Abstract
The spinocerebellar ataxias (SCAs) are devastating neurological diseases characterized by progressive cerebellar incoordination. While neurons bear the brunt of the pathology, a growing body of evidence suggests that glial cells are also affected. It has, however, been difficult to understand the role of glia, given the diversity of subtypes, each with their individual contributions to neuronal health. Using human SCA autopsy samples we have discovered that Bergmann glia—the radial glia of the cerebellum, which form intimate functional connections with cerebellar Purkinje neurons—display inflammatory JNK-dependent c-Jun phosphorylation. This phosphorylation defines a signaling pathway not observed in other activated glial populations, providing an opportunity to isolate the role of Bergmann glia in SCA inflammation. Turning to an SCA1 mouse model as a paradigmatic SCA, we demonstrate that inhibiting the JNK pathway reduces Bergmann glia inflammation accompanied by improvements in the SCA1 phenotype both behaviorally and pathologically. These findings demonstrate the causal role for Bergmann glia inflammation in SCA1 and point to a novel therapeutic strategy that could span several ataxic syndromes where Bergmann glia inflammation is a major feature.
Similar content being viewed by others
Introduction
The spinocerebellar ataxias (SCAs) are a group of autosomal dominant disorders characterized by adult-onset cerebellar and brainstem degeneration. They are progressive and untreatable, and patients eventually die from respiratory complications such as aspiration and pneumonia [1,2,3]. The more prevalent SCAs are caused by CAG trinucleotide genomic expansions. These mutations occur in the coding region of the relevant gene and therefore result in an expanded polyglutamine tract in the encoded protein. The polyglutamine ataxias include SCAs 1, 2, 3, 6, 7, and 17 and a related ataxic syndrome, dentatorubral–pallidoluysian atrophy [3, 4]. Together, they account for approximately 80% of the genetically elucidated SCAs [5]. The rest are caused by either microsatellite repeats or conventional mutations, such as deletions or point mutations that alter the coding region of the affected genes [2].
It is not entirely clear why the SCAs display regional vulnerability as reflected in the early involvement of the cerebellum that continues to degenerate as the disease progresses. The mutant proteins themselves are ubiquitously expressed and at largely similar levels in different cell type and tissues. To understand selective vulnerability, most of the focus has been on understanding cell autonomous changes in neurons, most notably Purkinje neurons that show dystrophic changes. But other neuronal populations, particularly those of the cerebellum and brainstem, also contribute to the syndrome. These include the inferior olivary neurons of the medulla, which provide glutamatergic excitatory inputs to Purkinje cells (PCs) by their dendritic connections, termed climbing fibers, and molecular layer interneurons in the cerebellar cortex, which provide GABAergic inhibitory inputs to further sculpt PC output [6, 7]. Other brainstem and cranial nerve nuclei including the vagus and hypoglossal are also involved to variable extents in the different SCAs. These studies have been performed in human autopsy samples and in genetically engineered mouse models of the disease defining the neuronal circuitry underlying the SCA syndromes [8, 9].
In addition to the neurons, however, there is also a growing awareness that non-neuronal cells such as endothelial cells and glia contribute to pathology [10,11,12,13,14]. Pathology in these non-neuronal cells could compromise neuronal function by interfering with their normal supportive role or triggering neuroinflammation. There is considerable empiric evidence for the latter from imaging modalities such as magnetic resonance spectroscopy, and pathological analysis of patients at autopsy [15,16,17]. The role of glia is particularly intriguing since they display alterations in gene expression which parallel those seen in neurons both based on the magnitude of alterations and longitudinal changes as the disease progresses [18, 19]. Indeed, gene expression changes are seen in all the major glial populations: oligodendrocytes that ensheath neurons: astrocytes that participate in complex neuronal–glial interactions to support neurons; and microglia, the resident macrophages, which protect neurons from stress and activating inflammatory responses [20,21,22]. Despite these findings, their causal role in pathogenesis has been difficult to decipher. Key obstacles to our understanding include the complex crosstalk of signals between glia and neurons, and the remarkable diversity beyond even the basic glial classification outlined above.
The cerebellum, for instance, has at least three distinct types of astrocytes—fibrous astrocytes in the deep white matter; protoplasmic or velate astrocytes in the granular layer; and Bergmann glia (BG), regionally specialized radial astrocytes that closely align with PCs [23,24,25]. Cerebellar microglia and oligodendrocytes also show distinct subpopulations whose diversity is still largely unexplored [26, 27]. One could in fact imagine a scenario where some glia participate in a pathogenic manner while yet others provide compensatory neuroprotective signals.
Here we describe that BG exhibit an increased activation of the transcription factor c-Jun in the context of inflammation. The activation of c-Jun is triggered by its phosphorylation that causes heterodimerization with other transcription factors such as Fos, ATF and CREB family members to trigger an inflammatory cascade [28]. The identification of this druggable pathway in SCA1 allowed us to intervene pharmacologically to tamp down JNK-dependent BG inflammation.
We decided to use SCA1 as a paradigm for these studies, since we and others have already established the presence of significant neurogliosis that affects BG [14, 21]. SCA1 also is the most severe of the polyglutamine ataxias in terms of disease progression in humans [29, 30]. Using SCA1 knock-in mice, we discovered that BG inflammation indeed plays a deleterious role in SCA that can be thwarted by a c-Jun N-terminal kinase (JNK) inhibitory drug that prevents c-Jun activation. These results unequivocally establish the harmful role of BG inflammation in abetting cerebellar degeneration and inspire the first glia-based strategy to treat the SCAs.
Results
SCA patients display BG-specific phosphorylation of the transcription factor c-Jun
Bacterial lipopolysaccharide (LPS), a potent inducer of inflammation, is a major component of the outer membrane of Gram-negative bacteria. It stimulates Toll-like receptors, which in turn signal through the MAP kinase receptor family of serine/threonine kinases to result in the phosphorylation of transcription factors [31]. This signaling module culminates in the transcriptional expression of downstream inflammatory factors [32, 33].
We used these properties of LPS to study cerebellar inflammation in an in vitro system. Specifically, we treated primary mixed cerebellar cultures derived from new mouse pups to LPS added to the culture medium. In the course of studying the signaling cascades downstream of LPS, we observed a robust phosphorylation of the inflammatory transcription factor c-Jun (Additional file 1: Fig. S1A). This phenomenon has been previously described [34,35,36]. The phosphorylation of c-Jun occurs on serine 63 (c-Jun-pS63), which is known to be a serine residue targeted by JNK [36]. However, c-Jun phosphorylation does not occur in all cells; it is predominantly confined to a subpopulation of glial cells that we later identified as BG based on co-staining with S100, a calcium binding protein that is solely expressed by these cells in the cerebellum [37]. Total c-Jun positive cells did not change significantly compared with control cultures, indicating that c-Jun phosphorylation, and not its levels, is increased with LPS activation. We confirmed BG predominant c-Jun phosphorylation upon LPS induction in vivo by intraperitoneal injection of mice with LPS and staining for phosphorylated c-Jun (Additional file 1: Fig. S1B).
Since gliosis is a major feature of the SCAs, we next asked whether the SCAs mirror the LPS-induced induction of c-Jun phosphorylation specifically in BG. At this juncture it is important to note that the pattern of gliosis varies in the different SCAs. For instance, in virtually all the SCAs gliosis occurs in the cerebellum; SCA3, however, is an exception in that inflammation is confined to the pons [38, 39]. To test c-Jun activation in different SCAs, we turned to autopsy samples from patients with SCAs. We focused on SCAs 1, 2, 3 and 7. We observed robust phosphorylation of c-Jun in cells residing in the Purkinje cell layer that are likely BG in several of the SCAs including 1, 2, and 7, but not SCA3 (Fig. 1). Together, these results demonstrate that BG activation as defined by c-Jun phosphorylation is a broad but not universal phenomenon across the SCAs, with the intensity of c-Jun activation corresponding to those SCAs with the most visible cerebellar inflammation.
Spinocerebellar ataxia patients exhibit Bergmann glia-specific c-Jun phosphorylation. A–D HRP-DAB immunostaining of human cerebellum from patients with A SCA1, B SCA2, C SCA7, and G SCA3 using c-Jun phosphorylation (c-Jun-pS63) antibody. Black-boxed regions represent the corresponding higher-magnification images shown below each photo. E–H HRP-DAB immunostaining of human cerebellum from patients with D SCA1, E SCA2, F SCA7, and H SCA3 using total c-Jun antibody. Black-boxed regions represent the corresponding higher-magnification images shown below each photo. In all panels nuclei are counterstained with hematoxylin. Scale bar = 100 μm. Representative images are shown. We performed staining on multiple SCA1 (n = 4), SCA2 (n = 3), SCA3 (n = 3), and SCA7 (n = 3) samples, as well as age-matched controls (n = 4). Arrowheads correspond to phosphorylated c-Jun staining in BG. I Quantification of percent of c-Jun-pS63 positive cells in BGL Bergmann glia layer; GCL granule cell layer; ML molecular layer. n = 3. **P < 0.01; ****P < 0.001, one-way ANOVA with Bonferroni’s multiple comparisons test
Characterizing c-Jun phosphorylation in SCA1 mice
To delve into BG inflammation in more detail, we turned to the SCA1 knock-in model. This mouse is engineered to express ATXN1 harboring 154 glutamine repeats. Even though all the functions of ATXN1 have yet to be deciphered, it appears to serve as a transcriptional regulator, whose functions and interactors are affected by the pathogenic expansion [40]. This SCA1 knock-in is an extremely precise model of human disease in that mutant ATXN1 is expressed under its endogenous promoter thus mirroring the spatial and temporal pattern of ATXN1 expression. It has also been extremely well-characterized with established timelines to monitor behavioral and pathological changes spanning the mouse lifespan [41, 42]. It is important to note that the polyglutamine tract in normal ATXN1 in humans is variable but does not extend beyond 40 repeats (whereas mouse ATXN1 has only 2 glutamines). The maximum glutamine repeat length described in human patients is 82 and causes a childhood onset of the disease, but this repeat must be further exaggerated to ensure a robust ataxic phenotype in the short lifespan of the mouse.
We stained for the BG-specific protein S100 along with c-Jun-pS63. The number of S100/c-Jun-pS63 double-positive cells was significantly increased in SCA1 cerebellum compared to wild-type controls (Fig. 2A–D). Phosphorylation of c-Jun was observed as early as 16 weeks of age, a time when BG also display glial activation evidenced by GFAP staining (Fig. 2E, F). These results extend our finding of BG-specific c-Jun activation to genetically engineered mice.
c-Jun phosphorylation in the activated Bergmann glia of SCA1 mice. A Immunostaining of 16-week-old SCA1 mouse cerebellum with Bergmann glia (BG)-specific S100 (red) along with anti-c-Jun-pS63 antibody (green). Scale bar = 100 μm. White-boxed regions represent the corresponding higher-magnification images shown in the “zoom” panels. B HRP-based DAB immunostaining of SCA1 mouse cerebellum with anti-c-Jun-pS63 antibody. Scale bar = 50 μm. C Quantification of percentage of BG cells positive for c-Jun-pS63 stain shown in A. D Immunostaining of SCA1 mouse cerebellum with total c-Jun (green). Sections are counterstained with DAPI (blue) to detect nuclei. Scale bar = 100 μm. E Immunostaining of 16-week-old SCA1 mouse cerebellum with glia-specific GFAP antibody (green) along with calbindin antibody to detect Purkinje cells (red). Scale bar = 100 μm. F Quantification of GFAP intensity. BGL Bergmann glia layer; GCL granule cell layer; ML molecular layer. Sections are stained for nuclei using DAPI (blue). n = 4 mice. **P < 0.01; ***P < 0.001, 2-tailed unpaired Student’s t test
We also confirmed elevated c-Jun phosphorylation specifically in BG in cerebellar dissociated cultures from SCA1 post-natal mice compared to cultures derived from wild-type mice (Additional file 2: Fig. S2).
JNK inhibitor treatment in SCA1 mice abolishes c-Jun phosphorylation and inhibits the levels of the cytokine IL-1β
Since there are well-characterized inhibitors of JNK catalytic activity, we turned to a pharmacological approach to inhibit JNK kinases. Encoded by three distinct genes, JNK kinases exist as three types: JNK 1, 2, and 3, each with further subtypes resulting from differential splicing [43]. As we yet do not know which JNK subtype is responsible for c-Jun activation in BG, we used a broad JNK inhibitor, SP600125 (inhibiting JNK1 and 2 with an IC50 of 40 nM, and JNK3 with an IC50 of 90 nM) [44]. This compound crosses the blood–brain barrier and has been used to study the role of JNK kinases in the CNS [45,46,47].
We treated SCA1 mice and wild-type littermates with intraperitoneal injections of SP600125 using a previously established delivery schedule, starting treatment at 2 months of age when mice are already symptomatic [45,46,47]. As expected, mice treated with this drug displayed a decrease in c-Jun phosphorylation in the BG layer detectable after 2 months of treatment (Fig. 3A–C).
Treatment of SCA1 mice with JNK inhibitor abolishes c-Jun phosphorylation in reactive Bergmann glia. A Immunostaining of WT (vehicle/JNK inhibitor) and SCA1 (vehicle/JNK inhibitor) treated cerebellum with Bergmann glia (BG)-specific S100 (red) along with c-Jun-pS63 antibody (green). Scale bar = 100 μm. B Immunostaining of WT (vehicle/JNK inhibitor) and SCA1 (vehicle/JNK inhibitor) treated cerebellum with Purkinje cell-specific calbindin (Calb) antibody along with c-Jun-pS63 antibody. C-Jun-pS63-positive BG cells (green) sit adjacent to the Purkinje cells (red). Scale bar = 100 μm. C Quantification of the percentage of BG cells positive for c-Jun-pS63, as shown in A. ***P < 0.001, one-way ANOVA with Bonferroni’s multiple comparison test. Inhib inhibitor
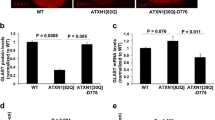
Since a major consequence of glial activation is cytokine release, we next asked whether the reduction of BG glial activation is associated with the reduction of cytokines, at least those known to play a role in neuroinflammation. We isolated RNA from experimental and control mice and performed real-time PCR (RT-PCR) to monitor the expression levels of IL-1β, IL-6, CCL2, and IL-18—four major proinflammatory molecules previously shown to be expressed in mice upon LPS treatment [48, 49]. We observed a significant increase in levels of IL-1β and IL-6 in SCA1 mice compared to wild-type controls (3.5-fold and twofold, respectively). Of the two, the increase in IL-1β mRNA but not IL-6 was reversed by JNK inhibition (Fig. 4A). These results point to the inflammatory factor IL-1β but not IL-6 as a BG-specific cytokine that is released upon activation.
JNK/c-Jun pathway is essential for cytokine release in SCA1 mice. A Quantitative real-time PCR analysis of IL-1β, IL-6, CCL2, and IL-18—four major proinflammatory cytokines from SCA1/WT cerebellum either treated with vehicle or JNK inhibitor as indicated in the bar graph legend. The data were normalized to GAPDH mRNA and are represented as the fold change. B Immunostaining of WT cerebellum using IL-1 receptor (IL-1RI) antibody (red) along with Purkinje cell-specific antibody against inositol-triphosphate receptor type I (IP3RI; green). Sections were also stained for nuclei using DAPI (blue). Scale bar = 50 μm. n = 3 mice. *P < 0.5; ****P < 0.0001, two-way ANOVA with Tukey’s multiple comparisons test. C Immunostaining of WT (JNK inhibitor) and SCA1 (vehicle/JNK inhibitor) treated cerebellum with glia-specific GFAP antibody (red). Sections were also stained for nuclei using DAPI (blue). Scale bar = 100 μm. D Quantification of GFAP fluorescence intensity shown in C. n = 4 mice. **P < 0.01; ***P < 0.001, one-way ANOVA with Bonferroni’s multiple comparison test. Inhib inhibitor
To identify which cells would be most affected by BG-specific release of IL-1β, we performed immunostaining of cerebellum for interleukin-1 receptor type I (IL-1RI) the predominant receptor for IL-1β ligand in the CNS. IL-1RI staining is not widespread in the cerebellum. We found the expression of this receptor in the cerebellum to be narrowly restricted to PCs (Fig. 4B). The inhibition of IL-1β was accompanied by a reduction of the glial activation marker GFAP, demonstrating that JNK activation is required for BG activation (Fig. 4C, D). These results suggest that in SCA1, the Bergmann glia release of IL-1β and possibly other inflammatory factors leads to PC dysfunction and death.
JNK inhibition ameliorates the SCA1 phenotype
Neuroinflammation, including that caused by the release of cytokines such as IL-1β, can be either neuroprotective or deleterious in a broad range of pathogenic contexts including the ataxias [50, 51]. To address the role of JNK activation, we studied the behavioral and pathological consequences of pharmacological inhibition of JNK in mice (Fig. 5A). For behavioral analysis, we turned to rotarod testing, a robust measure of cerebellar motor learning, which is compromised in mouse models of the SCAs. SCA1 mice treated with JNK inhibitor showed a significant improvement in their performance. It is notable that this improvement occurred only after 2 months of ongoing treatment in this treatment paradigm (*P < 0.05) (Fig. 5B, C). This improvement did not extend to non-cerebellar phenotypes; for instance, the weight loss in SCA1 mice—a composite sequela of neuromuscular wasting from spinal cord involvement and poor nutrition from swallowing defects—was not improved by the drug (Fig. 5D).
Treatment of SCA1 mice with JNK inhibitor ameliorates the motor coordination impairment. A Schematic representation of treatment and assessment course. Mice were treated with either 15 mg/kg of JNK inhibitor or vehicle (10% DMSO and 90% corn oil) intraperitoneally (IP) until 4 months of age starting from 2 months. Then mice were rested for behavioral assays followed by pathological and quantitative RT-PCR analysis. B, C Rotarod performance of mice at B 3 months and C 4 months of age. D Mouse weight before IP administration (at 2 months of age) and following administration at 3 and 4 months of age. The number of animals used is shown in the histogram legends. *P < 0.05; **P < 0.01, two-way ANOVA with Bonferroni’s multiple comparisons test
To study SCA1 cerebellar pathology, we performed experiments to address the health of PCs and their connections [52,53,54]. Staining for calbindin, a standard marker for PCs, we observed a significant increase in the thickness of the cerebellar molecular layer in SCA1 mice treated with the JNK inhibitor compared to mice treated with vehicle control (Fig. 6A, B).
JNK inhibitor treatment improves Purkinje cell pathology in SCA1 mice. A Cerebellar slices from 4-month-old mice treated with either JNK inhibitor or vehicle were stained with calbindin antibody specific for Purkinje cells (red). Images are taken from same lobules (VII, VIII and IX) in each condition. The corresponding higher-magnification images (×20) shown below each panel. Sections were also stained for nuclei using DAPI (blue). Scale bar = 100 μm. B Quantification of molecular layer thickness (red). n = 4 mice. *P < 0.05; ****P < 0.0001, one-way ANOVA with Bonferroni’s multiple comparison test
Thus, taken together our results point to a model where BG-specific inflammation, mediated by JNK kinase, results in the release of cytokines such as IL-1β that is deleterious to PCs. This process is ameliorated by JNK kinase inhibition, which in turn improves the SCA1 phenotype (Fig. 7).
Model of targeting Bergmann glia activation to combat SCAs. Top panel SCA patients and mouse cerebellums exhibit Bergmann glia (BG)-specific JNK-dependent c-Jun phosphorylation (black nuclei); the time when BG are known to have a reactive state is marked by enhanced GFAP intensity (red processes). These reactive BG release the enhanced proinflammatory cytokine IL-1β into the cerebellum in a JNK-dependent manner. Bottom panel Treatment of SCA1 mice with JNK inhibitor SP600125 abolishes the c-Jun phosphorylation in BG (light gray nuclei) and thereby tamps down the reactive GFAP staining and cytokine IL-1β release in the cerebellum. These changes in inflammation lead to a decrease in Purkinje cell pathology (green) and a rescue of motor deficits
Discussion
Neuroinflammation is a complex process reflecting an aggregate of interactions between glia, neurons, and the microvasculature. Some of these interactions are homeostatic and designed to be neuroprotective; others on the other hand are deleterious and contribute to pathogenicity [55, 56]. Dissecting and identifying these complex pathways in a cell type-specific manner will be crucial to finding therapies for patients suffering from neurodegeneration.
Here we describe a signaling pathway in SCAs that is seen predominantly in BG involving the JNK-dependent phosphorylation of c-Jun. BG are particularly well-placed to play a role in Purkinje cell dysfunction and degeneration given their location and interconnections with PCs in the cerebellar cortex; moreover they outnumber PCs 8:1 and they almost completely blanket the dendritic neuropil of PCs with their fine protrusions [23, 57, 58]. The fortuitous observation of c-Jun phosphorylation in activated BG allowed us to test the relevance of BG inflammation in the context of the SCAs. Using SCA1 as a model, we discovered that BG inflammation can be tamped down by inhibiting JNK proteins that are responsible for catalyzing this signaling pathway; this treatment in turn substantially ameliorates the disease.
As we demonstrate here, one likely mechanism for downstream toxicity of BG activation is the release of cytokines, such as IL-1β, under the control of JNK signaling. Indeed, direct injection of IL-1β into the cerebellum of wild-type mice is sufficient to induce Purkinje cell pathology and cerebellar ataxia [59]. But cytokine release need not be the only pathological event triggered by BG activation. BG inflammation could, for instance, affect the normal housekeeping functions critical for maintaining neuronal health. BG express the glutamate transporter EAAT2 (GLAST), which is responsible for the active reuptake of glutamate at excitatory synapses; this in turn modulates neurotransmission and prevents excitotoxicity. BG also express potassium Kir 4.1 channels, by which they regulate extracellular potassium levels in the vicinity of PCs and thus further fine-tune synaptic activity [21, 60]. Perturbation of some of these normal homeostatic functions has already been hinted at in the SCAs; in conditional mouse models of SCA7, for example, BG-specific expression of mutant ATXN7 is sufficient to cause non-cell-autonomous PC degeneration by reducing the protein GLAST and causing morphological consequences of excitotoxicity [61]. While similar conditional studies have yet to be performed for any of the other SCAs, SCA1 mice show a reduction in the number of BG [62], with individual glia expressing less GLAST [63]. Regardless, the importance of BG to PC function has been most vividly demonstrated by optogenetic manipulation of BG, where BG inactivation leads to virtually instantaneous alterations of PC firing and subsequent cerebellar behavioral deficits [64, 65].
Our findings raise two interesting questions. First, why do BG show relatively selective c-Jun-dependent activation? The reason could lie in their distinct developmental origin—BG arise from a stem cell niche in the rhombic lip whereas other cerebellar astrocytes arise from stem cells derived from the cerebellar ventricular zone [23, 24]. Alternatively, the role of BG in sustaining PC health may require distinct signaling pathways not shared by other glia. The second question is mechanistic, and that is: what are the proximate triggers for BG inflammation? Given that BG activation occurs even when the mutant protein is only expressed in Purkinje neurons [14]. We suspect that dysfunctional PCs, perhaps in conjunction with microglia, release signals that trigger BG activation. These signals are likely to be cytokines and chemokines, since these factors have been shown to activate astrocytes in other disease contexts [66]. One could thus envisage a scenario where PCs trigger BG activation, which in turn exacerbates PC dysfunction, resulting in a vicious feed-forward cycle of neurodegeneration.
An important translational contribution of this study is that it inspires a novel and eminently feasible treatment strategy for SCAs, namely, the use of JNK kinase inhibitors. While we have used a broad-specificity JNK inhibitor in these proof-of-principle studies, it would be important to determine which of the three JNK isoforms need to be targeted in order to reduce the potential side effects. It is also possible that interfering with downstream targets of c-Jun activation such as decreasing the levels or activity of IL-1β could also prove therapeutic, providing yet additional treatment avenues. Our findings are likely to be applicable to other SCAs such as SCAs 2 and 7, where gliosis occurs as in SCA1 even in the presymptomatic stages (as determined by magnetic resonance spectroscopy focusing on glial metabolites) [67]. Incidentally SCA3, the ataxia that does not show much JNK activation in our mouse models, is known for having less cerebellar cortical pathology in human patients. We should also emphasize that while we have focused on the polyglutamine SCAs, we suspect that JNK-dependent BG activation is likely a widespread phenomenon of other cerebellar syndromes both familial and sporadic where cerebellar gliosis has been described [68,69,70]. It will be important to further determine the extent to which cerebellar gliosis occurs in diverse ataxias using magnetic resonance studies or autopsy evaluations. These studies could provide the impetus for broadening this glial-based therapeutic approach.
Significance statement
We have identified a Bergmann glia signaling pathway contributing to cerebellar degeneration in the spinocerebellar ataxias. This pathway is defined by activation of JNK that phosphorylates the transcription factor c-Jun leading to the release of IL-1β and potentially other cytokines from Bergmann glia. Inhibiting c-Jun phosphorylation with pharmacological JNK inhibition could serve as therapeutic approach to treating cerebellar degeneration.
Materials and methods
Mouse lines
The Sca1154Q/2Q line was generated by inserting a small conserved region containing 154 CAG repeats of the human sequence into the mouse ATXN1 locus [41]. Animal experiments were performed in compliance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and the Northwestern University Institutional Animal Care and Use Committee.
Primary cultures of cerebellar neurons/glial cells
Neuronal/glial cerebellar cultures were derived from mice using an established protocol [36, 71]. Isoflurane anesthetized mice were killed by decapitation at post-natal day 4 (P4). The cerebella were dissected away from the meninges and choroid plexus. Minced cerebellar tissue was trypsinized for 15 min at 37 °C and then triturated in Hank’s balanced salt solution containing 10 U/mL DNAse I (Roche Diagnostics). The cells were centrifuged at 2000 rpm for 7 min and resuspended in Neurobasal media (Sigma) containing 4 mM glutamine, 10% FBS, 100 U/mL penicillin/streptomycin, and 25 mM KCl (Sigma-Aldrich). After counting, 7.5 × 105 cells were plated on precoated poly-d-lysine glass coverslips in 24-well plates. Cultures were maintained at 37 °C, 5% CO2, and media were changed every 2 days. On day 6 in culture, the cells were treated with LPS (Sigma #L2630) at 100 ng/mL concentration for 3 h. They were then fixed in 4% paraformaldehyde for immunohistochemical staining.
In vivo LPS treatment in wild-type mice was performed as previously described [36]. Briefly, LPS in PBS was administered intraperitoneally at a dose of 750 μg/kg for seven consecutive days. The control mice received the vehicle PBS alone. After 7 days of injections, mice were killed for immunohistochemical analysis.
Human brain immunohistochemistry
We obtained SCA autopsy samples: four SCA1, three SCA2, three SCA3, three SCA7, and four age-matched controls (from Arnulf Koeppen and Laura Ranum, with approval from their respective institutional review boards at the Veterans Affairs Medical Center, Albany, New York, and the University of Florida). Post-mortem cerebellar tissue from SCA patients was mounted in paraffin blocks, and 5-μm-thick slices were cut from each paraffin block, processed for HRP-DAB staining, and counterstained with hematoxylin. Antigen retrieval and antibody staining was optimized at the Northwestern University Pathology Core.
Experimental injections with pharmacological agents
The JNK inhibitor SP600125 (#HY-12041, MedChemExpress) was dissolved in 10% DMSO and 90% corn oil. It was injected intraperitoneally on an alternate day schedule at a dose of 15 mg/kg starting when mice were 2 months of age and continuing for 2 months. Control mice were treated with vehicle alone. The mice were then evaluated behaviorally and pathologically in a blinded fashion. Since SCA mice do not display sex-based differences in their cerebellar phenotype, the read-outs from males and females were pooled before statistical analysis [15, 41, 53, 72].
Rotarod assays
Rotarod testing was performed by placing mice on a motorized rotating rod that accelerates linearly from 4 to 40 rotations per minute over a maximum duration of 5 min (Ugo Basile, Comerio, Italy) [13]. The time it takes for a mouse to fall off was recorded. If mice passively clung to the rod for two consecutive rotations, that was also counted as a fall. Mice were subjected to four trials per day for 4 consecutive days. To ensure enough recovery time between trials, animals were given 10–15 min rest between the end of a trial and the subsequent trial.
Pathological assays/immunohistochemistry
Mice were killed by deep anesthesia (isoflurane) and transcardiac perfusion (first with PBS and then with 4% paraformaldehyde in PBS). The brains were dissected from the cranium and post-fixed with 4% paraformaldehyde in PBS in an overnight incubation at 4 °C. They were subsequently equilibrated in a 10–30% sucrose gradient and embedded in optimal cutting temperature medium. The cerebella were sliced into 30-μm-thick sections with a cryostat (Micron M505, Thermo Fisher Scientific) or Vibratome (Leica VT1000 S). Immunohistochemistry was then performed either by immunofluorescence or horseradish peroxidase (HRP)-based 3,3′-diaminobenzidine (DAB) detection.
For immunofluorescence, the sections were permeabilized and blocked with 10% normal goat serum and 0.25% Triton X-100 in 1× Tris-buffered saline for 1 h, after which the sections were incubated with primary antibodies (diluted in 1% BSA) overnight at 4 °C. The following day, the sections were washed three times with PBS, then incubated with fluorescently tagged secondary antibodies for 2 h at room temperature in the dark. Finally, the sections were washed three times with TBS (adding DAPI into the last wash) and mounted onto glass slides using Mowiol 4-88 (Sigma-Aldrich). The sections were imaged using a CTR6500 confocal microscope equipped with Leica LAS AF software (Leica, Buffalo Grove, IL).
For HRP-based DAB staining, the sections were processed for antigen retrieval using citrate-based buffer (pH 6.0) (Abcam #ab93678) and quenched for endogenous peroxidase activity by treating with 3% H2O2. The sections were then blocked in 10% normal blocking serum for 20 min, washed in PBS, and then incubated with primary antibody (diluted in 1% BSA) for 1 h. Sections were washed with PBS and incubated with biotinylated secondary antibody (rabbit IgG VECTASTAIN #PK-6101 or mouse IgG VECTASTAIN #PK-4002) for 30 min. After a wash with PBS, the sections were incubated with VECTASTAIN elite ABC reagent for 30 min followed by incubation with peroxidase substrate solution (VECTOR #SK-4100) for 2–10 min at room temperature until the desired brown color developed. Immediately, the slides were rinsed under tap water for 5 min. Slides were mounted using aqueous mounting medium (VectaMount AQ #H-5501).
Quantitative real-time PCR (RT-PCR)
Mice were killed by deep anesthesia (isoflurane) followed by decapitation. The cerebellar tissue was dissected from the cranium. Cerebellar RNA was extracted using a RNeasy Plus Universal mini kit (Qiagen #73404) that was then used to generate cDNA using a reverse-transcription kit (Biorad #1708840). Quantitative PCR was subsequently performed using TaqMan probes with iTaq Universal Probe Supermix on a CFX96 Real-Time thermocyler (Biorad C1000 Touch). For each sample, relative levels of target gene transcript were calculated as the ratio of Ct value of target gene (experimental to control sample) normalized to similarly derived GAPDH ratios.
The probes used were as follows: IL-1b: Catalog #4331182, ID: Mm00434228_m1. CCL2: Catalog #4331182, ID: Mm00441242_m1. IL-18: Catalog #4331182, ID: Mm00434225_m1. IL-6: Catalog #331182, ID: Mm00446190_m1. GAPDH: Catalog #4352661, Mm99999915_g1. All probes were fluorescein amidite-labeled.
Antibodies
The following primary antibodies were used: rabbit anti-c-Jun mAb (# 9165 Clone 60A8, Cell Signaling), rabbit anti-phospho-c-Jun (Ser63) II (#9261, Cell Signaling), mouse anti-GFAP mAb (#MCA-5C10, EnCor Biotechnology Inc), rabbit anti-GFAP (# Z0334, Dako), mouse anti-IL-1RI (#AF771, R&D Systems), rabbit anti-IP3R-I (#PA1-901, Thermo Fisher), mouse anti-S100B mAb (#S2532, Sigma-Aldrich).
Microscopy and image analyses
Nikon Eclipse TE2000-E fluorescence microscopes equipped with Intensilight C-HGFI (Nikon Inc., Melville, NY, USA) were used. Epifluorescence images were acquired using a Digital Sight DS-Qi1MC CCD camera (Nikon Inc., Melville, NY, USA), and light images were acquired using a Ds-Fi1 camera (Nikon Inc., Melville, NY, USA). Confocal images were collected using Leica TCS SP5 confocal microscopes (Leica Inc., Bensheim, Germany) and used to acquire low- and high-magnification images of fluorescent samples. To allow for the comparative quantification of fluorescence intensity between samples, all images were acquired with standardized settings and laser power. Maximum intensity projections for individual channels were generated and the fluorescence intensity of these projections were then measured using ImageJ (NIH, Bethesda, MD, USA). Mean intensity results of 3–5 images were plotted as histograms. For molecular layer thickness measurements line tool was used on the images J and average results of 3–5 images from each mouse were plotted as histogram. Quantification of cells staining positive for c-Jun-pS63 was done manually and presented as the percentage of immunostained cells to the total number of cells stained by hematoxylin (plotted as histograms). We used the cell counter tool of Image J; three images for each condition were used to count the cells positive for c-Jun-pS63 in the granule cell layer (GCL), Bergmann glia layer (BGL) and molecular layer (ML).
Statistical analysis
We performed all statistical tests using GraphPad Prism 4.0 (GraphPad Software). Data are presented as mean ± SEM The level of significance was set at P values less than 0.05. Two-tailed t-tests were used for comparison of the two data sets while two-way ANOVA and one-way ANOVA followed by Bonferroni correction were used for experiments with three or more data sets. Molecular and biochemical analyses were performed using a minimum of three biological replicates per condition.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Diallo A, Jacobi H, Cook A, Labrum R, Durr A, Brice A, Charles P, Marelli C, Mariotti C, Nanetti L, et al. Survival in patients with spinocerebellar ataxia types 1, 2, 3, and 6 (EUROSCA): a longitudinal cohort study. Lancet Neurol. 2018;17(4):327–34.
Klockgether T, Mariotti C, Paulson HL. Spinocerebellar ataxia. Nat Rev Dis Primers. 2019;5(1):24.
Ashizawa T, Öz G, Paulson HL. Spinocerebellar ataxias: prospects and challenges for therapy development. Nat Rev Neurol. 2018;14(10):590–605.
Sullivan R, Yau WY, O’Connor E, Houlden H. Spinocerebellar ataxia: an update. J Neurol. 2019;266(2):533–44.
Park JY, Joo K, Woo SJ. Ophthalmic manifestations and genetics of the polyglutamine autosomal dominant spinocerebellar ataxias: a review. Front Neurosci. 2020;14:892.
Edamakanti CR, Do J, Didonna A, Martina M, Opal P. Mutant ataxin1 disrupts cerebellar development in spinocerebellar ataxia type 1. J Clin Investig. 2018;128(6):2252–65.
Ebner BA, Ingram MA, Barnes JA, Duvick LA, Frisch JL, Clark HB, Zoghbi HY, Ebner TJ, Orr HT. Purkinje cell ataxin-1 modulates climbing fiber synaptic input in developing and adult mouse cerebellum. J Neurosci. 2013;33(13):5806–20.
Binda F, Pernaci C, Saxena S. Cerebellar development and circuit maturation: a common framework for spinocerebellar ataxias. Front Neurosci. 2020;14:293–293.
Robinson KJ, Watchon M, Laird AS. Aberrant cerebellar circuitry in the spinocerebellar ataxias. Front Neurosci. 2020;14:707.
Sheeler C, Rosa JG, Ferro A, McAdams B, Borgenheimer E, Cvetanovic M. Glia in neurodegeneration: the housekeeper, the defender and the perpetrator. Int J Mol Sci. 2020;21(23):9188.
Stevenson R, Samokhina E, Rossetti I, Morley JW, Buskila Y. Neuromodulation of glial function during neurodegeneration. Front Cell Neurosci. 2020;14:278.
Gleichman AJ, Carmichael ST. Glia in neurodegeneration: drivers of disease or along for the ride? Neurobiol Dis. 2020;142: 104957.
Hu YS, Do J, Edamakanti CR, Kini AR, Martina M, Stupp SI, Opal P. Self-assembling vascular endothelial growth factor nanoparticles improve function in spinocerebellar ataxia type 1. Brain. 2019. https://doi.org/10.1093/brain/awy328.
Cvetanovic M, Ingram M, Orr H, Opal P. Early activation of microglia and astrocytes in mouse models of spinocerebellar ataxia type 1. Neuroscience. 2015;289:289–99.
Cvetanovic M, Patel JM, Marti HH, Kini AR, Opal P. Vascular endothelial growth factor ameliorates the ataxic phenotype in a mouse model of spinocerebellar ataxia type 1. Nat Med. 2011;17(11):1445–7.
Zahr NM, Mayer D, Rohlfing T, Sullivan EV, Pfefferbaum A. Imaging neuroinflammation? A perspective from MR spectroscopy. Brain Pathol. 2014;24(6):654–64.
Joers JM, Deelchand DK, Lyu T, Emir UE, Hutter D, Gomez CM, Bushara KO, Eberly LE, Öz G. Neurochemical abnormalities in premanifest and early spinocerebellar ataxias. Ann Neurol. 2018;83(4):816–29.
Schuster KH, Zalon AJ, Zhang H, DiFranco DM, Stec NR, Haque Z, Blumenstein KG, Pierce AM, Guan Y, Paulson HL, et al. Impaired oligodendrocyte maturation is an early feature in SCA3 disease pathogenesis. J Neurosci. 2022;42(8):1604–17.
Tejwani L, Ravindra NG, Nguyen B, Luttik K, Lee C, Gionco J, Kim K, Yoon J, Haidery F, Ro H, et al. Longitudinal single-cell transcriptional dynamics throughout neurodegeneration in SCA1. bioRxiv. 2021. https://doi.org/10.1101/2021.10.22.465444.
Ferro A, Sheeler C, Rosa JG, Cvetanovic M. Role of microglia in ataxias. J Mol Biol. 2019;431(9):1792–804.
Kim JH, Lukowicz A, Qu W, Johnson A, Cvetanovic M. Astroglia contribute to the pathogenesis of spinocerebellar ataxia Type 1 (SCA1) in a biphasic, stage-of-disease specific manner. Glia. 2018;66(9):1972–87.
Qu W, Johnson A, Kim JH, Lukowicz A, Svedberg D, Cvetanovic M. Inhibition of colony-stimulating factor 1 receptor early in disease ameliorates motor deficits in SCA1 mice. J Neuroinflamm. 2017;14(1):107.
Buffo A, Rossi F. Origin, lineage and function of cerebellar glia. Prog Neurobiol. 2013;109:42–63.
Leung AW, Li JYH. The molecular pathway regulating Bergmann glia and folia generation in the cerebellum. Cerebellum. 2018;17(1):42–8.
Edamakanti CR, Opal P. Developmental alterations in adult-onset neurodegenerative disorders: lessons from polyglutamine diseases. Mov Disord. 2021. https://doi.org/10.1002/mds.28657.
Tan Y-L, Yuan Y, Tian L. Microglial regional heterogeneity and its role in the brain. Mol Psychiatry. 2020;25(2):351–67.
Hayashi C, Suzuki N, Takahashi R, Akazawa C. Development of type I/II oligodendrocytes regulated by teneurin-4 in the murine spinal cord. Sci Rep. 2020;10(1):8611.
Grondin B, Lefrancois M, Tremblay M, Saint-Denis M, Haman A, Waga K, Bédard A, Tenen DG, Hoang T. c-Jun homodimers can function as a context-specific coactivator. Mol Cell Biol. 2007;27(8):2919–33.
Diallo A, Jacobi H, Tezenas du Montcel S, Klockgether T. Natural history of most common spinocerebellar ataxia: a systematic review and meta-analysis. J Neurol. 2020. https://doi.org/10.1007/s00415-020-09815-2.
Jacobi H, du Montcel ST, Bauer P, Giunti P, Cook A, Labrum R, Parkinson MH, Durr A, Brice A, Charles P, et al. Long-term disease progression in spinocerebellar ataxia types 1, 2, 3, and 6: a longitudinal cohort study. Lancet Neurol. 2015;14(11):1101–8.
Miyake K. Innate recognition of lipopolysaccharide by Toll-like receptor 4-MD-2. Trends Microbiol. 2004;12(4):186–92.
Jin JJ, Kim HD, Maxwell JA, Li L, Fukuchi K. Toll-like receptor 4-dependent upregulation of cytokines in a transgenic mouse model of Alzheimer’s disease. J Neuroinflamm. 2008;5:23.
Ngkelo A, Meja K, Yeadon M, Adcock I, Kirkham PA. LPS induced inflammatory responses in human peripheral blood mononuclear cells is mediated through NOX4 and Giα dependent PI-3kinase signalling. J Inflamm (Lond). 2012;9(1):1.
Jang S, Kelley KW, Johnson RW. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc Natl Acad Sci USA. 2008;105(21):7534–9.
Waetzig V, Czeloth K, Hidding U, Mielke K, Kanzow M, Brecht S, Goetz M, Lucius R, Herdegen T, Hanisch UK. c-Jun N-terminal kinases (JNKs) mediate pro-inflammatory actions of microglia. Glia. 2005;50(3):235–46.
Albanito L, Reddy CE, Musti AM. c-Jun is essential for the induction of Il-1β gene expression in in vitro activated Bergmann glial cells. Glia. 2011;59(12):1879–90.
Dooves S, Bugiani M, Wisse LE, Abbink TEM, van der Knaap MS, Heine VM. Bergmann glia translocation: a new disease marker for vanishing white matter identifies therapeutic effects of Guanabenz treatment. Neuropathol Appl Neurobiol. 2018;44(4):391–403.
Evert BO, Vogt IR, Kindermann C, Ozimek L, de Vos RA, Brunt ER, Schmitt I, Klockgether T, Wullner U. Inflammatory genes are upregulated in expanded ataxin-3-expressing cell lines and spinocerebellar ataxia type 3 brains. J Neurosci. 2001;21(15):5389–96.
Dürr A, Stevanin G, Cancel G, Duyckaerts C, Abbas N, Didierjean O, Chneiweiss H, Benomar A, Lyon-Caen O, Julien J, et al. Spinocerebellar ataxia 3 and Machado-Joseph disease: clinical, molecular, and neuropathological features. Ann Neurol. 1996;39(4):490–9.
Tejwani L, Lim J. Pathogenic mechanisms underlying spinocerebellar ataxia type 1. Cell Mol Life Sci. 2020;77(20):4015–29.
Watase K, Weeber EJ, Xu B, Antalffy B, Yuva-Paylor L, Hashimoto K, Kano M, Atkinson R, Sun Y, Armstrong DL, et al. A long CAG repeat in the mouse Sca1 locus replicates SCA1 features and reveals the impact of protein solubility on selective neurodegeneration. Neuron. 2002;34(6):905–19.
Venkatraman A, Hu YS, Didonna A, Cvetanovic M, Krbanjevic A, Bilesimo P, Opal P. The histone deacetylase HDAC3 is essential for Purkinje cell function, potentially complicating the use of HDAC inhibitors in SCA1. Hum Mol Genet. 2014;23(14):3733–45.
Bogoyevitch MA. The isoform-specific functions of the c-Jun N-terminal Kinases (JNKs): differences revealed by gene targeting. BioEssays. 2006;28(9):923–34.
Hepp Rehfeldt SC, Majolo F, Goettert MI, Laufer S. c-Jun N-terminal kinase inhibitors as potential leads for new therapeutics for Alzheimer’s diseases. Int J Mol Sci. 2020;21(24):9677.
Wang W, Shi L, Xie Y, Ma C, Li W, Su X, Huang S, Chen R, Zhu Z, Mao Z, et al. SP600125, a new JNK inhibitor, protects dopaminergic neurons in the MPTP model of Parkinson’s disease. Neurosci Res. 2004;48(2):195–202.
Kim BJ, Silverman SM, Liu Y, Wordinger RJ, Pang IH, Clark AF. In vitro and in vivo neuroprotective effects of cJun N-terminal kinase inhibitors on retinal ganglion cells. Mol Neurodegener. 2016;11:30.
Vaishnav D, Jambal P, Reusch JE, Pugazhenthi S. SP600125, an inhibitor of c-jun N-terminal kinase, activates CREB by a p38 MAPK-mediated pathway. Biochem Biophys Res Commun. 2003;307(4):855–60.
Hong J, Yoon D, Nam Y, Seo D, Kim JH, Kim MS, Lee TY, Kim KS, Ko PW, Lee HW, et al. Lipopolysaccharide administration for a mouse model of cerebellar ataxia with neuroinflammation. Sci Rep. 2020;10(1):13337.
Zhao J, Bi W, Xiao S, Lan X, Cheng X, Zhang J, Lu D, Wei W, Wang Y, Li H, et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci Rep. 2019;9(1):5790.
Sochocka M, Diniz BS, Leszek J. Inflammatory response in the CNS: friend or foe? Mol Neurobiol. 2017;54(10):8071–89.
Hewett SJ, Jackman NA, Claycomb RJ. Interleukin-1β in central nervous system injury and repair. Eur J Neurodegener Dis. 2012;1(2):195–211.
Barnes JA, Ebner BA, Duvick LA, Gao W, Chen G, Orr HT, Ebner TJ. Abnormalities in the climbing fiber-Purkinje cell circuitry contribute to neuronal dysfunction in ATXN1 [82Q] mice. J Neurosci. 2011;31(36):12778.
Ruegsegger C, Stucki DM, Steiner S, Angliker N, Radecke J, Keller E, Zuber B, Ruegg MA, Saxena S. Impaired mTORC1-dependent expression of homer-3 influences SCA1 pathophysiology. Neuron. 2016;89(1):129–46.
Barnes J, Ebner BA, Duvick LA, Gao W, Chen G, Orr HT, Ebner TJ. Abnormalities in the climbing fiber-Purkinje cell circuitry contribute to neuronal dysfunction in ATXN1[82Q] mice. J Neurosci. 2011;31(36):12778–89.
Perez-Catalan NA, Doe CQ, Ackerman SD. The role of astrocyte-mediated plasticity in neural circuit development and function. Neural Dev. 2021;16(1):1.
Bernaus A, Blanco S, Sevilla A. Glia crosstalk in neuroinflammatory diseases. Front Cell Neurosci. 2020;14:209.
Cerrato V. Cerebellar astrocytes: much more than passive bystanders in ataxia pathophysiology. J Clin Med. 2020;9(3):757.
Araujo APB, Carpi-Santos R, Gomes FCA. The role of astrocytes in the development of the cerebellum. Cerebellum. 2019;18(6):1017–35.
Andoh T, Kishi H, Motoki K, Nakanishi K, Kuraishi Y, Muraguchi A. Protective effect of IL-18 on kainate- and IL-1β-induced cerebellar ataxia in mice. J Immunol. 2008;180(4):2322.
De Zeeuw CI, Hoogland TM. Reappraisal of Bergmann glial cells as modulators of cerebellar circuit function. Front Cell Neurosci. 2015;9:246.
Custer SK, Garden GA, Gill N, Rueb U, Libby RT, Schultz C, Guyenet SJ, Deller T, Westrum LE, Sopher BL, et al. Bergmann glia expression of polyglutamine-expanded ataxin-7 produces neurodegeneration by impairing glutamate transport. Nat Neurosci. 2006;9(10):1302–11.
Shiwaku H, Yagishita S, Eishi Y, Okazawa H. Bergmann glia are reduced in spinocerebellar ataxia type 1. NeuroReport. 2013;24(11):620–5. https://doi.org/10.1097/WNR.0b013e32836347b7.
Cvetanovic M. Decreased expression of glutamate transporter GLAST in Bergmann glia is associated with the loss of Purkinje neurons in the spinocerebellar ataxia type 1. Cerebellum. 2015;14(1):8–11.
Sasaki T, Beppu K, Tanaka KF, Fukazawa Y, Shigemoto R, Matsui K. Application of an optogenetic byway for perturbing neuronal activity via glial photostimulation. Proc Natl Acad Sci USA. 2012;109(50):20720–5.
Shuvaev AN, Belozor OS, Mozhei O, Yakovleva DA, Potapenko IV, Shuvaev AN, Smolnikova MV, Salmin VV, Salmina AB, Hirai H, et al. Chronic optogenetic stimulation of Bergman glia leads to dysfunction of EAAT1 and Purkinje cell death, mimicking the events caused by expression of pathogenic ataxin-1. Neurobiol Dis. 2021;154: 105340.
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung W-S, Peterson TC, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–7.
Emir UE, Brent Clark H, Vollmers ML, Eberly LE, Oz G. Non-invasive detection of neurochemical changes prior to overt pathology in a mouse model of spinocerebellar ataxia type 1. J Neurochem. 2013;127(5):660–8.
Rüb U, Schöls L, Paulson H, Auburger G, Kermer P, Jen JC, Seidel K, Korf HW, Deller T. Clinical features, neurogenetics and neuropathology of the polyglutamine spinocerebellar ataxias type 1, 2, 3, 6 and 7. Prog Neurobiol. 2013;104:38–66.
Iltis I, Hutter D, Bushara KO, Clark HB, Gross M, Eberly LE, Gomez CM, Oz G. (1)H MR spectroscopy in Friedreich’s ataxia and ataxia with oculomotor apraxia type 2. Brain Res. 2010;1358:200–10.
Oppenheimer DR. Brain lesions in Friedreich’s ataxia. Can J Neurol Sci. 1979;6(2):173–6.
Reddy CE, Albanito L, De Marco P, Aiello D, Maggiolini M, Napoli A, Musti AM. Multisite phosphorylation of c-Jun at threonine 91/93/95 triggers the onset of c-Jun pro-apoptotic activity in cerebellar granule neurons. Cell Death Dis. 2013;4: e852.
Fryer JD, Yu P, Kang H, Mandel-Brehm C, Carter AN, Crespo-Barreto J, Gao Y, Flora A, Shaw C, Orr HT, et al. Exercise and genetic rescue of SCA1 via the transcriptional repressor Capicua. Science. 2011;334(6056):690–3.
Acknowledgements
We thank members of the Opal lab for their suggestions throughout the course of the project, Melissa Stauffer for thoughtful comments on the manuscript, Abigail Brown for help with mouse husbandry and genotyping, and Arnulf H. Koeppen and Laura Ranum for providing SCA brain material. Autopsies were performed through a national tissue donation program supported by the National Ataxia Foundation. We thank Bella Shmaltsuyeva and the Pathology Core Facility of the Robert H. Lurie Comprehensive Cancer Center, Northwestern University, for helping with human brain immunohistochemistry.
Significance statement
We have identified a Bergmann glia signaling pathway contributing to cerebellar degeneration in the spinocerebellar ataxias. This pathway is defined by activation of JNK that phosphorylates the transcription factor c-Jun leading to the release of IL-1β and potentially other cytokines from Bergmann glia. Inhibiting c-Jun phosphorylation with pharmacological JNK inhibition could serve as therapeutic approach to treating cerebellar degeneration.
Funding
P.O. received support from the NIH (1R01NS082351 and 1R01NS127204). He has received funding from the following sources for clinical trials: Biohaven Pharmaceuticals, NIH U01NS104326 (site PI), and the National Ataxia Foundation (CRC‐SCA natural history study). C.R.E received support from a young investigator award from the National Ataxia Foundation, and from a Dixon Translational Research Grant award from Northwestern University.
Author information
Authors and Affiliations
Contributions
Design and conceptualization of study: CRE and PO; methodology and investigation: CRE, VM. Data curation: CRE, VM and PO. Writing, reviewing and editing the draft: CRE and PO. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All animal experiments were performed in compliance with the NIH’s Guide for the Care and Use of Laboratory Animals (National Academies Press, 2011) and were reviewed and approved by the Northwestern University IACUC.
Consent for publication
Not applicable.
Competing interests
PO and CRE filed a patent application (US Provisional Serial No. 63/201,779) using the data generated in this study. PO and CRE have declared that no competing interests exist pertaining to this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Lipopolysaccharide induces c-Jun phosphorylation in Bergmann glia in vitro and in vivo. A Immunostaining of S100 with c-Jun-pS63 in DIV6 neuronal/glial cerebellar cultures generated from P4 mice and treated with PBS or LPS. B Immunostaining of S100 with total c-Jun. Slides were stained for nuclei using DAPI that labeled all the cells including glia in this mixed population. Scale bars = 50 μm. C Immunostaining of the cerebellum for c-Jun-pS63 antibody in wild-type mice treated with LPS or vehicle by intraperitoneal injection daily for 5 days. Scale bar = 100 μm. D Quantification of S100/c-Jun-pS63 double-positive cells shown in A. Quantification of S100/c-Jun double-positive cells shown in B. n = 3 individual cultures. ***P < 0.001.
Additional file 2: Figure S2.
In vitro isolated Bergmann glial cultures exhibit enhanced c-Jun phosphorylation. A, B DIV6 neuronal/glial cerebellar cultures generated from P4 SCA1 or wild-type mice and immunostained with S100 either with A c-Jun-pS63 or B total c-Jun. White arrowheads indicate examples of S100/c-Jun-pS63 double-positive cells. Scale bar = 100 μm. C Quantification of S100/c-Jun-pS63 double positives shown in A. n = 3 individual cultures. ***P < 0.001. D Quantification of S100/c-Jun double-positive cells shown in B. n = 3 individual cultures.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Edamakanti, C.R., Mohan, V. & Opal, P. Reactive Bergmann glia play a central role in spinocerebellar ataxia inflammation via the JNK pathway. J Neuroinflammation 20, 126 (2023). https://doi.org/10.1186/s12974-023-02801-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12974-023-02801-1