Abstract
Chemotherapy-induced peripheral neuropathy (CIPN) is a primary dose-limiting side effect caused by antineoplastic agents, such as paclitaxel. A primary symptom of this neuropathy is pain. Currently, there are no effective treatments for CIPN, which can lead to long-term morbidity in cancer patients and survivors. Neuro-immune interactions occur in CIPN pain and have been implicated both in the development and progression of pain in CIPN and the resolution of pain in CIPN. We investigated the potential role of inducible co-stimulatory molecule (ICOS) in the resolution of CIPN pain-like behaviors in mice. ICOS is an immune checkpoint molecule that is expressed on the surface of activated T cells and promotes proliferation and differentiation of T cells. We found that intrathecal administration of ICOS agonist antibody (ICOSaa) alleviates mechanical hypersensitivity caused by paclitaxel and facilitates the resolution of mechanical hypersensitivity in female mice. Administration of ICOSaa reduced astrogliosis in the spinal cord and satellite cell gliosis in the DRG of mice previously treated with paclitaxel. Mechanistically, ICOSaa intrathecal treatment promoted mechanical hypersensitivity resolution by increasing interleukin 10 (IL-10) expression in the dorsal root ganglion. In line with these observations, blocking IL-10 receptor (IL-10R) activity occluded the effects of ICOSaa treatment on mechanical hypersensitivity in female mice. Suggesting a broader activity in neuropathic pain, ICOSaa also partially resolved mechanical hypersensitivity in the spared nerve injury (SNI) model. Our findings support a model wherein ICOSaa administration induces IL-10 expression to facilitate neuropathic pain relief in female mice. ICOSaa treatment is in clinical development for solid tumors and given our observation of T cells in the human DRG, ICOSaa therapy could be developed for combination chemotherapy—CIPN clinical trials.
Highlights
-
ICOS agonist antibody (ICOSaa) promotes paclitaxel-evoked pain resolution in female mice.
-
DRG T cells appear to enter an anti-inflammatory phenotype by ICOSaa treatment.
-
ICOSaa treatment increases DRG levels of IL-10 cytokine.
-
ICOSaa effects in female mice are blocked by IL-10 sequestering treatment.
Similar content being viewed by others
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a debilitating condition due to the dose-limiting side effects of antineoplastic agents. 30–70% of patients receiving chemotherapy treatment experience numbness, reduced proprioception, and pain in their extremities [1]. CIPN is predominately sensory-related, causing damage to the peripheral nervous system with some motor deficits [2, 3]. Paclitaxel is a chemotherapy agent primarily used to treat breast, lung, and ovarian cancers. It acts through the stabilization of microtubules which leads to mitotic arrest [4]. Paclitaxel causes neurotoxicity by accumulating in the dorsal root ganglia (DRG) and in the peripheral nerves impairing axonal transport and causing mitochondrial dysfunction that is linked to the neuropathy [5,6,7,8,9,10,11,12]. It is now well-accepted that the immune system can play an active role in modulating chronic pain progression and resolution [13, 14]. Neuro-immune interactions occur in CIPN, and both the adaptive and innate immune systems play an essential role in the progression and resolution of neuropathic pain [12, 13, 15]. For instance, immune cells have been shown to secrete both pro- and anti-inflammatory cytokines such as IL-6, IL-5, IL-4, IL-10, IFNγ and TNFα that can interact with neurons and regulate chronic pain [16,17,18,19].
Immune cells and their interactions with the peripheral nervous system (PNS) have emerged as a key factor in both promoting pain and in the resolution of pain in CIPN mouse models [13, 20]. While monocytes are likely important for driving pain in CIPN [21], distinct types of T cells have been shown to decrease pain in CIPN. T regulatory cells have been shown to reduce mechanical hypersensitivity [17], whereas CD8 + T cells need to be primed to promote pain resolution in CIPN models [22, 23].
T cell activation occurs when the T cell receptor (TCR) interacts with major histocompatibility complex I and II expressed on antigen-presenting cells (APC), dendritic cells (DC), macrophages, and B cells; however, secondary signaling is required for proper facilitation of T cell activation [24]. The CD28 co-stimulatory family of receptors provides this secondary signal for the activation and survival of T cells. Inducible co-stimulatory molecule (ICOS) is a member of the CD28 family, and an immune checkpoint receptor expressed on activated T cells [25]. It is known to induce an immune response by binding to its exclusive ICOS ligand (ICOSL) expressed on APC, DC, B cells, and tumor cells [24]. ICOS, upon activation, generates a signaling cascade on various subsets of T cells such as the CD4 T helper cells and CD8 cytotoxic T cells [25, 26]. ICOS induction enhances proliferation and differentiation of T cells as well as the secretion of cytokines such as IL-4 and IL-10 [26, 27]. Reducing ICOS expression or blocking its receptor-mediated action inhibits the production of anti-inflammatory cytokine IL-10 [28]. This makes ICOS-ICOSL an attractive therapeutic target by using the ICOS agonist antibody (ICOSaa) for cancer immunotherapy [26] and potentially for neuropathic pain.
Previous studies have demonstrated that cytokine signaling is an important driver of CIPN, and this is a primary mechanism of neuro-immune modulation [11, 13, 19]. Paclitaxel promotes an increase in pro-inflammatory cytokines such as TNFα and IL1β with the suppression of anti-inflammatory cytokine IL-10 and IL-4 [29,30,31]. IL-10, an anti-inflammatory cytokine, exerts a neuroprotective and pain-relieving effect in CIPN, osteoarthritis, and chronic constriction injury-induced neuropathic pain [32,33,34]. IL-10 is secreted by innate and adaptive immune cells during various challenges [35, 36]. The IL-10 receptor (IL-10R) is expressed in DRG neurons where its activation suppresses the excitability of nociceptors providing a plausible cellular mechanism for the relief of pain promoted by IL-10 [22, 33, 37,38,39]. Based on this foundation of evidence, we hypothesized that activation of ICOS signaling on T cells in the DRG, could lead to the secretion of IL-10 cytokine and alleviate paclitaxel-induced peripheral neuropathic pain.
Our hypothesis was that ICOSaa treatment would promote pain resolution in paclitaxel-induced peripheral neuropathic pain in mice. We found that paclitaxel leads to T cell infiltration in the DRG, which supports the idea of targeting ICOS signaling for pain resolution. Administration of ICOSaa attenuated hind paw mechanical hypersensitivity in female mice previously treated with paclitaxel. ICOSaa also reduced astrogliosis in the dorsal horn of the spinal cord and satellite cell gliosis in the DRG. ICOSaa treatment led to enhanced expression of anti-inflammatory cytokine IL-10 in the DRG. Consistent with this, IL-10 receptor blocking treatment occluded the beneficial effect of ICOSaa treatment in the CIPN model. Our findings demonstrate a new mechanism for stimulating T cells to promote pain resolution via ICOSaa treatment.
Materials and methods
Animals
ICR mice were maintained and bred at the animal facility at the University of Texas at Dallas. Experiments were performed using 8- to 12-week-old female and male littermates. The mice were housed (4 maximum/cage) with food and water ad libitum in a 12-h light–dark cycle and maintained at room temperature (21 ± 2 °C). All procedures were approved by the Institutional Animal Care and Use Committee at University of Texas at Dallas.
Injections
Paclitaxel was dissolved in a solvent containing a mix of Kolliphor EL (Sigma-Aldrich)–ethanol (1:1). Mice received 4 mg/kg of paclitaxel every other day, for a cumulative intraperitoneal dosage of 16 mg/kg, or vehicle control. The most widely used dosage of paclitaxel in humans is 175 mg/m2 given as a 3-h infusion, also prepared in Kolliphor EL, equating to just over 2 mg/kg in the average human [40]. Given differences in metabolism between mice and humans, these doses are similar. Intrathecal injections of Inducible co-stimulatory (ICOS) agonist antibody (C398.4A Biolegend, 0.5 µg/µl) in total volume of 5 µl volume or PBS as vehicle control was administered using a 30-gauge (0.5") needle and Hamilton syringe for four consecutive days under isoflurane anesthesia starting from day 8 after the last dosage of paclitaxel. A tail flick was observed in mice as evidence of successful entry into the intradural space for intrathecal injections [41]. This route of administration was used to target the fluid that bathes the DRG and because the systemic administration would result in widespread activation of T cells, which we wanted to avoid in our study. Since no previous intrathecal experiments have been done with ICOSaa we chose an injection concentration of antibody in the single digit μM range (estimated at 3 μM) expecting dilution in the intrathecal space into the nM range. For the IL-10 receptor blocking treatment, mice received intraperitoneally 250 µg of InVivoMAb anti-mouse IL-10R antibody (CD210, Bio X cell) or InVivoMAb rat IgG1 isotype control, anti-horseradish peroxidase antibody (Bio X cell) vehicle control twice weekly during the entire course of the experiment.
Behavioral testing
Mechanical withdrawal thresholds
Mice were habituated for 1 h before testing for mechanical hypersensitivity in a clear acrylic behavioral chamber. Mechanical paw withdrawal threshold was tested using the up-down method [42] using calibrated von Frey filaments (Stoelting) perpendicular to the mid plantar surface of the hind paw. A positive response consisted of an immediate flicking or licking behavior upon applying the filament to the hind paw. The investigator was blinded to treatment conditions during all days of testing.
Rotarod
A cohort of mice were assessed for motor coordination using a rotarod (IITC Life Science rotarod series 8). The animals were tested on a slowly rotating rod with ramp speed (20 rpm acceleration) for a maximum of 120 s. Prior to ICOSaa administration the animals were trained for 3 trials and then baselined by taking an average of 3 trails. Intrathecal administration of ICOSaa was injected for four consecutive days. All mice were tested until day 10 after administration of the ICOSaa and their scores were averaged.
Flow cytometry analysis
Mice were euthanized under isoflurane anesthesia by cervical dislocation on day 13 after intrathecal injection of ICOSaa. L3–L5 DRGs were dissected and minced using scissors in the lysis buffer containing 1.6 mg/mL collagenase (Worthington), 10 mM HEPES (Thermo Fisher Scientific), and 5 mg/ml of Bovine serum albumin (Thermo Fisher Scientific) at 37 ºC for 30 min followed by a gentle trituration. The digested tissue was passed through a 70 µm cell strainer, and the samples were centrifuged at 600 × g at 4 ºC for 5 min. The supernatant was discarded, and the cell pellet was incubated with red blood cell (RBC) lysis buffer (Biolegend) for 5 min at room temperature followed by a wash with 1% bovine serum albumin in Phosphate buffer saline (PBS) (Thermo Fisher Scientific). The resuspended cell pellet was stained for anti-mouse CD3-PE (1:200, 145-2C11 Biolegend), anti-mouse CD45-APC/Cy7 (1:200, 30-F11 Biolegend), anti-mouse CD8a-PE/Cy7 (53–6.7 Biolegend), anti-mouse CD4-PE-Cyanine 5.5 (RM4-5 Biolegend) in 1% BSA-PBS for 30 min in the dark at 4ºC followed by three washes with 1% bovine serum albumin in PBS. The cell samples were acquired using BD LSR Fortessa (BD Biosciences) flow cytometer using BDFACS DIVA software (BD Biosciences) and analyzed with FlowJo software (v.10, FlowJo). Flow cytometry analysis was performed by first gating for CD45-positive hematopoietic cells followed by gating for single-cell lymphocytes. We did not perform live/dead cell gating but all T cells were identified as CD45pos + CD3pos, subsets of T helper cells as CD45pos + CD3pos + CD4pos and cytotoxic T cell subset as CD45pos + CD3pos + CD8apos [43].
Immunohistochemistry
Mice were euthanized under isoflurane anesthesia by cervical dislocation on day 13 after the last administration of ICOSaa. DRGs and spinal cord were dissected and fresh-frozen in optimum cutting temperature (OCT) medium (Fisher Scientific). Human tissue issue procurement procedures were approved by the Institutional Review Boards at University of Texas at Dallas. Donor information is provided in Table 1. Human dorsal root ganglion were collected within 4 h of cross-clamp, frozen on dry ice and later embedded on OCT to cut on the cryostat. All tissues were sectioned at 20 µm using a cryostat (Leica) and placed on the SuperFrost charged side of the slides. The tissue sections were fixed in 4% cold formaldehyde (Thermo Fisher Scientific) for 10 min, followed by dehydration with increasing ethanol concentrations from 50%, 75%, and 100% for 5 min each. The sections were blocked for one hour using 10% normal goat serum (R&D systems) with 0.3% Triton-X100 (Sigma-Aldrich). The tissue sections were incubated with primary antibodies Glial fibrillary acidic protein (GFAP), Glutamine Synthetase (GS), Peripherin (PRPH), CD8a, CD4 (Table 2) diluted in blocking solution at 4 ºC overnight, followed by the corresponding secondary antibody with DAPI diluted in blocking solution for 1 h at room temperature. The slides were washed in 0.1 M PB and cover-slipped using Prolong Gold Antifade (Fisher Scientific P36930). Images were taken on Olympus FluoView 1200 confocal microscope or Olympus FluoView 3000 confocal microscope, using the same settings for all images per experiment.
Image analysis of the dorsal horn of the lumbar spinal cord were obtained by calculating the corrected total cell fluorescence (CTCF) intensity of GFAP using the formula CTCF = Integrated Density – (Area of selected cell × Mean fluorescence of background readings). CTCF values were normalized by the area the images captured and analyses of spinal cord images were done using ImageJ version 1.48 (National Institutes of Health, Bethesda, MD). A total of 3 sections per animal were analyzed.
L3–L5 DRG images were taken using the Olympus FluoView 3000 confocal microscope using the same setting for all images. A region of interest (ROI) was drawn around individual neurons to measure the mean grey intensity value (MGI). Peripherin was used as a guide to identify neurons, but all neurons were included in the calculation. Background fluorescence was also measured using negative control subjected to blocking and secondary antibody with no primary. An average intensity was calculated after subtracting the MGI values of negative control and normalized over the area of the individual ROI. Image analysis was performed using Olympus cellSens software.
Enzyme-linked immunosorbent assay (ELISA)
DRGs were dissected on day 13 after the last administration of ICOSaa, and flash frozen on dry ice. The frozen tissue was homogenized using a sonicator in lysis buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EDTA, and 1% Triton X-100) containing protease and phosphatase inhibitors (Sigma-Aldrich) and briefly centrifuged to extract the protein from the supernatant. Pierce BCA Protein Assay (Thermo Fisher Scientific) was performed by following the manufacturer’s protocol to identify the amount of protein in each sample. Enzyme-linked immunosorbent assay to detect IL-10 (Thermo Fisher Scientific—88-7105-22) cytokine was performed according to the manufacturer’s instructions.
Surgery
Spared nerve injury (SNI) was performed as previously described [44], sparing the sural branch and ligating and cutting the peroneal and tibial branches at the left sciatic nerve trifurcation. Sham control was performed the same way but without cutting any nerve. Mice were allowed to recover for 2 weeks post-surgery before administration of the ICOSaa treatment and testing for mechanical von Frey thresholds.
Data analysis and statistics
All analyses and data were generated using GraphPad Prism 8.4.1. Statistical analysis between groups were determined using one or two-way ANOVA, followed by Bonferroni, Sidak or Tukey post hoc tests. Differences between two groups were assessed using Student’s t-test. Statistical results can be found in the figure legends. Effect sizes were determined by subtracting behavior scores starting with ICOSaa administration (day 8) from baseline measures. Absolute values were summed from the beginning of ICOSaa treatment (starting from day 8) and plotted for each group. All data were represented as mean ± SEM with p < 0.05 considered significant. For determination of minimum sample sizes for behavioral experiments we did a power calculation using G-power. We estimated a minimum effect size of 2.063 between control and treated groups based on an expectation of nearly complete reversal of mechanical hypersensitivity based on previous studies from our lab where pain resolution was observed in this model [11]. Alpha was set at 0.05 and power (1-ß err) was set at 0.8. Based on this, a minimum sample size per sex for mechanical withdrawal threshold effect size was calculated at 4 mice per group. The sample size, which exceed 4 for each group in all experiments, and sex are noted in the graphs and figure legends.
Results
ICOSaa accelerates the resolution of paclitaxel-induced peripheral neuropathic mechanical hypersensitivity in female mice
T cells activation can occur with the engagement of ICOS similar to CD28 [45, 46]. For that reason, we first assessed the T cell response after paclitaxel administration using flow cytometry. We measured the number of CD3-positive T cells by gating for singlets with CD45-positive lymphocytes, followed by subsequent gating for CD3-positive cells (Fig. 1A, B). We observed a significant increase in the percentage of CD3-positive T cells in the DRG day 13 after the start of paclitaxel treatment compared to vehicle control (Fig. 1C). Having confirmed an increase in the number of T cells in the DRG in mice treated with paclitaxel, we then tested whether ICOSaa would have any effect on paclitaxel-induced mechanical hypersensitivity. To do this, we treated both male and female mice with paclitaxel every other day for a total of 4 injections and cumulative dose of 16 mg/kg, which produced mechanical hypersensitivity in both sexes (Fig. 2A). On the final day of paclitaxel treatment, we started once daily treatment of ICOSaa (0.5 μg/μL) or vehicle given intrathecally for 4 consecutive days. Qualitatively, animals of both sexes treated with ICOSaa showed a more rapid resolution of mechanical hypersensitivity, but a significant treatment effect was only seen in female mice by two-way ANOVA (Fig. 2B). There was a significant effect on the overall effect size of ICOSaa treatment in female mice (Fig. 2C). While there was a trend to faster resolution of mechanical hypersensitivity in male mice treated with ICOSaa, the effect was not significantly different from vehicle either at individual time points (Fig. 2D) or in effect size (Fig. 2E). Female mice treated with intrathecal injection of ICOSaa alone for four consecutive days showed no significant differences compared to their baseline paw withdrawal thresholds (Fig. 2F) and their motor function assessed by rotarod also showed no significant differences compared to their baseline measures (Fig. 2G). Given the clear effect seen in female mice of ICOSaa, and the far greater proportion of women treated with paclitaxel for cancer, we focused on the female mice for the remainder of the study.
Paclitaxel treatment promotes infiltration of T cells into the DRG. A Flow cytometry gating strategy for T cells in female mice gated for CD45pos singlets isolated from L3–L5 DRG on day 13 after paclitaxel treatment. B Representative flow cytometry plots for CD3pos T cells (previously gated for CD45pos singlets) on day 13 in mice treated with paclitaxel or vehicle. C Paclitaxel treatment was associated with a significant increase in the influx of T cells in the DRG measured by flow cytometry (unpaired t-test, t = 3.601, p-value = 0.0036, df = 12) N = 7/group. Data are represented as mean ± SEM
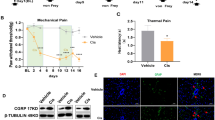
ICOS agonist antibody (ICOSaa) promotes the resolution of mechanical hypersensitivity in paclitaxel-induced peripheral neuropathic pain. A The cohorts of mice were subjected to intraperitoneal injection of 4 mg/kg paclitaxel every other day for a cumulative dosage of 16 mg/kg according to the schema shown followed by intrathecal injection of ICOSaa or vehicle for four consecutive days. Arrows represent days of von Frey testing. Paclitaxel group represented in yellow and Ptx + ICOSaa represented in blue. B Female mice reversed mechanical allodynia after intrathecal administration of ICOSaa (two-way ANOVA, F = 4.951, p-value < 0.0001, post hoc Sidak’s, Ptx + ICOSaa vs. Ptx, p-value = 0.0318 at day 8, Ptx + ICOSaa vs. Ptx, p-value = 0.0323 at day 15, Ptx + ICOSaa vs. Ptx, p-value = 0.0001 at day 28, Ptx + ICOSaa vs. Ptx, p-value = 0.0067 at day 32, Ptx + ICOSaa vs. Ptx, p-value = 0.0.0261 at day 36), N = 8/group C Effect size was determined by calculating the cumulative difference between the value for each time point and the baseline value. The effect size difference was significant in the female cohort of mice (effect size, unpaired t test, t = 4.963, p-value = 0.0002, df = 14). D Male mice showed a trend in resolution of mechanical hypersensitivity measured with von Frey filaments after administration of ICOSaa but it was not significant (two-way ANOVA, F = 2.020, p-value = 0.0544), N = 6/group. E We did not observe any statistically significant differences between the groups in male (Effect size, unpaired t test, t = 1.533, p-value = 0.1564, df = 10). F Paw withdrawal thresholds were determined with von Frey filaments after ICOSaa treatment alone and was not significant compared to baseline measures in female mice (one-way ANOVA, F = 2.049, p-value = 0.1529), N = 8. G Motor function was assessed using the rotarod after administration of ICOSaa and compared to baseline in female mice (one-way ANOVA, F = 1.28, p-value = 0.3075), N = 8. *p < 0.05, **p < 0.01, ***p < 0.001
ICOSaa reverses satellite cell gliosis in the DRG and astrogliosis in the spinal cord in paclitaxel-treated female mice
Since we observed a resolution of mechanical allodynia in the ICOS-treated animals, we next examined glial cell changes in the DRG and the spinal cord [12, 43, 47, 48]. Gliosis is a sign of injury or stress to the tissue, where the cells become hyperactive and change morphology [49]. We performed immunohistochemistry to quantify the expression of glutamine synthetase [43] in satellite glial cells (SGC) in the DRG and GFAP in astrocytes in the dorsal horn of lumbar spinal cord. We observed an increase in Glutamine Synthetase (GS) expression in paclitaxel treated animals and a trend towards a reduction in animals treated with ICOSaa (Fig. 3A, B). Expression of GFAP protein was increased in the dorsal horn of lumbar spinal cord in paclitaxel-treated animals and this was reduced in cohorts subjected to ICOSaa treatment (Fig. 4A–C).
ICOSaa inhibits satellite cell gliosis in paclitaxel treated mice in the DRG. A Representative images of GS that labels satellite glial cells in the DRG, PRPH (peripherin, purple) and Dapi (blue). B GS trends towards a reduction in female animals treated with ICOSaa and calculated using MGI (one-way ANOVA, F = 6.399, p-value = 0.0128, post hoc Tukey, Vehicle vs. Ptx, p-value = 0.0159, Ptx vs. Ptx + ICOSaa, p-value = 0.0612) N = 5/group. Data are represented as mean ± SEMs. Scale bar = 50 µm
ICOSaa reverses astrocyte gliosis in the spinal cord in paclitaxel treated mice. A Representative image of astrocyte labeling using GFAP antibody in the spinal cord of Vehicle, Ptx, and Ptx + ICOSaa groups from female mice. B Representative images of the dorsal horn of the spinal cord of vehicle, Ptx, and Ptx + ICOSaa animal cohorts stained with GFAP for astrocyte labeling and DAPI (blue). C Paclitaxel animals showed increased expression of GFAP in the dorsal horn of the spinal cord. This was significantly reduced in mice treated with ICOSaa and calculated using CTCF (one-way ANOVA, F = 18.42, p-value = 0.0002, post hoc Tukey, Vehicle vs. Ptx, p-value = 0.0002, Ptx vs. Ptx + ICOSaa, p-value = 0.0048, Vehicle vs. Ptx + ICOSaa, p-value = 0.2485) N = 5/group. Data are represented as mean ± SEMs. Scale bar = 100 µm and 200 µm, respectively
IL-10R antagonist blocks effects of ICOSaa on paclitaxel-induced mechanical hypersensitivity
Paclitaxel-treated female mice that were then treated with ICOSaa showed a significant reduction in mechanical hypersensitivity. We hypothesized that this effect could be driven by IL-10 based on multiple studies demonstrating the T cell derived IL-10 can resolve neuropathic pain, in particular in female mice [22, 33, 50]. We injected IL-10R antagonist antibody or isotype control twice every week to block the activity of IL-10 at IL-10R, using the same paclitaxel and ICOSaa dosing schedule as described above (Fig. 5A). We observed that while the IL-10R antagonist antibody had no effect on its own, it completely blocked the effect of ICOSaa treatment in paclitaxel-treated female mice (Fig. 5B, C). This finding suggests that the mechanism by which ICOSaa resolves paclitaxel-induced mechanical hypersensitivity is through the secretion of anti-inflammatory IL-10. Next, we assessed if IL-10 expression could be increased in the DRG after ICOSaa administration. We measured IL-10 concentration in the DRG on day 13, the day after the end of ICOSaa treatment, using an ELISA assay. We observed a significant increase in production of IL-10 cytokine in the ICOSaa-treated cohort compared to paclitaxel or vehicle control (Fig. 5D) [51].
IL-10R antagonist reverses the effect of ICOSaa treatment in mice treated with paclitaxel. A Schematic representation of experimental design. Arrow represents days with von Frey testing. Groups are shown as: Vehicle + IL-10Ra (grey), Ptx + IgG (yellow), Ptx + ICOSaa + IgG (blue), Ptx + ICOSaa + IL-10Ra (pink), Ptx + IL-10Ra [32]. B Mechanical nociceptive thresholds are shown for each group with statistical differences represented in the graph (two-way ANOVA, F = 1.921, p-value = 0.0048, post hoc Bonferroni, Vehicle + IL-10Ra vs. Ptx + IgG, p-value = 0.0362, Vehicle + IL-10Ra vs. Ptx + ICOSaa + IgG, p-value = ns, Vehicle + IL-10Ra vs. Ptx + IL-10Ra, p-value = 0.0485, Vehicle + IL-10Ra vs. Ptx + ICOSaa + IL-10Ra, p-value = 0.0452, $Ptx + IgG vs Ptx + ICOSaa + IgG, p-value = 0.0574, Ptx + IgG vs Ptx + IL-10Ra, p-value = ns, Ptx + IgG vs. Ptx + ICOSaa + IL-10Ra, p-value = ns, #Ptx + ICOSaa + IgG vs. Ptx + IL-10Ra, p-value = 0.0697, *Ptx + ICOS + IgG vs. Ptx + ICOS + IL-10Ra, p-value = 0.0697, Ptx + ICOSaa + IL-10Ra vs. Ptx + IL-10Ra, p-value = ns). N = 4, 5, 5, 5, 5 per group, respectively. C The effect size was significant between Ptx + ICOSaa + IL-10Ra and Ptx + ICOSaa + IgG1 (one-way ANOVA, F = 7.211, p-value = 0.0010, post hoc Tukey, Vehicle + IL-10Ra vs. Ptx + IgG, p-value = 0.0108, Vehicle + IL-10Ra vs. Ptx + ICOSaa + IgG, p-value = ns, Vehicle + IL-10Ra vs. Ptx + IL-10Ra p-value = 0.0173, Vehicle + IL-10Ra vs. Ptx + ICOSaa + IL-10Ra, p-value = 0.0079, Ptx + IgG vs. Ptx + ICOSaa + IgG, p-value = 0.0301, Ptx + IgG vs. Ptx + IL-10Ra, p-value = ns, Ptx + ICOSaa + IgG vs.Ptx + IL-10Ra, p-value = 0.0485, Ptx + IL-10Ra + IgG vs.Ptx + ICOSaa + IL-10Ra, p-value = ns). D A significant increase in IL-10 expression was observed in mice subjected to intrathecal injection of ICOSaa using ELISA (one-way ANOVA, F = 8.845, p-value = 0.0075, post hoc Tukey, Vehicle vs. Ptx, p-value = ns, Vehicle vs. Ptx + ICOSaa, p-value = 0.0079, Ptx vs. Ptx + ICOSaa, p-value = 0.0293) N = 4/group. E Representative flow cytometry plots of subset of T cells CD4-positive and CD8-positive (previously gated on CD45posCD3pos) in each group after treatment, isolated on day 13 from L3–L5 DRG. F Percentage of CD4-positive T cells was increased in Ptx and Ptx + ICOSaa groups compared to vehicle treated cohort and no significant changes were observed between Ptx and Ptx + ICOSaa groups (one-way ANOVA, F = 10.86, p-value = 0.004, post hoc Tukey, Vehicle vs. Ptx,p-value = 0.0073, Vehicle vs. Ptx + ICOSaa, p-value = 0.076, Ptx vs. Ptx + ICOSaa, p-value = ns) N = 4/group. G Percentage of CD8-positive T cells were increased only in Ptx + ICOSaa group compared to vehicle control (one-way ANOVA, F = 4.881, p-value = 0.0367, post hoc Tukey, Vehicle vs. Ptx, p-value = ns, Vehicle vs. Ptx + ICOSaa, p-value = 0.0301, Ptx vs. Ptx + ICOSaa p-value = ns), N = 4/group, *#$p < 0.05
Building on our observation that paclitaxel administration recruits T cells into the DRG, we sought to further examine the subsets of T cells infiltrating the DRG after administration of ICOSaa. We performed flow cytometry analysis by gating for T cell subsets as CD45posCD3posCD4pos and CD45posCD3posCD8apos (Fig. 5E). We saw no significant differences in the percentage of CD4 and CD8 positive T cells between ICOSaa-treated cohort and paclitaxel-treated mice (Fig. 5F and G). We observed an increase in the frequency of CD8-positive T cells in ICOSaa mice. This cell type is known to promote upregulation of IL-10 in the DRG [22, 23]. This further suggests that ICOSaa either directly activates T cells or does so indirectly to increase endogenous IL-10 expression promoting the resolution of paclitaxel-induced neuropathic mechanical hypersensitivity in female mice.
ICOS agonist antibody alleviates mechanical hypersensitivity in the SNI model
We next investigated if ICOSaa treatment could have an effect on mechanical hypersensitivity in the SNI neuropathic pain model. We performed SNI or sham surgery and administered four consecutive doses of ICOSaa 2 weeks post-surgery (Fig. 6A). We observed an inhibition of mechanical hypersensitivity with ICOSaa treatment in female SNI mice on days 17 and 19 post SNI. This effect was transient and lasted only until the last dose of ICOSaa administration (Fig. 6B). The effect size was also significant in animals treated with ICOSaa compared to vehicle (Fig. 6C).
ICOSaa partially reverses mechanical allodynia in female SNI mice. A Schematic representation of groups subjected to SNI on day one followed by intrathecal injection of ICOSaa on days 14–17. Arrows represent days with von Frey testing. B Mechanical hypersensitivity was partially reversed only on days 17 and 19 in the group administered with ICOSaa group (two-way ANOVA, F = 3.473, p-value < 0.0001, post hoc Bonferroni, ***SNI vs. SNI + ICOSaa at day 17, p-value = 0.0006, ***SNI vs. SNI + ICOSaa at day 19, p-value = 0.0003), N = 7/group. C Effect size was determined by calculating the cumulative difference between the value for each time point and the baseline value. The effect size was significant between SNI and SNI + ICOSaa-treated mice (one-way ANOVA, F = 18.0, p-value < 0.0001, post hoc Tukey, Sham vs. SNI + ICOSaa, p-value = 0.0265, Sham vs. SNI, p = value < 0.0001, SNI vs. SNI + ICOS, p-value = 0.0151). ***p < 0.001
Presence of T cells in human DRG
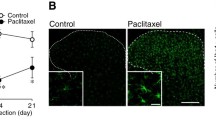
Our findings suggest that T cells in the DRG can be manipulated by ICOSaa treatment to increase IL-10 expression and alleviate neuropathic pain. To examine the translational potential of this approach, we investigated whether T cells are found in the human DRG using DRGs recovered from organ donors. We performed IHC using markers for CD4 and CD8 T cells. We observed that both CD4 and CD8 T cells were present in the DRG of organ donors, with many of these cells clustered around neurons (Fig. 7). This is in contrast with findings in naïve mouse DRG, where T cells are not present or are found only in low numbers [22]. This finding supports the translational potential of ICOSaa treatment for neuropathic pain.
T cell expression in human DRG. A representative image of CD4 (white) peripherin (purple), DAPI (blue) T cell expression in human dorsal root ganglion. B representative image of CD8 (white) peripherin (purple), DAPI (cyan) T cell expression in human DRG. The white arrows point towards CD4 or CD8 T cell. Scale bar = 50 µm and 20 µm, respectively
Discussion
Our results support the hypothesis that targeting ICOS molecule facilitates the resolution of neuropathic pain in female mice. ICOS treatment enhanced the production of the anti-inflammatory cytokine IL-10 in the DRG of paclitaxel treated female mice, that subsequently, led to reversal of mechanical allodynia. Our findings are in line with literature that T cells can play a beneficial role in promoting neuropathic pain resolution [13, 22, 52]. Our study suggests a path for development of a completely new strategy for exogenously modulating T cells to facilitate pain resolution. Our work also shows that T cells infiltrate into the DRG after paclitaxel treatment. This is likely critical for pain resolution induced by ICOSaa as ICOS is thought to only be present on the surface of activated T cells [53, 54]. In a recent study, CD8 T cells were shown to play a key role in alleviating CIPN induced by cisplatin via an increase in the expression of IL-10R in the DRG [22, 23]. These observations are consistent with our results that administration of paclitaxel promotes T cell infiltration into the DRG and that ICOSaa treatment likely directs these cells towards a phenotype that promotes pain resolution.
ICOSaa treatment has been shown to mitigate disease severity in autoimmune disorders, lupus and cancers [25]. ICOS-ICOSL signaling has dual functions. On the one hand, it can suppress T regulatory cells and on the other it can cause T effector cells to express and secrete anti-inflammatory cytokines such as IL-4 and IL-10 [55]. Here we show that administration of ICOSaa increases anti-inflammatory cytokine IL-10 in the female mouse DRG. We suggest this administration activated the ICOS-ICOSL signaling cascade [56,57,58] to promote the release of anti-inflammatory, pro-pain resolution factors. ICOSaa treatment led to resolution of mechanical hypersensitivity in paclitaxel-treated female mice and also trended towards the reversal of satellite cell gliosis in the DRG and reversed astrocyte gliosis in the dorsal horn of the spinal cord. This action on cellular measures of CIPN suggests the possibility of disease-modifying properties of ICOSaa treatment. Further studies will be needed to ascertain if ICOSaa treatment can also prevent the development of CIPN with paclitaxel treatment and/or if it is effective in other species. The observation of T cells in the DRGs of organ donors is promising from the perspective of clinical translation for these findings.
Cytokines are an important group of molecules that regulate the excitability of nociceptors and for this reason are intensively investigated for the treatment of pain where agonist and antagonist cytokine therapies are under development [59]. IL-10 has been shown to alleviate CIPN and rheumatoid arthritis [32]. In vitro studies show that ICOSaa activates the secretion of anti-inflammatory cytokines such as IL-4 and IL-10 [60]. Our results are in line with this literature as we have shown here that ICOSaa administration likely leads to secretion of the anti-inflammatory cytokine IL-10 in the DRG because expression of the cytokine was increased and effects of ICOSaa were blocked by IL-10 sequestering treatment. IL-10R is expressed on sensory neurons in the rodent DRG, and also on human DRG neurons [61], and its activation in rodent nociceptors reduces their excitability [33]. We are not aware of evidence that IL-10R is expressed by SGCs or astrocytes in the spinal cord, and single-cell sequencing suggests that this receptor is exclusively found in neurons in the DRG [62]. Based on this, we speculate that the effect on astrogliosis and SGC gliosis is caused by the direct effects of IL-10 on DRG neurons. Reversing spontaneous activity in these neurons may allow for resolution of gliosis in the DRG and dorsal horn. Although IL-10 therapy can alleviate neuropathic pain, IL-10 has a short half-life under physiological conditions and the plasmid-based DNA therapy which showed promising results on canines with osteoarthritis is not yet FDA approved [32, 63]. Clinical trials with systemic administration of IL-10 did not downregulate inflammation due to the low concentration accumulated at the site of inflammation and higher dose IL-10 administration can be detrimental [64]. Our hypothesized model of educating T cells to release IL-10 with ICOSaa may be able to overcome some of these limitations with a therapeutic approach that is nearing the clinic for other indications [65].
We observed a transient anti-nociceptive effect with the administration of ICOSaa in the SNI model of neuropathic pain. A possible explanation for this result could be that the mechanisms for the development and maintenance of pain in the SNI model is not entirely T cell dependent and that plasticity is developed with nerve injury. The role of T cells in trauma-induced neuropathic pain is still somewhat controversial. A previous study using the SNI model in Rag1−/− mice (T cell deficient mice) reported less mechanical allodynia [66]. However, another study using the same model showed that T cells likely play a more important role in promoting neuropathic pain in female mice than in male mice [14]. Since this time, it has become clear that the type of T cell is critical for understanding the impact of these cells on neuropathic pain and neuropathic pain resolution [67]. Another potential reason for this difference in the duration of effect is that the SNI model creates a situation where nociceptor spontaneous activity is continuously driven by the ligation injury to the injured nerves. In the CIPN model the chemotherapy treatment is ceased prior to the ICOSaa treatment and there is no ongoing injury to drive the reemergence of neuropathic pain in this model following cessation of ICOSaa treatment.
A key finding of our study is the presence of CD8 + T cells in human DRG collected from healthy donors. This strengthens the case for the translational potential of ICOSaa. In naïve mice there are few T cells present in the DRG but the efficacy of ICOSaa treatment is presumably facilitated by the paclitaxel challenge promoting the infiltration of T cells into the DRG [22]. Our work demonstrates that both CD4 + and CD8 + T cells are present in human, healthy DRGs recovered from organ donors and are in the vicinity of neurons. This immunohistochemical finding agrees with our previous spatial transcriptomic data from organ donor DRGs where we also detected T cell gene expression [61]. It also agrees with bulk sequencing data from DRG recovered from thoracic vertebrectomy patients [68], indicating that the presence of T cells is not an artifact of post-mortem tissue recovery. To date, multiple Phase I and II clinical trials are being conducted for ICOS agonists and ICOS antagonists due to the high expression of ICOS in the tumor microenvironment and duality of function for the treatment of advance solid tumors [69, 70]. We think that our findings support the possibility that ICOS agonists could be used for pain treatment.
There are some limitations to the work described here. The first is that we cannot completely rule out a beneficial effect of ICOSaa treatment on male mice. In our study, paclitaxel treated male mice demonstrated a far more rapid resolution of mechanical hypersensitivity than female mice with vehicle treatment, so it is difficult to ascertain whether there is a treatment effect or not over the time course of the experiment in males. The faster resolution of CIPN signs in these male mice creates a problem of statistical power as the expected effect size was smaller in this cohort of mice. Another weakness is that we have not tested the effect of ICOSaa treatment in mice depleted of T cells or looked at its effect on meningeal T regulatory cells. Experiments using T cell depleted mice or examining meningeal immune cell populations could further delineate the mechanism of action of ICOSaa in neuropathic pain. Finally, our study was only done in mice. Replicating this work in another species and using human DRGs to better understand the clinical translational potential of ICOSaa treatment will be important to fully understand the translational potential of this novel approach.
Availability of data and materials
Data and materials are available upon request to the corresponding author.
References
Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155:2461–70.
Boyette-Davis JA, Cata JP, Driver LC, Novy DM, Bruel BM, Mooring DL, Wendelschafer-Crabb G, Kennedy WR, Dougherty PM. Persistent chemoneuropathy in patients receiving the plant alkaloids paclitaxel and vincristine. Cancer Chemother Pharmacol. 2013;71:619–26.
Krishnan AV, Goldstein D, Friedlander M, Kiernan MC. Oxaliplatin-induced neurotoxicity and the development of neuropathy. Muscle Nerve. 2005;32:51–60.
Malacrida A, Meregalli C, Rodriguez-Menendez V, Nicolini G. Chemotherapy-induced peripheral neuropathy and changes in cytoskeleton. Int J Mol Sci. 2019;20:2287.
Gornstein E, Schwarz TL. The paradox of paclitaxel neurotoxicity: mechanisms and unanswered questions. Neuropharmacology. 2014;76 Pt A:175–83.
Sahenk Z, Barohn R, New P, Mendell JR. Taxol neuropathy. Electrodiagnostic and sural nerve biopsy findings. Arch Neurol. 1994;51:726–9.
Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J, Koltzenburg M, Kiernan MC. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013;63:419–37.
McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 2009;8:10–6.
Duggett NA, Griffiths LA, McKenna OE, de Santis V, Yongsanguanchai N, Mokori EB, Flatters SJ. Oxidative stress in the development, maintenance and resolution of paclitaxel-induced painful neuropathy. Neuroscience. 2016;333:13–26.
Flatters SJ, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain. 2006;122:245–57.
Megat S, Ray PR, Moy JK, Lou TF, Barragan-Iglesias P, Li Y, Pradhan G, Wanghzou A, Ahmad A, Burton MD, et al. Nociceptor translational profiling reveals the ragulator-Rag GTPase complex as a critical generator of neuropathic pain. J Neurosci. 2019;39:393–411.
Agalave NM, Mody PH, Szabo-Pardi TA, Jeong HS, Burton MD. Neuroimmune consequences of eIF4E phosphorylation on chemotherapy-induced peripheral neuropathy. Front Immunol. 2021;12: 642420.
Laumet G, Ma J, Robison AJ, Kumari S, Heijnen CJ, Kavelaars A. T cells as an emerging target for chronic pain therapy. Front Mol Neurosci. 2019;12:216.
Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18:1081–3.
Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science. 2016;354:572–7.
Peters CM, Jimenez-Andrade JM, Jonas BM, Sevcik MA, Koewler NJ, Ghilardi JR, Wong GY, Mantyh PW. Intravenous paclitaxel administration in the rat induces a peripheral sensory neuropathy characterized by macrophage infiltration and injury to sensory neurons and their supporting cells. Exp Neurol. 2007;203:42–54.
Liu XJ, Zhang Y, Liu T, Xu ZZ, Park CK, Berta T, Jiang D, Ji RR. Nociceptive neurons regulate innate and adaptive immunity and neuropathic pain through MyD88 adapter. Cell Res. 2014;24:1374–7.
Zhang H, Li Y, de Carvalho-Barbosa M, Kavelaars A, Heijnen CJ, Albrecht PJ, Dougherty PM. Dorsal root ganglion infiltration by macrophages contributes to paclitaxel chemotherapy-induced peripheral neuropathy. J Pain. 2016;17:775–86.
Brandolini L, d'Angelo M, Antonosante A, Allegretti M, Cimini A. Chemokine signaling in chemotherapy-induced neuropathic pain. Int J Mol Sci. 2019;20(12):2904.
Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14:217–31.
Montague K, Malcangio M. The therapeutic potential of monocyte/macrophage manipulation in the treatment of chemotherapy-induced painful neuropathy. Front Mol Neurosci. 2017;10:397.
Krukowski K, Eijkelkamp N, Laumet G, Hack CE, Li Y, Dougherty PM, Heijnen CJ, Kavelaars A. CD8+ T cells and endogenous IL-10 are required for resolution of chemotherapy-induced neuropathic pain. J Neurosci. 2016;36:11074–83.
Laumet G, Edralin JD, Dantzer R, Heijnen CJ, Kavelaars A. Cisplatin educates CD8+ T cells to prevent and resolve chemotherapy-induced peripheral neuropathy in mice. Pain. 2019;160:1459–68.
Wikenheiser DJ, Stumhofer JS. ICOS co-stimulation: friend or foe? Front Immunol. 2016;7:304.
Amatore F, Gorvel L, Olive D. Inducible co-stimulator (ICOS) as a potential therapeutic target for anti-cancer therapy. Expert Opin Ther Targets. 2018;22:343–51.
Amatore F, Gorvel L, Olive D. Role of inducible co-stimulator (ICOS) in cancer immunotherapy. Expert Opin Biol Ther. 2020;20:141–50.
Maeda S, Fujimoto M, Matsushita T, Hamaguchi Y, Takehara K, Hasegawa M. Inducible costimulator (ICOS) and ICOS ligand signaling has pivotal roles in skin wound healing via cytokine production. Am J Pathol. 2011;179:2360–9.
Kohyama M, Sugahara D, Sugiyama S, Yagita H, Okumura K, Hozumi N. Inducible costimulator-dependent IL-10 production by regulatory T cells specific for self-antigen. Proc Natl Acad Sci. 2004;101:4192–7.
Lees JG, Makker PG, Tonkin RS, Abdulla M, Park SB, Goldstein D, Moalem-Taylor G. Immune-mediated processes implicated in chemotherapy-induced peripheral neuropathy. Eur J Cancer. 2017;73:22–9.
Tonini G, Santini D, Vincenzi B, Borzomati D, Dicuonzo G, La Cesa A, Onori N, Coppola R. Oxaliplatin may induce cytokine-release syndrome in colorectal cancer patients. J Biol Regul Homeost Agents. 2002;16:105–9.
Singh AK, Mahalingam R, Squillace S, Jacobson KA, Tosh DK, Dharmaraj S, Farr SA, Kavelaars A, Salvemini D, Heijnen CJ. Targeting the A3 adenosine receptor to prevent and reverse chemotherapy-induced neurotoxicities in mice. Acta Neuropathol Commun. 2022;10:11.
Watkins LR, Chavez RA, Landry R, Fry M, Green-Fulgham SM, Coulson JD, Collins SD, Glover DK, Rieger J, Forsayeth JR. Targeted interleukin-10 plasmid DNA therapy in the treatment of osteoarthritis: Toxicology and pain efficacy assessments. Brain Behav Immun. 2020;90:155–66.
Laumet G, Bavencoffe A, Edralin JD, Huo XJ, Walters ET, Dantzer R, Heijnen CJ, Kavelaars A. Interleukin-10 resolves pain hypersensitivity induced by cisplatin by reversing sensory neuron hyperexcitability. Pain. 2020;161:2344–52.
Milligan ED, Sloane EM, Langer SJ, Hughes TS, Jekich BM, Frank MG, Mahoney JH, Levkoff LH, Maier SF, Cruz PE. Repeated intrathecal injections of plasmid DNA encoding interleukin-10 produce prolonged reversal of neuropathic pain. Pain. 2006;126:294–308.
Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 2012;32:23–63.
Ng TH, Britton GJ, Hill EV, Verhagen J, Burton BR, Wraith DC. Regulation of adaptive immunity; the role of interleukin-10. Front Immunol. 2013;4:129.
Shen K-F, Zhu H-Q, Wei X-H, Wang J, Li Y-Y, Pang R-P, Liu X-G. Interleukin-10 down-regulates voltage gated sodium channels in rat dorsal root ganglion neurons. Exp Neurol. 2013;247:466–75.
Prado J, Westerink RHS, Popov-Celeketic J, Steen-Louws C, Pandit A, Versteeg S, van de Worp W, Kanters DHAJ, Reedquist KA, Koenderman L, Hack CE, Eijkelkamp N. Cytokine receptor clustering in sensory neurons with an engineered cytokine fusion protein triggers unique pain resolution pathways. Proc Natl Acad Sci. 2021;118(11):e2009647118.
Yanik BM, Dauch JR, Cheng HT. Interleukin-10 reduces neurogenic inflammation and pain behavior in a mouse model of type 2 diabetes. J Pain Res. 2020;13:3499.
Stage TB, Bergmann TK, Kroetz DL. Clinical pharmacokinetics of paclitaxel monotherapy: an updated literature review. Clin Pharmacokinet. 2018;57:7–19.
Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–6.
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63.
Makker PG, Duffy SS, Lees JG, Perera CJ, Tonkin RS, Butovsky O, Park SB, Goldstein D, Moalem-Taylor G. Characterisation of immune and neuroinflammatory changes associated with chemotherapy-induced peripheral neuropathy. PLoS ONE. 2017;12: e0170814.
Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–58.
Löhning M, Hutloff A, Kallinich T, Mages HW, Bonhagen K, Radbruch A, Hamelmann E, Kroczek RA. Expression of ICOS in vivo defines CD4+ effector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. J Exp Med. 2003;197:181–93.
Mahajan S, Cervera A, MacLeod M, Fillatreau S, Perona-Wright G, Meek S, Smith A, MacDonald A, Gray D. The role of ICOS in the development of CD4 T cell help and the reactivation of memory T cells. Eur J Immunol. 2007;37:1796–808.
Leo M, Schmitt L-I, Kutritz A, Kleinschnitz C, Hagenacker T. Cisplatin-induced activation and functional modulation of satellite glial cells lead to cytokine-mediated modulation of sensory neuron excitability. Exp Neurol. 2021;341: 113695.
Robinson CR, Zhang H, Dougherty PM. Astrocytes, but not microglia, are activated in oxaliplatin and bortezomib-induced peripheral neuropathy in the rat. Neuroscience. 2014;274:308–17.
Pekny M, Pekna M. Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol Rev. 2014;94:1077–98.
Ledeboer A, Jekich BM, Sloane EM, Mahoney JH, Langer SJ, Milligan ED, Martin D, Maier SF, Johnson KW, Leinwand LA, et al. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun. 2007;21:686–98.
Sakthivel P, Gereke M, Breithaupt A, Fuchs D, Gigliotti L, Gruber AD, Dianzani U, Bruder D. Attenuation of immune-mediated influenza pneumonia by targeting the inducible co-stimulator (ICOS) molecule on T cells. PLoS ONE. 2014;9: e100970.
Mao L, Li P, Zhu W, Cai W, Liu Z, Wang Y, Luo W, Stetler RA, Leak RK, Yu W, et al. Regulatory T cells ameliorate tissue plasminogen activator-induced brain haemorrhage after stroke. Brain. 2017;140:1914–31.
Lees JG, Duffy SS, Perera CJ, Moalem-Taylor G. Depletion of Foxp3+ regulatory T cells increases severity of mechanical allodynia and significantly alters systemic cytokine levels following peripheral nerve injury. Cytokine. 2015;71:207–14.
Austin PJ, Kim CF, Perera CJ, Moalem-Taylor G. Regulatory T cells attenuate neuropathic pain following peripheral nerve injury and experimental autoimmune neuritis. Pain. 2012;153:1916–31.
Watanabe M, Takagi Y, Kotani M, Hara Y, Inamine A, Hayashi K, Ogawa S, Takeda K, Tanabe K, Abe R. Down-regulation of ICOS ligand by interaction with ICOS functions as a regulatory mechanism for immune responses. J Immunol. 2008;180:5222–34.
Da Mesquita S, Papadopoulos Z, Dykstra T, Brase L, Farias FG, Wall M, Jiang H, Kodira CD, de Lima KA, Herz J, et al. Meningeal lymphatics affect microglia responses and anti-Abeta immunotherapy. Nature. 2021;593:255–60.
Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, Herod SG, Knopp J, Setliff JC, Lupi AL, et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci. 2018;21:1380–91.
Salvador AF, de Lima KA, Kipnis J. Neuromodulation by the immune system: a focus on cytokines. Nat Rev Immunol. 2021;21:526–41.
Inyang KE, Folger JK, Laumet G. Can FDA-approved immunomodulatory drugs be repurposed/repositioned to alleviate chronic pain? J Neuroimmune Pharmacol. 2021;16:531–47.
Arimura Y, Kato H, Dianzani U, Okamoto T, Kamekura S, Buonfiglio D, Miyoshi-Akiyama T, Uchiyama T, Yagi J. A co-stimulatory molecule on activated T cells, H4/ICOS, delivers specific signals in T(h) cells and regulates their responses. Int Immunol. 2002;14:555–66.
Tavares-Ferreira D, Shiers S, Ray PR, Wangzhou A, Jeevakumar V, Sankaranarayanan I, Cervantes AM, Reese JC, Chamessian A, Copits BA, et al. Spatial transcriptomics of dorsal root ganglia identifies molecular signatures of human nociceptors. Sci Transl Med. 2022;14:eabj8186.
Zeisel A, Hochgerner H, Lonnerberg P, Johnsson A, Memic F, van der Zwan J, Haring M, Braun E, Borm LE, La Manno G, et al. Molecular architecture of the mouse nervous system. Cell. 2018;174(999–1014): e1022.
Soderquist RG, Milligan ED, Harrison JA, Chavez RA, Johnson KW, Watkins LR, Mahoney MJ. PEGylation of interleukin-10 for the mitigation of enhanced pain states. J Biomed Mater Res A. 2010;93:1169–79.
Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy—review of a new approach. Pharmacol Rev. 2003;55:241–69.
Solinas C, Gu-Trantien C, Willard-Gallo K. The rationale behind targeting the ICOS-ICOS ligand costimulatory pathway in cancer immunotherapy. ESMO Open. 2020;5(1):e000544.
Costigan M, Moss A, Latremoliere A, Johnston C, Verma-Gandhu M, Herbert TA, Barrett L, Brenner GJ, Vardeh D, Woolf CJ, Fitzgerald M. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J Neurosci. 2009;29:14415–22.
Singh SK, Krukowski K, Laumet GO, Weis D, Alexander JF, Heijnen CJ, Kavelaars A. CD8+ T cell-derived IL-13 increases macrophage IL-10 to resolve neuropathic pain. JCI Insight. 2022;7(5):e154194.
Ray PR, Shiers S, Caruso JP, Tavares-Ferreira D, Sankaranarayanan I, Uhelski ML, Li Y, North RY, Tatsui C, Dussor G, Burton MD, Dougherty PM, Price TJ. RNA profiling of human dorsal root ganglia reveals sex-differences in mechanisms promoting neuropathic pain. Brain. 2022:awac266. https://doi.org/10.1093/brain/awac266.
Le Tourneau C, Rischin D, Groenland S, Lim A, Martin-Liberal J, Moreno V, Trigo J, Mathew M, Cho D, Hansen A. 1O Inducible T cell co-stimulatory (ICOS) receptor agonist, GSK3359609 (GSK609) alone and combination with pembrolizumab: preliminary results from INDUCE-1 expansion cohorts in head and neck squamous cell carcinoma (HNSCC). Ann Oncol. 2020;31:S1.
Garber K. Immune agonist antibodies face critical test. Nat Rev Drug Discov. 2020;19:3–5.
Funding
NIH grants NS065926 and NS111929 to T.J.P. and DK130015 to M.D.B.
Author information
Authors and Affiliations
Contributions
I.S. and T.J.P. wrote the paper. I.S., M.D.B. and T.J.P. designed the study. I.S., D.T.-F., J.M.M. and G.L.M conducted experiments. I.S. and D.T.-F. analyzed data and made the figures. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Animal procedures were approved by UT Dallas IACUC and human tissue use was approved by UT Dallas IRB.
Consent for publication
All authors have agreed to publication of the final version of the manuscript.
Competing interests
The authors declare no conflicts of interest for the work described in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sankaranarayanan, I., Tavares-Ferreira, D., Mwirigi, J.M. et al. Inducible co-stimulatory molecule (ICOS) alleviates paclitaxel-induced neuropathic pain via an IL-10-mediated mechanism in female mice. J Neuroinflammation 20, 32 (2023). https://doi.org/10.1186/s12974-023-02719-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12974-023-02719-8