age-subgroup analyses were conducted to evaluate the effect of inflammation, glycation, or both on ACD risk. The participants’ characteristics and the research design in recruitment regarding the TwSHHH and NHIRD were described in detail previously and maintained by the Health and Welfare Data Science Center, Ministry of Health and Welfare (HWDC, MOHW) [25, 26]. This study was reviewed and approved by the Joint Institutional Review Board at Taipei Medical University.
Study sample
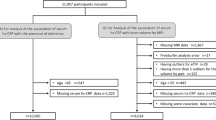
Baseline participants in the cohort study were from the TwSHHH in 2007, with a total of 4682 samples aged 21–99 years. Those without complete data on blood pressure and biochemical laboratory data and those who could not be linked to the NHIRD were excluded (n = 281). Additionally, we excluded subjects with a history of ACD (n = 42) or who died (n = 1) before 2007. Moreover, in order to reduce the bias and increase the validity of the diagnosis, data sources with missing data on age, sex, laboratory measurements, lifestyle, and comorbidities, and participants with only a single diagnosis of ACD were also excluded (n = 245). Finally, 4113 samples were included in the analysis (Fig. 1).
Definition of high levels of inflammation (hs-CRP) and glycation (HbA1c)
The subjects’ hs-CRP and HbA1c levels were examined during the second TwSHHH visit in 2007. The biomarker of inflammation, represented by hs-CRP, was measured using the particle-enhanced immunoturbidmetric principle. Glycation, represented by HbA1c, was assessed using high-performance liquid chromatography. The specimens were collected by trained technicians. All measurements, derived from 5% duplicated blood samples, were obtained with blinded quality control specimens in the central laboratory.
The cut-off points of high levels of inflammation and glycemic control were defined as values greater than or equal to the 66th percentile from the final sample. Therefore, the study participants were separated into three categories according to the top tertiles of hs-CRP and HbA1c levels, including both hs-CRP and HbA1c levels above the 66th percentile, either hs-CRP levels or HbA1c levels above the 66th percentile, and neither hs-CRP nor HbA1c levels above the 66th percentile. Additionally, participants with either hs-CRP or HbA1c levels above the 66th percentile were then divided into two groups: only hs-CRP above the 66th percentile and only HbA1c above the 66th percentile.
Study outcome
The endpoint of ACD in this study was identified in the NHIRD according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. A diagnosis of ACD was defined as senile dementia, uncomplicated dementia (290.0), presenile dementia (290.1x), senile dementia with delusional or depressive features (290.2x), senile dementia with delirium (290.3), arteriosclerotic dementia (290.4x), dementia in conditions classified elsewhere (294.1), AD (331.0), Pick’s disease (331.1), and senile degeneration of the brain (331.2).
Covariables
The covariates in this study were age, sex, and associated comorbidities that might affect the relationship between hs-CRP and HbA1c levels and the risk of ACD. Baseline demographic characteristics, including age and sex, were defined using the questionnaire. Laboratory data regarding systolic blood pressure, diastolic blood pressure, glucose, total cholesterol, triglycerides, and body mass index were obtained from the TwSHHH in 2007. However, the TwSHHH did not identify the illnesses associated with inflammatory disorders and infections. Therefore, we used the NHIRD to determine the variables of chronic infection or inflammation (ICD-9-CM 042–044, 010–018, and 090–099). In addition, these covariables were adjusted using statistical models.
Statistical analysis
Statistical analysis system software (SAS System for Windows, version. 9.4; SAS Institute, Cary, NC, USA) was used to perform all statistical analyses. Those with only one ACD diagnosis were excluded to ensure the validity of the diagnosis. In this study, the index date to explore the joint effect of hs-CRP and HbA1C on ACD risk was set as the date of the second TwSHHH visit in 2007. In the final study sample, each subject was tracked from the index date to whichever came first: the development of ACD, death, or the end of 2015 (year).
Continuous and dichotomous variables were expressed as the mean ± standard deviation (SD) and as numbers with percentages, respectively. The comparison of the differences in the distributions of the demographic characteristics among the 4 groups was performed using the Chi-squared test and Kruskal-Wallis H test. Moreover, the risk of ACD was examined using a Cox proportional hazard regression model with hazard ratios (HRs) and 95% confidence intervals (CIs). In addition, a multivariate Cox proportional hazard regression was used to explore relationships between the combination of hs-CRP and HbA1c levels with ACD risk after adjusting for potential confounders such as age, sex, systolic blood pressure (SBP), diastolic blood pressure (DBP), glucose, total cholesterol, triglycerides, body mass index (BMI), exercise status, smoking status, alcohol consumption, heart disease, stroke, and chronic infection or inflammation. In all of the Cox models, the proportional hazard assumptions were not violated.
Furthermore, a subgroup analysis was carried out to investigate the impact of the joint effect of hs-CRP and HbA1c with the endpoints, including the primary endpoint of ACD among those without DM, those aged less than 65 years based on their age at baseline, and those aged 65 years and older; and secondary endpoints of AD and vascular dementia among the total sample, which tested the consistency of the results. Statistical significance was set at P < 0.05.
Results
A total of 4113 individuals aged 21–99 years were enrolled, with a mean age of 47.11 years; 47% were male, and the participants had mean hs-CRP and hbA1c values of 0.22 mg/dL and 5.62%, respectively. During 32,926.90 person-years, 106 persons developed ACD in up to eight years of follow-up. These participants were classified into four categories, including both hs-CRP and HbA1c levels above the 66th percentile (756 subjects), only hs-CRP levels above the 66th percentile (682 subjects), only HbA1c levels above the 66th percentile (772 subjects), and neither hs-CRP nor HbA1c levels above the 66th percentile (1903 subjects).
The distributions of all demographic characteristics, except for lifestyle factors in alcohol consumption (P = 0.110) and comorbidities in chronic infection or inflammation (P = 0.147), differed significantly according to the status of the combination of hs-CRP and HbA1c. The mean age, systolic blood pressure, diastolic blood pressure, glucose, total cholesterol, triglycerides, and body mass index (all P < 0.001) of the participants and the proportion of men (P = 0.002), smoking behavior (P < 0.001), heart disease (P < 0.001), and stroke (P < 0.001) were all higher among those with high levels of both hs-CRP and HbA1c, as presented in Table 1.
During the 8 years of follow-up, subjects with high levels of both hs-CRP and HbA1c (crude HR = 5.69; 95% CI = 3.08 ~ 10.51; P < 0.001), individuals with a high level of only hs-CRP (crude HR = 3.66; 95% CI = 1.86 ~ 7.19; P < 0.001), and those with a high level of HbA1c (crude HR = 6.85; 95% CI = 3.79 ~ 12.40; P < 0.001; Table 2) were associated with a higher risk of developing ACD than those with no high levels. However, after controlling for age, sex, SBP, DBP, glucose, total cholesterol, triglycerides, BMI, regular exercise, smoking status, alcohol consumption, heart disease, stroke, and chronic infection or inflammation, the results in Table 2 demonstrated that those with only a high level of hs-CRP had a higher risk of developing ACD (adjusted HR = 2.58; 95% CI = 1.29 ~ 5.17; P = 0.007), followed by the group with only a high level of HbA1c (adjusted HR = 2.52; 95% CI = 1.34 ~ 4.74; P = 0.004) and the groups with high levels of both hs-CRP or HbA1c (adjusted HR = 2.36; 95% CI = 1.20 ~ 4.62; P = 0.012; Table 2).
Additionally, the results of the subgroup analysis presented inconsistent findings compared to the whole study sample. Among those without diabetes mellitus, groups with only a high level of hs-CRP (adjusted HR = 2.89; 95% CI = 1.27 ~ 6.56; P = 0.011) and HbA1c (adjusted HR = 2.27; 95% CI = 1.11 ~ 4.67; P = 0.026; Table 3) both had a greater risk of ACD, whereas those with high levels of both hs-CRP and HbA1c showed borderline significant results (P = 0.055). Nonetheless, Table 3 shows that only those in the group with a high level of inflammation, as measured by hs-CRP, presented a significant result for the development of ACD (adjusted HR = 11.33; 95% CI = 1.05 ~ 122.39; P = 0.046) in participants aged less than 65 years, but not in the group with high levels of both inflammation and hyperglycemia (P = 0.205) and the group with a high level of only HbA1c (P = 0.172). However, the adjusted hazard ratios for ACD risk were not significantly different among the four groups in those aged 65 years or older, and the same was seen for the secondary endpoints of Alzheimer’s disease and vascular dementia among the total sample (all P > 0.05).
Discussion
After 8 years of follow-up, the results of the current study revealed that high levels of inflammation (represented with hs-CRP) and hyperglycemia (represented with hbA1c) were risk factors that predict a higher risk of ACD. Moreover, the participants with high levels of only inflammation presented a significant ACD risk among adults aged less than 65 years, whereas no such relationships were found in elderly people.
The results of the present study found that subjects with only high levels of hs-CRP had a higher risk of ACD, followed by those with only high levels of HbA1c and their combination. These findings are similar to those of published studies [27,28,29,30,31,32]. Those with high levels of inflammation and glycation had at least 2.4-fold (range, 2.4 to 3.8) [27,28,29] and 1.9-fold (range, 1.9–2.9) [30,31,32] changes, respectively, in their cognitive impairment. These published results showed that the risk of dementia appears to be associated with higher hs-CRP levels as opposed to HbA1c levels, which is comparable to our findings. The major role of systemic inflammation in the development of type 2 diabetes mellitus may be considered as a possible pathogenesis [33]. In addition, the mechanisms underlying the relationship between inflammation or hyperglycemia and cognitive dysfunction might be explained by impaired endothelial function, [34, 35] which can result in cerebral white matter hyperintensities [36, 37]. The pathologic stimuli contributing to the response of endothelial cells resulted in the initiation of vascular compromise via breakdown of the blood-brain barrier and could lead to subsequent leukoaraiosis in the brain [38].
Several studies have examined the combined impact of inflammation and glycated hemoglobin on subsequent adverse consequences, such as severity of coronary artery disease, [20] cardiovascular events, [21] progression of carotid atherosclerosis, [22] and hyperglycemia [39]. To the best of our knowledge, this is the first study to assess the causal association of the combined effect of hs-CRP and HbA1c simultaneously with cognitive decline, although their combined effect presented a significant but non-multiplicative effect with regard to ACD risk. A negative association between hs-CRP and HbA1c might be a potential interpretation, in which hs-CRP levels might be influenced by multiple factors and cannot be explained by HbA1c alone [40]. Although contradictory to the results of prior studies, HbA1c levels increased when hs-CRP levels increased [33, 41].
With regard to the subgroup analysis shown in Table 3, the participants in the group with only a high level of hs-CRP had an increased risk of developing ACD among adults aged less than 65 years, which is consistent with the results of other studies. An adverse relationship between hs-CRP and incident Alzheimer’s disease was observed in adults aged between 60 and 70.5 years [27]. However, most studies have not examined the association between Hba1c and dementia under 65 years of age. Notably, there were no significant difference in ACD risk in the elderly population (age ≥ 65 years) among the four groups when using a cutoff point of 0.23 mg/dL for hs-CRP and ≥ 6.00% for Hba1c. The findings of this study are comparable to those of previous studies [32, 42]. There was not an adverse association between hs-CRP level and cognitive function in older women, [42] and in seniors aged greater than 70.6 years, an inverse association was found [27]. Moreover, those with HbA1c levels between 5.7 and 6.4% showed a higher but not significant risk of ACD, while an HbA1c level ≥ 7% presented an increased risk of incident ACD [32]. Although the small sample size and small number of events might result in insignificant findings, this study still provided adequate statistical power (> 80%) with which to elucidate the relationship between hs-CRP or HbA1c and cognitive decline among younger and elderly individuals. In addition, all of the diabetic individuals in the TwSHHH survey were reported to have type 2 DM. In order to test the consistency of the results, a subgroup analysis among those without DM was performed. The analysis presented findings comparable to those reported in the total sample. Therefore, the diagnosis of DM may not have influenced our main findings. In addition, treatment for DM or diabetes control might reduce the possibility of developing dementia.
This study had several strengths. First, to the best of our knowledge, this is the first study to investigate the combined effect of hs-CRP and HbA1c on the subsequent risk of ACD. Second, the present study used a nationwide population-based dataset, which increased the representativeness and generalizability of the study sample. Third, the subtypes of dementia, including Alzheimer’s disease and vascular dementia, were included in the subgroup analysis, whereas a lower incidence of cognitive impairment might result in non-significant results. Finally, a cohort study design was adopted in this study. Thus, a clear temporal causality was well established. Moreover, some potential limitations of the present study should be mentioned. First, the confounders regarding lifestyle factors were based on self-reporting instruments. Therefore, the possibility of selection and recall bias may have occurred, and the findings were limited. Second, the claims data from Taiwan’s NHIRD did not provide detailed information about the severity of cognitive decline. The ascertainment of diagnosed dementia might have led to an underestimation. Third, although this study did not define dementia by including the prescription of anti-dementia medications, subjects with dementia who had claims data of at least two confirmed visits were included to increase the validity of the diagnosis. Finally, the exposure assessments of inflammation and glycation were identified by a single screening, while the determination of the dynamic changes in hs-CRP and HbA1c could not be defined. In addition, exposure misclassification may occur.
Conclusions
In summary, a higher risk of developing ACD was associated not only with high levels of inflammation but also with high levels of glycated hemoglobin during the 8-year follow-up period in a nationwide population-based cohort. In addition, high-sensitivity C-reactive protein and glycated hemoglobin A1c are useful prognostic markers for detecting cognitive dysfunction. Future studies should focus on the clinical implementation of hs-CRP or HbA1c to monitor cognitive deficits.
Availability of data and materials
The data described in the manuscript, code book, and analytic code will not be made available because the data source used in this study is managed by the Health and Welfare Data Science Center, Ministry of Health and Welfare (HWDC, MOHW), for which researchers need to submit an application to acquire it for scientific purposes; thus, these data were not publicly accessed.
Abbreviations
- ACD:
-
all-cause dementia
- DM:
-
diabetes mellitus
- TwSHHH:
-
Taiwanese Survey on Prevalence of Hypertension, Hyperglycemia, and Hyperlipidemia
- NHIRD:
-
National Health Insurance Research Database
- ICD-9-CM:
-
International Classification of Diseases, Ninth Revision, Clinical Modification
- AD:
-
Alzheimer’s disease
- IR:
-
Insulin resistance
- HR:
-
Hazard ratio
- CI:
-
Confidence Interval
- HWDC:
-
Health and Welfare Data Science Center
- MOHW:
-
Ministry of Health and Welfare
- SD:
-
standard deviation
- SBP:
-
systolic blood pressure
- DBP:
-
diastolic blood pressure
- BM:
-
body mass index
References
Talwar P, Grover S, Sinha J, Chandna P, Agarwal R, Kushwaha S, et al. Multifactorial analysis of a biomarker pool for Alzheimer disease risk in a north Indian population. Dement Geriatr Cogn Disord. 2017;44(1–2):25–34. https://doi.org/10.1159/000477206.
Liu Y, Zhang S, Tomata Y, Nurrika D, Sugawara Y, Tsuji I. The impact of risk factors for dementia in China. Age Ageing. 2020;49(5):850–5. https://doi.org/10.1093/ageing/afaa048.
Deckers K, van Boxtel MP, Schiepers OJ, de Vugt M, Muñoz Sánchez JL, Anstey KJ, et al. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int J Geriatr Psychiatry. 2015;30(3):234–46. https://doi.org/10.1002/gps.4245.
Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. 2020;396(10248):413–46. https://doi.org/10.1016/s0140-6736(20)30367-6.
Dhana K, Evans DA, Rajan KB, Bennett DA, Morris MC. Healthy lifestyle and the risk of Alzheimer dementia: findings from 2 longitudinal studies. Neurology. 2020;95(4):e374–e83. https://doi.org/10.1212/wnl.0000000000009816.
Sharma N, Singh AN. Exploring biomarkers for alzheimer's disease. J Clin Diagn Res. 2016;10(7):Ke01–6. https://doi.org/10.7860/jcdr/2016/18828.8166.
Mushtaq G, Khan JA, Kumosani TA, Kamal MA. Alzheimer's disease and type 2 diabetes via chronic inflammatory mechanisms. Saudi J Biol Sci. 2015;22(1):4–13. https://doi.org/10.1016/j.sjbs.2014.05.003.
Anjum I, Fayyaz M, Wajid A, Sohail W, Ali A. Does obesity increase the risk of dementia: a literature review. Cureus. 2018;10(5):e2660. https://doi.org/10.7759/cureus.2660.
Straub RH. The brain and immune system prompt energy shortage in chronic inflammation and ageing. Nat Rev Rheumatol. 2017;13(12):743–51. https://doi.org/10.1038/nrrheum.2017.172.
Liu Y, Li H, Wang J, Xue Q, Yang X, Kang Y, et al. Association of cigarette smoking with cerebrospinal fluid biomarkers of neurodegeneration, neuroinflammation, and oxidation. JAMA Netw Open. 2020;3(10):e2018777. https://doi.org/10.1001/jamanetworkopen.2020.18777.
Hayley S, Hakim AM, Albert PR. Depression, dementia and immune dysregulation. Brain: J Neurol. 2021;144(3):746–60. https://doi.org/10.1093/brain/awaa405.
Toppala S, Ekblad LL, Tuisku J, Helin S, Johansson JJ, Laine H, et al. Association of Early β-amyloid accumulation and Neuroinflammation measured with [(11) C]PBR28 in elderly individuals without dementia. Neurology. 2021;96(12):e1608–e19. https://doi.org/10.1212/wnl.0000000000011612.
Fan YC, Hsu JL, Tung HY, Chou CC, Bai CH. Increased dementia risk predominantly in diabetes mellitus rather than in hypertension or hyperlipidemia: a population-based cohort study. Alzheimers Res Ther. 2017;9(1):7. https://doi.org/10.1186/s13195-017-0236-z.
Saha S, Schwarz PE. Impact of glycated hemoglobin (HbA1c) on identifying insulin resistance among apparently healthy individuals. J Public Health. 2017;25(5):505–12. https://doi.org/10.1007/s10389-017-0805-4.
Marden JR, Mayeda ER, Tchetgen Tchetgen EJ, Kawachi I, Glymour MM. High hemoglobin A1c and diabetes predict memory decline in the health and retirement study. Alzheimer Dis Assoc Disord. 2017;31(1):48–54. https://doi.org/10.1097/wad.0000000000000182.
Umeno A, Yoshida Y. Utility of hemoglobin A1c in detecting risk of type 2 diabetes: comparison of hemoglobin A1c with other biomarkers. J Clin Biochem Nutr. 2019;65(1):59–64. https://doi.org/10.3164/jcbn.19-16.
Hobday AL, Parmar MS. The link between diabetes mellitus and tau hyperphosphorylation: implications for risk of Alzheimer's disease. Cureus. 2021;13(9):e18362. https://doi.org/10.7759/cureus.18362.
Vinuesa A, Pomilio C, Gregosa A, Bentivegna M, Presa J, Bellotto M, et al. Inflammation and insulin resistance as risk factors and potential therapeutic targets for Alzheimer's disease. Front Neurosci. 2021;15:653651. https://doi.org/10.3389/fnins.2021.653651.
Bednarz K, Siuda J. Alzheimer's disease and type 2 diabetes mellitus: similarities in pathomechanisms lead to therapeutic opportunities. Neurol Neurochir Pol. 2021;55(5):418–28. https://doi.org/10.5603/PJNNS.a2021.0056.
Ahmed M, Islam MM, Rahman MA, Rubaiyat KA, Khuda CKE, Ferdous KAF, et al. Association of C-reactive protein and HbA1c in the severity of coronary artery disease in patients with ischemic heart disease. Cardiovasc J. 2018;11(1):53–8. https://doi.org/10.3329/cardio.v11i1.38243.
Schillinger M, Exner M, Amighi J, Mlekusch W, Sabeti S, Rumpold H, et al. Joint effects of C-reactive protein and glycated hemoglobin in predicting future cardiovascular events of patients with advanced atherosclerosis. Circulation. 2003;108(19):2323–8. https://doi.org/10.1161/01.CIR.0000095267.24234.00.
Sander D, Schulze-Horn C, Bickel H, Gnahn H, Bartels E, Conrad B. Combined effects of hemoglobin A1c and C-reactive protein on the progression of subclinical carotid atherosclerosis: the INVADE study. Stroke. 2006;37(2):351–7. https://doi.org/10.1161/01.STR.0000199034.26345.bc.
Alfaddagh A, Martin SS, Leucker TM, Michos ED, Blaha MJ, Lowenstein CJ, et al. Inflammation and cardiovascular disease: from mechanisms to therapeutics. Am J Prevent Cardiol. 2020;4:100130. https://doi.org/10.1016/j.ajpc.2020.100130.
Prasad K. Does HbA1cc play a role in the development of cardiovascular diseases? Curr Pharm Des. 2018;24(24):2876–82. https://doi.org/10.2174/1381612824666180903121957.
Hsieh C-Y, Su C-C, Shao S-C, Sung S-F, Lin S-J, Yang Y-HK, et al. Taiwan’s National Health Insurance Research Database: past and future. Clin Epidemiol. 2019;11:349–58. https://doi.org/10.2147/CLEP.S196293.
Fan YC, Chou CC, You SL, Sun CA, Chen CJ, Bai CH. Impact of worsened metabolic syndrome on the risk of dementia: a nationwide cohort study. J Am Heart Assoc. 2017;6(9):e004749. https://doi.org/10.1161/jaha.116.004749.
Gabin JM, Saltvedt I, Tambs K, Holmen J. The association of high sensitivity C-reactive protein and incident Alzheimer disease in patients 60 years and older: the HUNT study. Norway Immun Ageing. 2018;15(1):4. https://doi.org/10.1186/s12979-017-0106-3.
Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia aging study. Ann Neurol. 2002;52(2):168–74. https://doi.org/10.1002/ana.10265.
Noble JM, Manly JJ, Schupf N, Tang MX, Mayeux R, Luchsinger JA. Association of C-reactive protein with cognitive impairment. Arch Neurol. 2010;67(1):87–92. https://doi.org/10.1001/archneurol.2009.308.
Yaffe K, Blackwell T, Whitmer RA, Krueger K, Barrett CE. Glycosylated hemoglobin level and development of mild cognitive impairment or dementia in older women. J Nutr Health Aging. 2006;10(4):293–5.
Wium-Andersen IK, Rungby J, Jørgensen MB, Sandbæk A, Osler M, Wium-Andersen MK. Risk of dementia and cognitive dysfunction in individuals with diabetes or elevated blood glucose. Epidemiol Psychiatr Sci. 2019;29:e43. https://doi.org/10.1017/s2045796019000374.
Ramirez A, Wolfsgruber S, Lange C, Kaduszkiewicz H, Weyerer S, Werle J, et al. Elevated HbA1c is associated with increased risk of incident dementia in primary care patients. J Alzheimers Dis. 2015;44(4):1203–12. https://doi.org/10.3233/jad-141521.
Ahmadi-Abhari S, Kaptoge S, Luben RN, Wareham NJ, Khaw KT. Longitudinal association of C-reactive protein and Haemoglobin A1c over 13 years: the European prospective investigation into Cancer--Norfolk study. Cardiovasc Diabetol. 2015;14(1):61. https://doi.org/10.1186/s12933-015-0224-1.
Markus HS, Hunt B, Palmer K, Enzinger C, Schmidt H, Schmidt R. Markers of endothelial and hemostatic activation and progression of cerebral white matter hyperintensities: longitudinal results of the Austrian stroke prevention study. Stroke. 2005;36(7):1410–4. https://doi.org/10.1161/01.STR.0000169924.60783.d4.
Torimoto K, Okada Y, Mori H, Tanaka Y. Relationship between fluctuations in glucose levels measured by continuous glucose monitoring and vascular endothelial dysfunction in type 2 diabetes mellitus. Cardiovasc Diabetol. 2013;12(1):1. https://doi.org/10.1186/1475-2840-12-1.
Tamura Y, Kimbara Y, Yamaoka T, Sato K, Tsuboi Y, Kodera R, et al. White matter Hyperintensity in elderly patients with diabetes mellitus is associated with cognitive impairment, functional disability, and a high Glycoalbumin/Glycohemoglobin ratio. Front Aging Neurosci. 2017;9:220. https://doi.org/10.3389/fnagi.2017.00220.
Low A, Mak E, Malpetti M, Passamonti L, Nicastro N, Stefaniak JD, et al. In vivo neuroinflammation and cerebral small vessel disease in mild cognitive impairment and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2020;92(1):45–52. https://doi.org/10.1136/jnnp-2020-323894.
Johnson NF, Gold BT, Brown CA, Anggelis EF, Bailey AL, Clasey JL, et al. Endothelial function is associated with White matter microstructure and executive function in older adults. Front Aging Neurosci. 2017;9:255. https://doi.org/10.3389/fnagi.2017.00255.
Yan Y, Li S, Liu Y, Bazzano L, He J, Mi J, et al. Temporal relationship between inflammation and insulin resistance and their joint effect on hyperglycemia: the Bogalusa heart study. Cardiovasc Diabetol. 2019;18(1):109. https://doi.org/10.1186/s12933-019-0913-2.
Bahrami A, Zarghami N, Khajehali L. Association between C-reactive protein and HbA1C among patients with type 2 diabetes mellitus. Iran J Diabetes Lipid Disord. 2007;6(3):59–65.E32.
Seo YH, Shin HY. Relationship between hs-CRP and HbA1c in diabetes mellitus patients: 2015-2017 Korean National Health and nutrition examination survey. Chonnam Med J. 2021;57(1):62–7. https://doi.org/10.4068/cmj.2021.57.1.62.
Weuve J, Ridker PM, Cook NR, Buring JE, Grodstein F. High-sensitivity C-reactive protein and cognitive function in older women. Epidemiology. 2006;17(2):183–9. https://doi.org/10.1097/01.ede.0000198183.60572.c9.
Acknowledgements
The authors would like to thank the Health Promotion Administration and Health and Welfare Data Science Center, Ministry of Health and Welfare, Taiwan. The interpretation and conclusions of this research may not represent the opinion of the Health Promotion Administration and Health and Welfare Data Science Center, Ministry of Health and Welfare, Taiwan.
Funding
This work was funded by the Health Promotion Administration, Ministry of Health and Welfare (D1060103), and the Ministry of Science and Technology of Taiwan (MOST-103-2314-B038–033-MY3).
Author information
Authors and Affiliations
Contributions
YCF conceived the idea, performed the statistical analysis, and drafted the manuscript. CCC, BSB, and KLC contributed to providing clinical knowledge and reviewing the manuscript. CHB reviewed and revised the idea and study design, supported the grants, and helped edit the manuscript. All authors read and approved the final manuscript. YCF and CHB are the guarantors of this work and take responsibility for this study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was reviewed and approved by the Joint Institutional Review Board of Taipei Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fan, YC., Chou, CC., Bintoro, B.S. et al. High sensitivity C-reactive protein and glycated hemoglobin levels as dominant predictors of all-cause dementia: a nationwide population-based cohort study. Immun Ageing 19, 10 (2022). https://doi.org/10.1186/s12979-022-00265-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12979-022-00265-0