Abstract
Background
Although prior reports have evaluated the clinical and cost impacts of cardiovascular magnetic resonance (CMR) for low-to-intermediate-risk patients with suspected significant coronary artery disease (CAD), the cost-effectiveness of CMR compared to relevant comparators remains poorly understood. We aimed to summarize the cost-effectiveness literature on CMR for CAD and create a cost-effectiveness calculator, useable worldwide, to approximate the cost-per-quality-adjusted-life-year (QALY) of CMR and relevant comparators with context-specific patient-level and system-level inputs.
Methods
We searched the Tufts Cost-Effectiveness Analysis Registry and PubMed for cost-per-QALY or cost-per-life-year-saved studies of CMR to detect significant CAD. We also developed a linear regression meta-model (CMR Cost-Effectiveness Calculator) based on a larger CMR cost-effectiveness simulation model that can approximate CMR lifetime discount cost, QALY, and cost effectiveness compared to relevant comparators [such as single-photon emission computed tomography (SPECT), coronary computed tomography angiography (CCTA)] or invasive coronary angiography.
Results
CMR was cost-effective for evaluation of significant CAD (either health-improving and cost saving or having a cost-per-QALY or cost-per-life-year result lower than the cost-effectiveness threshold) versus its relevant comparator in 10 out of 15 studies, with 3 studies reporting uncertain cost effectiveness, and 2 studies showing CCTA was optimal. Our cost-effectiveness calculator showed that CCTA was not cost-effective in the US compared to CMR when the most recent publications on imaging performance were included in the model.
Conclusions
Based on current world-wide evidence in the literature, CMR usually represents a cost-effective option compared to relevant comparators to assess for significant CAD.
Similar content being viewed by others
Introduction
For patients with suspected coronary artery disease (CAD), cardiovascular magnetic resonance (CMR) offers a non-invasive and accurate diagnostic option. However, there are other diagnostic alternatives for such patients at low-to-intermediate risk, including ergometry, single-photon emission computed tomography (SPECT), or coronary computed tomography angiography (CCTA). Even conservative management, i.e. waiting for symptomatic disease to worsen and to manifest by complications, or more aggressive management, i.e., or immediate invasive coronary angiography (ICA), could represent options [1]. Depending on certain factors, such as imaging costs, local availability of imaging modalities and expertise and prevalence of underlying disease, tradeoffs may be realized among these options that can influence clinical decision-making [2].
Cost-effectiveness analysis can be used to quantitatively weigh tradeoffs between length of life, quality of life, and incurred costs, across different diagnostic strategies, allowing payers and physician decision-makers to choose a higher-value pathways [3, 4]. Most health technology assessments in high-income countries use cost-per-quality-adjusted life-year (QALY) to quantify value from cost-effectiveness analyses, with estimated costs including both immediate imaging costs and all downstream costs (including both additional care stemming from test results and saved costs from averted coronary heart disease events, follow-up tests, and procedures) [5]. Although there have been prior publications evaluating the clinical and cost impacts of CMR-based diagnostic strategies in patients with suspected CAD, there has been less clarity on the specific cost-effectiveness profile of CMR compared to its relevant comparators across both clinical settings and patient types, particularly as evidence of imaging performance continues to evolve [6,7,8].
In a 2-step approach, we first analyzed and summarized the comparative cost effectiveness surrounding use of CMR imaging for the assessment of patients presenting with stable chest pain syndromes compared to its relevant comparators based on the existing medical literature. Then we created a unique cost-effectiveness calculator that could be used globally to estimate lifetime discounted costs and QALYs for CMR versus its relevant comparator techniques. To facilitate global use for different international geographic regions or referral populations, adjustment of context-specific patient and system-level inputs (such as disease prevalence and imaging costs) was incorporated into the cost-effectiveness calculator.
Methods
Search strategy of existing literature and data extraction
We conducted a systematic literature review using the Tufts Cost-Effectiveness Analysis Registry (CEA Registry, www.cearegistry.org) and PubMed for English-language cost-effectiveness published from 2005 to 2020 [9, 10]. The CEA Registry contains 8000 English-language cost-per-QALY studies. The CEA Registry uses keywords such as QALYs, quality adjusted, and cost-utility analysis to search PubMed for English-language publications. The reference lists of every identified CMR cost-per-QALY or relevant review study were searched to identify additional CMR cost-effectiveness studies missed by our other methods. We only included studies that used CMR as an imaging strategy to assess for CAD as the primary clinical condition. Two reviewers (YY and AP) independently reviewed each study to extract relevant data, and resolved any differences in data extractions at in-person meetings; a third author (SK) was contacted when consensus could not be reached at the in-person meetings. Detailed information on the search strategy and data extraction for the CEA Registry is reported elsewhere [10, 11].
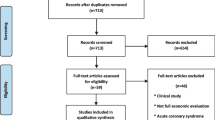
The search terms used to identify cost-per-QALY studies were combinations of methodological terms (cost-effectiveness, QALY, incremental cost-effective ratio) and clinical terms (cardiac magnetic resonance, CMR, coronary angiography). Articles other than cost-effectiveness studies that were related to the overall value of CMR (reviews, meta-analyses, diagnostic performance, editorials, etc.) were identified but not included in our systematic review (Appendix Fig. 3). We reviewed the cost-effectiveness studies that included CMR as a strategy and summarized the following information among these articles: setting of the analysis, comparators included, analytic perspective taken, analytic time horizon taken, main conclusion on the cost effectiveness of CMR (where “cost-effective” was defined as either health-improving and cost saving [“dominant”] [3] or having a cost-per-QALY or cost-per-life-year result lower than the cost-effectiveness threshold [4]), and key drivers of the results. We performed a sensitivity analysis excluding papers that reported cost-effectiveness outcomes other than cost-per-QALY or cost-per-life year (such as cost-per-case detected).
We used author assessments to determine whether CMR represented a cost-effective option or cost-ineffective option for a given paper, which could depend on country-specific cost-effectiveness thresholds. In the United States (US), for example, the American College of Cardiology (ACC) and the American Heart Association (AHA) issued a joint statement on health care “value” in 2014 that specified that cost-per-QALY results below $50,000/QALY indicate high-value care, cost-per-QALY estimates between $50,000/QALY and $150,000/QALY indicate intermediate-value care, and cost-per-QALY estimates greater than $150,000/QALY indicate low-value care [12]. We also categorized some papers as showing unclear CMR cost-effectiveness when there was considerable uncertainty around the CMR cost-effectiveness results.
Development of the CMR cost effectiveness calculator (meta-model)
We built a user-friendly CMR cost effectiveness meta-model based on a larger CMR Markov (state-transition cohort) model developed for a prior CMR cost-per-QALY study (by study co-authors AP, YG, RK) performed for a US health care system perspective [13]. The model projects lifetime discounted QALY and cost outputs for five strategies: (1) no imaging; (2) CMR; (3) SPECT; (4) CCTA; (5) ICA. Other strategies, such as echocardiography, were not included in the larger Markov model. In this model, patients in the CMR, SPECT, and CCTA groups underwent ICA only if noninvasive imaging demonstrated abnormal findings. Those with positive ICA results (with some having also received FFR) were assumed to undergo both medical and revascularization therapies, and this combination led to overall improved health outcomes (quantified using lifetime discounted QALYs). Patients with normal findings were presumed to be free of obstructive CAD and were managed accordingly. In the no imaging strategy patients were initially managed without any investigations. Assuming escalating symptoms in 58% of patients with obstructive CAD who did not receive treatment in their first year after assessment (i.e., patients with false negative results) would return within the first year and undergo ICA, leading to medical and revascularization therapies (i.e., 58% of false negatives would experience the same outcomes as true positives within 1 year) [14]. The Markov model had four major health states: no clinical major cardiovascular events [MACE, defined as: cardiovascular death, acute nonfatal myocardial infarction (MI), hospitalization for unstable angina or heart failure], history of one MACE, history of more than one MACE, and all-cause death [13]. In the Markov model, QALYs are estimated by combining the length of life patients spend in each of the health states with a quality-of-life value (“utility”) ranging between 0 (representing death) and 1 (perfect health), with no MACE having an average utility value of 0.84 and MACE having an average utility of 0.78 [15]. Life costs estimated by the Markov model depend on the cost of the imaging strategies used, procedures performed, and the acute and long-term healthcare-related costs associated with each health state (with MACE states having 1st-year costs between $11,000–18,000 and subsequent annual costs of $3400) [13]. Both QALY and cost outcomes can be discounted at an annual rate (such as 3%, recommended for cost-effectiveness analyses performed for the US setting) [16].
The linear regression-based meta-model was trained (i.e., coefficients were estimated) on 100,000 model input–output combinations from the probabilistic sensitivity analysis (2nd-order Monte Carlo simulation) from the larger Markov simulation model [17]. Previous studies have shown that simple linear regression-based meta-models can approximate larger disease models with high accuracy (r-squared values that often exceed 0.95). The meta-model allows users to replicate the larger Markov model results and change 10–20 key meta-model inputs to view corresponding meta-model outputs to create customized results for specific scenarios or populations of interest [18, 19]. Meta-model variable selection was based on a 0.05 level of significance for beta coefficients in the linear regressions. We validated our meta-model on a separate 1000 model input–output combinations from the probabilistic sensitivity analysis (2nd-order Monte Carlo simulation) from the larger Markov simulation model that were not used to train the meta-model (i.e., the test set) [17]. Meta-model goodness-of-fit was assessed using adjusted r-squared and percentage deviation (for external validation) metrics for the test set.
The meta-model approximates the lifetime discounted QALY and cost results for a given imaging strategy based on user-entered population- and system-level inputs, such as prevalence of CAD, costs of imaging and procedures, and other model inputs listed in Appendix Table 3. We used the meta-model to explain observed differences in conclusions from published cost-effectiveness analyses included in our literature review that were performed in the US setting. Specifically, we used our meta-model to replicate the cost-effectiveness results of the US study it was based on Ge et al. [13], which found CMR to be cost-effective compared to CCTA, SPECT, ICA, and no imaging strategies [13]. Then we changed key input parameters of the meta-model (such as imaging performance and cost parameters, CAD prevalence, treatment costs, etc.) to replicate the cost-effectiveness results of another US study (with a similar model structure and decision problem) that found CCTA to be cost-effective compared to CMR [20]. Using the meta-model, we then changed only the imaging performance inputs from the values in Genders et al. [20] cost-effectiveness study to the more recent imaging performance inputs used by Ge et al. [13] to determine whether these imaging performance inputs alone could explain the difference in CMR cost-effectiveness results.
The larger Markov model was programmed in TreeAge Pro 2019 software (version 19.2.1; TreeAge Software, LLC, Williamstown, Massachusetts, USA), the meta-model was created using RStudio (version 1.2.5042; RStudio Software, Boston, Massachusetts, USA), and the user-friendly cost-effectiveness calculator is programmed in Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA) and is included as a downloadable spreadsheet in Additional file 1 and as a web-based tool at: https://docs.google.com/spreadsheets/d/12TMgbIS6sDpXkafSYfNaNw8vSx-LCTCE4K_yAa7mdyY/edit?usp=sharing.
Results
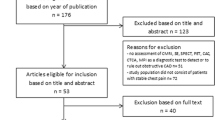
Our search yielded 39 studies of which 15 ultimately met our inclusion criteria (shown in Table 1 and Appendix Fig. 3) [13, 14, 20,21,22,23,24,25,26,27,28,29,30]. We excluded 17 studies because they were not original research (such as perspective articles or literature reviews), they focused on the cost-effectiveness of CMR for heart failure patients (as opposed to CAD), or they did not include CMR as a comparator. Of the remaining studies, an additional seven were excluded for not being a cost-effectiveness analysis (such as studies evaluating only costs or clinical effectiveness). Further two studies were excluded in our sensitivity analysis restricting our analysis to cost-per-QALY or cost-per-life-year studies [24, 29].
Summary of methods used for CMR cost-effectiveness studies
Table 1 shows that most of the 15 studies that met our inclusion criteria were performed in a US (five studies) [13, 20, 24, 28, 29] or European setting (seven included the United Kingdom [14, 20, 23, 24, 28, 30, 31], three included Germany [22, 24, 25]). One study (Bertoldi et al.) was performed for the Brazilian public health system [21] and another (Kozer et al.) was performed for the Australian health care system [32]. In 11 of the 15 studies CMR was compared to SPECT strategies, in 10/15 immediate ICA, in 5/15 to CCTA, and 4/15 to stress electrocardiography. Simulation models (such as decision trees or state transition models) were used for 10/15 studies [13, 14, 20,21,22,23,24, 26, 29, 32], and seven of these ten studies extrapolated outcomes for a lifetime time horizon; the five studies not using simulation models relied on empirical data [25, 27, 28, 30, 31] resulting in time horizons of less than 10 years.
Summary of results of CMR cost-effectiveness studies
CMR was found to be cost-effective versus its relevant comparator in 10/15 studies [13, 14, 22, 24,25,26,27, 29, 31, 32]. Among these ten studies, the most common comparators to CMR were strategies that used ICA [13, 14, 22, 24,25,26, 31, 32] or SPECT [13, 14, 22, 26, 29, 31, 32], while three studies compared CMR versus strategies that used CCTA [13, 27, 31], and one directly compared CMR to a no imaging strategy [13].
Two studies concluded that CCTA was more cost-effective (Bertoldi et al. and Genders et al.) compared to CMR [20, 21]. In sensitivity analyses, Bertoldi et al. found that a cost reduction of 79% was required for the CMR strategy to be cost-effective versus the CCTA from the Brazilian public health system perspective [21]. This result was driven by only slightly higher QALYs from the CMR strategy compared to the CCTA strategy based on sensitivity and specificity inputs from meta analyses published in 2010 [33] (for CMR) and 2008 [34] (for CCTA). Genders et al. focused on a low-to-moderate risk population (performing separate analyses for men and women) and found that CCTA as a first-line test (combined with baseline echocardiography in all patients and additional invasive diagnostic work-up in patients with positive CCTA) almost always dominated CMR in the three settings analyzed (the United States, the Netherlands, and the United Kingdom) [20]. These results were primarily driven by assuming superior accuracy for CCTA (sensitivity of 0.98 and specificity of 0.89) [34,35,36] compared to CMR (0.89 and 0.76, respectively) [37]. Model-based cost-effectiveness studies by Walker et al. and Ge et al. used similar model structures as applied by Genders et al., but with CMR operating characteristics based on more recent trials and meta-analyses [7, 8, 38]. With this approach CMR was cost-effective compared to other imaging strategies such as ICA [13, 14], SPECT [13, 14], and CCTA (with CT-derived fractional flow reserve) [13].
Three studies found unclear cost-effectiveness among the imaging strategies analyzed [23, 28, 30], including two studies that were cost-effectiveness analyses conducted alongside a randomized controlled trial, which were not powered to show statistically significant differences in cost-effectiveness outcomes [28, 30]. Campbell et al. developed a simulation model that included positron emission tomography (PET) for the United Kingdom health care payer perspective and found in probabilistic sensitivity analyses that there were similar probabilities of CMR or PET being optimal in the relevant cost-effectiveness threshold ranges for the United Kingdom [23].
Across the 15 studies included in our review, nine found that underlying prevalence of CAD was a key driver of the cost-effectiveness findings [13, 14, 20, 22, 24, 26, 29, 30, 32], and four found that these results were sensitive to changes in imaging prices [21, 24, 26, 28]. In our sensitivity analysis restricting inclusion to cost-per-QALY or cost-per-life-year studies, 8/13 found CMR to be cost-effective [13, 14, 22, 25,26,27, 31, 32], 3/13 showed uncertain cost-effectiveness rankings [23, 28, 30], and 2/13 concluded that CCTA was superior [20, 21]. We did not find evidence that CMR was more or less likely to be cost-effective across settings; among the 5 studies that were performed for the US setting [13, 20, 24, 28, 29], 3 found that CMR was cost-effective [13, 24, 29], which was similar to the proportion of all studies that found CMR to be cost-effective (10 out of 15).
CMR cost effectiveness calculator (meta-model) results
The fitted coefficients for the lifetime discounted QALY and cost results for ‘No Imaging’ strategy, and incremental QALY and cost results for the ‘CMR’, ‘CCTA’, ‘SPECT’, and ‘Immediate ICA’ strategies are shown in Appendix Table 3. Validation using the test sets showed good fits for each model, with r-squared values ranging from 0.846 to 0.999 across the ten meta-models, with percentage deviation varying from − 0.117% to 0.028%, which are also shown in Appendix Table 3. Table 2 shows the cost-effectiveness analysis results of our meta-model compared to: (1) the model originally used to derive it (i.e., a replication of the Ge et al. cost-effectiveness results using our meta-model); (2) the Genders et al. study that concluded that CCTA was more cost-effective than CMR in the US (i.e., a replication of the Genders et al. cost-effectiveness results using our meta-model); (3) and meta-model results using key input values from the Genders et al. paper (such as CMR and CCTA sensitivity and specificity, CMR and CCTA costs, underlying prevalence of CAD, and other selected inputs) with Ge et al. model inputs for all other inputs (see Appendix Table 4 for full list) [13, 20].
We were able to use the meta-model to closely replicate the incremental cost-effectiveness results for CCTA compared to a ‘No Imaging’ strategy and to CMR (Table 2); when the Genders et al. model inputs values were used, CCTA dominated CMR (i.e. CCTA had greater QALYs and lower costs) [20]. When the Ge et al. model input values were used, CMR dominated CCTA, replicating the cost-effectiveness comparisons from the Ge et al. analysis [13, 20]. When the meta-model was fed with Genders et al. inputs except for the updated sensitivity and specificity values as used by Ge et al., CMR dominated CCTA (Table 2, Figs. 1 and 2). CMR would have to cost 182% of our base-case estimate ($807 to $1465) before the CCTA strategy would be considered more cost-effective (using a willingness-to-pay of $100,000 per QALY). Appendix Table 5 shows other threshold values for imaging performance, prevalence, and age inputs for CMR and the other imaging strategies we evaluated using the meta-model.
Meta-model lifetime discounted incremental net monetary benefit results (compared to ‘No Imaging’); higher incremental net monetary benefit indicates better cost effectiveness profile. Net Monetary Benefit (NMB) is a single metric that monetizes QALYs (using willingness-to-pay of $100,000/QALY) and subtracts costs. CMR cardiovascular magnetic resonance, CCT coronary computed tomography angiography, ICA invasive coronary angiography; Genders et al. [20]; Ge et al. [13]
Meta-model lifetime discounted quality-adjusted life year and cost results (compared to ‘No Imaging’). Squares indicate CMR, circles indicate CCT, colors indicate meta-model input sources, arrows represent comparisons of CMR vs. CCT for a given inputs source (strategies to the bottom and right to their comparators have higher quality adjusted life years (QALYs) and lower costs, i.e. they are dominant strategies), dotted lines represent cost-effectiveness thresholds (strategies below cost-effectiveness thresholds are good value compared to ‘No Imaging’)
Discussion
Our systematic literature review found that most (62%) studies formally evaluating the cost-effectiveness of CMR compared to other relevant imaging options for patients with suspected CAD world-wide concluded that:
-
CMR-based diagnostic strategies produced health at reasonable value compared to setting-specific cost-effectiveness thresholds.
-
When CMR is not available, CCTA represents a cost-effective alternative compared to a no imaging strategy or immediate ICA strategy, which is consistent with current recommendations of major international cardiac societies [39].
These cost-effectiveness outcomes depend on the operating characteristics of the imaging modality of interest and the underlying prevalence of CAD in the population of interest. It is therefore of importance for readers of such cost-effectiveness analyses to scrutinize the sources of these key variables when interpreting the resulting cost-effectiveness outcomes. Publications which concluded that CCTA dominated CMR used inputs in the cost-effectiveness models which were outside the range of the majority of reported results. We also found that relying on a single data source to estimate the cost-effectiveness of CMR, such as a cost-effectiveness analysis conducted alongside a clinical trial, could lead to uncertain results (e.g. when such trials are underpowered for cost and QALY outcomes).
Considering uncertain and setting-specific inputs that could drive cost-effectiveness results, we developed a CMR cost-effectiveness calculator that end-users (physicians, hospital decision-makers, guidelines writers, payers, health economist researchers) can use to approximate setting-specific cost-effectiveness results. The ability to iteratively generate these estimates is crucial given the importance of weighting potential tradeoffs between length of life, quality of life, and local costs across imaging modalities available to physicians aiming to diagnosis and treat ischemic heart disease, and the setting-specific and evolving nature of these inputs. The calculator also allows to determine strategies, which allow to achieve diagnosis at the lowest possible cost, or to treat significant CAD with the lowest cumulative cost of care. As expected, changing the sensitivity and specificity of CMR or CCTA changes the relative cost effectiveness of each of these modalities. Our cost-effectiveness calculator allows users to update these inputs as newer evidence and meta-analyses are published. Our replication and adaptation of the Genders et al. study showed this explicitly. Other key drivers of CMR cost-effectiveness, such as disease prevalence and imaging or treatment costs, can also be setting-specific, and we found there are many settings (almost all non-US/non-European countries, with the exceptions of one study for Brazil and one study for Australia) without any formal cost-effectiveness analyses published. Users can use our tool world-wide to better align their local understanding of model inputs to cost-effectiveness results.
Our study focused on cost-per-QALY or cost-per-life-year-saved studies, but there are other types of economic evaluation studies beyond formal cost-effectiveness analyses (such as cost-minimization analyses) that we identified comparing CMR to other imaging strategies used to diagnose CAD [40]. Data from the European CMR registry [41], which contains data from 59 medical centers across 18 countries, were used for two such analyses that compared CMR to ICA-based strategies; these studies both found that the CMR-based strategy would result in cost savings compared to inpatient ICA, driven by the costs differences between strategies and reduced revascularization procedures in the CMR-based strategies [42, 43]. A randomized controlled trial assigning 109 patients to either a CMR observation unit arm or usual inpatient care arm found that the CMR-based care arm reduced cardiac-related costs during the hospitalization and over the first year post-discharge [44]. These studies were not included in our literature review as they did not include a QALY or life-year effectiveness measure, but they add to our overall study conclusion that CMR can represent high- or intermediate-value care, or even produce cost-savings, depending on the imaging strategy CMR is being compared to. Future clinical studies providing sex-specific inputs (on imaging performance, for instance) could also help reveal whether the cost-effectiveness of CMR differs for men and women.
Limitations
Our study has limitations that should be noted. For our systematic literature review, we were limited to the existing published literature. There is publication bias in terms of what settings cost-effectiveness of CMR studies are performed for (with US, Germany, and the United Kingdom relatively overrepresented), and there is the possibility of financial or non-financial bias in the study authors that could affect which model inputs they choose (thus affecting model results). Our cost-effectiveness calculator tool can somewhat mitigate this bias, if users have non-biased inputs they believe would better reflect the current state of the evidence or their local populations or settings. The cost-effectiveness calculator is based on a meta-model of a larger Markov model, and therefore the calculator contains many model limitations of the larger model in addition to its own imperfect ability to replicate the model results. To overcome this limitation, we performed a model validation analysis on the meta-model using data that were specifically not used to generate the model. Our meta-model was also limited to the strategies evaluated in the larger Markov model [13], which is why echocardiography is not included as a comparator in the meta-model. Due to data limitations, we also did not model potential side effects from contrast agents used for CCTA or CMR or radiation exposure from CCTA, which would have amplified our lifetime discount QALY meta-model results comparing CCTA to CMR [45]. Finally, our review and cost calculator focus on cost-effectiveness outcomes, which might not capture all dimension that are relevant for health policy or clinical decision-making, missing elements such as equity and patients’ preference considerations.
Despite those limitations, cost-effectiveness analysis represents the field’s best attempt to systematically and fairly quantify the value of health care interventions, a notion supported by the 2014 ACC-AHA policy statement that uses cost-effectiveness metrics to differentiate high-value care (health gains are worth their costs) from low-value care (prices should be lowered to be in line with the health gains that are produced by the intervention of interest) [12].
Conclusions
The majority of cost-effectiveness evidence evaluating CMR-based diagnostic strategies for patients with suspected CAD identify CMR to be a cost-effective imaging strategy, delivering high-value care in many settings. When comparing diagnostic techniques, the optimal strategy depends on factors that change over time and across clinical settings, such as imaging performance, imaging costs, and disease prevalence. Therefore, decision-makers should contextualize existing literature with additional information, whether from their local data when available or through use of a tool like the cost-effectiveness calculator we present here. Such tools can assist in obtaining the most realistic estimates of overall value from options available to diagnose and treat significant CAD.
Availability of data and materials
The data used to calculate all meta-model results is contained in the “CMR CEA Calculator_Demo” Excel file, which is included as a Additional file for this paper and at: https://docs.google.com/spreadsheets/d/12TMgbIS6sDpXkafSYfNaNw8vSx-LCTCE4K_yAa7mdyY/edit?usp=sharing.
Change history
16 March 2022
A Correction to this paper has been published: https://doi.org/10.1186/s12968-022-00847-3
Abbreviations
- ACC:
-
American College of Cardiology
- AHA:
-
American Heart Association
- AHRQ:
-
Agency for Healthcare Research and Quality
- CABG:
-
Coronary artery bypass grafting
- CAD:
-
Coronary artery disease
- CCTA:
-
Coronary computed tomographic angiography
- CMR:
-
Cardiovascular magnetic resonance
- CPT:
-
Current procedural terminology
- ESC:
-
European Society of Cardiology
- FFR:
-
Fractional flow reserve
- ICA:
-
Invasive coronary angiography
- ICER:
-
Incremental cost-effectiveness ratio
- MACE:
-
Major adverse cardiovascular event(s)
- MI:
-
Myocardial infarction
- PCI:
-
Percutaneous coronary intervention
- PET:
-
Positron emission tomography
- QALY:
-
Quality-adjusted life year
- SPECT:
-
Single-photon emission computed tomography
- XCA:
-
X-ray coronary angiography
References
Mark DB, Anderson JL, Brinker JA, Brophy JA, Casey DE Jr, Cross RR, Edmundowicz D, Hachamovitch R, Hlatky MA, Jacobs JE, Jaskie S, Kett KG, Malhotra V, Masoudi FA, McConnell MV, Rubin GD, Shaw LJ, Sherman ME, Stanko S, Ward RP. ACC/AHA/ASE/ASNC/HRS/IAC/Mended Hearts/NASCI/RSNA/SAIP/SCAI/SCCT/SCMR/SNMMI 2014 health policy statement on use of noninvasive cardiovascular imaging: a report of the American College of Cardiology Clinical Quality Committee. J Am Coll Cardiol. 2014;63:698–721.
Francis SA, Daly C, Heydari B, Abbasi S, Shah RV, Kwong RY. Cost-effectiveness analysis for imaging techniques with a focus on cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2013;15:52.
Singer ME, Applegate KE. Cost-effectiveness analysis in radiology. Radiology. 2001;219:611–20.
Pandya A. Adding cost-effectiveness to define low-value care. JAMA. 2018;319:1977–8.
Neumann PJ, Cohen JT. QALYs in 2018-advantages and concerns. JAMA. 2018;319:2473–4.
Turchetti G, Kroes MA, Lorenzoni V, Trieste L, Chapman AM, Sweet AC, Wilson GI, Neglia D. The cost-effectiveness of diagnostic cardiac imaging for stable coronary artery disease. Expert Rev Pharmacoecon Outcomes Res. 2015;15:625–33.
Danad I, Szymonifka J, Twisk JWR, Norgaard BL, Zarins CK, Knaapen P, Min JK. Diagnostic performance of cardiac imaging methods to diagnose ischaemia-causing coronary artery disease when directly compared with fractional flow reserve as a reference standard: a meta-analysis. Eur Heart J. 2017;38:991–8.
Knuuti J, Ballo H, Juarez-Orozco LE, Saraste A, Kolh P, Rutjes AWS, Juni P, Windecker S, Bax JJ, Wijns W. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: a meta-analysis focused on post-test disease probability. Eur Heart J. 2018;39:3322–30.
http://healtheconomics.tuftsmedicalcenter.org/cear4/Home.aspx.
Neumann PJ, Thorat T, Shi J, Saret CJ, Cohen JT. The changing face of the cost-utility literature, 1990–2012. Value Health. 2015;18:271–7.
Thorat T, Cangelosi M, Neumann PJ. Skills of the trade: the tufts cost-effectiveness analysis registry. J Benefit-Cost Anal. 2012;3:1–9.
Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ, Halperin JL, Hlatky MA, Jacobs AK, Mark DB, Masoudi FA, Peterson ED, Shaw LJ. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304–22.
Ge Y, Pandya A, Steel K, Bingham S, Jerosch-Herold M, Chen YY, Mikolich JR, Arai AE, Bandettini WP, Patel AR, Farzaneh-Far A, Heitner JF, Shenoy C, Leung SW, Gonzalez JA, Shah DJ, Raman SV, Ferrari VA, Schulz-Menger J, Hachamovitch R, Stuber M, Simonetti OP, Kwong RY. Cost-effectiveness analysis of stress cardiovascular magnetic resonance imaging for stable chest pain syndromes. JACC Cardiovasc Imaging. 2020;13(7):1505–17.
Walker S, Girardin F, McKenna C, Ball SG, Nixon J, Plein S, Greenwood JP, Sculpher M. Cost-effectiveness of cardiovascular magnetic resonance in the diagnosis of coronary heart disease: an economic evaluation using data from the CE-MARC study. Heart. 2013;99:873–81.
Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Mak. 2006;26:410–20.
Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, Kuntz KM, Meltzer DO, Owens DK, Prosser LA, Salomon JA, Sculpher MJ, Trikalinos TA, Russell LB, Siegel JE, Ganiats TG. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–103.
Degeling K, IJzerman MJ, Lavieri MS, Strong M, Koffijberg H. Introduction to metamodeling for reducing computational burden of advanced analyses with health economic models: a structured overview of metamodeling methods in a 6-step application process. Med Decis Mak. 2020;40:348–63.
Degeling K, Ijzerman MJ, Koffijberg H. A scoping review of metamodeling applications and opportunities for advanced health economic analyses. Expert Rev Pharmacoecon Outcomes Res. 2019;19:181–7.
Jalal H, Dowd B, Sainfort F, Kuntz KM. Linear regression metamodeling as a tool to summarize and present simulation model results. Med Decis Mak. 2013;33:880–90.
Genders TS, Petersen SE, Pugliese F, Dastidar AG, Fleischmann KE, Nieman K, Hunink MG. The optimal imaging strategy for patients with stable chest pain: a cost-effectiveness analysis. Ann Intern Med. 2015;162:474–84.
Bertoldi EG, Stella SF, Rohde LE, Polanczyk CA. Long-term cost-effectiveness of diagnostic tests for assessing stable chest pain: modeled analysis of anatomical and functional strategies. Clin Cardiol. 2016;39:249–56.
Boldt J, Leber AW, Bonaventura K, Sohns C, Stula M, Huppertz A, Haverkamp W, Dorenkamp M. Cost-effectiveness of cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary artery disease in Germany. J Cardiovasc Magn Reson. 2013;15:30.
Campbell F, Thokala P, Uttley LC, Sutton A, Sutton AJ, Al-Mohammad A, Thomas SM. Systematic review and modelling of the cost-effectiveness of cardiac magnetic resonance imaging compared with current existing testing pathways in ischaemic cardiomyopathy. Health Technol Assess. 2014;18:1–120.
Moschetti K, Favre D, Pinget C, Pilz G, Petersen SE, Wagner A, Wasserfallen JB, Schwitter JJ. Comparative cost-effectiveness analyses of cardiovascular magnetic resonance and coronary angiography combined with fractional flow reserve for the diagnosis of coronary artery disease. J Cardiovasc Magn Reson. 2014;16:13.
Petrov G, Kelle S, Fleck E, Wellnhofer E. Incremental cost-effectiveness of dobutamine stress cardiac magnetic resonance imaging in patients at intermediate risk for coronary artery disease. Clin Res Cardiol. 2015;104:401–9.
Pletscher M, Walker S, Moschetti K, Pinget C, Wasserfallen JB, Greenwood JP, Schwitter J, Girardin FR. Cost-effectiveness of functional cardiac imaging in the diagnostic work-up of coronary heart disease. Eur Heart J Qual Care Clin Outcomes. 2016;2:201–7.
Pontone G, Andreini D, Guaricci AI, Rota C, Guglielmo M, Mushtaq S, Baggiano A, Beltrama V, Fusini L, Solbiati A, Segurini C, Conte E, Gripari P, Annoni A, Formenti A, Petulla M, Lombardi F, Muscogiuri G, Bartorelli AL, Pepi M. The STRATEGY study (stress cardiac magnetic resonance versus computed tomography coronary angiography for the management of symptomatic revascularized patients): resources and outcomes impact. Circ Cardiovasc Imaging. 2016;9: e005171.
Sharples L, Hughes V, Crean A, Dyer M, Buxton M, Goldsmith K and Stone D. Cost-effectiveness of functional cardiac testing in the diagnosis and management of coronary artery disease: a randomised controlled trial. The CECaT trial. Health Technol Assess. 2007;11:iii–iv, ix–115.
Stojanovic I, Schneider JE, Cooper J. Cost-impact of cardiac magnetic resonance imaging with Fast-SENC compared to SPECT in the diagnosis of coronary artery disease in the US. J Med Econ. 2019;22:430–8.
Thom H, West NE, Hughes V, Dyer M, Buxton M, Sharples LD, Jackson CH, Crean AM, group CEs. Cost-effectiveness of initial stress cardiovascular MR, stress SPECT or stress echocardiography as a gate-keeper test, compared with upfront invasive coronary angiography in the investigation and management of patients with stable chest pain: mid-term outcomes from the CECaT randomised controlled trial. BMJ Open. 2014;4: e003419.
Walker S, Cox E, Rothwell B, Berry C, McCann GP, Bucciarelli-Ducci C, Dall’Armellina E, Prasad A, Foley JRJ, Mangion K, Bijsterveld P, Everett C, Stocken D, Plein S, Greenwood JP, Sculpher M. Cost-effectiveness of cardiovascular imaging for stable coronary heart disease. Heart. 2020;107(5):381–8.
Kozor R, Walker S, Parkinson B, Younger J, Hamilton-Craig C, Selvanayagam JB, Greenwood JP, Taylor AJ. Cost-effectiveness of cardiovascular magnetic resonance in diagnosing coronary artery disease in the Australian health care system. Heart Lung Circ. 2020;30(3):380–7.
Hamon M, Fau G, Nee G, Ehtisham J, Morello R, Hamon M. Meta-analysis of the diagnostic performance of stress perfusion cardiovascular magnetic resonance for detection of coronary artery disease. J Cardiovasc Magn Reson. 2010;12:29.
Mowatt G, Cook JA, Hillis GS, Walker S, Fraser C, Jia X, Waugh N. 64-Slice computed tomography angiography in the diagnosis and assessment of coronary artery disease: systematic review and meta-analysis. Heart. 2008;94:1386–93.
von Ballmoos MW, Haring B, Juillerat P, Alkadhi H. Meta-analysis: diagnostic performance of low-radiation-dose coronary computed tomography angiography. Ann Intern Med. 2011;154:413–20.
Schuetz GM, Zacharopoulou NM, Schlattmann P, Dewey M. Meta-analysis: noninvasive coronary angiography using computed tomography versus magnetic resonance imaging. Ann Intern Med. 2010;152:167–77.
Jaarsma C, Leiner T, Bekkers SC, Crijns HJ, Wildberger JE, Nagel E, Nelemans PJ, Schalla S. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2012;59:1719–28.
Greenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, Everett CC, Bijsterveld P, Ridgway JP, Radjenovic A, Dickinson CJ, Ball SG, Plein S. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet. 2012;379:453–60.
Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ, Group ESCSD. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–77.
Raman SV, Hachamovitch R, Scandling D, Mazur W, Kwong RY, Wong TC, Schelbert EB, Moore S, Truong V, Simonetti OP. Lower ischemic heart disease diagnostic costs with treadmill stress CMR versus SPECT: a multicenter, randomized trial. JACC Cardiovasc Imaging. 2020;13:1840–2.
Bruder O, Wagner A, Lombardi M, Schwitter J, van Rossum A, Pilz G, Nothnagel D, Steen H, Petersen S, Nagel E, Prasad S, Schumm J, Greulich S, Cagnolo A, Monney P, Deluigi CC, Dill T, Frank H, Sabin G, Schneider S, Mahrholdt H. European cardiovascular magnetic resonance (EuroCMR) registry—multi national results from 57 centers in 15 countries. J Cardiovasc Magn Reson. 2013;15:9.
Moschetti K, Muzzarelli S, Pinget C, Wagner A, Pilz G, Wasserfallen JB, Schulz-Menger J, Nothnagel D, Dill T, Frank H, Lombardi M, Bruder O, Mahrholdt H, Schwitter J. Cost evaluation of cardiovascular magnetic resonance versus coronary angiography for the diagnostic work-up of coronary artery disease: application of the European Cardiovascular Magnetic Resonance registry data to the German, United Kingdom, Swiss, and United States health care systems. J Cardiovasc Magn Reson. 2012;14:35.
Moschetti K, Petersen SE, Pilz G, Kwong RY, Wasserfallen JB, Lombardi M, Korosoglou G, Van Rossum AC, Bruder O, Mahrholdt H, Schwitter J. Cost-minimization analysis of three decision strategies for cardiac revascularization: results of the “suspected CAD” cohort of the European cardiovascular magnetic resonance registry. J Cardiovasc Magn Reson. 2016;18:3.
Miller CD, Hwang W, Case D, Hoekstra JW, Lefebvre C, Blumstein H, Hamilton CA, Harper EN, Hundley WG. Stress CMR imaging observation unit in the emergency department reduces 1-year medical care costs in patients with acute chest pain: a randomized study for comparison with inpatient care. JACC Cardiovasc Imaging. 2011;4:862–70.
Richards CE, Obaid DR. Low-dose radiation advances in coronary computed tomography angiography in the diagnosis of coronary artery disease. Curr Cardiol Rev. 2019;15:304–15.
Kwong RY, Ge Y, Steel K, Bingham S, Abdullah S, Fujikura K, Wang W, Pandya A, Chen YY, Mikolich JR, Boland S, Arai AE, Bandettini WP, Shanbhag SM, Patel AR, Narang A, Farzaneh-Far A, Romer B, Heitner JF, Ho JY, Singh J, Shenoy C, Hughes A, Leung SW, Marji M, Gonzalez JA, Mehta S, Shah DJ, Debs D, Raman SV, Guha A, Ferrari VA, Schulz-Menger J, Hachamovitch R, Stuber M, Simonetti OP. Cardiac magnetic resonance stress perfusion imaging for evaluation of patients with chest pain. J Am Coll Cardiol. 2019;74:1741–55.
O’Sullivan AK, Rubin J, Nyambose J, Kuznik A, Cohen DJ, Thompson D. Cost estimation of cardiovascular disease events in the US. Pharmacoeconomics. 2011;29:693–704.
Nieman K, Galema T, Weustink A, Neefjes L, Moelker A, Musters P, de Visser R, Mollet N, Boersma H, de Feijter PJ. Computed tomography versus exercise electrocardiography in patients with stable chest complaints: real-world experiences from a fast-track chest pain clinic. Heart. 2009;95:1669–75.
Acknowledgements
CBD is in part supported by the NIHR Biomedical Research Centre at University Hospitals Bristol NHS Foundation Trust and the University of Bristol. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care.
Funding
This work was funded in part by a research grant provided by Novartis to S. Kelle at the German Heart Institute Berlin, Germany.
Author information
Authors and Affiliations
Contributions
AP and SK designed the study. AP and YY conducted the literature review. AP, YY, EN, JS, OPS, CD, LMS, and SK interpreted the results from the literature review. AP, YG, and RK constructed the simulation model used to create the meta-model. AP programmed the meta-model. AP, YY, and SK drafted the manuscript. All authors revised the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not needed as this research only involved data from published sources and simulation modeling of hypothetical populations (i.e., no human subjects research).
Consent for publication
Not applicable.
Competing interests
S. Kelle receives research funding by Novartis and Philips Healthcare. S. E. Petersen provides consultancy to and is a shareholder of Circle Cardiovascular Imaging Inc, Calgary, Canada. J. Schwitter and E. Nagel receive research funding by Bayer Healthcare. J. White is a shareholder of Cohesic Inc. S. Kelle and E. Nagel are supported by the DZHK (German Centre for Cardiovascular Research) and by the BMBF (German Ministry of Education and Research). CBD is the Chief Executive Officer (part time) of the Society for Cardiovascular Magnetic Resonance (SCMR). JC was president of the Society for Cardiovascular Magnetic Resonance (SCMR) at the time this work was performed, receives research support from Bayer, Siemens, Guerbet, advisory board/lectures for Bayer, Siemens, Bracco. The other co-authors have no disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been updated to correct the affiliation of Chiara Bucciarelli-Ducci.
Supplementary Information
Additional file 1.
Users can enter model inputs that relate to their population of interest (e.g., age, probability of patient having treatable CAD), imaging strategies (sensitivity, specificity, cost), treatments (e.g., effectiveness, risks, and costs of revascularization procedures), and other variables (e.g., willingness-to-pay for health, discount rate), to then see how these collection of inputs translates to lifetime per-person net monetary benefit (i.e., an overall metric of value using cost-effectiveness analysis), lifetime discounted quality-adjusted life years (i.e., the main measure of effectiveness), and lifetime discounted costs. Default input values for the US are provided in the model file.
Appendix
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pandya, A., Yu, YJ., Ge, Y. et al. Evidence-based cardiovascular magnetic resonance cost-effectiveness calculator for the detection of significant coronary artery disease. J Cardiovasc Magn Reson 24, 1 (2022). https://doi.org/10.1186/s12968-021-00833-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12968-021-00833-1