Abstract
Objective
Systemic elevations in pro-inflammatory cytokines are a marker of non-functional over reaching, and betaine has been shown to reduce the secretion of pro-inflammatory cytokines in vitro. The aim of this study was to investigate the effects of betaine supplementation on tumor necrosis factor alpha (TNF-α), interleukins-1 beta (IL-1β), − 6 (IL-6) and the complete blood cell (CBC) count in professional youth soccer players during a competitive season.
Methods
Twenty-nine soccer players (age, 15.5 ± 0.3 years) were randomly divided into two groups based on playing position: betaine group (BG, n = 14, 2 g/day) or placebo group (PG, n = 15). During the 14-week period, training load was matched and well-being indicators were monitored daily. The aforementioned cytokines and CBC were assessed at pre- (P1), mid- (P2), and post- (P3) season.
Results
Significant (p < 0.05) group x time interactions were found for TNF-α, IL-1β, and IL-6. These variables were lower in the BG at P2 and P3 compared to P1, while IL-1β was greater in the PG at P3 compared to P1 (p = 0.033). The CBC count analysis showed there was significant group by time interactions for white blood cells (WBC), red blood cells (RBC), hemoglobin (Hb), and mean corpuscular hemoglobin concentration (MCHC). WBC demonstrated increases at P3 compared to P2 in PG (p = 0.034); RBC was less at P3 compared to P1 in BG (p = 0.020); Hb was greater at P2 compared to P1, whilst it was less at P3 compared to P3 for both groups. MCHC was greater at P3 and P2 compared to P1 in BG, whereas MCHC was significantly lower at P3 compared to P2 in the PG (p = 0.003).
Conclusion
The results confirmed that 14 weeks of betaine supplementation prevented an increase in pro-inflammatory cytokines and WBC counts. It seems that betaine supplementation may be a useful nutritional strategy to regulate the immune response during a fatiguing soccer season.
Similar content being viewed by others
Introduction
Soccer is the most popular sport in the world, performed by men and women of varying ages and skills [1]. Youth participation in soccer has increased world-wide in the past two decades, and many youth players aspire to become professional soccer players [2]. Soccer is a high effort intermittent sport with highly varied metabolic and movement patterns. Youth soccer players cover an average of 6 to 8 km per game, with sprints occurring approximately every 90 s, comprising 1–11% of total distance, and approximately 81 accelerations (<− 1 m.s− 2) and 82 decelerations (> − 1 m.s− 2) performed per game [3,4,5,6,7]. The average heart rate during a professional youth game has been reported between 82 and 87% HRmax [8, 9] and lactate levels between 3 to 8 mmol/L [10].
Monitoring training load, recovery, and changes in psychological status provides useful information for coaches to manage variations in intensity and individualize training in order to reduce the risk of injury and the development of non-functional overreaching syndrome (NFOR) [11, 12]. When the volume of training/competition increases in conjunction with insufficient recovery, players may enter into an NFOR marked by decrements in performance and greater incidences of injury and illness due to cumulative fatigue during the season [13]. On the other hand, regular monitoring of endocrine hormones may be used as biomarkers of physiological stress which may influence recovery and performance throughout the season. Particularly, changes in growth hormone, cortisol, and the testosterone/cortisol ratio, in addition to biomarkers of the immune system (i.e.: interleukins), have all been reported as reliable biomarkers that may illustrate NFOR in athletes [13, 14]. In some studies as well, to monitor the training load of rating of perceived exertion [7, 15] and Hooper index (i.e.: perception of fatigue, stress, delayed onset muscle soreness, or sleep quality) has also been used to identify the well-being status of players and their variation across critical periods of the season, which can be other options for identifying NFOR [16, 17].
Due to intense training, competitions, and match-related stress, soccer players experience homeostatic, biochemical, and hematological changes following a soccer match and across a competitive season [18]. Large effect sizes have been reported for the inflammatory and immunological response immediately and up to 72 h following a soccer match. This response includes an elevation in tumor necrosis factor alpha (TNF)-α and pro-inflammatory interleukins (IL) [19] that are produced by skeletal muscle, T-cells, and natural killer cells [20]. The best-studied cytokine that is related to exercise is interleukin-6 (IL-6), which can act as both a pro- and anti-inflammatory cytokine [21], and it is sensitive to both the intensity and volume of exercise in addition to metabolic stress and muscular injury [22, 23]. TNF-α is part of the nuclear factor kappa b (NFκB) pathway, and produced predominantly by macrophages in response to injured skeletal muscle tissue [24]. In addition to inflammatory cytokines, hemoglobin (Hb), hematocrit (Hct), and mean corpuscular hemoglobin concentration (MCHC) are diminished following a soccer match [25], and a large increase in circulating leukocytes and specific monocytes, macrophages and lymphocytes occurs [26] as these cells rapidly migrate to injured muscle tissue.
Regular bloodwork, including measuring complete blood cell count (CBC) with differential and inflammatory cytokines, may be used as indicators of training stress associated with NFOR [27]. Walker et al. reported that IL-6 significantly increased over the duration of a soccer season, and the greatest elevations in IL-6 were observed during the highest states of physiological demand occurring at the end of the season [28]. This pro-inflammatory state was suggested to disrupt the hypothalamic-pituitary axis leading to greater circulating cortisol concentrations, and it is an indicator of NFOR [28, 29]. Red blood cells (RBC) and platelets (Pts) are also sensitive to systematic inflammatory changes, and may be used to monitor NFOR [30]. Huggins et al. reported a significant decrease in Hct and Hb from pre-season to the fourth week that remained depressed throughout the season [31]. Huggins et al. also reported a regression in anemia indicators, such as mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and red cell distribution width (RDW) at the end of the season, in addition to an increase in MCHC levels between pre-season and the fourth week [31].
Dietary supplements have become popular in the sports community over the past few decades to support adaptation and manage fatigue, however, research suggests that only a small group of supplements are safe and effective for athletes [32, 33]. Betaine is a zwitterionic quaternary ammonium compound, a byproduct isolated from molasses during sugar beet refinement, and is naturally occurring in spinach, whole grains, and seafood. The daily average betaine intake is approximately 100–400 mg [34], and several studies suggest that 2.0–2.5 g of betaine supplementation per day may be consider as an ergogenic aid [35, 36]. Although the mechanisms by which betaine may be ergogenic are not fully understood, chronic betaine supplementation may enhance recovery between training sessions by protecting against protein denaturation and promoting the secretion of insulin growth factor-1 and protein kinase B phosphorylation [37].
Inverse correlations between betaine ingestion and markers of inflammation (C reactive protein and TNF-α) were first reported in 2008 [38], and since several studies have demonstrated a causal relationship [39]. In regards to exercise recovery, IL-1β accumulation leads to intramuscular inflammation following eccentric exercise [40]. Betaine has been shown to inhibit NFκB activity [41], potentially dampening the inflammatory response to exercise by lowering the production and secretion of IL-1β, IL-6 and TNF-α [42]. We recently reported that betaine supplementation prevented a reduction in the testosterone to cortisol ratio associated with the demands of a competitive season in professional youth soccer players [13]. Currently there is limited research examining the influence of betaine supplementation on markers of inflammation or blood cell parameters. Given the positive effects of betaine supplementation on hormones associated with NFOR, and in vitro evidence demonstrating an anti-inflammatory effect of betaine, betaine supplementation may also offset the development of NFOR by ameliorating the inflammatory response to chronic, strenuous exercise, such as that required by professional soccer players.
This study is an extension of an ongoing study exploring the effects of betaine supplementation on markers of NFOR in professional youth soccer players [13]. The purpose of this study was to investigate the effect of betaine supplementation on pro-inflammatory cytokines and CBC in professional youth soccer players during a 14-week competitive season. We hypothesize that betaine supplementation will reduce markers of inflammation and indicators of anemia.
Material and method
Selection of study groups
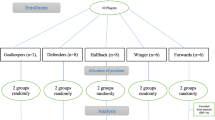
Professional youth soccer players (n = 29), from the Foolad Mobarakeh Sepahan Sport Club competing in the Iranian Youth Premier League, participated in this study. Demographics, inclusion, and exclusion criteria associated with this sample has been previously published [13]. In brief, subjects were required to attend all training sessions, not consume any dietary supplements during the study period, not perform any non-team training, and have no records of sensitivity to dietary supplements in the team medical records. Subjects were matched according to position prior to randomization into either a betaine (BG, n = 14) or placebo (PG, n = 15) group (Table 1). Players, as well as their parents, signed a consent letter to participate. The Ethics Committee of the University of Isfahan approved the study (IR.UI.REC.1398.102), and the recommendations of Human Ethics in Research were followed by the Helsinki Declaration (2013).
Experimental approach to the problem
The present study was an independent group, pre-test, mid-test, and post-test of an experimental design. Studies in humans show that betaine is rapidly absorbed from the intestine and reach peak serum concentrations within 1–2 h [43]. Based on this reason, subjects consumed one capsule, betaine or placebo, twice daily (2 g/day), with 300 ml water approximately, 2 h prior to training and one-hour post-training, or with lunch and dinner on non-training days. Anthropometric measurements, body composition, and blood tests were conducted before week 1 (P1), seventh week (P2) and after 14 weeks (P3) of the intervention, and were performed 48 h apart from the last training session. Subjects recorded their nutrition for three full days and delivered it to the researchers at each testing time-point. All players participated in the same standardized training sessions during the study (i.e., four training sessions and one match per week). In order to match the load of the weekly workouts, players who did not participate in competitions performed post-match, small side games, individual training, or friendly competition. The subjects presented individual wellness questionnaires before the start of each training session, and reported internal training load via rating of perceived exertion (RPE) 30 min after the end of each training session.
Procedures
Blood analysis
Subjects reported to the Alzahra Hospital’s lab for blood sampling following a 12 h fast, and at least 48 h following the last training session. To account for circadian rhythms, 10 cc of blood was collected from the antecubital vein at 8–9 a.m. Samples were immediately centrifuged, the serum was separated, and then used to measure CBC, IL-1β, IL-6, and TNF-α on the same day.
Analyzing hematology levels
The CBC count consisted of neutrophils, lymphocytes, and the combination of eosinophils, basophils, and monocytes (MIX). To obtain the ratio of neutrophils to lymphocytes (NLR) these two variables were divided together. Total RBCs were measured in addition to MCV, MCH, RDW. The Hb, Hct, and Pts determinations were performed on plasma anticoagulated with EDTA using a fully-automated hematology analyzer (Sysmex kx-21 N Kobe, JAPAN) according to the manufacturer’s recommended protocols.
Analyzing cytokine levels
Inflammatory markers were measured using enzyme-like immune-sorbent assay (ELISA reader, awareness technology, USA). All variables were measured with kits obtained from Diaclone, Besançon, France. Serum concentrations were performed for IL-1β with the sensitivity of the kit 6.5 pg/mL. The sensitivity of IL-6 was 2 pg/mL and average inter- and intra-assay coefficients of variability (CVs) were 7.7 and 3.6%, respectively. TNF-α had a sensitivity 8 pg/mL and average inter- and intra-assay CVs were 10.9 and 3.2%, respectively. All analyses were performed in duplicates.
Anthropometric and body composition
Detailed methods regarding the measurement of anthropometrics and body composition have been previously reported for this sample [13]. In brief, standing height with a stadiometer (Seca 213, Germany), weight with a balance scale (Seca 813, UK), and body composition with 7 site skin folds (Lafayette Instrument Company, Lafayette, IN, USA) and Brozek’s formula [44]. To determine the maturity offset and age at peak height velocity of the subjects the following formula was used [45]: Maturity offset = − 9.236 + 0.0002708 (leg length × sitting height) − 0.001663 (age × leg length) + 0.007216 (age × sitting height) + 0.02292 (Weight by Height ratio).
Aerobic power test
An Intermittent Fitness Test 30–15 (30-15IFT) was used to estimate the maximal oxygen uptake (VO2max) and the readiness level of the subjects. This test was performed after the P1 evaluations and before the start of the competition season (i.e., week 1). All subjects performed 10 min of standard warm-up, include jogging, dynamic stretching, ABC run drills (e.g., high knee, A-Skip, B-Skip, carioca, etc.), and submaximal short runs (2–3 rep), under the guidance of the team fitness coach.
After the warm-up, subjects were placed in four-person groups. The 30-15IFT includes a 40-m shuttle with 30 s of activity and 15 s of passive recovery. The first stage was 30 s and initial speed started with 8 km/h− 1 and increased by 0.5 km/h− 1 every 45 s [46]. This test was terminated when subjects could not continue or for three consecutive shuttles could not maintain the appropriate pace. The following formula was used to estimate the VO2max [46]: VO2max (ml.kg− 1.min− 1) = 28.3 – (2.15 × 1) – (0.741 × 16 years) – (0.0357 x Weight) + (0.0586 × 16 years x VIFT) + (1.03 x VIFT). Where VIFT is the final running speed. The test-retest of this assessment at the intra-class correlation coefficient (ICC) of this test was 0.91. This test has shown high validity (ICC = 0.96) in soccer players [47].
Control of food intake
Detailed methods regarding the dietary standards and measurement of energy and macronutrient intake have been previously described and published for this sample [13]. In brief, subjects were provided with nutrition recommendations of Iranian native foods that provided energy equal to 1.55 times the individual subjects basal metabolic rate by a nutritionist. Subjects were required to consume the same foods and record these intakes 72 h prior to each blood sample, and total calorie intake was measured with Nutrition 4 version 3.5.2 software [48].
Monitoring internal training loads
Internal training loads in soccer players were assessed with a 10-piont session RPE and have been described in detail and previously reported for this sample [13]. Training load monitoring was performed 30 min after each training session. This is the standardized approximate time according to previous studies [15, 49,50,51].
Wellness monitoring
Hopper index was used to assess fatigue, recovery, soreness, quality of sleep, and health status of soccer players in each session [52,53,54]. Detailed methods and results have been previously described and published for this sample in relation to the Hooper index [13]. Players had an average of 5 training sessions per week for 14 weeks. Immediately after attending the training, they answered Hooper’s 7-score questionnaire with the above variables mentioned [16, 17].
Statistical analysis
Descriptive statistics are reported as mean ± standard deviation. Shapiro-Wilk test and Levene’s test were used to check the normality and homogeneity of variables of data, respectively. All variables were assessed with a mixed factorial 2 × 2 analysis of covariance (ANCOVA) with repeated measures. The pre-season value was used as the covariate, time (mid-season and post-season) the within subject factor, and BG and PG the between subject factor. When a significant time x group interaction was found a one-way repeated-measures analysis of variance (ANOVA) was conducted for each group separately with the Bonferroni correction. If the results of the one-way ANOVA were similar for each group, then the percent changes were computed. Hedge’s g effect size (95% confidence interval) was computed to define the magnitude of comparisons pre- and post-season for both groups by separately. Threshold’s include: trivial (< 0.2), small (≥ 0.2), moderate (≥ 0.5) and large (≥ 0.8). All analyses were conducted with SPSS 22.0 (IBM) and the significant level was set at p < 0.05. also, Excel was used for the training workload and hopper data and the charts were drawn with GraphPad Prism 8.0.1.
Results
There were no significant main effects of time for changes in IL-6 (p = 0.88, F = 0.022, ηp2 = 0.001) and TNF-α (p = 0.84, F = 0.04, ηp2 = 0.002), however, there were significant group by time interactions (p = 0.008, F = 8.11, ηp2 = 0.24) and (p = 0.038, F = 4.78, ηp2 = 0.16), respectively. Post hoc analysis revealed the IL-6 and TNF-α significantly decreased from P1 to P2 and P3, as well as from P2 to P3 in the BG, whereas there were no significant differences between P1, P2, or P3 in the PG. There were no significant (p = 0.69, F = 0.16, ηp2 = 0.01) main effects of time, however, there was a group by time interaction (p ≤ 0.001, F = 16.02, ηp2 = 0.38) for changes in IL-1β. The IL-1β at P3 and P2 were significantly lower than P1, as well as significantly lower than from P2 to P3 only in BG. In PG the IL-1β was significantly greater at P3 compared to P1 (p = 0.033) (Table 2). Figure 1a shows the comparison of the mean difference between-group results of the one-way ANOVA at the three assessments stages. For the BG it was observed that the level of IL-6 (p < 0.05), IL-1β and TNF-α (p ≤ 0.001) were significantly lower for P2 and P3.
Change in all blood sampling variables assessment for each group and assessment stage. a Indicates changes in pro-inflammatory cytokines, and NLR; b Indicates changes in types of leukocytes, Plts, and Hct; c Indicates changes to the rest of RBC variables. * significant difference compared to P1 with the superiority of the BG (p < 0.05); # significant difference compared to P1 with the superiority of the PG (p < 0.05); ∞ significant difference compared to P2 with the superiority of the BG (p < 0.05); α significant difference compared to P2 with the superiority of the PG (p < 0.05). BG: Betaine Group; PG: Placebo Group; IL-6: Interleukin-6; IL-1β: Interleukin-1 beta; TNF-α: Tumor necrosis factor-alpha; WBC: White blood cells; MIX: Plural of eosinophils, basophils, and monocytes; NLR: Neutrophils to lymphocytes ratio; RBC: Red blood cells; MCH: Mean corpuscular hemoglobin; MCHC: Mean corpuscular hemoglobin concentration; RDW: Red cell distribution width; Hb: Hemoglobin; Hct: Hematocrit; Plts: Platelets; P1: Pre-season; P2: Mid-season; P3: Post-season
There were no significant (p = 0.36, F = 0.86, ηp2 = 0.03) main effects of time, however, there was a group by time interaction (p = 0.002, F = 11.49, ηp2 = 0.31) for changes in WBC. This variable demonstrated a significant increase at P3 compared to P2 in PG (p = 0.034). There were no significant main effects of time for changes in neutrophils (p = 0.60, F = 0.28, ηp2 = 0.01), lymphocytes (p = 0.69, F = 0.17, ηp2 = 0.01), NLR (p = 0.76, F = 0.10, ηp2 = 0.004) and MIX (p = 0.09, F = 3.02, ηp2 = 0.10) or group by time interaction (p = 0.22, F = 1.56, ηp2 = 0.06), (p = 0.14, F = 2.38, ηp2 = 0.08), (p = 0.23, F = 1.55, ηp2 = 0.06) and (p = 0.44, F = 0.61, ηp2 = 0.02), respectively.
There were no significant (p = 0.71, F = 0.14, ηp2 = 0.01) main effects of time, however, there was a group by time interaction (p = 0.02, F = 6.22, ηp2 = 0.19) for changes in RBC. This variable was significantly lower at P3 compared to P1 in BG (p = 0.020). There was no significant main effect of time for MCV (p = 0.83, F = 0.05 ηp2 = 0.002) or group by time interactions (p = 0.64, F = 0.23, ηp2 = 0.01). There were no significant (p = 0.50, F = 0.46, ηp2 = 0.02) main effects of time, however, there was a group by time interaction (p = 0.03, F = 5.36, ηp2 = 0.17) for changes in MCH. Post hoc analysis showed the MCH were significantly greater at P3 and P2 compared to P1 for both groups, but MCH was significantly less at P3 compared to P2 in the PG (p = 0.003). For MCHC there was significant main effect of time (p = 0.04, F = 4.79, ηp2 = 0.16) and significant group by time interactions (p = 0.001, F = 14.73, ηp2 = 0.36). Post hoc analysis showed the MCHC were significantly greater at P3 and P2 compared to P1 in BG, whereas there were significantly less from P2 to P3 in the PG (p = 0.003). There were no significant (p = 0.89, F = 0.02, ηp2 = 0.001) main effects of time, however, there was a group by time interaction (p = 0.002, F = 11.22, ηp2 = 0.30) for changes in RDW. This variable was significantly less at P3 compared to P2 in PG (p = 0.016).
There were no significant (p = 0.08, F = 3.45, ηp2 = 0.12) main effects of time, however, there was a group by time interaction (p = 0.004, F = 10.12, ηp2 = 0.28) for changes in Hb. Post hoc showed that the Hb was significantly greater at P2 compared to P1, whilst, there were significant decreases at P3 compared to P2 for both groups. There were no significant (p = 0.96, F = 0.002, ηp2 ≤ 0.001) main effects of time, however, there was a group by time interaction (p = 0.004, F = 9.99, ηp2 = 0.28) for changes in Pts. Post hoc analysis showed a significant increase at P3 compared to P2 in PG (p = 0.001). There were no significant main effects of time for changes in Hct (p = 0.39, F = 0.76, ηp2 = 0.03) or group by time interaction (p = 0.24, F = 1.45, ηp2 = 0.05) (Table 3).
Figure 1b-c shows the comparison of the mean difference between-group results with one-way ANOVA at the three assessments stages for CBC. WBC showed a difference in levels that were lower in the BG compared to the PG from P2 to P3 (p = 0.002) and P1 to P3 (p = 0.007). Neutrophils and NLR demonstrated that there was a difference in levels which were lower in the BG compared to PG from P1 to P2 (p ≤ 0.001) and P3 (p = 0.002). Lymphocytes demonstrated that there was a difference in levels which were greater compared to the BG with PG from P1 to P2 (p ≤ 0.001) and P3 (p = 0.001) (Fig. 1b). Pts levels decreased in P3 to P2 (p = 0.003) and P1 (p = 0.048) in BG compared to the PG. BG decreased in RDW levels in P2 to P1 (p = 0.014) compared to the PG, however, the PG decreased in P3 to P2 (p = 0.002) compared to the BG (Fig. 1b). BG decreased RBC levels in P3 to P2 (p = 0.019) and P1 (p = 0.003) compared to the PG. The reduction in Hb levels from P3 to P2 (p = 0.008) was less in the PG compared to the BG. MCH reduction levels in P3 to P2 (p = 0.019) was less in the BG compared to the PG, and increased levels in comparison at P3 to P1 (p = 0.007). MCHC demonstrated that there was a difference in levels which were greater compared to the BG with PG from P1 to P3 (p = 0.001) and P2 to P3 (p = 0.001) (Fig. 1c).
Discussion
The aim of this study was to analyze the effect of betaine supplementation on the status of pro-inflammatory cytokines IL-1β, IL-6 and TNF-α, and the CBC counts in professional youth soccer players. We hypothesized that betaine supplementation would decrease the secretion of pro-inflammatory cytokines associated with a competitive soccer season. The results confirmed that 14 weeks of betaine supplementation prevented an increase in pro-inflammatory cytokines and WBC counts. These changes occurred despite both groups reporting a similar internal workload, consuming similar energy and macronutrients, and having similar levels of fatigue, stress, and sleep, as previously reported [13].
The competitive demands of soccer involve continuously performing high intensity eccentric actions, such as direction changes, accelerations, and decelerations, which, seems particularly damaging to the muscle [55,56,57]. When these demands are required chronically, fluctuations in hematological parameters occur [58, 59]. In the present study, inflammatory cytokines and WBCs were increased, and Hb, MCH, and MCHC were reduced at the end of the season in the PG. These changes in inflammatory markers are in line with another study that evaluated IL-6, at 5 different times during a season, in senior female soccer players and showed a linear increase [28]. These changes in basic indicators of pro-inflammatory status in athletes, when combined with changes in hematological parameters, point to a connection between NFOR and incomplete recovery and/or fatigue accumulation.
In the present study, inflammatory cytokines in the BG were 24–40% less at the end of the season compared to the start, whereas IL-1β (26%) and IL-6 (6.8%) were increased in the PG. IL-1β is produced in response to infection and injury, and its accumulation leads to intramuscular inflammation after eccentric exercise (17). Of the inflammatory cytokines assessed, IL-1β showed the greatest difference in response, decreasing by 40% between pre- and post-season in BG, but increasing by 26% in the PG. Betaine has been shown to suppress the NFκB pathway, and as a result, downregulates IL-1β and TNF-α gene expression and secretion [42]. The anti-inflammatory properties of betaine have been suggested to be beneficial for several diseases, including diabetes, cancer, and Alzheimer’s [39]. The changes in immune cell and inflammatory status in the present study suggest, for the first time, that betaine supplementation may also be a useful nutritional strategy to counter some of the negative immunological changes that are associated with NFOR.
Reductions in RBC, MCH, and Hb are the result of physical stress compounded over repeated competitions [60, 61], and have also been suggested to be indicative of the development of NFOR [27]. Previously published data from this ongoing study found an increase in testosterone and the testosterone to cortisol ratio during a competitive soccer season in this sample with betaine supplementation [13]. Testosterone has been shown to stimulate erythrocytosis [62] and cortisol increases Na (+), K (+)-ATPase activity to reduce erythrocyte volume [63]. Additionally, the addition of betaine to an in vitro medium in physiological concentrations has been shown to decrease hypoosmotic stress induced hemolysis by 42% via inhibition of erythrocyte membrane ATPases [64]. Given the aforementioned findings, we expected to see an improvement compared to placebo in anemia indicators over the course of the season. In the present study, betaine supplementation prevented a reduction in Hb, MCH, and RDW compared to placebo at the end of the season, but a decrease in RBC was observed in the BG. We are unable to speculate on what may account for these divergent results at this time. The improvement in Hb, MCH, and RDW suggests that betaine supplementation may protect erythrocytes against hemolysis during a soccer season, however, future research is necessary to further explore this hypothesis.
This study has limitations and proposals for future lines of research. First, although we instructed subjects to consume the same quantity and type of foods, we cannot discount that within subject differences in micronutrient intake may have influenced inflammatory and hematological parameters. Second, although we were able to track internal workload and indices of recovery, we did not have access to tools to measure external workloads, such as Global Positioning System devices [65,66,67]. Future research is necessary to investigate more closely the temporal changes in markers of inflammation over time with betaine supplementation, and to determine if the changes in inflammatory cytokines are sustainable with longer supplementation periods. Finally, additional research is necessary to measure changes in metrics of physical performance to better correlate the improvements in makers of inflammation, immune cells, and the testosterone to cortisol ratio [13] observed in response to betaine supplementation with NFOR.
Conclusions
Fourteen weeks of betaine supplementation ameliorated an increase in IL-1β, IL-6, and TNF-α associated with the physical stressors of a competitive season in professional youth soccer players. These changes in pro-inflammatory cytokines and WBC suggest that betaine supplementation may be a useful nutritional strategy to regulate the immune system, and, together with the differences in Hb and MCH compared to PG, and the increase in the testosterone to cortisol ratio reported in this same sample [13], suggest that betaine supplementation may be used as part of a nutritional strategy to counter NFOR.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- NFκB:
-
nuclear factor kappa b pathway
- IL-6:
-
Interleukin-6
- IL-1β:
-
Interleukin-1 beta
- TNF-α:
-
Tumor necrosis factor-alpha
- WBC:
-
White blood cells
- MIX:
-
Plural of eosinophils, basophils, and monocytes
- NLR:
-
Neutrophils to lymphocytes ratio
- RBC:
-
Red blood cells
- MCH:
-
Mean corpuscular hemoglobin
- MCHC:
-
Mean corpuscular hemoglobin concentration
- RDW:
-
Red cell distribution width
- Hb:
-
Hemoglobin
- Hct:
-
Hematocrit
- Plts:
-
Platelets
- NFOR:
-
non-functional over reaching
- ELISA:
-
enzyme-like immune-sorbent assay
- BG:
-
betaine group
- PG:
-
placebo group
- P1:
-
pre-season
- P2:
-
mid-season
- P3:
-
post-season
References
Bangsbo J. The physiology of soccer--with special reference to intense intermittent exercise. Acta Physiol Scand Suppl. 1994;619:1–155.
Taşkin C, KarakoÇ Ö, Acaroglu E, Budak C. Futbolcu Çocuklarda seÇİlmİş motorİk Özellİkler arasindakİ İlİşkİnİn İncelenmesİ. Spor ve Performans Araştırmaları Dergisi. 2015;6(2):101–7.
Stølen T, Chamari K, Castagna C, Wisløff U. Physiology of soccer. Sports Med. 2005;35(6):501–36. https://doi.org/10.2165/00007256-200535060-00004.
Vigh-Larsen JF, Dalgas U, Andersen TB. Position-specific acceleration and deceleration profiles in elite youth and senior soccer players. J Strength Cond Res. 2018;32(4):1114–22. https://doi.org/10.1519/JSC.0000000000001918.
Arruda AF, Carling C, Zanetti V, Aoki MS, Coutts AJ, Moreira A. Effects of a very congested match schedule on body-load impacts, accelerations, and running measures in youth soccer players. Int J Sports Physiol Perform. 2015;10(2):248–52. https://doi.org/10.1123/ijspp.2014-0148.
Nobari H, Alves AR, Clemente FM, Pérez-Gómez J, Clark CCT, Granacher U, et al. Associations between variations in accumulated workload and physiological variables in young male soccer players over the course of a season. Front Physiol. 2021;12638180. https://doi.org/10.3389/fphys.2021.638180.
Nobari H, Barjaste A, Haghighi H, Clemente FM, Carlos-Vivas J, Perez-Gomez J. Quantification of training and match load in elite youth soccer players: a full-season study. J Sports Med Phys Fitness. 2021. https://doi.org/10.23736/S0022-4707.21.12236-4.
Helgerud J, Engen LC, Wisloff U, Hoff J. Aerobic endurance training improves soccer performance. Med Sci Sports Exerc. 2001;33(11):1925–31. https://doi.org/10.1097/00005768-200111000-00019.
Strøyer J, Hansen L, Klausen K. Physiological profile and activity pattern of young soccer players during match play. Med Sci Sports Exerc. 2004;36(1):168–74. https://doi.org/10.1249/01.MSS.0000106187.05259.96.
Capranica L, Tessitore A, Guidetti L, Figura F. Heart rate and match analysis in pre-pubescent soccer players. J Sports Sci. 2001;19(6):379–84. https://doi.org/10.1080/026404101300149339.
Gabbett TJ, Nassis GP, Oetter E, Pretorius J, Johnston N, Medina D, et al. The athlete monitoring cycle: a practical guide to interpreting and applying training monitoring data. In: BMJ publishing group Ltd and British Association of Sport and Exercise Medicine; 2017.
Wrigley R, Drust B, Stratton G, Scott M, Gregson W. Quantification of the typical weekly in-season training load in elite junior soccer players. J Sports Sci. 2012;30(15):1573–80. https://doi.org/10.1080/02640414.2012.709265.
Nobari H, Kargarfard M, Minasian V, Cholewa JM, Pérez-Gómez J. The effects of 14-week betaine supplementation on endocrine markers, body composition and anthropometrics in professional youth soccer players: a double blind, randomized, placebo-controlled trial. J Int Soc Sports Nutri. 2021;18(1):20. https://doi.org/10.1186/s12970-021-00417-5.
Schmikli SL, de Vries WR, Brink MS, Backx FJ. Monitoring performance, pituitary–adrenal hormones and mood profiles: how to diagnose non-functional over-reaching in male elite junior soccer players. Br J Sports Med. 2012;46(14):1019–23. https://doi.org/10.1136/bjsports-2011-090492.
Nobari H, Silva R, Clemente FM, Akyildiz Z, Ardigò LP, Pérez-Gómez J. Weekly variations in the workload of Turkish National Youth Wrestlers: a season of complete preparation. Int J Environ Res Public Health. 2021;18(7):3832. https://doi.org/10.3390/ijerph18073832.
Nobari H, Akyildiz Z, Fani M, Oliveira R, Pérez-Gómez J, Clemente FM. Weekly wellness variations to identify non-functional overreaching syndrome in Turkish National Youth Wrestlers: a pilot study. Sustainability. 2021;13(9):4667. https://doi.org/10.3390/su13094667.
Nobari H, Fani M, Clemente FM, Carlos-Vivas J, Pérez-Gómez J, Ardigò LP. Intra-and inter-week variations of well-being across a season: a cohort study in elite youth male soccer players. Front Psychol. 2021;12671072. https://doi.org/10.3389/fpsyg.2021.671072.
Banfi G, Morelli P. Relation between values of haemoglobin, erythrocytes and reticulocytes and body mass index in elite athletes of different sports disciplines. Int J Lab Hematol. 2007;29(6):484–5. https://doi.org/10.1111/j.1751-553X.2007.00908.x.
Silva J, Rumpf M, Hertzog M, Castagna C, Farooq A, Girard O, et al. Acute and residual soccer match-related fatigue: a systematic review and meta-analysis. Sports Med. 2018;48(3):539–83. https://doi.org/10.1007/s40279-017-0798-8.
Lu Y, Mak KK, Van Bever HP, Ng TP, Mak A, Ho RCM. Prevalence of anxiety and depressive symptoms in adolescents with asthma: a meta-analysis and meta-regression. Pediatr Allergy Immunol. 2012;23(8):707–15. https://doi.org/10.1111/pai.12000.
Steinbacher P, Eckl P. Impact of oxidative stress on exercising skeletal muscle. Biomolecules. 2015;5(2):356–77. https://doi.org/10.3390/biom5020356.
Fischer CP. Interleukin-6 in acute exercise and training: what is the biological relevance. Exerc Immunol Rev. 2006;12(6–33):41.
Pedersen BK, Febbraio M. Muscle-derived interleukin-6—a possible link between skeletal muscle, adipose tissue, liver, and brain. Brain Behavior Immunity. 2005;19(5):371–6. https://doi.org/10.1016/j.bbi.2005.04.008.
Parameswaran N, Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010;20(2):87–103. https://doi.org/10.1615/critreveukargeneexpr.v20.i2.10.
Sporiš G, Vlahović T, Trajković N, Milanović Z, Madić D. Haematological and iron status following a soccer match. Facta Universitatis Series: Physical Educ Sport. 2016;14(2):289–95.
Silva J, Rumpf M, Hertzog M, Castagna C, Farooq A, Girard O, et al. Acute and residual soccer match-related fatigue: a systematic review and meta-analysis. Sports Med. 2018;48(3):539–83.
Lee EC, Fragala MS, Kavouras SA, Queen RM, Pryor JL, Casa DJ. Biomarkers in sports and exercise: tracking health, performance, and recovery in athletes. J Strength Cond Res. 2017;31(10):2920–37. https://doi.org/10.1519/JSC.0000000000002122.
Walker AJ, McFadden BA, Sanders DJ, Rabideau MM, Hofacker ML, Arent SM. Biomarker response to a competitive season in division I female soccer players. J Strength Cond Res. 2019;33(10):2622–8. https://doi.org/10.1519/JSC.0000000000003264.
SMITH LL. Cytokine hypothesis of overtraining: a physiological adaptation to excessive stress? Med Sci Sports Exerc. 2000;32(2):317–31. https://doi.org/10.1097/00005768-200002000-00011.
Bester J, Pretorius E. Effects of IL-1β, IL-6 and IL-8 on erythrocytes, platelets and clot viscoelasticity. Sci Rep. 2016;6(1):1–10.
Huggins RA, Fortunati AR, Curtis RM, Looney DP, West CA, Lee EC, et al. Monitoring blood biomarkers and training load throughout a collegiate soccer season. J Strength Cond Res. 2019;33(11):3065–77. https://doi.org/10.1519/JSC.0000000000002622.
Oliveira CC, Ferreira D, Caetano C, Granja D, Pinto R, Mendes B, et al. Nutrition and supplementation in soccer. Sports. 2017;5(2):28. https://doi.org/10.3390/sports5020028.
Heaton LE, Davis JK, Rawson ES, Nuccio RP, Witard OC, Stein KW, et al. Selected in-season nutritional strategies to enhance recovery for team sport athletes: a practical overview. Sports Med. 2017;47(11):2201–18. https://doi.org/10.1007/s40279-017-0759-2.
Meyer KA, Shea JW. Dietary choline and betaine and risk of CVD: a systematic review and meta-analysis of prospective studies. Nutrients. 2017;9(7):711. https://doi.org/10.3390/nu9070711.
Cholewa JM, Wyszczelska-Rokiel M, Glowacki R, Jakubowski H, Matthews T, Wood R, et al. Effects of betaine on body composition, performance, and homocysteine thiolactone. J Int Soc Sports Nutri. 2013;10(1):39. https://doi.org/10.1186/1550-2783-10-39.
Pryor JL, Craig SA, Swensen T. Effect of betaine supplementation on cycling sprint performance. J Int Soc Sports Nutri. 2012;9(1):1–7.
Apicella JM, Lee EC, Bailey BL, Saenz C, Anderson JM, Craig SA, et al. Betaine supplementation enhances anabolic endocrine and Akt signaling in response to acute bouts of exercise. Eur J Appl Physiol. 2013;113(3):793–802. https://doi.org/10.1007/s00421-012-2492-8.
Detopoulou P, Panagiotakos DB, Antonopoulou S, Pitsavos C, Stefanadis C. Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: the ATTICA study. Am J Clin Nutr. 2008;87(2):424–30. https://doi.org/10.1093/ajcn/87.2.424.
Zhao G, He F, Wu C, Li P, Li N, Deng J, et al. Betaine in inflammation: mechanistic aspects and applications. Front Immunol. 2018;9:1070. https://doi.org/10.3389/fimmu.2018.01070.
Fielding R, Manfredi T, Ding W, Fiatarone M, Evans W, Cannon JG. Integrative, Physiology C: Acute phase response in exercise. III. Neutrophil and IL-1 beta accumulation in skeletal muscle. Am J Physiol. 1993;265(1):R166–72.
Go EK, Jung KJ, Kim JM, Lim H, Lim HK, Yu BP, et al. Betaine modulates age-related NF-κB by thiol-enhancing action. Biol Pharm Bull. 2007;30(12):2244–9. https://doi.org/10.1248/bpb.30.2244.
Xia Y, Chen S, Zhu G, Huang R, Yin Y, Ren W. Betaine inhibits interleukin-1β production and release: potential mechanisms. Front Immunol. 2018;9:2670.
Craig SA. Betaine in human nutrition. Am J Clin Nutr. 2004;80(3):539–49. https://doi.org/10.1093/ajcn/80.3.539.
Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978;40(3):497–504. https://doi.org/10.1079/BJN19780152.
Mirwald RL, Baxter-Jones AD, Bailey DA, Beunen GP. An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc. 2002;34(4):689–94.
Buchheit M. The 30–15 intermittent fitness test: 10 year review. Myorobie J. 2010;1(9):278.
Buchheit M. The 30–15 intermittent fitness test: reliability and implication for interval training of intermittent sport players. In: 10th European Congress of Sport Science Belgrade, Serbia; 2005.
Nobari H, Ahmadi M, SÁ M, Pérez-Gómez J, Manuel Clemente F, Adsuar JC, Minasian V, Afonso J. The effect of two types of combined training on bio-motor ability adaptations in sedentary females. J Sports Med Phys Fitness. 2020. https://doi.org/10.23736/S0022-4707.20.11736-5.
Nobari H, Vahabidelshad R, Pérez-Gómez J, Ardigò LP. Variations of training workload in micro- and meso-cycles based on position in elite young soccer players: a competition season study. Front Physiol. 2021;12:668145. https://doi.org/10.3389/fphys.2021.668145.
Nobari H, Silva AF, Clemente FM, Siahkouhian M, García-Gordillo MÁ, Adsuar JC and Pérez-Gómez J. Analysis of Fitness Status Variations of Under-16 Soccer Players Over a Season and Their Relationships With Maturational Status and Training Load. Front. Physiol. 2021;11:597697. https://doi.org/10.3389/fphys.2020.597697.
Nobari H, Tubagi Polito LF, Clemente FM, Pérez-Gómez J, Ahmadi M, Garcia-Gordillo MÁ, et al. Relationships between training workload parameters with variations in anaerobic power and change of direction status in elite youth soccer players. Int J Environ Res Public Health. 2020;17(21):7934. https://doi.org/10.3390/ijerph17217934.
Moalla W, Fessi MS, Farhat F, Nouira S, Wong DP, Dupont G. Relationship between daily training load and psychometric status of professional soccer players. Res Sports Med. 2016;24(4):387–94.
Nobari H, Aquino R, Clemente FM, et al. Description of acute and chronic load, training monotony and strain over a season and its relationships with well-being status: A study in elite under-16 soccer players. Physiology & Behavior. 2020;225:113117. https://doi.org/10.1016/j.physbeh.2020.113117.
Hooper SL, Mackinnon LT. Monitoring overtraining in athletes. Sports Med. 1995;20(5):321–7. https://doi.org/10.2165/00007256-199520050-00003.
Raastad T, Owe SG, Paulsen G, Enns D, Overgaard K, Crameri R, et al. Changes in calpain activity, muscle structure, and function after eccentric exercise. J Med Sci Sport Exerc. 2010;42(1):86–95. https://doi.org/10.1249/MSS.0b013e3181ac7afa.
Silva JR, Rebelo A, Marques F, Pereira L, Seabra A, Ascensão A, et al. Biochemical impact of soccer: an analysis of hormonal, muscle damage, and redox markers during the season. Appl Physiol Nutri Metab. 2014;39(4):432–8. https://doi.org/10.1139/apnm-2013-0180.
Young WB, Hepner J, Robbins DW. Movement demands in Australian rules football as indicators of muscle damage. J Strength Cond Res. 2012;26(2):492–6. https://doi.org/10.1519/JSC.0b013e318225a1c4.
Kocakulak NA, Polat Y, Karakükcü M, Sucan S, Çelenk Ç. The Effects Of Match Conditions On The Shaped Elements Of Blood And Iron Level Of Football Players. Hacettepe J Biol Chem. 48(3):275–82.
Cinar V, Cengiz S, Pala R, Dundar A. Effect of football practices on certain blood values of athletes. Adv Environ Biol. 2013:924–8. https://www.semanticscholar.org/paper/Effect-of-Football-Practices-on-Certain-Blood-of-Cengiz-D%C3%BCndar/774f3e58ab5c2d085308766d0a79429d967cb015.
Malcovati L, Pascutto C, Cazzola M. Hematologic passport for athletes competing in endurance sports: a feasibility study. Haematologica. 2003;88(5):570–81.
Ostojic S, Ahmetovic Z. Indicators of iron status in elite soccer players during the sports season. Int J Lab Hematol. 2009;31(4):447–52. https://doi.org/10.1111/j.1751-553X.2008.01064.x.
Bachman E, Travison TG, Basaria S, Davda MN, Guo W, Li M, et al. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. J Gerontol A Biol Sci Med Sci. 2014;69(6):725–35. https://doi.org/10.1093/gerona/glt154.
Mokrushnikov PV, Panin LE, Zaitsev BN. The action of stress hormones on the structure and function of erythrocyte membrane. General Physiol Biophysics. 2015;34(3):311–21. https://doi.org/10.4149/gpb_2014041.
Moeckel GW, Shadman R, Fogel JM, Sadrzadeh SM. Organic osmolytes betaine, sorbitol and inositol are potent inhibitors of erythrocyte membrane ATPases. Life Sci. 2002;71(20):2413–24. https://doi.org/10.1016/S0024-3205(02)02035-0.
Nobari H, Oliveira R, Brito JP, Pérez-Gómez J, Clemente FM, Ardigò LP. Comparison of running distance variables and body load in competitions based on their results: a full-season study of professional soccer players. Int J Environ Res Public Health. 2021;18(4):2077. https://doi.org/10.3390/ijerph18042077.
Nobari H, Oliveira R, Clemente FM, Adsuar JC, Pérez-Gómez J, Carlos-Vivas J, et al. Comparisons of accelerometer variables training monotony and strain of starters and non-starters: a full-season study in professional soccer players. Int J Environ Res Public Health. 2020;17(18):6547. https://doi.org/10.3390/ijerph17186547.
Clemente FM, Silva R, Chen Y-S, Aquino R, Praça GM, Castellano J, et al. Accelerometry-workload indices concerning different levels of participation during congested fixture periods in professional soccer: a pilot study conducted over a full season. Int J Environ Res Public Health. 2021;18(3):1137. https://doi.org/10.3390/ijerph18031137.
Acknowledgments
The authors would like to thank the players who participated in this study, Deputy Vice-Chancellor for Research and Technology, University of Isfahan and the Sepahan Professional Club. We also thank Dr. Jenna Bartley for her valuable guidance.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, HN, JPG, and JMC, methodology, HN, JMC, and JPG, data collection, HN, analysis, HN and JMC, writing—original draft preparation, HN, and ACR, writing—review and editing, HN, JMC, ACR, and JPG. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Players, as well as their parents, signed a consent letter to participate in the project. Prior to the start of the study, the Ethics Committee of the University of Isfahan approved the study (IR.UI.REC.1398.102), and the recommendations of Human Ethics in Research were followed by the Helsinki Declaration.
Consent for publication
No individual or indemnifiable data is being published as part of this manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nobari, H., Cholewa, J.M., Pérez-Gómez, J. et al. Effects of 14-weeks betaine supplementation on pro-inflammatory cytokines and hematology status in professional youth soccer players during a competition season: a double blind, randomized, placebo-controlled trial. J Int Soc Sports Nutr 18, 42 (2021). https://doi.org/10.1186/s12970-021-00441-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12970-021-00441-5