Abstract
Few years after HTLV-1 identification and isolation in humans, STLV-1, its simian counterpart, was discovered. It then became clear that STLV-1 is present almost in all simian species. Subsequent molecular epidemiology studies demonstrated that, apart from HTLV-1 subtype A, all human subtypes have a simian homolog. As HTLV-1, STLV-1 is the etiological agent of ATL, while no case of TSP/HAM has been described. Given its similarities with HTLV-1, STLV-1 represents a unique tool used for performing clinical studies, vaccine studies as well as basic science.
Similar content being viewed by others
Background
The first human oncogenic retrovirus was discovered in the USA, in a T cell line obtained from blood cells of a patient suffering from a disease then called “cutaneous T-cell lymphoma” [1, 2]. Few years earlier, Adult T-cell Leukemia/Lymphoma or ATLL (i.e. an aggressive malignancy of CD4+ T-cells) had been described in Japan [3, 4]. In 1982, Japanese researchers also reported the presence of a retrovirus among ATLL patients. They named it Adult T cell leukemia virus (ATLV). Further work demonstrated that HTLV-1 specific antibodies were present among Japanese ATLL patients, thus allowing identification of the first HTLV-1 endemic area [5]. Later, it was decided to name this virus HTLV-1 for Human T-cell Leukemia Virus type 1.
Few years later, Tropical Spastic Paraparesis/HTLV-1 associated myelopathy (TSP/HAM), a severe neuromyelopathy, was also identified as another disease caused by HTLV-1 [6]. Thus, ATLL and TSP/HAM are the main pathologies present among HTLV-1 infected individuals. It was recently estimated that 5 to 10 million people are infected by HTLV-1 worldwide, although HTLV-1 prevalence is likely to be underestimated. Two to 4% of HTLV-1 carriers will develop either ATLL or TSP/HAM, while most of them will remain asymptomatic [7]. HTLV-1 is endemic in areas such as Japan, central Africa, the Caribbean region and South America [8]. Because HTLV-1 mostly replicates through clonal expansion of infected cells even in asymptomatic carriers [9], its retroviral genome displays a remarkable genetic stability. HTLV-1 molecular epidemiology studies have been carried out throughout the world. The very low genetic variability allowed identification of different HTLV-1 subtypes. All but one of these subtypes, i.e. Cosmopolitan subtype A that is present all over the world, are specific to a given African or Asian region [8]. ATL cases were described in HTLV-1 carriers infected by HTLV-1 subtype A but also subtype B and subtype C [10, 11], thus suggesting that ATL occurrence is not linked to the most frequent HTLV-1 subtype. Of note, HTLV-1 subtype B and subtype C lack p12 and/or p30 auxiliary protein. Whether the lower ATL frequency in type B and C infected individuals is linked to the absence of these proteins remains to be determined.
In 1982, lymphocytes from a Japanese monkey (Macaca fuscata) were co-cultured with chronically and productively infected T-cells from the MT-2 cells, an HTLV-1-transformed cell line. This allowed the authors to obtain a simian cell line persistently infected by HTLV-1, thus suggesting that Japanese monkeys might be susceptible to HTLV-1 natural infection [12]. Later, seroepidemiological studies were performed in Japan and demonstrated that many Japanese monkeys were infected by HTLV-1-like viruses [13]. Sera from New World Monkeys (NWM), Old World Monkeys (OWM) and Apes were then tested and revealed the presence of antibodies reacting against HTLV-1 antigens. Such antibodies were detected in OWM and Apes, but not in NWM, suggesting endemicity of HTLV-1-related viruses in African and Asian monkeys, but not in American animals [14]. Sequence analyses characterized these viruses as Simian T-cell Leukemia Viruses (STLVs) [15, 16]. To date, it is well established that Old World Non-Human Primates (NHPs) and Apes are naturally infected with a great variety of STLV-1 viruses and that HTLV-1 appeared in Humans following STLV-1 cross-species transmission approximately 27,300 years ago (95% CI 19,100–35,500) in Africa, even if interspecies transmission episodes still occur [17,18,19]. Given the high degree of similarity between HTLV-1 and STLV-1 sequences, it was suggested to cluster these viruses in the single PTLV (Primate T lymphotropic virus) family [20,21,22]. Because STLV-1 induces ATLL in naturally infected NHPs [23, 24], and even if some auxiliary proteins are lacking [25], it represents a suitable tool that contributes to our understanding of HTLV-1 pathogenesis. This review will compare HTLV-1 and STLV-1 retroviruses from different aspects and will focus on the use of STLV-1 as a model of HTLV-1 infection.
STLV-1 epidemiology
Around 132 non-human primate species represent Old World Monkeys (OWM). They are divided in two subfamilies, Cercopithecinae and Colobinae, distributed in African and Asian continents [26].
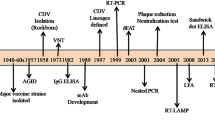
To determine which simian species carry STLV-1, seroepidemiological studies were performed using kits that had been previously developed for the detection of anti-HTLV-1 human antibodies, as well as by PCR (Fig. 1). Sera from Japanese monkeys were tested, and 25% scored seropositive. As in humans, STLV-1 incidence increased with age and was higher in females than males. Other species were tested later. A high seroprevalence was observed in African Green monkeys (AGM). Two studies then reported STLV-1 infection in captive Old World NHPs and Apes [27, 28]. Ishikawa et al. [29] performed an STLV-1 survey using 567 NHPs’ blood samples covering 30 species caught in the wild or kept in zoos, institutes or private owners from Kenya, Gabon, Ghana, Cameroon, Ethiopia and Indonesia. STLV-1 was detected in African Green monkeys and Sykes’ monkeys, in Olive baboons, Patas monkeys, Mandrills and Gorillas. STLV-1 was also found in different species of macaques from Indonesia, with a seroprevalence ranging from 11 to 25%. Other studies reported natural STLV-1 infections in AGM, Vervet monkeys and among baboon species (Papio anubis, Papio hamadryas, Papio papio and Papio cynocephalus) originating from South Africa and Ethiopia [30,31,32,33]. As in Japan, the infection status positively correlates with age, and disease incidence is higher in females than males. Other seroepidemiological studies were also performed [34,35,36,37,38,39,40,41,42,43,44] (Fig. 1). Thirty-one Old World NHP species were reported as naturally infected with STLV-1 [33, 45,46,47,48,49,50].
Epidemiology of Simian T-Leukemia Virus Type-1 in wild-caught or captive non-human primates (NHPs) from Asia and Africa. All studies which reported STLV-1 infection in NHPs are listed. Orange and purple colors represent Asian and African STLV-1 infected NHPs, respectively. Countries with both colors and hatching represent Asian and African NHPs hosted in geographical areas where they are not naturally present
STLV-1 sequence analyses were then performed in order to determine relationship between STLV-1 and HTLV-1 and whether HTLV-1 originated from a non-human primate virus.
STLV-1 phylogeny
Since the first publication of a complete HTLV-1 proviral genome [51], phylogenetic studies enabled to identify several HTLV-1 subtypes: Cosmopolitan subtype A, which is found all over the world; subtypes B, D, E, F, G, which are restricted to Central Africa; and Australo-Melanesian subtype C which is the most divergent HTLV-1 subtype [8]. Based on molecular clock and phylogenetic analyses, origin of HTLV-1 subtypes A, B, D, E was inferred in a time frame of 27,300 ± 8200 years, whereas subtype F arose more than 10,000 years ago.
In 1984, Watanabe et al. [52] demonstrated similarities between restriction maps obtained using HTLV-1 from Robert Gallo’s laboratory or using Japanese simian Adult T-cell Leukemia Virus (ATLV). These results suggested that HTLV-1 and simian ATLV shared a common ancestor. Other studies reported that HTLV-1 and STLV-1 from Japanese monkeys, Red-faced monkeys, Pig-tailed monkeys, AGM, Chimpanzees and baboons (Papio cynocephalus) had the same genomic organization i.e. LTR-gag-pol-env-pX-LTR [15, 20]. Sequence analyses comparing Pig-tailed (Asian NHP) and AGM (African NHP) STLV-1 sequences to HTLV-1 revealed 90% and 95% identity respectively. These results suggested that (1) STLV-1 could be separated into two subgroups: Asian and African and that (2) HTLV-1 originated from the African STLV-1 subgroup [16].
Phylogenetic studies revealed that HTLV-1 subtype B is very closely related to STLV-1 strains infecting chimpanzees (98% identity), Allen’s swamp monkeys (around 96% identity) and gorillas from Zaïre, Central African Republic and Cameroon [45, 53,54,55]. STLV-1 strains infecting Mandrillus sphinx, Cercopithecus cephus, C. agilis, C. pogonias, G. agilis and C. nictitans share close relationships with HTLV-1D and -F from Cameroon and Gabon [49, 56,57,58]. Regarding HTLV-1 subtype E, the Env region clusters with STLV-1 isolated from two baboon species, Papio ursinus and Papio cynocephalus [59]. No data has been so far reported about a simian counterpart of HTLV-1G and HTLV-1A. Altogether, the diversity of STLV-1 strains found in different NHPs species and related to a given HTLV-1 subtype from the same geographical areas is strongly supporting the concept of multiple cross-species transmissions between NHPs but also from NHPs to humans.
Most divergent STLV-1 strains were described in Asian Macaca tonkeana (living in Indonesia) and Macaca arctoides (living in India, Thailand and China) [60,61,62]. Macaca tonkeana virus is related to the most divergent HTLV-1 subtype C that is present in Melanesia and Australia. Molecular clock data inferred STLV-1 introduction around 156,000 to 269,000 years ago on the Asian continent [59]. These results suggest that macaque infection with STLV-1 might have led to the emergence of HTLV-1 in Asian human population.
Finally, Calvignac et al. [63] demonstrated that STLV-1 sequences could be amplified from bones samples originating from an early 20th century Chlorocebus pygerythrus sample. Therefore, it should now be possible to use this technique to determine STLV-1 virus evolution over time using available Egyptian or Asian NHP mummies.
STLV-1 interspecies transmission
Prevalence of HTLV-1 may reach 1 to 40% in adults depending on age, sex and geographic location [8]. It is well known that HTLV-1 can be transmitted under different routes: sexual, mother-to-child and contact with infected blood. However, STLV-1 transmission occurs mostly through aggressive contacts instead of mother to infant or sexual transmissions [64,65,66,67,68], even if sexual transmission of STLV-1 is more important in NHPs such as vervet [40].
STLV-1 associated-disease in naturally infected animals
As it is the case for HTLV-1-infected individuals, most STLV-1-infected monkeys remain lifelong asymptomatic hosts [69]. For some unexplained reasons, TSP/HAM cases have never been observed in infected NHPs, even when those animals were living in animal facilities for a long period. Phylogenetic studies performed using samples from an African human TSP/HAM patient showed that the viral sequence was highly related to an STLV-1 sequence obtained from asymptomatic West-African sooty mangabey [70]. Other strains obtained from HTLV-1 African TSP/HAM patients also clustered with STLV-1 strains obtained from asymptomatic animals [71, 72]. It is well established that there is no specific mutation in HTLV-1 genome that would be associated with a given disease. Altogether, these data suggest that the lack of TSP/HAM described cases in NHPs might only be linked to the mode of viral transmission rather than the age of infection.
On the contrary, a number of ATLL-like diseases sharing clinical and pathological features with human ATLL were reported in NHPs [24, 69, 73,74,75,76,77,78,79]. The first report was made in STLV-1 infected macaques which developed malignant lymphoma [80]. Subsequent studies reported similar symptoms in captive Papio anubis, Gorillas and AGM [75,76,77,78, 81, 82]. In a recent study, Tax-positive cells were detected in lymphoid and non-lymphoid organs, mesenteric and axillary lymph nodes and lung, but not in the blood from an infected Papio anubis suffering from ATL [24]. In that case, skin lesion biopsies also showed a massive dermal, hypodermic and muscular cell infiltrates of positive CD3+ CD25+ T cells, as described in human ATL.
Using STLV-1 infected animals
After natural STLV-1 infection
Given the high degree of sequence similarities between STLV-1 and HTLV-1 genomes and the fact that both viruses cause ATL, STLV-1 infected NHPs (Japanese macaques, Mandrillus sphinx and Papio anubis) have been used for performing molecular studies [79, 83,84,85,86,87,88,89] (Table 1). As HTLV-1, STLV-1 infection is mostly occurring in CD4+ T-cells, although STLV-1 Tax expression was also detected in bone marrow hematopoietic stem cells in vivo, and viral DNA was retrieved in all myeloid and lymphoid cells derived from these infected progenitors [86].
STLV-1 natural infection leads to Tax and SBZ (simian equivalent of HBZ) expression. Simian SBZ and Tax amino-acid sequences are highly similar to human HBZ and Tax (see Tables 2 and 3). These viral proteins also display activating properties on viral LTR and NF-κB signaling pathways. As an example, a high STLV-1 proviral load (PVL) is linked to IL-2, IL-6, IL-10, IFNγ and TNF-α elevated expression in asymptomatic STLV-1-infected Mandrillus sphinx [90]. Given well-established results published in the HTLV-1 situation, this is likely due to STLV-1 Tax expression, although this hypothesis has not been formally demonstrated. IL-2 and IFNγ results were also obtained in asymptomatic STLV-1-positive Macaca mulatta [87], while anti IFNγ and TNF-α responses against Tax expressing cells were also observed in STLV-1 infected baboons [85]. STLV-1 infection also promotes CTL response against STLV-1 Tax protein [84, 85].
Interestingly, TCF1 and LEF1, two T-cell specific proteins, prevent Tax effect on viral LTR. Their expression is high in thymocytes and thus counteract STLV-1 replication in thymus. On the opposite, their expression and thus their effect is down-regulated in peripheral blood T-cells (both in human and simian cells), thanks to a Tax effect on STAT5a. This might explain why Tax is more potent in these cells, and why HTLV-1 induces ATL in the periphery [83].
Depending upon STLV-1 strain, SBZ protein sequence is highly similar or contain insertions and deletion compared to HBZ (see Table 2). Nevertheless, in both cases, animals can develop ATL [24, 79]. This might be due to conservation of the N-terminal region as well as of C-terminus basic leucin zipper domain between human and simian viral proteins.
As its human counterpart, STLV-1 replication occurs through clonal expansion of infected cells, both in asymptomatic and ATL animals [24, 79]. Antiviral therapy based on the use of azidothymidine (AZT) combined with interferon-α (IFN-α) improves the survival rate of ATL patients suffering from acute and chronic/smoldering forms. A confirmation clinical trial using these compounds was reported in an STLV-1 infected Papio anubis suffering from ATL. The animal was treated with a combination of AZT and interferon-α. However, and contrary to human ATL, no clinical improvement was observed. It would now be interesting to determine post-mortem whether, this absence of remission was linked to p53 mutation already present when treatment started as shown in human ATL cases who were not responding to AZT [91].
Given the fact that treating ATL patients is difficult, and because an elevated PVL is a characteristic of ATL, a study tested whether PVL decreases when valproate and AZT were delivered to asymptomatic STLV-1-infected animals [92]. This was indeed the case and it was associated to an increased anti-Tax CTL response, thus confirming the importance of immune response for controlling viral infection [92]. In another study, STLV-1 infected asymptomatic Japanese monkey were inoculated with mogamulizumab (anti-CCR4), a component that is also used for human relapsed ATL cases. This led to a strong reduction of STLV-1 proviral load [79, 89]. Altogether, these results support the fact that STLV-1 infected animals represent a useful tool for testing drugs.
Finally, a recent study was performed in two asymptomatic STLV-1-infected animals. This showed that immunization using recombinant vaccinia viruses expressing either Tax-22 (which cannot activate the NF-kB pathway) or an HBZ LL/AA mutant (which is partially impaired for blocking Tax ability to induce transcription) was linked to a temporary decrease of STLV-1 PVL [89].
After STLV-1 interspecies transmission
A limited number of reports described STLV-1 inter-simian species transmission [32, 53, 93, 94] (Table 1). In one report and following an unknown mode of transmission, it was shown that baboons accidentally infected with a rhesus macaque STLV-1 virus, developed leukemia/lymphoma at a high frequency [93]. This is the only reported case suggesting that inter-simian species transmission might impact viral pathogenesis. Experimental infection of pig-tailed macaques with sooty mangabey STLV-1 was also tested. Animals maintained low antibody titers and displayed a high mortality rate without any identified cause [95]. Finally, another work reported tantalus and patas animals artificially infected with STLV-1 from other species. All animals became infected, as shown by PCR results, even if one stayed seronegative due to mutations in the genome [94]. Why were these pol mutant viruses still able to infect animals remains unexplained.
After artificial HTLV-1 infection
Finally, given the high degree of similarity between HTLV-1 and STLV-1 genomes and the abundance of molecular tools available in the HTLV-1 field, some laboratories decided to use the HTLV-1 molecular clone or HTLV-1 infected cells to perform studies in non-human primates (Table 1). Artificial infection after inoculation of HTLV-1 to primates provides an inestimable tool to study primo-infection and viral dissemination, in vivo, a process that is inaccessible in humans. HTLV-1 infection of Saimiri sciureus, i.e. non-human primates that are not naturally infected with STLV-1 [96], demonstrated that lymphoid organs represent the major viral reservoir [97]. As in HTLV-1 infected humans and STLV-1 naturally-infected animals, IL-2, IL-10, IFNγ levels also increased after HTLV-1 infection [98]. In Saimiri sciureus, the virus also replicates through clonal expansion after having used reverse transcription (RT) at the initial stages [99] and it causes ATL [100]. As in baboons treated with AZT/IFN [24], arsenic combined to IFN-α was not able to lead to HTLV-1 proviral load reduction, even if the number of circulating ATL flower cells decreased for some unexplained reason [101].
Studies were also performed in pig-tailed and rhesus macaques inoculated with autologous cells previously transfected with the HTLV-1 ACH molecular clone [102,103,104]. Following infection with wild-type HTLV-1, pig-tailed macaques developed a series of extremely aggressive diseases that were different from ATL. These results therefore suggest that this animal model cannot be used for studying events that are resulting from HTLV-1 infection.
Consequences of rhesus macaque infection with the same molecular clone were different since animals remained asymptomatic. HTLV-1 p12 and p8 proteins have been shown previously to increase NFAT activity, IL-2 production and STAT-5 activity, while p30 controls viral expression at the post-transcriptional level in vitro (for a review, see [105, 106]). Thus, this simian model was useful to investigate the role of p12, p13, and p30 auxiliary proteins in vivo [102, 103]. This allowed researchers to show that p12 and p30 are required for allowing HTLV-1 presence and replication in dendritic cells [103], while p12 and p8 are necessary for allowing a viral resistance to CTL responses. These studies provided the first in vivo evidence on the mechanisms that HTLV-1 uses to establish chronic infection and on the crucial role of myeloid cells in that process.
Interestingly, the authors also demonstrated that the results obtained in rhesus macaques were different from those obtained in rabbits infected with the same viral clones, thus reinforcing the fact that NHPs are the more relevant system for studying HTLV-1 pathogenesis.
PTLV retroviral coinfection in NHPs and in humans
In addition to STLV-1, other retroviruses, i.e. Simian Immunodeficiency Virus (SIV) and Simian Foamy Virus (SFV) infect NHPs. Cases of natural coinfection have been reported both in humans and in NHPs: HTLV-1/HIV-1, HTLV-1/HFV, STLV-1/SFV or STLV-1/SIV-1 [67, 107,108,109,110,111,112,113,114,115]. HIV-1/HTLV-1 coinfection leads to significant increase of HTLV-1 PVL as well as on a possible delay in HIV-1 pathogenesis in humans [107, 108, 116]. Anti-HIV-1 therapy promotes an increase in HTLV-1 PVL in HIV-1/HTLV-1 coinfected carriers. These results strongly suggest that both retroviruses compete for CD4+ T-cell infection. However, it is worth noting that opposite results were obtained in other studies [117,118,119,120,121].
Natural STLV-1/SIV-1 co-infection induces the development of a neoplastic disease in sooty mangabey [122] and of a lymphoproliferative disease in AGM [123]. Souquière et al. described pathological manifestations, i.e. infective dermatitis and scabies, in two STLV-1/SIV-1 co-infected mandrills [111], while no clinical sign has been reported previously in STLV-1 naturally infected mandrills [90]. Thus, these symptoms could be due to co-infection. Ongoing experiments should allow us to determine whether STLV-1 clonal expansion impacts SIV replication in vivo.
Finally, blood SFV proviral load from STLV-1/SFV naturally co-infected Papio anubis, was recently shown to be much higher compared to SFV mono-infected animals [124]. These results either suggest that cells might be co-infected with both retroviruses, with STLV-1 promoting clonal expansion, or that soluble STLV-1 Tax transactivator enters SFV-infected cells where it promotes viral replication. Ongoing experiments should allow us to answer this question.
Altogether, these data demonstrate that STLV-1 is a useful tool to understand mechanisms of HTLV-1 transmission and ATL pathogenesis. PTLV-1 mono-infected as well as SIV co-infected animals could also be used to develop possible new anti-HTLV-1 clinical approaches and to modify anti-HIV treatment.
Availability of data and materials
Not applicable.
References
Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–9.
Poiesz BJ, Ruscetti FW, Reitz MS, Kalyanaraman VS, Gallo RC. Isolation of a new type C retrovirus (HTLV) in primary uncultured cells of a patient with Sézary T-cell leukaemia. Nature. 1981;294:268–71.
Takatsuki K, Uchiyama T, Sagawa K. Hattori T [Lymphoma and immunoglobulin abnormalities, with special reference to M proteinemia]. Nippon Rinsho. 1977;35:3757–67.
Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481–92.
Gallo RC. History of the discoveries of the first human retroviruses: HTLV-1 and HTLV-2. Oncogene. 2005;24:5926–30.
Gessain A, Francis H, Sonan T, Giordano C, Akani F, Piquemal M, et al. HTLV-I and tropical spastic paraparesis in Africa. Lancet. 1986;2:698.
Futsch N, Mahieux R, Dutartre H. HTLV-1, the other pathogenic yet neglected human retrovirus: from transmission to therapeutic treatment. Viruses. 2017;10:1.
Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol. 2012;3:388.
Wattel E, Vartanian JP, Pannetier C, Wain-Hobson S. Clonal expansion of human T-cell leukemia virus type I-infected cells in asymptomatic and symptomatic carriers without malignancy. J Virol. 1995;69:2863–8.
Mahieux R, Ibrahim F, Mauclere P, Herve V, Michel P, Tekaia F, et al. Molecular epidemiology of 58 new African human T-cell leukemia virus type 1 (HTLV-1) strains: identification of a new and distinct HTLV-1 molecular subtype in Central Africa and in pygmies. J Virol. 1997;71:17.
Einsiedel L, Cassar O, Bardy P, Kearney D, Gessain A. Variant human T-cell lymphotropic virus type 1c and adult T-cell leukemia, Australia. Emerg Infect Dis. 2013;19:1639–41.
Miyoshi I, Taguchi H, Fujishita M, Yoshimoto S, Kubonishi I, Ohtsuki Y, et al. Transformation of monkey lymphocytes with adult T-cell leukaemia virus. Lancet. 1982;1:1016.
Miyoshi I, Yoshimoto S, Fujishita M, Taguchi H, Kubonishi I, Niiya K, et al. Natural adult T-cell leukemia virus infection in Japanese monkeys. Lancet. 1982;2:658.
Hayami M, Komuro A, Nozawa K, Shotake T, Ishikawa K, Yamamoto K, et al. Prevalence of antibody to adult T-cell leukemia virus-associated antigens (ATLA) in Japanese monkeys and other non-human primates. Int J Cancer. 1984;33:179–83.
Komuro A, Watanabe T, Miyoshi I, Hayami M, Tsujimoto H, Seiki M, et al. Detection and characterization of simian retroviruses homologous to human T-cell leukemia virus type I. Virology. 1984;138:373–8.
Watanabe T, Seiki M, Hirayama Y, Yoshida M. Human T-cell leukemia virus type I is a member of the African subtype of simian viruses (STLV). Virology. 1986;148:385–8.
Mossoun A, Calvignac-Spencer S, Anoh AE, Pauly MS, Driscoll DA, Michel AO, et al. Bushmeat hunting and zoonotic transmission of simian T-lymphotropic virus 1 in tropical West and Central Africa. J Virol. 2017;91:e02479-16.
Kazanji M, Mouinga-Ondémé A, Lekana-Douki-Etenna S, Caron M, Makuwa M, Mahieux R, et al. Origin of HTLV-1 in hunters of nonhuman primates in Central Africa. J Infect Dis. 2015;211:361–5.
Filippone C, Betsem E, Tortevoye P, Cassar O, Bassot S, Froment A, et al. A severe bite from a nonhuman primate is a major risk factor for HTLV-1 infection in hunters from Central Africa. Clin Infect Dis. 2015;60:1667–76.
Guo HG, Wong-Stall F, Gallo RC. Novel viral sequences related to human T-cell leukemia virus in T cells of a seropositive baboon. Science. 1984;223:1195–7.
Watanabe T, Seiki M, Tsujimoto H, Miyoshi I, Hayami M, Yoshida M. Sequence homology of the simian retrovirus genome with human T-cell leukemia virus type I. Virology. 1985;144:59–65.
Sherman MP, Saksena NK, Dube DK, Yanagihara R, Poiesz BJ. Evolutionary insights on the origin of human T-cell lymphoma/leukemia virus type I (HTLV-I) derived from sequence analysis of a new HTLV-I variant from Papua New Guinea. J Virol. 1992;66:2556–63.
Locatelli S, Peeters M. Cross-species transmission of simian retroviruses: how and why they could lead to the emergence of new diseases in the human population. AIDS. 2012;26:659–73.
Turpin J, Alais S, Marçais A, Bruneau J, Melamed A, Gadot N, et al. Whole body clonality analysis in an aggressive STLV-1 associated leukemia (ATLL) reveals an unexpected clonal complexity. Cancer Lett. 2017;389:78–85.
Afonso PV, Fagrouch Z, Deijs M, Niphuis H, Bogers W, Gessain A, et al. Absence of accessory genes in a divergent simian T-lymphotropic virus type 1 isolated from a bonnet macaque (Macaca radiata). PLoS Negl Trop Dis. 2019;13:e0007521.
Lawrence JM, Cords M. Old World Monkeys. Nat Educ Knowl. 2012;3(7):13.
Lowenstine LJ, Pedersen NC, Higgins J, Pallis KC, Uyeda A, Marx P, et al. Seroepidemiologic survey of captive Old-World primates for antibodies to human and simian retroviruses, and isolation of a lentivirus from sooty mangabeys (Cercocebus atys). Int J Cancer. 1986;38:563–74.
Hunsmann G, Schneider J, Schmitt J, Yamamoto N. Detection of serum antibodies to adult T-cell leukemia virus in non-human primates and in people from Africa. Int J Cancer. 1983;32:329–32.
Ishikawa K, Fukasawa M, Tsujimoto H, Else JG, Isahakia M, Ubhi NK, et al. Serological survey and virus isolation of simian T-cell leukemia/T-lymphotropic virus type I (STLV-I) in non-human primates in their native countries. Int J Cancer. 1987;40:233–9.
Becker WB, Becker ML, Homma T, Brede HD, Kurth R. Serum antibodies to human T-cell leukaemia virus type I in different ethnic groups and in non-human primates in South Africa. S Afr Med J. 1985;67:445–9.
Ishida T, Yamamoto K, Shotake T, Nozawa K, Hayami M, Hinuma Y. A field study of infection with human T-cell leukemia virus among African primates. Microbiol Immunol. 1986;30:315–21.
Voevodin A, Samilchuk E, Allan J, Rogers J, Broussard S. Simian T-lymphotropic virus type 1 (STLV-1) infection in wild yellow baboons (Papio hamadryas cynocephalus) from Mikumi National Park, Tanzania. Virology. 1997;228:350–9.
Moné J, Whitehead E, Leland M, Hubbard G, Allan JS. Simian T-cell leukemia virus type I infection in captive baboons. AIDS Res Hum Retroviruses. 1992;8:1653–61.
Hayami M, Ishikawa K, Komuro A, Kawamoto Y, Nozawa K, Yamamoto K, et al. ATLV antibody in cynomolgus monkeys in the wild. Lancet. 1983;2:620.
Yamamoto N, Hinuma Y, zur Hausen H, Schneider J, Hunsmann G. African green monkeys are infected with adult T-cell leukaemia virus or closely related agent. Lancet. 1983;1:240–1.
Yamamoto N, Okada M, Hinuma Y, Hirsch FW, Chosa T, Schneider J, et al. Human adult T-cell leukaemia virus is distinct from a similar isolate of Japanese monkeys. J Gen Virol. 1984;65(Pt 12):2259–64.
Yamamoto N, Kobayashi N, Takeuchi K, Koyanagi Y, Hatanaka M, Hinuma Y, et al. Characterization of African green monkey B-cell lines releasing an adult T-cell leukemia-virus-related agent. Int J Cancer. 1984;34:77–82.
Botha MC, Jones M, de Klerk WA, Yamamoto N. Spread and distribution of human T-cell leukaemia virus type I-reactive antibody among baboons and monkeys in the northern and eastern Transvaal. S Afr Med J. 1985;67:665–8.
Coursaget P, Barres JL, Yvonnet B, Chiron JP, Cornet M, Ferrara L, et al. Antibodies to human T-cell leukemia virus (HTLV-1) in non human primates from Senegal. Biomed Pharmacother. 1985;39:198–9.
Dracopoli NC, Turner TR, Else JG, Jolly CJ, Anthony R, Gallo RC, et al. STLV-I antibodies in feral populations of East African vervet monkeys (Cercopithecus aethiops). Int J Cancer. 1986;38:523–9.
Daniel MD, Letvin NL, Sehgal PK, Schmidt DK, Silva DP, Solomon KR, et al. Prevalence of antibodies to 3 retroviruses in a captive colony of macaque monkeys. Int J Cancer. 1988;41:601–8.
Fultz PN, Gordon TP, Anderson DC, McClure HM. Prevalence of natural infection with simian immunodeficiency virus and simian T-cell leukemia virus type I in a breeding colony of sooty mangabey monkeys. AIDS. 1990;4:619–25.
Estaquier J, Peeters M, Bedjabaga L, Honoré C, Bussi P, Dixson A, et al. Prevalence and transmission of simian immunodeficiency virus and simian T-cell leukemia virus in a semi-free-range breeding colony of mandrills in Gabon. AIDS. 1991;5:1385–6.
Courgnaud V, Van Dooren S, Liegeois F, Pourrut X, Abela B, Loul S, et al. Simian T-cell leukemia virus (STLV) infection in wild primate populations in Cameroon: evidence for dual STLV type 1 and type 3 infection in agile mangabeys (Cercocebus agilis). J Virol. 2004;78:4700–9.
Koralnik IJ, Boeri E, Saxinger WC, Monico AL, Fullen J, Gessain A, et al. Phylogenetic associations of human and simian T-cell leukemia/lymphotropic virus type I strains: evidence for interspecies transmission. J Virol. 1994;68:2693–707.
Mahieux R, Pecon-Slattery J, Chen GM, Gessain A. Evolutionary inferences of novel simian T lymphotropic virus type 1 from Wild-Caught Chacma (Papio ursinus) and olive baboons (Papio anubis). Virology. 1998;251:71–84.
Saksena NK, Herve V, Durand JP, Leguenno B, Diop OM, Digouette JP, et al. Seroepidemiologic, molecular, and phylogenetic analyses of simian T-cell leukemia viruses (STLV-I) from various naturally infected monkey species from central and western Africa. Virology. 1994;198:297–310.
Schatzl H, Yakovleva L, Lapin B, Rose D, Inzhiia L, Gaedigk-Nitschko K, et al. Detection and characterization of T-cell leukemia virus-like proviral sequences in PBL and tissues of baboons by PCR. Leukemia. 1992;6(Suppl 3):158S–60S.
Sintasath DM, Wolfe ND, LeBreton M, Jia H, Garcia AD, Diffo JLD, et al. Simian T-lymphotropic virus diversity among nonhuman primates, Cameroon. Emerg Infect Dis. 2009;15:175–84.
Van Dooren S, Verschoor EJ, Fagrouch Z, Vandamme A-M. Phylogeny of primate T lymphotropic virus type 1 (PTLV-1) including various new Asian and African non-human primate strains. Infect Genet Evol. 2007;7:374–81.
Seiki M, Hattori S, Hirayama Y, Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci USA. 1983;80:3618–22.
Watanabe T, Seiki M, Yoshida M. HTLV type I (U. S. isolate) and ATLV (Japanese isolate) are the same species of human retrovirus. Virology. 1984;133:238–41.
Voevodin AF, Johnson BK, Samilchuk EI, Stone GA, Druilhet R, Greer WJ, et al. Phylogenetic analysis of simian T-lymphotropic virus type I (STLV-I) in common chimpanzees (Pan troglodytes): evidence for interspecies transmission of the virus between chimpanzees and humans in Central Africa. Virology. 1997;238:212–20.
Meertens L, Rigoulet J, Mauclère P, Van Beveren M, Chen GM, Diop O, et al. Molecular and phylogenetic analyses of 16 novel simian T cell leukemia virus type 1 from Africa: close relationship of STLV-1 from Allenopithecus nigroviridis to HTLV-1 subtype B strains. Virology. 2001;287:275–85.
Ayouba A, Michem A, Peeters M, Vercammen F. Full-genome characterization of simian T-cell leukemia virus type 1 subtype b from a wild-born captive gorilla gorilla gorilla with T-cell lymphoma. Genome Announc. 2017;5:e01117-17.
Mahieux R, Chappey C, Georges-Courbot MC, Dubreuil G, Mauclere P, Georges A, et al. Simian T-cell lymphotropic virus type 1 from Mandrillus sphinx as a simian counterpart of human T-cell lymphotropic virus type 1 subtype D. J Virol. 1998;72:10316–22.
Makuwa M, Souquière S, Clifford SL, Telfer PT, Sallé B, Bourry O, et al. Two distinct STLV-1 subtypes infecting Mandrillus sphinx follow the geographic distribution of their hosts. AIDS Res Hum Retroviruses. 2004;20:1137–43.
Liégeois F, Lafay B, Switzer WM, Locatelli S, Mpoudi-Ngolé E, Loul S, et al. Identification and molecular characterization of new STLV-1 and STLV-3 strains in wild-caught nonhuman primates in Cameroon. Virology. 2008;371:405–17.
Van Dooren S, Salemi M, Vandamme AM. Dating the origin of the African human T-cell lymphotropic virus type-i (HTLV-I) subtypes. Mol Biol Evol. 2001;18:661–71.
Mahieux R, Pecon-Slattery J, Gessain A. Molecular characterization and phylogenetic analyses of a new, highly divergent simian T-cell lymphotropic virus type 1 (STLV-1marc1) in Macaca arctoides. J Virol. 1997;71:6253–8.
Ibrahim F, de Thé G, Gessain A. Isolation and characterization of a new simian T-cell leukemia virus type 1 from naturally infected celebes macaques (Macaca tonkeana): complete nucleotide sequence and phylogenetic relationship with the Australo-Melanesian human T-cell leukemia virus type 1. J Virol. 1995;69:6980–93.
Van Dooren S, Meertens L, Lemey P, Gessain A, Vandamme A-M. Full-genome analysis of a highly divergent simian T-cell lymphotropic virus type 1 strain in Macaca arctoides. J Gen Virol. 2005;86:1953–9.
Calvignac S, Terme J-M, Hensley SM, Jalinot P, Greenwood AD, Hänni C. Ancient DNA identification of early 20th century simian T-cell leukemia virus type 1. Mol Biol Evol. 2008;25:1093–8.
Niphuis H, Verschoor EJ, Bontjer I, Peeters M, Heeney JL. Reduced transmission and prevalence of simian T-cell lymphotropic virus in a closed breeding colony of chimpanzees (Pan troglodytes verus). J Gen Virol. 2003;84:615–20.
Leendertz FH, Junglen S, Boesch C, Formenty P, Couacy-Hymann E, Courgnaud V, et al. High variety of different simian T-cell leukemia virus type 1 strains in chimpanzees (Pan troglodytes verus) of the Taï National Park, Côte d’Ivoire. J Virol. 2004;78:4352–6.
d’Offay JM, Eberle R, Sucol Y, Schoelkopf L, White MA, Valentine BD, et al. Transmission dynamics of simian T-lymphotropic virus type 1 (STLV1) in a baboon breeding colony: predominance of female-to-female transmission. Comp Med. 2007;57:105–14.
Nerrienet E, Amouretti X, Müller-Trutwin MC, Poaty-Mavoungou V, Bedjebaga I, Nguyen HT, et al. Phylogenetic analysis of SIV and STLV type I in mandrills (Mandrillus sphinx): indications that intracolony transmissions are predominantly the result of male-to-male aggressive contacts. AIDS Res Hum Retroviruses. 1998;14:785–96.
Roussel M, Pontier D, Ngoubangoye B, Kazanji M, Verrier D, Fouchet D. Modes of transmission of simian T-lymphotropic virus type 1 in semi-captive mandrills (Mandrillus sphinx). Vet Microbiol. 2015;179:155–61.
Allan JS, Leland M, Broussard S, Mone J, Hubbard G. Simian T-cell lymphotropic viruses (STLVs) and lymphomas in african nonhuman primates. Cancer Invest. 2001;19:383–95.
Enose-Akahata Y, Caruso B, Haner B, Charlip E, Nair G, Massoud R, et al. Development of neurologic diseases in a patient with primate T lymphotropic virus type 1 (PTLV-1). Retrovirology. 2016;13:56.
Salemi M, Van Dooren S, Audenaert E, Delaporte E, Goubau P, Desmyter J, et al. Two new human T-lymphotropic virus type I phylogenetic subtypes in seroindeterminates, a Mbuti pygmy and a Gabonese, have closest relatives among African STLV-I strains. Virology. 1998;246:277–87.
Touzé E, Gessain A, Lyon-Caen O, Gout O. Tropical spastic paraparesis/HTLV-I-associated myelopathy in Europe and in Africa: clinical and epidemiologic aspects. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13(Suppl 1):S38–45.
Noda Y, Ishikawa K, Sasagawa A, Honjo S, Mori S, Tsujimoto H, et al. Hematologic abnormalities similar to the preleukemic state of adult T-cell leukemia in African green monkeys naturally infected with simian T-cell leukemia virus. Jpn J Cancer Res. 1986;77:1227–34.
Sakakibara I, Sugimoto Y, Sasagawa A, Honjo S, Tsujimoto H, Nakamura H, et al. Spontaneous malignant lymphoma in an African green monkey naturally infected with simian T-lymphotropic virus (STLV). J Med Primatol. 1986;15:311–8.
Tsujimoto H, Noda Y, Ishikawa K, Nakamura H, Fukasawa M, Sakakibara I, et al. Development of adult T-cell leukemia-like disease in African green monkey associated with clonal integration of simian T-cell leukemia virus type I. Cancer Res. 1987;47:269–74.
McCarthy TJ, Kennedy JL, Blakeslee JR, Bennett BT. Spontaneous malignant lymphoma and leukemia in a simian T-lymphotropic virus type I (STLV-I) antibody positive olive baboon. Lab Anim Sci. 1990;40:79–81.
Hubbard GB, Moné JP, Allan JS, Davis KJ, Leland MM, Banks PM, et al. Spontaneously generated non-Hodgkin’s lymphoma in twenty-seven simian T-cell leukemia virus type 1 antibody-positive baboons (Papio species). Lab Anim Sci. 1993;43:301–9.
d’Offay JM, Eberle R, Wolf RF, Kosanke SD, Doocy KR, Ayalew S, et al. Simian T-lymphotropic Virus-associated lymphoma in 2 naturally infected baboons: T-cell clonal expansion and immune response during tumor development. Comp Med. 2013;63:288–94.
Miura M, Yasunaga J, Tanabe J, Sugata K, Zhao T, Ma G, et al. Characterization of simian T-cell leukemia virus type 1 in naturally infected Japanese macaques as a model of HTLV-1 infection. Retrovirology. 2013;10:118.
Homma T, Kanki PJ, King NW, Hunt RD, O’Connell MJ, Letvin NL, et al. Lymphoma in macaques: association with virus of human T lymphotrophic family. Science. 1984;225:716–8.
Srivastava BI, Wong-Staal F, Getchell JP. Human T-cell leukemia virus I provirus and antibodies in a captive gorilla with non-Hodgkin’s lymphoma. Cancer Res. 1986;46:4756–8.
Voevodin AF, Lapin BA, Yakovleva LA, Ponomaryeva TI, Oganyan TE, Razmadze EN. Antibodies reacting with human T-lymphotropic retrovirus (HTLV-I) or related antigens in lymphomatous and healthy hamadryas baboons. Int J Cancer. 1985;36:579–84.
Ma G, Yasunaga J, Akari H, Matsuoka M. TCF1 and LEF1 act as T-cell intrinsic HTLV-1 antagonists by targeting Tax. Proc Natl Acad Sci USA. 2015;112:2216–21.
Castro I, Giret TM, Magnani DM, Maxwell HS, Umland O, Perry JK, et al. Cellular immune responses against simian T-lymphotropic virus type 1 target tax in infected baboons. J Virol. 2016;90:5280–91.
Termini JM, Magnani DM, Maxwell HS, Lauer W, Castro I, Pecotte J, et al. Simian T lymphotropic virus 1 infection of Papio anubis: tax sequence heterogeneity and T cell recognition. J Virol. 2017;91:e00950-17.
Furuta R, Yasunaga J-I, Miura M, Sugata K, Saito A, Akari H, et al. Human T-cell leukemia virus type 1 infects multiple lineage hematopoietic cells in vivo. PLoS Pathog. 2017;13:e1006722.
Yee JL, Montiel NA, Ardeshir A, Ardeshr A, Lerche NW. Constitutive release of IFNγ and IL2 from peripheral blood mononuclear cells of rhesus macaques (Macaca mulatta) infected with simian T-lymphotropic virus type 1. Comp Med. 2013;63:508–14.
Souquière S, Mouinga-Ondemé A, Makuwa M, Hermine O, Kazanji M. Dynamic interaction between STLV-1 proviral load and T-cell response during chronic infection and after immunosuppression in non-human primates. PLoS ONE. 2009;4:e6050.
Sugata K, Yasunaga J-I, Miura M, Akari H, Utsunomiya A, Nosaka K, et al. Enhancement of anti-STLV-1/HTLV-1 immune responses through multimodal effects of anti-CCR4 antibody. Sci Rep. 2016;6:27150.
Souquière S, Mouinga-Ondeme A, Makuwa M, Beggio P, Radaelli A, De Giuli Morghen C, et al. T-cell tropism of simian T-cell leukaemia virus type 1 and cytokine profiles in relation to proviral load and immunological changes during chronic infection of naturally infected mandrills (Mandrillus sphinx). J Med Primatol. 2009;38:279–89.
Datta A, Bellon M, Sinha-Datta U, Bazarbachi A, Lepelletier Y, Canioni D, et al. Persistent inhibition of telomerase reprograms adult T-cell leukemia to p53-dependent senescence. Blood. 2006;108:1021–9.
Afonso PV, Mekaouche M, Mortreux F, Toulza F, Moriceau A, Wattel E, et al. Highly active antiretroviral treatment against STLV-1 infection combining reverse transcriptase and HDAC inhibitors. Blood. 2010;116:3802–8.
Voevodin A, Samilchuk E, Schätzl H, Boeri E, Franchini G. Interspecies transmission of macaque simian T-cell leukemia/lymphoma virus type 1 in baboons resulted in an outbreak of malignant lymphoma. J Virol. 1996;70:1633–9.
Dube S, Saksena N, Spicer T, Healey J, Benz P, Dube DK, et al. Delayed seroconversion to STLV-1 infection is associated with mutations in the pol and rex genes. Virol J. 2013;10:282.
McGinn TM, Tao B, Cartner S, Schoeb T, Davis I, Ratner L, et al. Association of primate T-cell lymphotropic virus infection of pig-tailed macaques with high mortality. Virology. 2002;304:364–78.
Kazanji M, Moreau JP, Mahieux R, Bonnemains B, Bomford R, Gessain A, et al. HTLV-I infection in squirrel monkeys (Saïmiri sciureus) using autologous, homologous, or heterologous HTLV-I-transformed cell lines. Virology. 1997;231:258–66.
Kazanji M, Ureta-Vidal A, Ozden S, Tangy F, de Thoisy B, Fiette L, et al. Lymphoid organs as a major reservoir for human T-cell leukemia virus type 1 in experimentally infected squirrel monkeys (Saimiri sciureus): provirus expression, persistence, and humoral and cellular immune responses. J Virol. 2000;74:4860–7.
Kazanji M, Heraud J-M, Merien F, Pique C, de Thé G, Gessain A, et al. Chimeric peptide vaccine composed of B- and T-cell epitopes of human T-cell leukemia virus type 1 induces humoral and cellular immune responses and reduces the proviral load in immunized squirrel monkeys (Saimiri sciureus). J Gen Virol. 2006;87:1331–7.
Mortreux F, Kazanji M, Gabet AS, de Thoisy B, Wattel E. Two-step nature of human T-cell leukemia virus type 1 replication in experimentally infected squirrel monkeys (Saimiri sciureus). J Virol. 2001;75:1083–9.
Debacq C, Héraud J-M, Asquith B, Bangham C, Merien F, Moules V, et al. Reduced cell turnover in lymphocytic monkeys infected by human T-lymphotropic virus type 1. Oncogene. 2005;24:7514–23.
Heraud JM, Mortreux F, Merien F, Contamin H, Mahieux R, Pouliquen JF, et al. The efficacy of combined therapy of arsenic trioxide and alpha interferon in human T-cell leukemia virus type-1-infected squirrel monkeys (Saimiri sciureus). Antiviral Res. 2006;70:132–9.
Pise-Masison CA, de Castro-Amarante MF, Enose-Akahata Y, Buchmann RC, Fenizia C, Washington Parks R, et al. Co-dependence of HTLV-1 p12 and p8 functions in virus persistence. PLoS Pathog. 2014;10:e1004454.
Valeri VW, Hryniewicz A, Andresen V, Jones K, Fenizia C, Bialuk I, et al. Requirement of the human T-cell leukemia virus p12 and p30 products for infectivity of human dendritic cells and macaques but not rabbits. Blood. 2010;116:3809–17.
McGinn TM, Wei Q, Stallworth J, Fultz PN. Immune responses to HTLV-I(ACH) during acute infection of pig-tailed macaques. AIDS Res Hum Retroviruses. 2004;20:443–56.
Bai XT, Nicot C. Overview on HTLV-1 p12, p8, p30, p13: accomplices in persistent infection and viral pathogenesis. Front Microbiol. 2012;3:400.
Anupam R, Doueiri R, Green PL. The need to accessorize: molecular roles of HTLV-1 p30 and HTLV-2 p28 accessory proteins in the viral life cycle. Front Microbiol. 2013;4:275.
Beilke MA, Theall KP, O’Brien M, Clayton JL, Benjamin SM, Winsor EL, et al. Clinical outcomes and disease progression among patients coinfected with HIV and human T lymphotropic virus types 1 and 2. Clin Infect Dis. 2004;39:256–63.
Oo Z, Barrios CS, Castillo L, Beilke MA. High levels of CC-chemokine expression and downregulated levels of CCR5 during HIV-1/HTLV-1 and HIV-1/HTLV-2 coinfections: CC-chemokines and CCR5 levels during HIV/HTLV coinfections. J Med Virol. 2015;87:790–7.
Switzer WM, Garcia AD, Yang C, Wright A, Kalish ML, Folks TM, et al. Coinfection with HIV-1 and simian foamy virus in West Central Africans. J Infect Dis. 2008;197:1389–93.
Switzer WM, Tang S, Zheng H, Shankar A, Sprinkle PS, Sullivan V, et al. Dual simian foamy virus/human immunodeficiency virus type 1 infections in persons from Côte d’Ivoire. PLoS ONE. 2016;11:e0157709.
Souquière S, Makuwa M, Sallé B, Lepelletier Y, Mortreux F, Hermine O, et al. Immunological alterations and associated diseases in mandrills (Mandrillus sphinx) naturally co-infected with SIV and STLV. Virology. 2014;454–455:184–96.
Traina-Dorge VL, Martin LN, Lorino R, Winsor EL, Beilke MA. Human T cell leukemia virus type 1 up-regulation after simian immunodeficiency virus-1 coinfection in the nonhuman primate. J Infect Dis. 2007;195:562–71.
Harrison LH, Quinn TC, Schechter M. Human T cell lymphotropic virus type I does not increase human immunodeficiency virus viral load in vivo. J Infect Dis. 1997;175:438–40.
Goldberg TL, Sintasath DM, Chapman CA, Cameron KM, Karesh WB, Tang S, et al. Coinfection of ugandan red colobus (Procolobus [Piliocolobus] rufomitratus tephrosceles) with novel, divergent delta-, lenti-, and spumaretroviruses. J Virol. 2009;83:11318–29.
Durand JP, Tuppin P, Maison P, Galat G, Galat-Luong A, Jeannel D, et al. Increased risk for a second retroviral infection (SIV or STLV type I) for wild African green monkeys already infected by one retrovirus in Senegal (West Africa). AIDS Res Hum Retroviruses. 1995;11:985–8.
Beilke MA, Dorge VLT, Sirois M, Bhuiyan A, Murphy EL, Walls JM, et al. Relationship between human T lymphotropic virus (HTLV) type 1/2 viral burden and clinical and treatment parameters among patients with HIV type 1 and HTLV-1/2 coinfection. Clin Infect Dis. 2007;44:1229–34.
Beilke MA, Japa S, Moeller-Hadi C, Martin-Schild S. Tropical spastic paraparesis/human T leukemia virus type 1-associated myelopathy in HIV type 1-coinfected patients. Clin Infect Dis. 2005;41:e57–63.
Casseb J, Posada-Vergara MP, Montanheiro P, Fukumori LMI, Olah I, Smid J, et al. T CD4+ cells count among patients co-infected with human immunodeficiency virus type 1 (HIV-1) and human T-cell leukemia virus type 1 (HTLV-1): high prevalence of tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP/HAM). Revista do Instituto de Medicina Tropical de São Paulo. 2007;49:231–3.
Casseb J, de Oliveira ACP, Vergara MPP, Montanheiro P, Bonasser F, Meilman Ferreira C, et al. Presence of tropical spastic paraparesis/human T-cell lymphotropic virus type 1-associated myelopathy (TSP/HAM)-like among HIV-1-infected patients. J Med Virol. 2008;80:392–8.
Casoli C, Pilotti E, Bertazzoni U. Molecular and cellular interactions of HIV-1/HTLV coinfection and impact on AIDS progression. AIDS Rev. 2007;9:140–9.
Rahimi H, Rezaee SA, Valizade N, Vakili R, Rafatpanah H. Assessment of HTLV-I proviral load, HIV viral load and CD4 T cell count in infected subjects; with an emphasis on viral replication in co-infection. Iran J Basic Med Sci. 2014;17:49–54.
Fultz PN, Su L, May P, West JT. Isolation of sooty mangabey simian T-cell leukemia virus type I [STLV-I(sm)] and characterization of a mangabey T-cell line coinfected with STLV-I(sm) and simian immunodeficiency virus SIVsmmPBj14. Virology. 1997;235:271–85.
Traina-Dorge V, Blanchard J, Martin L, Murphey-Corb M. Immunodeficiency and lymphoproliferative disease in an African green monkey dually infected with SIV and STLV-I. AIDS Res Hum Retroviruses. 1992;8:97–100.
Alais S, Pasquier A, Jegado B, Journo C, Rua R, Gessain A, et al. STLV-1 co-infection is correlated with an increased SFV proviral load in the peripheral blood of SFV/STLV-1 naturally infected non-human primates. PLoS Negl Trop Dis. 2018;12:e0006812.
Acknowledgements
BJ is supported by Labex Ecofect, RM is supported by Ecole Normale Supérieure de Lyon. RM is part of the French Laboratory of Excellence project ECOFECT (ANR-11-LABX-0048). The authors acknowledge the support Fondation pour la recherche médicale (équipe Labellisée). The authors thank Dr C. Journo for her helpful comments.
Funding
RM is part of the French Laboratory of Excellence project ECOFECT (ANR-11-LABX-0048). The authors acknowledge the support Fondation pour la recherche médicale (équipe Labellisée DEQ20180339200). BJ is supported by Labex Ecofect, RM is supported by Ecole Normale Supérieure de Lyon. HD is funded by INSERM.
Author information
Authors and Affiliations
Contributions
BJ, HD, FK and RM wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jégado, B., Kashanchi, F., Dutartre, H. et al. STLV-1 as a model for studying HTLV-1 infection. Retrovirology 16, 41 (2019). https://doi.org/10.1186/s12977-019-0503-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12977-019-0503-0