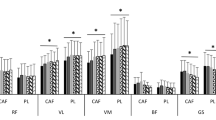Abstract
Background
To examine the effects of varying doses of caffeine on autonomic reactivation following anaerobic exercise.
Methods
Recreationally active males (N = 20; 24 ± 2y) participated in a randomized, double-blind, placebo-controlled, crossover study where participants ingested: [1] Control (CON; no supplement), [2] a non-caffeinated placebo (PLA), [3] 3-mg∙kg− 1 of caffeine (CAF3) or [4] 6-mg∙kg− 1 of caffeine (CAF6) prior to Wingate testing. Parasympathetic (lnRMSSD, primary outcome) and global HRV (lnSDNN, secondary outcome) were assessed at rest (i.e., pre-ingestion), 45-min post-ingestion, and 5-min and 35-min post-exercise recovery. We used a GLM to assess mean (95% CI) changes from pre-ingestion baseline.
Results
Overall, we observed a significant trend for lnRMSSD and lnSDNN (both, p = 0.001, ηp2 = 0.745). Forty-five minutes after treatment ingestion, we observed a significant increase in lnRMSSD for CAF3 (0.15 ms, 95%CI, 0.07,0.24) and CAF6 (0.16 ms, 95%CI, 0.06,0.25), both being significant (both, p < 0.004) vs. CON (− 0.02 ms, 95%CI, − 0.09,0.04). Five-minutes after exercise, all treatments demonstrated significant declines in lnRMSSD vs. baseline (all, p < 0.001). After 35-min of recovery, lnRMSSD returned to a level not significantly different than baseline for CAF3 (0.03 ms, 95%CI, − 0.05, 0.12) and CAF6 (− 0.03 ms, 95%CI, − 0.17, 0.10), while PLA (− 0.16 ms, 95%CI, − 0.25, − 0.06) and CON (− 0.17 ms, 95%CI, − 0.28, − 0.07) treatments remained significantly depressed. A similar pattern was also observed for SDNN.
Conclusion
Caffeine ingestion increases resting cardiac autonomic modulation and accelerates post-exercise autonomic recovery after a bout of anaerobic exercise in recreationally active young men. However, no differences between caffeine doses on cardiac autonomic reactivity were observed.
Similar content being viewed by others
Introduction
Adaptations to exercise training require an appropriate training stimulus accompanied by sufficient recovery [1]. One factor associated with exercise training and recovery is the balance between the sympathetic and parasympathetic nervous branches of the autonomic nervous system (ANS) [2]. Accordingly, an imbalance in ANS between the training stimuli and recovery can may lead to ANS dysregulation and negatively impact exercise performance [1, 3]. Fortunately, ANS can be assessed easily and non-invasively assess heart rate variability (HRV) via heart rate monitors, thus providing for a useful tool for sports performance and recovery from strenuous exercise.
During exercise, heart rate increases via parasympathetic withdrawal and increased sympathetic activity [2]. HRV becomes useful during training and as it a validated tool to assess internal training load during and following exercise allowing for the individual evaluation surrounding training stimuli [1, 3, 4]. Despite the potential implications of HRV, the time course of HRV following exercise is multifactorial and varies based on genetics, and the intensity, duration, and mode of exercise [2, 4,5,6]. Accordingly, exercise intensity seems to play the greatest role relative an athlete during acute post exercise HRV recovery, in particular parasympathetic reactivation, is delayed following high intensity exercise [4, 5, 7]. A potential, yet relatively unexplored means of improving parasympathetic activity following strenuous exercise is the ingestion of caffeine.
Caffeine is one of the most popular ergogenic compounds used in sport and supported by a large body of scientific evidence for improving anaerobic and aerobic activities [8,9,10,11]. The ingestion of caffeine influences the ANS via an increase in catecholamine secretion, which subsequently increases heart rate and mean arterial blood pressure at rest and during exercise [12]. Caffeine acts as a sympathetic stimulus during exercise and has been shown to attenuate autonomic recovery post-exercise [13, 14] . However, research findings are mixed, with some studies showing that 300–400 mg of caffeine delays post-exercise parasympathetic reactivation [12, 14, 15], while others have found no effect from caffeine at doses associated with < 3 mg∙kg− 1 body mass [16, 17]. While it is difficult to compare and contrast results across studies, these inconsistent findings may be related to the caffeine dosage used, which warrants further investigation within athletic populations. The purpose of this study was to examine the effects of two dosages of caffeine (3 mg∙kg− 1 and 6 mg∙kg− 1) on HRV indices of the ANS following a single bout of high intensity exercise. The primary outcome measure was root mean square of the successive differences (rMSSD), a time domain HRV index reflecting parasympathetic tone. The secondary outcome for our study was the standard deviation of the NN (R-R) intervals (SDNN). We hypothesized that caffeine ingestion would reactivate parasympathetic markers following a reduction immediately following exercise.
Methods
Participants
We enrolled healthy, recreationally active males (N = 20, age: 24 ± 2 y; body mass: 74.70 ± 7.07 kg; height: 178.8 ± 4.64 cm) who engaged in ≥3 sessions per week or 200–300 min∙wk.− 1 of exercise into the study. Inclusion criteria required participants to have been physically active for the 6-months preceding the study and not habitually consuming caffeine (< 120 mg∙day− 1; < 3 days/wk). All participants signed an informed consent following verbal and written information of the study design and potential risks before beginning the study. A health history questionnaire (HHQ) and physical activity readiness questionnaire (PAR-Q) were administered in order to ensure participants were eligible to do high intensity physical activity safely [18]. Individuals having had orthopedic complications or cardiovascular, pulmonary, or metabolic disease were excluded from the study. For all testing sessions, participants wore light and comfortable clothing, avoided strenuous exercise for 48 h and caffeine for 24 h. The study was approved by the Ethics Committee of Islamic Azad University of Karaj.
Experimental design
The study used a double-blind, placebo-controlled, randomized cross-over design. An independent investigator not involved in data collection performed randomization and supplementation preparation. Participants visited the laboratory on six separate occasions within a 20-day period, with a minimum of 72 h between visits. During the first visit, the study procedures were explained, informed consent was obtained, and participants completed various questionnaires, such as the PAR-Q and HHQ. Participants also recorded their dietary intake prior to the first experimental day and were asked to replicate this diet on subsequent visits. Height (cm) was measured with an electronic stadiometer (SECA 217, Seca Ltd., Hamburg, Germany) to the nearest 0.01 cm without shoes and with each participant standing erect against a wall and body mass (kg) was measured to the nearest 0.01 kg using a calibrated digital scale (Seca 770-floor, Seca Ltd., Hamburg, Germany). During the second visit participants completed a 30-s all-out Wingate Anaerobic Test (WAnT) for familiarization and to reduce the learning effect [19, 20].
During the remaining experimental visits [3,4,5,6], participants performed the experimental treatments based on computer generated randomization of four treatments: [1] control (no treatment), [2] placebo (PLA, non-caffeinated treatment matched for taste and color), [3] 3 mg∙kg− 1 of caffeine (CAF3) and [4] 6 mg∙kg− 1 of caffeine (CAF6). All experimental visits were conducted at the same time of day (between 11:00 and 14:30). To minimize potential gastrointestinal distress, participants consumed a standardized snack (white bread and boiled eggs) containing 3 g∙kg− 1 of carbohydrates, 20 g of protein, and 10 g fat 180–240 min before each session. A schematic of the testing protocol is presented in Fig. 1 and is detailed below:
-
(Time 1) Resting: Pre-Ingestion. Prior to treatment ingestion, participants sat quietly for 5-min before providing a resting blood sample to assess blood lactate (BLa; Lactate Scout plus analyzer, SensLab GmbH, Germany). A resting HRV was also measured. Following these baseline measurements participants then ingested their respective treatments.
-
(Time 2) Resting: 45-min Post Ingestion. Forty-five minutes after treatment ingestion, participants were assessed again for HRV and BLa. Following this assessment, participants engaged in a standardized warm-up before performing WAnT testing (detailed below).
-
(Time 3) Post Exercise: 5-min Post WAnT. Following their WAnT, participants were tested immediately for BLa, ratings of perceived exertion (RPE), and another HRV measurement was completed 5-min after exercise.
-
(Time 4) Post Exercise: 35-min post WAnT. Following the 5-min post exercise assessment, participants engaged in a passive recovery period, and were subsequently assessed a final time for HRV and BLa 35 min post exercise.
Wingate anaerobic test
Participants performed a Wingate Anaerobic Test (WAnT) using a friction-loaded cycle ergometer (MONARK 894E, Stockholm, Sweden) connected to a computer as previously described [19,20,21]. The ergometer was calibrated before each test. Briefly, the WAnT was a 30-s test requiring participants to pedal as fast as possible against a fixed resistance. Each test began with a 4-min standardized warm-up against a fixed load of 1 kilopond and three separate 2 s sprints were performed against a load of 0.075 kp∙kg− 1 of body mass were interspersed with 45-s of active recovery [20]. After the warm-up, one minute of light dynamic stretching was performed. The test protocol began with a verbal command of “faster, faster, 3,2,1, go”, during which the load of 0.075 kp∙kg− 1 body mass [22] was applied. At “go” the participants were pedaling as fast as possible and each participant was verbally encouraged to maintain as high of a cadence as possible over the entire 30-s. No visual or verbal feedback regarding the time to complete the test was provided.
Power output in watts was calculated as the product of resistance and flywheel revolutions, which was recorded every 1-s, peak power output was determined from the average of the first 5-s a mean power output was assessed as the average over the entire 30-s of the test. Fatigue index was calculated from the difference between peak 5 s power output and the power output that occurred during the final 5-s of the test divided by the peak power output multiplied by 100 [21]. The seat height and handlebars were adjusted such that the knee would be slightly bent at maximal leg extension and kept constant throughout the remaining experimental sessions.
Supplementation protocol
Caffeine powder (Cat. No. C0750; Sigma Aldrich) and PLA (dextrose) treatments were packaged in identical gelatin capsules (Iran Gelatin Capsule Co. Iran). The capsules’ ingredients were unknown to participants and investigators who performed data collection. The PLA contained 200 mg dextrose, while the caffeine was provided at two doses: 3 mg∙kg− 1 of body mass or 6 mg∙kg− 1 of body mass.
Heart rate variability measurement
Heart rate variability is a non-invasive tool used to quantify cardiovascular autonomic function based on the measurement of the timing between consecutive R-R intervals [23, 24]. Briefly, the heart rate monitor strap was placed on the participant according to the manufacture instructions. All HRV measurements were collected in a seated position, within a quiet and dimly lit room with no external stimuli. The R-R interval data were recorded at a sampling frequency of 1000 Hz for 5-min and was synchronized with the Polar Flow web service (Polar Flow software). Raw unfiltered R-R data was exported as a space delimitedtext file for analysis of time (Kubios V 2.2, Joensuu, Finland), as previously described [25]. Any segments that contained three or more irregular R-R intervals were excluded from analysis, and possible artifact noise was replaced with the interpolated adjacent R-R interval values (filter power < low). R-R interval markers were measured using a window width of 256-s and overlap of 50% through the specialized HRV software (Kubios V 2.2, Joensuu, Finland). The dependent variables in the time domain included the standard deviation of normal-to-normal (SDNN) intervals and the root mean squared of successive difference (RMSSD) of R-R intervals [26]. Log transformed data for all time were used in statistical analysis to avoid outliers.
Statistical analysis
The primary outcome for our study was parasympathetic tone as measured via lnRMSSD as RMSSD is a singular index measuring parasympathetic nervous system tone. Our secondary outcome was lnSDNN, which reflects mixed ANS modulation and is considered a global index of HRV. We used general linear models to examine changes from the baseline, pre-ingestion HRV assessment at 45-min post-ingestion, 5-min post exercise and 45-min post exercise. Statistical analyses were performed using general linear models. Post-hoc analyses were performed using Bonferonni adjusted, paired t-tests in order to prevent the likelihood of Type I error (SPSS® version 25, IBM North America, New York, NY, USA). Normality was examined using Kolmogorov-Smirnov test and confirmed for all exercise related variables. However, we observed that HRV indices were not normally distributed. Therefore, we performed a natural log transformation for all HRV measures. Effect sizes are presented as partial Eta squared (ηp2) for the general linear models and Cohen’s D for simple effects. All calculations were accomplished using) and the probability level for statistical significance was pre-set at P = 0.05, while ES were calculated using means and pooled standard deviations (SD). Effect sizes for partial eta squared were interpreted as: 0.01, small; 0.06, medium and > .14, large [26]. Effect sizes for Cohen’s D were interpreted as 0.20 = small, 0.50 = moderate, 0.80 = large [27]. Data throughout the manuscript are presented as mean (SD) or mean change (95% CI).
Results
We have presented the results for WAnT testing in Table 1, inclusive of RPE and blood lactate. Further, we have presented a schematic representation of the study procedures in Fig. 1 and our findings for lnRMSSD and lnSDNN in Fig. 2. In brief, we observed that overall, participants generated a peak power output of 773.84 ± 178.41 W, a mean power of 502.96 ± 86.46, achieved a maximum exercise heart rate of 155.04 ± 7.36 b/min− 1, a maximal RPE of 16.37 ± 1.68, a maximal blood lactate level of 4.80 ± 0.65 mmol/L at peak exercise and 5-min post exercise 8.26 ± 1.08). Between group comparisons showed a significantly greater peak power output (157 W, 95% CI, 10.17, 304.42, p = 0.03), maximal HR (7.45 b/min− 1, 95% CI, 1.46, 13.44, p = 0.007). Both the CAF3 (− 1.80, 95% CI, − 3.05, − 0.55, p = 0.001) and CAF6 (− 2.25, 95% CI, − 3.60, − 1.10, p < 0.001) exhibited a lower maximal RPE vs. CON and PLA. No significant between group differences were observed for mean power, minimum power or fatigue index.
Data present change from baseline for lnRMSSD (panel a) and lnSDNN (panel b). Significance notations within each time period are as follows: (a) Significant vs. Baseline pre-ingestion (p < 0.001), (b) Significant vs. CON (p ≤ 0.004), (c) Significant vs. PLA (p = 0.011), (d) Significant vs. PLA and Control (p ≤ 0.033), and (e) Significant (p ≤ 0.003)
Primary outcome: lnRMSSD
We observed a significant trend for changes in lnRMSSD (Fig. 2a) and lnSDNN (Fig. 2b), from baseline, following treatment ingestion (both, p = 0.001, ηp2 = 0.745). For lnRSSD, significant treatment effects were observed 45-min following ingestion (p < 0.009, ηp2 = 0.140) and 35-min post exercise (p = 0.014, ηp2 = 0.129). While all groups demonstrated a significant reduction in 5-min lnRMSSD vs. baseline (all, p < 0.001); no significant between group (i.e., treatment) were observed at this time (p = 0.809, ηp2 = 0.013). Specific between group comparisons findings demonstrated that the CAF3 and CAF6 treatments increased lnRMSSD significantly 45-min after treatment ingestion (both, p < 0.001) both CAF treatments to be significant vs. CON (p < 0.004). Thirty-five minutes after the completion exercise, both CAF3 and CAF6 treatments demonstrated a return of lnRMSSD to values not significantly different to baseline, while the CON and PLA treatments remained significantly depressed (both, p < 0.001). For this latter assessment, the CAF3 treatment was significantly higher than CON (p < 0.004) and PLA (p < 0.011) treatments (Fig. 2).
Secondary outcome: lnSDNN
For lnSDNN, we observed significant treatment effects 45-min post-treatment ingestion (p < 0.023, ηp2 = 0.117) and 35-min post exercise performance (p = 0.017, ηp2 = 0.124), but not at 5-min post exercise (p = 0.784, ηp2 = 0.014), BMI 23.3 (SD). Forty-five minutes following treatment ingestion and before exercise testing, we observed a significant increase from baseline for lnSDNN in the CAF3 and CAF6 treatment conditions (both, p < 0.001). Both the CAF3 and CAF6 treatments were significant vs. the CON treatment (p < 0.004). Following WAnT testing, we observed a significant reduction in lnSDNN for all treatments after 5-min of recovery compared to baseline, pre-ingestion (all, p < 0.001). After 35-min of exercise, we observed a continued reduction in lnSDNN for the CON and PLA treatments (both, p < 0.001); however, no significant reductions in lnSDNN vs. baseline, pre-ingestion were noted for the CAF3 and CAF6 treatments. For this latter assessment, lnSDNN was significantly greater for the CAF3 and CAF6 treatments vs. PLA (p < 0.003). In summary, the HRV indices measured in this study returned to baseline conditions for both caffeine treatments, while the same indices remain reduced compared to baseline following a single bout of strenuous exercise.
Discussion
The primary objective of this study was to analyze the effects of different caffeine dosages on resting and post-exercise cardiac autonomic modulation. While higher cardiac parasympathetic and global modulations were observed after CAF3 and CAF6 ingestion during the resting condition, no such effects were noted for the PLA and CON groups. Further, while all treatment groups demonstrated a significant reduction in lnRMSSD and lnSDNN 5-min following exercise, no between treatment effects were noted. Finally, given the continual HRV suppression for the PLA and CON groups at 35-min post-exercise, compared to the restoration of said indices for the CAF3 and CAF6 treatments to levels not significantly different from baseline, we conclude that the CAF ingestion in the quantities used in this study are sufficient to accelerates post-exercise autonomic recovery following a single bout of strenuous exercise. Based on these observations we accept our research hypothesis.
The effects of caffeine ingestion on resting HRV are conflicting, with studies reporting increases [28,29,30], reduction [31] and no changes [32] of resting parasympathetic and/or global modulation markers. Establishing a cause of these divergences is not an easy task since several variables can affect HRV analysis, such as sex [33], body position [34], body mass index [35], nutritional status [36], functional condition [37], corresponding heart rate [38], cardiorespiratory fitness [39] and age [40]. In that same sense, the physiological and functional response to caffeine ingestion also depends on various factors such as individual caffeine habituation [41], caffeine dosage [42], sex [43], functional condition adopted to analysis [32], genetic profile [44], caffeine expectancies [45] and some other neuromuscular characteristics [46]. Thus, it is plausible to infer that the autonomic response to caffeine ingestion is dependent on several independent variables, and the increase of cardiac parasympathetic and global modulations observed in this study may be limited to our study design and participants’ characteristics.
Regarding the caffeine dosage effect, no differences between CAF3 and CAF6 on resting autonomic dynamics were observed by this study. Previous studies showed that both 2 [28] and 5 mg∙kg− 1 [30] of caffeine, dosage close to those adopted in this study, were able to increase cardiac parasympathetic modulation. In this scenario, our results reinforce the possibility of increasing parasympathetic modulation after caffeine intake and add important information suggesting that the relationship between caffeine dosage and parasympathetic reactivity is not linear. No changes on lnRMSSD and lnSDNN were observed after 45 min of resting on PLA and CON groups, suggesting no effect of resting time on rest cardiac autonomic modulation. In opposition, a significant effect of resting time on supine and orthostatic cardiac parasympathetic and global modulation was previously observed after 60 min of resting in the supine position in young men [32]. In this same scenario, Zimmermann-Viehoff et al. (2015) observed a significant effect of resting time on HRV parameters in a sample of young men and women from 30 to 50 min of rest at the seated position [47] . Despite ours and Zimmermann-Viehoff et al. (2015) studies using the seated position to analyze HRV, some differences between them and may explain the conflicting results observed. These differences include the amplitude of R-R interval segments used for HRV analysis, rest time before the nutritional intervention and sample characteristics. Thus, these data indicate that the effect of resting time on HRV may be protocol-dependent and should be considered in studies involving the effect of different pharmacological and non-pharmacological interventions on HRV.
In the initial post-exercise analysis, all treatment groups demonstrated a significant reduction in lnRMSSD and lnSDNN, but no differences between treatments were noted. However, after 35 min of passive recovery no differences between rest and post-exercise lnRMSSD and lnSDNN were identified in CAF3 and CAF6 protocols, while a persistent depression of these autonomic markers was identified in CON and PLA groups. Thus, these results confirm our initial hypothesis (please, check it) that caffeine intake can boosts post-exercise cardiac autonomic recovery. Corroborating our results, Rolim et al. (2018) observed a higher post-exercise cardiac parasympathetic reactivation after a submaximal exercise test in young men after caffeine uptake (3 mg∙kg− 1), despite no changes in resting markers of cardiac autonomic modulation [32]. On the other hand, Kliszczewicz et al. (2018) underwent ten physically active young males to Wingate anaerobic test, and no effect of a complex containing caffeine (100 mg) + Citrus aurantium (100 mg) was observed on post-exercise parasympathetic and sympathetic activity, despite higher resting sympathetic activity compared to the placebo condition [17]. Otherwise, Bunsawat et al. (2014) suggest that caffeine can promote a sympathetic over activation after maximal exercise, a hypothesis based mainly on higher absolute heart rate and blood pressure after an exercise test [14]. However, higher training load and maximum heart rate were observed in Bunsawat’s study after caffeine intake, with no differences in heart rate recovery at the first and the second-minute post-exercise, which makes the caffeine-induced sympathetic over activation hypothesis questionable. In other words, higher post-exercise absolute heart rate and blood pressure may occur due to a higher training load and not necessarily a direct effect of caffeine ingestion.
From a physiological perspective, the autonomic response to caffeine uptake is complex and may be bilateral. Was previously reported that caffeine ingestion could promote a significant increase in plasma levels of catecholamines [48, 49] and inhibits the enzymatic degradation of cyclic adenosine monophosphate by phosphodiesterases, which potentiates postsynaptic neurotransmission in the sympathetic nervous system [50] On the other hand, despite parasympathetic response to caffeine uptake remain underexplored, it has been shown that caffeine can stimulate acetylcholine receptors and acts as an inhibitor of acetylcholinesterase [51, 52], which explains, at least in part, the caffeine-induced increase in parasympathetic activity reported in our and previous studies. In addition, it has been hypothesized that the caffeine-induced parasympathetic activation may be a result of baroreflex activation due to an increase in peripheral vascular resistance and blood pressure resulting from antagonistic caffeine effect on adenosine receptors [32], which need to be confirmed in future studies.
Notwithstanding a lack of difference between group mean power observed in caffeine and placebo protocols, higher peak power was observed in CAF3 and CAF6 compared to PLA and control reveal an ergogenic effect of caffeine on anaerobic performance. Also, higher peak power in CAF6 compared to CAF3 indicate that this ergogenic effect is dose dependent. Previously, a lack of effect and even reduction in anaerobic performance after caffeine consumption has already been reported in the literature [48]. However, a recent meta-analysis using studies of good and excellent methodological quality reveal that caffeine intake can augment mean and peak power output on the Wingate anaerobic test by 3 and 4%, respectively [11] . Interestingly, in our study, higher cardiac parasympathetic reactivation after caffeine intake was observed even in the face of higher peak power in CAF3 and CAF6 compared to control and PLA protocols. This finding strengthens the favorable effect of caffeine on post-exercise parasympathetic reactivation since an inverse relationship between exercise intensity and the magnitude of parasympathetic reactivation is expected [53, 54].
While a higher fatigue index was found following caffeine compared to control, but there were no differences compared to placebo. Of note, examining the effect of caffeine supplementation on repeated bouts of Wingate tests (four 30-s Wingate tests with 4 min of rest between each exercise) after caffeine (6 mg∙kg− 1) or placebo ingestion, Greer, McLean, and Graham (1998) observed that caffeine ingestion had an ergolytic effect in the latter two exercise bouts [48]. Otherwise, it was recently reported that caffeine supplementation (6 mg∙kg− 1) increased the peak power during Wingate anaerobic test and diminished neuromuscular fatigue, shown by attenuation of decrease in countermovement jump performance after Wingate test [55] . Thus, since increase [55, 56] and reduction [48, 57] of different markers of exercise tolerance after caffeine supplementation already been reported, the recommendation of caffeine supplementation to improve recreational or athletic performance should be made cautiously.
Despite no observed difference between RPE in caffeine and placebo during warm-up, the main effect of treatment and lower RPE observed after CAF6 compared to control and PLA indicates that caffeine may reduce the exercise-induced psychological stress. Interestingly, Duncan et al. (2019) observed a reduction of RPE during Wingate test for the upper-body, but not for the lower-body segment, suggesting that caffeine’s effect on RPE depends on body segment exercised [58]. Despite the absence of caffeine effect on RPE during lower-body Wingate test observed in some studies [58,59,60], our findings reveal that this benefit can be acquired with caffeine supplementation in this condition. We note that lower RPE identified in CAF6 protocol was accompanied by high peak power and mean power, which reinforce the psychostimulant effect of caffeine. It is an interesting approach since increases in exercise performance without altering RPE mean a higher power output without the increase in psychological stress per se; this positive effect should also be investigated in future studies.
As expected, an increase of BLA was observed after WAnT in all protocols indicating the vital contribution of anaerobic metabolism to the energy requirements during the exercise test. Despite increase [61] and maintenance [58] of BLA levels are commonly reported after caffeine intake, lower BLA concentration was observed in CAF3 compared to other protocols after five minutes of recovery. Unfortunately, the only lactate analysis performed in the initial phase of recovery does not permit to detect the exact moment with the highest lactate concentration, which makes any inference about the effect of caffeine on lactate production or clearance questionable. In the final phase of post-exercise recovery, we observed higher BLA levels in CAF6 compared to PLA, but the absence of difference between CAF6 and control prevents the attribution of higher blood lactate to caffeine supplementation. Of note, blood lactate reflects the balance between lactate production and clearance and the precise mechanisms that explain the small differences observed in this study is unclear and it may be just a inter day variation of BLA response to exercise, hypothesis previously reported in the literature [62, 63].
Limitations
A major strength of our study is our randomized, crossover design. A limitation of our study is the use of a single, acute bout of WAnT testing. Therefore, we cannot generalize our findings to higher exercise volume conditions, such as multiple WAnT testing, multiple sets of resistance training, interval style workouts. We also cannot generalize our findings to women. The absence of ventilatory, sympathetic activity, and post-exercise blood pressure analysis, variables that influence HRV could also contribute to a better physiological interpretation of our data. We believe that a particular strength of our study was the use of a non-supplemented CON condition in addition an inert PLA and support this contention that a number of between group comparisons in our study were significant vs. the CON, but not the PLA treatments. Lastly, we assessed cardiac parasympathetic reactivation during 35 min of recovery, which limits our conclusions to this time window. However, despite the mentioned limitations, the analysis of autonomic response to different caffeine supplementation dosages on resting and post-exercise conditions adopted in this study adds robust information to current scientific debate about the autonomic effect of caffeine ingestion. The post-exercise time window adopted in this study allow fast and slow parasympathetic reactivation analysis and is within of window of opportunity for sudden death in young observed 30 min after vigorous exercise, which can be partially attributed to post-exercise cardiac autonomic dysfunction [64] and add clinical relevance to our results.
Conclusion
We conclude that caffeine ingestion increases resting cardiac autonomic modulation and accelerates post-exercise autonomic recovery after a bout of anaerobic exercise in recreationally active young men. However, no differences between caffeine doses (3 or 6 mg∙kg− 1) on cardiac autonomic reactivity were observed.
Availability of data and materials
Data and publication materials are available from the corresponding author on reasonable request.
Abbreviations
- ACSM:
-
American College of Sports Medicine
- ANS:
-
Autonomic nervous system
- Bla:
-
Blood lactic acid
- CAF3:
-
Caffeine treatment (3 mg/kg)
- CAF6:
-
Caffeine treatment (6 mg/kg)
- CI:
-
Confidence interval
- CON:
-
Control treatment
- GLM:
-
General linear model
- HHQ:
-
Health history questionnaire
- hr.:
-
Hour
- HRV:
-
Heart rate variability
- kg:
-
Kilogram
- lnRMSSD:
-
Log normalized root mean square of the successive differences
- lnSDNN:
-
Log normlized standard deviation of the NN (R-R) intervals
- mg:
-
Milligram
- min:
-
Minute
- ms:
-
Millisecond
- NN (R-R):
-
ECG intervals between R-waves
- PAR-Q:
-
Physical activity readiness questionnaire
- PLA:
-
Placebo treatment
- RMSSD:
-
Root mean square of the successive differences
- RPE:
-
Rate of Perceived Exertion
- SD:
-
Standard deviation
- SDNN:
-
Standard deviation of the NN (R-R) intervals
- WAnT:
-
Wingate anaerobic test
- wk.:
-
Week
References
Bellenger CR, Fuller JT, Thomson RL, Davison K, Robertson EY, Buckley JD. Monitoring athletic training status through autonomic heart rate regulation: a systematic review and meta-analysis. Sports Med. 2016;46(10):1461–86..
Vitale JA, Bonato M, La Torre AL, Banfi G. Heart rate variability in sport performance: do time of day and Chronotype play a role? J Clin Med. 2019;8(5):723..
Aubert AE, Seps B, Beckers F. Heart rate variability in athletes. Sports Med. 2003;33(12):889–919.
Michael S, Graham KS, Davis GM. Cardiac autonomic responses during exercise and post-exercise recovery using heart rate variability and systolic time intervals—a review. Frontiers in physiology. 2017;8:301.
Stuckey MI, Tordi N, Mourot L, Gurr LJ, Rakobowchuk M, Millar PJ, et al. Autonomic recovery following sprint interval exercise. Scand J Med Sci Sports. 2012;22(6):756–63.
Myllymäki T, Rusko H, Syväoja H, Juuti T, Kinnunen M-L, Kyröläinen H. Effects of exercise intensity and duration on nocturnal heart rate variability and sleep quality. Eur J Appl Physiol. 2011;112(3):801–9.
Goulopoulou S, Heffernan KS, Fernhall BO, Yates G, Baxter-Jones ADG, Unnithan VB. Heart rate variability during recovery from a Wingate test in adolescent males. Med Sci Sports Exerc. 2006;38(5):875–81.
Maughan RJ, Burke LM, Dvorak J, Larson-Meyer DE, Peeling P, Phillips SM, et al. IOC consensus statement: dietary supplements and the high-performance athlete. Br J Sports Med. 2018;52(7):439–55.
Naderi A, de Oliveira EP, Ziegenfuss TN, Willems MET. Timing, optimal dose and intake duration of dietary supplements with evidence-based use in sports nutrition. J Exerc Nutr Biochem. 2016;20(4):1–12.
Southward K, Rutherfurd-Markwick KJ, Ali A. Correction to: the effect of acute caffeine ingestion on endurance performance: a systematic review and meta-analysis. Sports Med. 2018;48(10):2425–41.
Grgic J. Caffeine ingestion enhances Wingate performance: a meta-analysis. Eur J Sport Sci. 2017;18(2):219–25.
Gonzaga LA, Vanderlei LC, Gomes RL, Valenti VE. Caffeine affects autonomic control of heart rate and blood pressure recovery after aerobic exercise in young adults: a crossover study. Scientific Reports. 2017;7(1):1–8.
Graham TE, Spriet LL. Metabolic, catecholamine, and exercise performance responses to various doses of caffeine. J Appl Physiol. 1995;78(3):867–74.
Bunsawat K, White DW, Kappus RM, Baynard T. Caffeine delays autonomic recovery following acute exercise. Eur J Prev Cardiol. 2014;22(11):1473–9.
Gonzaga LA, Vanderlei LCM, Gomes RL, Garner DM, Valenti VE. Involvement of cardiorespiratory capacity on the acute effects of caffeine on autonomic recovery. Medicina. 2019;55(5):196.
An SM, Park JS, Kim SH. Effect of energy drink dose on exercise capacity, heart rate recovery and heart rate variability after high-intensity exercise. J Exerc Nutr Biochem. 2014;18(1):31–9.
Kliszczewicz B, Bechke E, Williamson C, Bailey P, Hoffstetter W, McLester J, McLester C. The influence of citrus aurantium and caffeine complexversus placebo on the cardiac autonomic response: a double blind crossover design. J Int Society Sports Nutr. 2018;15(1):34.
ACSM’s Exercise Testing and Prescription. 10 ed: Lippincott Williams & Wilkins; 2017.
Peterson MD. NSCA’s guide to tests and assessments. Champaign, IL: Human Kinetics; 2012. p. 217–52.
Kavaliauskas M, Phillips SM. Reliability and sensitivity of the 6 and 30 second Wingate tests in physically active males and females. Isokinet Exerc Sci. 2016;24(3):277–84.
Bar-Or O. The Wingate anaerobic test. An update on methodology, reliability and validity. Sports Med. 1987;4(6):381–94.
Nindl BC, Mahar MT, Harman EA, Patton JF. Lower and upper body anaerobic performance in male and female adolescent athletes. Med Sci Sports Exerc. 1995;27(2):235–41.
Thayer JF, Åhs F, Fredrikson M, Sollers JJ III, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36(2):747–56.
Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task force of the European Society of Cardiology and the north American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–65.
Tarvainen MP, Niskanen J-P, Lipponen JA, Ranta-aho PO, Karjalainen PA. Kubios HRV – heart rate variability analysis software. Comput Methods Prog Biomed. 2014;113(1):210–20.
Karim N, Hasan JA, Ali SS. Heart rate variability-a review. Aust J Basic Appl Sci. 2011;7(1).
Cohen J. A power primer. Psychol Bull. 1992;112(1):155–9.
Hibino G, Moritani T, Kawada T, Fushiki T. Caffeine enhances modulation of parasympathetic nerve activity in humans: quantification using power spectral analysis. J Nutr. 1997;127(7):1422–7.
Monda M, Viggiano A, Vicidomini C, Viggiano A, Iannaccone T, Tafuri D, et al. Expresso coffee increases parasympathetic activity in young, healthy people. Nutr Neurosci. 2013;12(1):43–8.
Yeragani VK, Krishnan S, Engels HJ, Gretebeck R. Effects of caffeine on linear and nonlinear measures of heart rate variability before and after exercise. Depress Anxiety. 2005;21(3):130–4.
Sondermeijer HP, van Marle AGJ, Kamen P, Krum H. Acute effects of caffeine on heart rate variability. Am J Cardiol. 2002;90(8):906–7.
da Silva RP, da Costa Matos RA, EdMK VKS, Molina GE, CJG d C. Caffeine increases parasympathetic reactivation without altering resting and exercise cardiac parasympathetic modulation: a balanced placebo design. Eur J Sport Sci. 2018;19(4):490–8.
Koenig J, Thayer JF. Sex differences in healthy human heart rate variability: a meta-analysis. Neurosci Biobehav Rev. 2016;64:288–310.
da Cruz CJ, Porto LG, da Silva Rolim P, de Souza Pires D, Garcia GL, Molina GE. Impact of heart rate on reproducibility of heart rate variability analysis in the supine and standing positions in healthy men. Clinics. 2019;74.
Koenig J, Jarczok MN, Warth M, Ellis RJ, Bach C, Hillecke TK, et al. Body mass index is related to autonomic nervous system activity as measured by heart rate variability — a replication using short term measurements. J Nutr Health Aging. 2014;18(3):300–2.
Hammoud S, Mourad R, Karam R, Saad I, van den Bemt BJ, Kurdi M. Effect of Ramadan fasting on heart rate variability as a measure of cardiac stress in a Lebanese cohort. Eur J Clin Nutr. 2020:1–3.
Molina GE, Fontana KE, Porto LGG, Junqueira LF. Post-exercise heart-rate recovery correlates to resting heart-rate variability in healthy men. Clin Auton Res. 2016;26(6):415–21.
Sacha J. Interplay between heart rate and its variability: a prognostic game. Front Physiol. 2014;5:347.
Porto LGG, Schmidt ACB, de Souza JM, Nogueira RM, Fontana KE, Molina GE, et al. Firefighters’ basal cardiac autonomic function and its associations with cardiorespiratory fitness. Work. 2019;62(3):485–95.
Hernandez AV, Voss A, Schroeder R, Heitmann A, Peters A, Perz S. Short-term heart rate variability—influence of gender and age in healthy subjects. PLoS One. 2015;10(3):e0118308.
Huertas F, Blasco E, Moratal C, Lupiañez J. Caffeine intake modulates the functioning of the attentional networks depending on consumption habits and acute exercise demands. Sci Rep. 2019;9(1).
Turley KR, Eusse PA, Thomas MM, Townsend JR, Morton AB. Effects of different doses of caffeine on anaerobic exercise in boys. Pediatr Exerc Sci. 2015;27(1):50–6.
Mielgo-Ayuso J, Marques-Jiménez D, Refoyo I, Del Coso J, León-Guereño P, Calleja-González J. Effect of caffeine supplementation on sports performance based on differences between sexes: a systematic review. Nutrients. 2019;11(10):2313.
Grgic J, Pickering C, Bishop DJ, Del Coso J, Schoenfeld BJ, Tinsley GM, et al. ADORA2A C allele carriers exhibit ergogenic responses to caffeine supplementation. Nutrients. 2020;12(3):741.
Shabir A, Hooton A, Spencer G, Storey M, Ensor O, Sandford L, et al. The influence of caffeine expectancies on simulated soccer performance in recreational individuals. Nutrients. 2019;11(10):2289.
Soares EMKVK, Garcia GL, Molina GE, Fontana KE. Muscle strength and caffeine supplementation: are we doing more of the same? Rev Bras Med Esporte. 2019;25(2):168–74.
Zimmermann-Viehoff F, Thayer J, Koenig J, Herrmann C, Weber CS, Deter H-C. Short-term effects of espresso coffee on heart rate variability and blood pressure in habitual and non-habitual coffee consumers – a randomized crossover study. Nutr Neurosci. 2015;19(4):169–75.
Greer F, McLean C, Graham TE. Caffeine, performance, and metabolism during repeated Wingate exercise tests. J Appl Physiol. 1998;85(4):1502–8.
Van Soeren MH, Sathasivam P, Spriet LL, Graham TE. Caffeine metabolism and epinephrine responses during exercise in users and nonusers. J Appl Physiol. 1993;75(2):805–12.
Glade MJ. Caffeine—not just a stimulant. Nutrition. 2010;26(10):932–8.
Fabiani C, Murray AP, Corradi J, Antollini SS. A novel pharmacological activity of caffeine in the cholinergic system. Neuropharmacology. 2018;135:464–73.
Pohanka M. The effects of caffeine on the cholinergic system. Mini-Rev Med Chem. 2014;14(6):543–9.
Buchheit M, Laursen PB, Ahmaidi S. Parasympathetic reactivation after repeated sprint exercise. Am J Phys Heart Circ Phys. 2007;293(1):H133–H41.
Gladwell VF, Sandercock GRH, Birch SL. Cardiac vagal activity following three intensities of exercise in humans. Clin Physiol Funct Imaging. 2010;30(1):17–22.
San Juan AF, López-Samanes Á, Jodra P, Valenzuela PL, Rueda J, Veiga-Herreros P, et al. Caffeine supplementation improves anaerobic performance and neuromuscular efficiency and fatigue in Olympic-level boxers. Nutrients. 2019;11(9):2120.
Pethick J, Winter SL, Burnley M. Caffeine ingestion attenuates fatigue-induced loss of muscle torque complexity. Med Sci Sports Exerc. 2018;50(2):236–45.
Lee C-L, Cheng C-F, Lin J-C, Huang H-W. Caffeine’s effect on intermittent sprint cycling performance with different rest intervals. Eur J Appl Physiol. 2011;112(6):2107–16.
Duncan MJ, Eyre E, Grgic J, Tallis J. The effect of acute caffeine ingestion on upper and lower body anaerobic exercise performance. Eur J Sport Sci. 2019;19(10):1359–66.
Lara B, Gutiérrez Hellín J, Ruíz-Moreno C, Romero-Moraleda B, Del Coso J. Acute caffeine intake increases performance in the 15-s Wingate test during the menstrual cycle. Br J Clin Pharmacol. 2020;86(4):745–52.
Woolf K, Bidwell WK, Carlson AG. The effect of caffeine as an ergogenic aid in anaerobic exercise. Int J Sport Nutr Exerc Metab. 2008;18(4):412–29.
Glaister M, Gissane C. Caffeine and physiological responses to submaximal exercise: a meta-analysis. Int J Sports Physiol Perform. 2018;13(4):402–11.
Fric J, Fric J, Boldt F, Stoboy H, Meller W, Feldt F, et al. Reproducibility of post-exercise lactate and anaerobic threshold. Int J Sports Med. 2008;09(05):310–2.
S G, K M, J N, L W, S K, D S, et al. Reproducibility of the blood lactate threshold, 4 mmol·l −1 marker, heart rate and ratings of perceived exertion during incremental treadmill exercise in humans. Eur J Appl Physiol. 2002;87(2):159–66.
Albert CM, Mittleman MA, Chae CU, Lee IM, Hennekens CH, Manson JE. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med. 2000;343(19):1355–61.
Acknowledgements
We would like to make a special note regarding Dr. Conrad Earnest’s contributions to this manuscript, the co-authors, and the scientific community. Dr. Earnest made an enormous impact in sport nutrition and was instrumental as a mentor and friend to many across the world.
We would also like to thank all the participants of this study for participating in the study.
Funding
Any funding source was used during the research and all expenses were paid by the seventh, eighth, ninth author.
Author information
Authors and Affiliations
Contributions
AS: Data Collection, Supervisor of the project, manuscript preparation. AN: Data Collection and manuscript preparation. CJGD: Data analysis, interpretation and manuscript preparation. FF: PhD: Data Collection. SCF: Data analysis, interpretation and manuscript preparation. DGC: Data analysis, interpretation and manuscript preparation. EM, MA, NJ, AR, EA: Data Collection.MA: Data Collection NJ: Data Collection. CPE: Data analysis, interpretation and manuscript preparation. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of the Islamic Azad University, Boroujerd Branch, Boroujerd, Iran, and the protocol was approved by the University Ethics Committee (IR.IAU.B.REC.1398.7.7). All participants were informed about the procedures and signed an informed consent form prior to commencement of the research.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sarshin, A., Naderi, A., da Cruz, C.J.G. et al. The effects of varying doses of caffeine on cardiac parasympathetic reactivation following an acute bout of anaerobic exercise in recreational athletes. J Int Soc Sports Nutr 17, 44 (2020). https://doi.org/10.1186/s12970-020-00373-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12970-020-00373-6