Abstract
Background
Propofol is a widely used anesthetic and sedative, which has been reported to exert an anti-inflammatory effect. TLR4 plays a critical role in coordinating the immuno-inflammatory response during sepsis. Whether propofol can act as an immunomodulator through regulating TLR4 is still unclear. Given its potential as a sepsis therapy, we investigated the mechanisms underlying the immunomodulatory activity of propofol.
Methods
The effects of propofol on TLR4 and Rab5a (a master regulator involved in intracellular trafficking of immune factors) were investigated in macrophage (from Rab5a−/− and WT mice) following treatment with lipopolysaccharide (LPS) or cecal ligation and puncture (CLP) in vitro and in vivo, and peripheral blood monocyte from sepsis patients and healthy volunteers.
Results
We showed that propofol reduced membrane TLR4 expression on macrophages in vitro and in vivo. Rab5a participated in TLR4 intracellular trafficking and both Rab5a expression and the interaction between Rab5a and TLR4 were inhibited by propofol. We also showed Rab5a upregulation in peripheral blood monocytes of septic patients, accompanied by increased TLR4 expression on the cell surface. Propofol downregulated the expression of Rab5a and TLR4 in these cells.
Conclusions
We demonstrated that Rab5a regulates intracellular trafficking of TLR4 and that propofol reduces membrane TLR4 expression on macrophages by targeting Rab5a. Our study not only reveals a novel mechanism for the immunomodulatory effect of propofol but also indicates that Rab5a may be a potential therapeutic target against sepsis.
Similar content being viewed by others
Background
Sepsis has been defined as life-threatening organ dysfunction caused by a dysregulated host inflammatory immune response to infection, and it remains the leading cause of death in critically ill patients [1]. Many clinical trials have focused on seeking an effective therapeutic target for sepsis, but these have met with very limited success [2, 3]. Therefore, it is imperative to gain a better understanding of the molecular mechanisms involved in the pathogenesis of sepsis and develop novel therapeutic strategies.
Propofol is most widely used in anesthesia induction and maintenance in the operating room and also in sedation for ICU patients including those with sepsis [4]. Previous studies by our group and others have revealed that propofol possesses anti-inflammatory properties in many conditions including sepsis, ischemia/reperfusion injuries of organs, and cardiopulmonary bypass [5,6,7]. Nevertheless, the precise mechanism that governs these protective effects of propofol is still not well understood.
Sepsis, an inflammatory response triggered by infection, predominantly relies on the innate immune system as its foremost line of defense against foreign bacteria. Macrophages play a crucial role in the clearance of bacteria and responding to the heightened inflammatory state induced by bacterial invasion. Among various immune cells, macrophages play an indispensable role throughout all phases of sepsis due to their ubiquitous presence and comprehensive effects in maintaining immune homeostasis and regulating inflammatory processes. Hence, the scrupulous attention and regulation of macrophage immune function bear immense significance in the realm of sepsis treatment [8, 9]. The toll-like receptor 4 (TLR4) receptor plays an important role in regulating the innate immune response to bacterial infection during sepsis [10, 11]. The activation and upregulation of TLR4 expression on the macrophage cell membrane surface are critical in mediating immuno-inflammatory responses [12]. Most TLR4 molecules are stored in subcellular compartments such as the Golgi apparatus and endosomes, indicating that the expression of cell surface TLR4 is determined by receptor trafficking between the subcellular compartments and the cell membrane [12]. However, the molecular mechanisms of TLR4 intracellular trafficking are unclear. The relationship between propofol and TLR4 has been documented in the literature [13, 14], primarily focusing on the regulatory effects of propofol on the downstream inflammatory pathway associated with TLR4. However, there is a dearth of studies investigating the impact of propofol on TLR4 intracellular trafficking. As propofol is an immunomodulating agent with anti-inflammatory effects, it is reasonable to hypothesize that it may influence cell surface TLR4 expression.
Rab GTPases are a large family of small GTPases that are known as key coordinators of vesicle traffic [15]. Rab5a, one of the Rab GTPases, has been recognized as a master regulator of endocytosis. Rab5a is also involved in the intracellular trafficking of cell surface receptors including TLR4 [16, 17]. However, whether propofol inhibits cell surface TLR4 expression via targeting Rab5a, has not yet been reported.
We hypothesized that Rab5a regulates intracellular trafficking of TLR4 in macrophages and that propofol exerts its anti-inflammatory role by reducing membrane TLR4 expression on macrophages via targeting Rab5a. To investigate these hypotheses, we employed a series of experiments with macrophages following LPS treated in vitro and CLP in vivo. We found that Rab5a is critical in TLR4 intracellular trafficking, and regulates membrane TLR4 expression on macrophages. Propofol exerts its anti-inflammatory role by reducing TLR4 expression on macrophages via inhibiting Rab5a expression and the interaction between Rab5a and TLR4.
Methods
Animals
C57/BL mice were obtained from Guangdong Medical Laboratory Animal Center (Guangzhou, China). Rab5a knockout mice were purchased from Cyagen Biosciences (Guangzhou, China). All animal experiments were reviewed and approved by the Ethics Committee of Southern Medical University (approval number NFYY-2019-24).
BMDM isolation and culture
BMDMs were isolated and cultured as previously described [18]. Briefly, 6–8-week-old male C57BL mice were executed by neck breakage and the tibia and femur were isolated. Bones were kept on ice and rinsed in a sterile dish with DMEM (11971025, Gibco, CA, USA), and bone marrow was then flushed out with DMEM using a 30-gauge needle. Cells were harvested and plated at 106 cell/ml with DMEM containing 10% fetal bovine serum (FBS) (30044333, Gibco, CA, USA), 20% L929 supernatant, 100 U/ml penicillin, and 100 µg/ml streptomycin (15070063, Gibco, CA, USA). The cells were cultured at 37 °C in 5% CO2 for 7 days to differentiate into macrophages before further experiments. BMDMs were treated with LPS (1 μg/ml) (L3012, sigma-Aldrich, CA, USA), and propofol (50 μm) (D126608, sigma-Aldrich, CA, USA) were added 30 min before LPS treated according to previous research [19].
CLP procedure
The CLP-induced mouse sepsis model was performed according to general guidelines [20, 21]. The CLP model involves the perforation of the cecum, which allows for the release of fecal material into the peritoneal cavity, thereby inducing an exaggerated immune response triggered by polymicrobial infection. Male 6–8-week-old C57BL mice were anesthetized with isoflurane. For the CLP procedure, in brief, a 2-cm midline laparotomy was performed to expose the cecum. The distal 5 mm of the tip was tightly ligated with 3.0 silk suture and punctured once using an 18-gauge needle. A small amount of fecal material was then squeezed to extrude into the peritoneal cavity. The cecum was returned to the abdominal cavity, and the abdomen and skin were respectively closed using 4.0 silk. Following the surgery, 1 ml of saline was subcutaneously administered to the neck. In the sham group, mice underwent the same procedure, but without the cecal ligation or puncture. In the propofol-treated group, mice were pretreated with 50 mg/kg propofol (dissolved in the fat emulsion) intraperitoneally 30 min before CLP according to previous research [19, 22]. Imipenem (a carbapenem antibiotic with a broader antimicrobial spectrum) was administered subcutaneously at a dose of 0.5 mg/d after CLP [23].
Tissue histology
After animals were euthanized, segments of the small intestine, lung, liver, and kidney were fixed using 4% paraformaldehyde, embedded in paraffin, used to prepare 5 μm sections, and hematoxylin and eosin (H&E) stained. Two pathologists blinded to study groups then independently evaluated and scored the injury severity of stained sections as in prior studies [21].
Plasmid transfection
Mouse Rab5a (GV141) plasmid was constructed by Genechem (Shanghai, China). BMDMs were transfected with control GV141, or GV141-mRab5a (2.5 μg/105 cells) using jetPEI® reagent (101000053, Polyplus, Strasbourg, France) according to the manufacturer’s protocol. Sequences for plasmids and primers were listed in Additional file 6: Tables S3 and S4. Twenty-four hours after transfection, cells were stimulated with LPS (1 μg/ml) for 24 h and then harvested for membrane TLR4 detection using flow cytometry.
Immunoprecipitation and immunoblot
Immunoprecipitation and immunoblot were performed as previously described [24]. Cells were lysed in IP lysis buffer (87787, Thermo Scientific, MA, USA) at 4 °C for 30 min. After centrifugation at 12,000×g for 20 min, 500 μl supernatant (500 μg protein) was collected and 1 μg TLR4 (ab8376, Abcam, Shanghai, China) antibody was added, followed by incubation overnight at 4 °C. Protein A/G Magnetic beads (20 μl) (88803, Thermo Scientific, MA, USA) were used to capture the protein and antibody complex. The beads were incubated for 4–6 h and washed three times in PBS. Proteins were subjected to SDS-PAGE (6–20% gel) and then transferred to Immobilon-P membranes for Western blotting. For Western blotting, cells were lysed in a radioimmunoprecipitation buffer (P0013B, Beyotime Institute of Biotechnology, Jiangsu, China). The supernatants were obtained by centrifugation at 13,440×g for 15 min at 4 °C. The proteins (30 µg/lane) were separated and subsequently transferred onto a polyvinylidene difluoride membrane (IPVH00010, Millipore, MA, USA). The membranes were blocked with 5% non-fat milk at room temperature for 1 h. Following blocking, the membranes were incubated overnight at 4 °C with specific primary antibodies and then incubated with secondary antibodies at room temperature for 1 h. Antibody concentrations used for Western blotting were as follows: rabbit anti-TLR4 (ab22048, Abcam, Shanghai, China) 1:1000; rabbit anti-Na/K ATPase (ab7671, Abcam, shanghai, China) 1:2000; mouse anti-GAPDH (60004-1-Ig, proteintech, Wuhan, China) 1:5000; rabbit anti-p-JNK (4668, Cell Signaling Technology, MA, USA) 1:1000; rabbit anti-p-ERK (4370, Cell Signaling Technology, MA, USA) 1:1000; rabbit anti-RAB5A (11947-1-AP, proteintech, Wuhan, China) 1:1000; The HRP-conjugated anti-mouse secondary antibody (12-349, Sigma-Aldrich, CA, USA) was used at 1:10,000 and the HRP-conjugated anti-rabbit secondary antibody (AP160P, Sigma-Aldrich, CA, USA) was used at 1:5000.
Immunofluorescence
Cells were fixed and permeabilized with 0.5% TritonX-100, followed by blocking with 1% BSA for 30 min at room temperature. Thereafter, the primary antibodies for TLR4 (ab8376, Abcam, shanghai, China) 1:100, Rab5a (11947-1-AP, Proteintech, Wuhan, China) 1:100, or GM130 (ab52649, Abcam, shanghai, China) 1:200, were added and incubated at 4 °C overnight. After washed 3 times in PBS, cells were incubated with secondary antibody (AlexaFluor 488, 594 goat anti-mouse or anti-rabbit IgG, Invitrogen, 1:200) for 1 h at 37 °C. After being washed 3 times in PBS again and stained with DAPI, immunostaining was observed using an LSM510 (Zeiss) confocal microscope [25].
Flow cytometric analysis
To detect membrane TLR4 expression and other markers, cells were incubated with fluorescently labeled antibodies against mouse TLR4 (12-9041-80, eBioscience, CA, USA, 1:100) or F4/80 (11-4801-82, eBioscience, CA, USA, 1:500) for 30 min on ice, washed 3 times in PBS, and analyzed in a FACScalibur flow cytometer (BD Bioscience, NJ, USA).
PCR array
Cytokines and Chemokines PCR Array kit was purchased from Wcgene Biotech (Shanghai China). Each 96-well plate contained containing two housekeeping genes (Gapdh and Actb), negative controls, and testing genes. The design of the RT-qPCR array is summarized in Additional file 6: Table S1. The PCR array reaction was carried out in an ABI Q6 Real-Time PCR System (Thermo Fisher Scientific) with the SYBR Green detection protocol (TOYOBO, Osaka, Japan), and analyzed by the standard method of 2−ΔΔCt (Additional file 7).
Clinical samples
Patients were included if they were at least 18 years of age, and met sepsis criteria within the first 24 h after being admitted to the Intensive Care Unit of Nanfang Hospital. Exclusion criteria were age younger than 18 years, immunosuppressed, treatment with hemodialysis, chemotherapy within 4 weeks, and unable to sign informed consent. A total of 19 septic patients were enrolled. The clinical characteristics, including SOFA score (Sequential Organ Failure Assessment score, for screening sepsis and assessing prognosis), APACHE II score (Acute Physiology and Chronic Health Evaluation, prognostic factors for critically ill patients), causes of sepsis, length of Intensive Care Unit stay, and the 28-day mortality, were recorded in Additional file 6: Table S2. Eleven healthy donors with no medical problems in the medical examination center of Nanfang Hospital were included as controls. Peripheral blood was collected within the first 24 h of Intensive Care Unit admission. Monocytes were prepared as previously described [26]. Human monocyte isolation solution kits (TBD2011H, TBDscience, Tianjin, China) were used for the preliminary isolation of monocytes from peripheral blood before flow cytometry analysis. PE rat anti-human CD14 antibody (301805, Biolegend, CA, USA) was used to mark and sort monocytes. monocytes from healthy volunteers were treated with LPS (1 μg/ml) for the indicated time with or without pretreatment of propofol (50 μM) for 30 min. monocytes from sepsis patients were treated with propofol (50 μM) for 3 h. Expression of TLR4 (APC rat anti-human TLR4 antibody, 312815, Biolegend, CA, USA) on monocyte surface was detected using flow cytometry, and expression of Rab5a mRNA was detected using qPCR. The study protocol was approved by the Ethical Committee of Nanfang Hospital, Southern Medical University (approval number NFEC-202009-k2-01). All individuals gave informed consent to participate.
Statistical analysis
Results are presented as mean ± SD. Differences between the two groups were analyzed by Student’s t-test. For multi-group comparisons, One-way ANOVA was used followed by Tukey’s post hoc test. Correlation analysis was performed using Pearson correlation. P < 0.05 was considered statistically significant. Graphs and figures were made with Graphpad Prism 5.
Results
Propofol inhibits membrane TLR4 expression on macrophages in response to LPS stimulation and CLP
To analyze the expression pattern of cytokines in BMDM upon propofol treatment, we employed cytokines and chemokines PCR array to detect differential expression levels of cytokines in BMDM (the design of the RT-qPCR array is summarized in Additional file 6: Table S1).
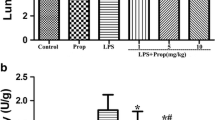
As shown in Additional file 1: Fig. S1, the genes with the most significant changes were pro-inflammatory cytokines, including Il1a, il6, Il1b, and tnf, which were consistent with previous studies. We further confirmed by qPCR that propofol can effectively inhibit the expression of pro-inflammatory cytokines in BMDMs after LPS treatment, and can reduce the level of pro-inflammatory cytokines in the serum of mice challenged with CLP (Fig. 1A, B). Recent evidence suggests that the amount of TLR4 on the surface of macrophages significantly increases following LPS treatment, reflecting involvement in the inflammatory response [27, 28]. To determine whether propofol reduces plasma membrane TLR4 in macrophages, we measured plasma membrane TLR4 after LPS stimulation using flow cytometry. At 6, 12, and 24 h after LPS stimulation, TLR4 expression on BMDM cell membranes was significantly increased compared to the control group. However, when propofol was added 30 min before LPS stimulation, membrane TLR4 expression on BMDMs decreased significantly at all time points (Fig. 1C). To further confirm these findings, we isolated cytomembrane from BMDMs and then detected the expression of TLR4 by western blotting. As shown in Fig. 1D, propofol significantly inhibited the cell surface expression of TLR4 in response to LPS. Interestingly, propofol does not affect the total expression of TLR4 as shown in Additional file 2: Fig. S2.
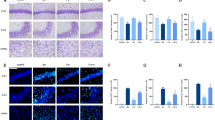
Propofol inhibits membrane TLR4 expression of macrophages after LPS stimulation or CLP. BMDMs were treated with LPS (1 μg/ml) for the indicated time with or without pretreatment of propofol (50 μM) for 30 min. C57BL mice that underwent CLP were pretreated with propofol (50 mg/kg) or equal volume of fat emulsion for 30 min and sacrificed after 12 h. A Proinflammatory factor mRNA levels in BMDMs were measured by qPCR. B Serum proinflammatory factor levers of CLP mice were assessed by ELISA. C Membrane TLR4 expression of BMDMs was analyzed by flow cytometry. D Western blot analysis of membrane TLR4 expression of BMDMs. E Membrane TLR4 expression of peritoneal macrophages from CLP mice was detected by flow cytometry. Data are expressed as the mean ± SD, n = 5–8, *p < 0.05, **p < 0.01, ***p < 0.001, 1-way ANOVA with Tukey’s post hoc test. PPF propofol
To explore the influence of propofol on macrophage plasma membrane TLR4 in vivo, we performed standardized CLP on male mice, the most reliable model in sepsis research. Propofol (50 mg/kg) was injected intraperitoneally 30 min before CLP. Six hours after surgery, peritoneal macrophages were collected and marked with the cell surface marker F4/80. Similar to the vitro results, CLP induced an increase in the amount of TLR4 on the peritoneal macrophage surface, and propofol eliminated the TLR4 response otherwise induced by CLP (Fig. 1E). Moreover, propofol significantly reduced organ injury in CLP mice, indicated by hematoxylin and eosin [H&E] staining and serum markers (Additional file 3: Fig. S3A, B). Propofol also increased the survival of CLP mice (Additional file 3: Fig. S3C). Taken together these results indicate that propofol reduces the amount of cell surface TLR4 induced by immune challenge.
Knockout of Rab5a attenuates membrane TLR4 expression on macrophages and inhibits the activation of macrophages in response to LPS and CLP
Since propofol reduces the amount of cell surface TLR4 without affecting the total expression of TLR4, we hypothesize that propofol may regulate the intracellular transport of TLR4. It is well established that Rab5a is a key regulator of endocytosis and transportation of plasma membrane compartments [29, 30]. Therefore, we asked whether Rab5a participates in the transport of TLR4 and/or regulates the expression of TLR4 on the cell membrane. Flow cytometry results revealed that TLR4 expression on the cell surface of BMDMs from Rab5a−/− mice was significantly lower than that in the WT group after LPS stimulation (Fig. 2A). Furthermore, knockout of Rab5a inhibited the expression of proinflammatory cytokines and activation of the JNK and ERK (belong to the MAPK pathway and play a key role in inflammatory response during sepsis) in BMDMs upon LPS treated (Fig. 2B, C). In vivo, we also found a decreased release of proinflammatory cytokines in the serum of Rab5a−/− mice compared to WT mice subjected to CLP (Fig. 3B). We further identified an interaction between TLR4 and Rab5a by co-immunoprecipitation and confocal immune-fluorescence microscopy. LPS promoted interaction and co-localization between TLR4 and Rab5a (Fig. 3B–D). To confirm the role of Rab5a in TLR4 intracellular trafficking, we labeled the Golgi apparatus in BMDMs with GM130 antibody. We found that TLR4 was partially localized in the Golgi before LPS stimulation. LPS weakened the co-localization of TLR4 and Golgi, indicating a translocation of TLR4 from Golgi to the cell membrane, and this response was attenuated by Rab5a knockout (Fig. 2D).
Knockout of Rab5a attenuates membrane TLR4 expression on macrophages and inhibits the activation of macrophages. BMDMs from wild-type and Rab5a−/− C57BL mice were treated with LPS (1 μg/ml) for the indicated time. A Membrane TLR4 expression of BMDMs was analyzed by flow cytometry. B Proinflammatory factor mRNA levels in BMDMs were measured by qPCR. C Western blotting of MAPK levels in BMDMs. D Representative image of TLR4 (red) and GM130 (green) immunofluorescence staining in BMDMs, scale bars: 5 μm. Data are expressed as the mean ± SD, n = 5, *p < 0.05, **p < 0.01, ***p < 0.001, 1-way ANOVA with Tukey’s post hoc test
Knockout of Rab5a attenuates membrane TLR4 expression on macrophages. Wild type and Rab5a−/− mice that underwent CLP were sacrificed after 12 h. A Membrane TLR4 expression of peritoneal macrophages from CLP mice was detected by flow cytometry. B Serum proinflammatory factor levers of CLP mice were assessed by ELISA. C The survival curve of Wild type and Rab5a−/− mice underwent CLP. Kaplan Meier analysis was used to evaluate the survival rate of CLP mice (n = 15). D H&E staining and histological score of lung, liver, kidney, and intestinal in wild type and RAB5a−/− mice, Scale bars: 50 μm. Data are expressed as the mean ± SD, n = 8, *p < 0.05, **p < 0.01, ***p < 0.001, unpaired t test or 1-way ANOVA with Tukey’s post hoc test
To further investigate the role of Rab5a in septic mice, we performed CLP on Rab5a−/− mice. Peritoneal macrophages from Rab5a−/− CLP mice showed reduced cell surface TLR4 expression compared to the WT group (Fig. 3A). The expression of serum cytokines in Rab5a−/− CLP mice was also significantly lower than in WT CLP mice (Fig. 3B). Further, the survival of Rab5a−/− CLP mice was significantly increased compared to that of the WT group, and histopathological sections suggested that the damage to lung, liver, kidney, and intestinal tissue in Rab5a−/− mice with CLP surgery was significantly alleviated compared to WT mice (Fig. 3C, D). Taken together, these data indicate that Rab5a is essential for the intracellular trafficking of TLR4 from Golgi to the cell membrane, and for the upregulation of cell surface TLR4 upon LPS treatment. Knockdown of Rab5a can attenuate the inflammatory response and organ damage in septic mice, suggesting it is a potential target for treating sepsis.
Propofol inhibits Rab5a expression and the interaction between Rab5a and TLR4 in macrophages in response to LPS and CLP
Rab5a has previously been shown to play a significant role in membrane receptor trafficking [31]. We hypothesized that Rab5a may bind to TLR4 and help it to traffic between membrane and cytoplasm during LPS treatment and that this may be suppressed by propofol. We first evaluated the expression of Rab5a in BMDMs treated with LPS, with or without propofol pretreatment. Western blotting analysis showed that LPS increased the expression of Rab5a, and this effect was suppressed by propofol pretreatment (Fig. 4A) and we found that propofol did not affect Rab7 and Rab10 (Additional file 2: Fig. S2C). Co-immunoprecipitation revealed that propofol pretreatment weakened the interaction between TLR4 and Rab5a (Fig. 4B). Collectively, these data reveal that propofol inhibits both Rab5a expression and the interaction between Rab5a and TLR4.
Propofol inhibits Rab5a expression and the interaction between Rab5a and TLR4 in macrophages. BMDMs were treated with LPS (1 μg/ml) for the indicated time with or without pretreatment of propofol (50 μM) for 30 min. A Western blotting of Rab5a in BMDMs. B Interaction of TLR4 withRab5a determined by Co-IP analyses in BMDMs. Immunoprecipitation (IP) and immunoblotting (IB) were performed with anti-TLR4 and anti-Rab5a antibodies. Data are expressed as the mean ± SD, n = 3, *p < 0.05, **p < 0.01, ***p < 0.001, 1-way ANOVA with Tukey’s post hoc test
Propofol reduces membrane TLR4 expression on macrophages upon LPS treatment through downregulating Rab5a
To gain further insight into whether propofol inhibits cell surface TLR4 expression by LPS-treated macrophages through regulating Rab5a, we analyzed the amount of cell surface TLR4 in Rab5a-overexpressing BMDMs in response to LPS in the presence or absence of propofol pretreatment. As shown in Additional file 4: Fig. S4A, B, the amount of cell surface TLR4 was increased by Rab5a upregulation and abolished by propofol pretreatment in BMDM treated with LPS compared to an empty-vector-transfected group (Additional file 5: Fig. S5). Furthermore, propofol could not effectively reduce the expression of pro-inflammatory factors in Rab5a-overexpressing BMDMs treated with LPS compared to the control group (Additional file 4: Fig. S4C).
In summary, these results reveal that propofol attenuates the expression of cell surface TLR4 by downregulating Rab5a.
Propofol inhibits the expression of Rab5a and TLR4 on LPS-treated peripheral blood monocytes from healthy volunteers as well as septic patients
In order to validate the results detailed above with in vitro and in vivo experiments, and to exclude differences between mice and humans, we extracted peripheral blood monocytes from healthy volunteers and verified the effects of propofol on Rab5a and TLR4 following LPS stimulation. We found that the expression of Rab5a and TLR4 on the surface of human peripheral blood monocytes increased after LPS stimulation and propofol pretreatment inhibited the expression of Rab5a and cell surface TLR4 (Fig. 5A, B). In addition, lower levels of mRNA expression of proinflammatory cytokines were also observed in the propofol pretreatment group (Fig. 5C). Further, we extracted peripheral blood mononuclear cells in sepsis patients and found a significant increase in both Rab5a and cell surface TLR4 expression compared with Health volunteers. Propofol was able to inhibit the expression of Rab5a and cell surface TLR4, and the release of inflammatory factors (Fig. 5D–F). Moreover, Rab5a and membrane TLR4 expressions were positively associated with the Sequential Organ Failure Assessment score of sepsis patients (Fig. 6A, B), and more importantly, the septic non-survivors had significantly increased Rab5a mRNA levels when compared to the septic survivors (Fig. 6C). The above findings showed that the expression levels of Rab5a and TLR4 are elevated in peripheral blood mononuclear cells of patients with sepsis, and the mechanisms by which propofol exerts its anti-inflammatory effects include inhibiting the expression of Rab5a and TLR4, which is in accordance with the results of our in vitro and in vivo experiments.
Propofol inhibits the expression of Rab5a and TLR4 on LPS-treated peripheral blood monocytes. peripheral blood monocytes from healthy volunteers were treated with LPS treated with LPS (1 μg/ml) for the indicated time with or without pretreatment of propofol (50 μM) for 30 min. A Membrane TLR4 expression of peripheral blood monocytes from healthy volunteers was analyzed by flow cytometry. CD14 antibody was used to mark monocytes. B Western blotting of Rab5a in peripheral blood monocytes from healthy volunteers. C Proinflammatory factor mRNA levels in peripheral blood monocytes from healthy volunteers were measured by qPCR. D Western blotting of Rab5a in peripheral blood monocytes from healthy volunteers and sepsis patients. N1 to N4 represent healthy volunteers and S1 to S4 represent sepsis patients. E Membrane TLR4 expression of peripheral blood monocytes from healthy volunteers and sepsis patients were measured by flow cytometry. Peripheral blood monocytes from sepsis patients were treated with propofol (50 μM) for 3 h. F Proinflammatory factor mRNA levels in peripheral blood monocytes from healthy volunteers and sepsis patients were measured by qPCR. Peripheral blood monocytes from sepsis patients were treated with propofol (50 μM) for 3 h. Data are expressed as the mean ± SD, n = 3–5, *p < 0.05, **p < 0.01,***p < 0.001, 1-way ANOVA with Tukey’s post hoc test. PPF propofol
Rab5a expression in peripheral blood monocytes from sepsis patients and its clinical relevance. A The correlation between Rab5a mRNA level in peripheral blood monocytes from sepsis patients and SOFA scores. The data were analyzed using the Pearson correlation test (r = 0.565, P < 0.05, n = 19). B The correlation between membrane TLR4 in peripheral blood monocytes from sepsis patients and SOFA scores. The data were analyzed using the Pearson correlation test (r = 0.537, P < 0.05, n = 19). C Rab5a mRNA level in peripheral blood monocytes from health control, septic survivor, and septic non-survivor (n = 11, 15, 4). D Membrane TLR4 in peripheral blood monocytes from health control, septic survivor, and septic non-survivor (n = 11, 15, 4). Data are expressed as the mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001, 1-way ANOVA with Tukey’s post hoc test
Discussion
In this study, we found that possibly as a result of its involvement in TLR4 intracellular trafficking, Rab5a is critical for macrophage activation and TLR4-mediated inflammatory reaction.
Another important finding of this study was that propofol exerts its anti-inflammatory role by reducing membrane TLR4 expression on macrophages via inhibiting Rab5a expression and the interaction between Rab5a and TLR4. Consistently, we also showed that both Rab5a, and cell surface TLR4 expression, were upregulated in LPS-treated peripheral blood monocytes from healthy volunteers, and in peripheral blood monocytes from septic patients, and that expression of both factors was downregulated by propofol. The correlation between the expression of both factors and the SOFA score of sepsis patients and the high expression of Rab5a in septic non-survivors peripheral blood monocytes further enhanced the clinical relevance of our study. In this study, we demonstrated for the first time, that propofol, acting as an immunomodulating agent, exerts its anti-inflammatory role in sepsis by targeting Rab5a and thereby downregulating membrane TLR4 expression by macrophages.
TLR4 is a key signaling molecule in initiating the immune response to exogenous bacteria and endogenous damage-associated molecular patterns during endotoxemia or sepsis. It can be activated by LPS, a major component of the cell walls of gram-negative bacteria, heat shock proteins released by necrotic cells, heparin sulfate, polysaccharide degraded from hyaluronate, and other factors [32]. Numerous studies have suggested that TLR4 is a “double-edged sword” during sepsis [32, 33]. On the one hand, it can mediate the inflammatory reaction to remove exogenous microorganisms and damage cells in the body. On the other hand, excessive inflammation can cause damage to normal tissue and may lead to multiple organ failure. Therefore, as a crucial regulation molecule of endotoxemia or sepsis, TLR4 expression on the cell membrane surface is tightly regulated [34, 35]. Here, we showed that propofol prevents excessive activation of inflammatory responses by reducing TLR4 expression on the surface of macrophages exposed to exogenous bacteria. Therefore, propofol may be a useful immunomodulating agent to return an aberrant immune response to homeostasis in sepsis.
It has been demonstrated that the expression of cell-surface TLR4 is determined by both endocytosis of cell-surface TLR4 into endosomes and receptor trafficking from the Golgi apparatus to the cell membrane [36]. However, the molecular events involved in the membrane trafficking of TLR4 are still not well understood. Many studies have shown that LPS not only causes endocytic degradation of TLR4 but also promotes intracellular TLR4 transport to the cell membrane [37, 38]. Rab5a has been recognized as a master regulator of endocytosis and is involved in the intracellular trafficking of cell surface receptors [16]. Of interest, we showed that knockout of the Rab5a gene not only reduces endocytosis of TLR4 but also abolishes the replenishment of TLR4 from Golgi to the cell membrane. This indicates that Rab5a is a key molecule in the membrane trafficking of TLR4 for its role in bridging TLR4 endocytosis and replenishment. Rab5a has been documented to participate in the trafficking of various cell surface receptors such as TLR2, C5ar1, and EGFR, among others, via endosomes and other intracellular vesicles [39,40,41]. However, limited studies have explored the interplay between receptors regulated by Rab5a. Is there a competitive inhibition relationship between different receptors in binding with Rab5a, for example, TLR2 and TLR4. Alternatively, it can be achieved through synergistic effects, such as the existing literature suggesting a correlation between EGFR receptor trafficking and TLR4 receptor activation [42]. Moreover, distinct receptor trafficking may be governed by the same signaling pathway. Existing literature suggests that both TLR2 and C5ar1 receptors are regulated by the Rab5a-PI3K signaling axis [39, 40]. CD14 and integrin β3 are also involved in the intracellular trafficking of TLR4, especially in endocytosis. The requirement of Rab5a involvement in the transport of those receptors remains to be further investigated [43]. The utilization of immuno-electron microscopy can enable observation of TLR4 trafficking between intracellular vesicles and organelles while further investigating how Rab5a facilitates its trafficking mechanism. Furthermore, employing Co-IP combined mass spectrometry might help identify additional signaling molecules involved in Rab5a and TLR4 trafficking.
Sepsis is defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection [44]. In sepsis, the immune response that is initiated by an invading pathogen is refractory to a return to homeostasis, thus culminating in a pathological syndrome that is characterized by sustained excessive inflammation and immune suppression [45]. Immunomodulation has been considered an important avenue of treatment for sepsis, but anti-inflammatory agents against inflammatory factors have been shown to fail to improve patient outcomes [46]. New therapeutic targets and individualized treatment options are urgently needed. Our study revealed that propofol inhibits the interaction between TLR4 and Rab5a and abolishes Rab5a-mediated TLR4 membrane trafficking, indicating that propofol may enact its anti-inflammatory properties by mediating TLR4 expression via targeting of Rab5a. Moreover, higher expression of Rab5a and surface TLR4 were found in peripheral blood monocytes of patients with sepsis than in the normal control group, and both were correlated with the SOFA score of sepsis patients, with a higher expression of Rab5a found in septic non-survivors. Given the importance of these factors to the immune response, therapeutic methods focusing on exploring the possible mechanisms involved in TLR4 trafficking, the regulation of Rab5a expression, and the amount of TLR4 on macrophage surfaces, may be effective strategies for treating sepsis.
However, our clinical data suggest that TLR4 expression is not associated with patient survival, and studies of eritoran (a TLR-4 antagonist) have failed in clinical trials [47], which may raise questions about the clinical application of our findings. As TLR4 not only mediates inflammatory responses leading to organ damage but also plays an important role in maintaining the normal function of the immune system, direct blocking of TLR4 may not benefit all patients. Based on the failure of the eritoran clinical trial, some scholars proposed new strategies for enrolling only patients who are likely to respond to the drug based on appropriate biochemical screening [48]. Rab5a is elevated and is associated with survival in sepsis patients. Given that Rab5a is critical in TLR4 trafficking, high expression of Rab5a may indicate abnormal transport and overactivation of TLR4. Therefore, Rab5a may be a good marker for screening patients sensitive to TLR4 inhibitors.
Limitations of this study include our inability to explore the exact mechanisms of propofol’s effect on Rab5a. Propofol was initially defined as a gamma-aminobutyric acid (GABA) receptor antagonist. Recent studies have demonstrated that immune cells, including macrophages, also express GABA receptors [49]. The function of GABA in the immune system is at an early stage of study, but inhibitory effects have been observed in autoimmune diseases. The question of whether propofol regulates Rab5a through GABA receptors, is of interest for future research.
Conclusions
In conclusion, we demonstrated that Rab5a regulates intracellular trafficking of TLR4 and propofol reduces membrane TLR4 expression on macrophages by targeting Rab5a (Fig. 7). Our study sheds light on the mechanism underlying the anti-inflammatory effect of propofol from the aspects of receptor transport and immune regulation. This informs the potential clinical application of propofol to septic patients and indicates that Rab5a may be a potential therapeutic target against sepsis.
Schematic representation of the potential mechanism of propofol in regulating membrane TLR4 expression on macrophages. Rab5a participates in intracellular trafficking of TLR4 between Golgi and cell membrane in macrophages upon LPS treated and propofol reduces membrane TLR4 expression on macrophages by inhibiting Rab5a expression and the interaction between Rab5a and TLR4
Data availability
All datasets used and/or analyzed supporting the conclusions are available from the corresponding author upon reasonable request.
Abbreviations
- CLP:
-
Cecal ligation and puncture
- LPS:
-
Lipopolysaccharide
- PBS:
-
Phosphate-buffered saline
- GABA:
-
Gamma-aminobutyric acid
- TLR4:
-
The toll-like receptor 4
- BMDM:
-
Bone marrow-derived macrophages
- PBMC:
-
Peripheral blood monocyte
- MAPK:
-
Mitogen-activated protein kinases
- H&E:
-
Hematoxylin and eosin
- PCR:
-
Polymerase chain reaction
References
Deng F, Chen Y, Sun QS, Lin ZB, Min Y, Zhao BC, Huang ZB, Liu WF, Li C, Hu JJ, Liu KX. Gut microbiota dysbiosis is associated with sepsis-induced cardiomyopathy in patients: a case-control study. J Med Virol. 2023;95:e28267.
Jensen JU, Bouadma L. Why biomarkers failed in sepsis. Intensive Care Med. 2016;42:2049–51.
Marshall JC. Why have clinical trials in sepsis failed? Trends Mol Med. 2014;20:195–203.
Habre C, Tramer MR, Popping DM, Elia N. Ability of a meta-analysis to prevent redundant research: systematic review of studies on pain from propofol injection. BMJ. 2014;348: g5219.
An K, Shu H, Huang W, Huang X, Xu M, Yang L, Xu K, Wang C. Effects of propofol on pulmonary inflammatory response and dysfunction induced by cardiopulmonary bypass. Anaesthesia. 2008;63:1187–92.
Liu KX, Chen SQ, Huang WQ, Li YS, Irwin MG, Xia Z. Propofol pretreatment reduces ceramide production and attenuates intestinal mucosal apoptosis induced by intestinal ischemia/reperfusion in rats. Anesth Analg. 2008;107:1884–91.
Liu KX, Rinne T, He W, Wang F, Xia Z. Propofol attenuates intestinal mucosa injury induced by intestinal ischemia-reperfusion in the rat. Can J Anaesth. 2007;54:366–74.
Chen X, Wu R, Li L, Zeng Y, Chen J, Wei M, Feng Y, Chen G, Wang Y, Lin L, et al. Pregnancy-induced changes to the gut microbiota drive macrophage pyroptosis and exacerbate septic inflammation. Immunity. 2023;56:336-352.e339.
Patoli D, Mignotte F, Deckert V, Dusuel A, Dumont A, Rieu A, Jalil A, Van Dongen K, Bourgeois T, Gautier T, et al. Inhibition of mitophagy drives macrophage activation and antibacterial defense during sepsis. J Clin Invest. 2020;130:5858–74.
Ryu JK, Kim SJ, Rah SH, Kang JI, Jung HE, Lee D, Lee HK, Lee JO, Park BS, Yoon TY, Kim HM. Reconstruction of LPS transfer cascade reveals structural determinants within LBP, CD14, and TLR4-MD2 for efficient LPS recognition and transfer. Immunity. 2017;46:38–50.
West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–80.
Wang D, Lou J, Ouyang C, Chen W, Liu Y, Liu X, Cao X, Wang J, Lu L. Ras-related protein Rab10 facilitates TLR4 signaling by promoting replenishment of TLR4 onto the plasma membrane. Proc Natl Acad Sci USA. 2010;107:13806–11.
Dai QD, Wu KS, Xu LP, Zhang Y, Lin N, Jiang Y, Shao CY, Su LD. Toll-like receptor 4 deficiency ameliorates propofol-induced impairments of cognitive function and synaptic plasticity in young mice. Mol Neurobiol. 2023;61(1):519–32.
Gao Y, Han T, Han C, Sun H, Yang X, Zhang D, Ni X. Propofol regulates the TLR4/NF-κB pathway through miRNA-155 to protect colorectal cancer intestinal barrier. Inflammation. 2021;44:2078–90.
Langemeyer L, Frohlich F, Ungermann C. Rab GTPase function in endosome and lysosome biogenesis. Trends Cell Biol. 2018;28:957–70.
Gong B, Li Z, Xiao W, Li G, Ding S, Meng A, Jia S. Sec14l3 potentiates VEGFR2 signaling to regulate zebrafish vasculogenesis. Nat Commun. 2019;10:1606.
Tang J, Zhou B, Scott MJ, Chen L, Lai D, Fan EK, Li Y, Wu Q, Billiar TR, Wilson MA, et al. EGFR signaling augments TLR4 cell surface expression and function in macrophages via regulation of Rab5a activation. Protein Cell. 2020;11:144–9.
Li Z, Scott MJ, Fan EK, Li Y, Liu J, Xiao G, Li S, Billiar TR, Wilson MA, Jiang Y, Fan J. Tissue damage negatively regulates LPS-induced macrophage necroptosis. Cell Death Differ. 2016;23:1428–47.
Yi S, Tao X, Wang Y, Cao Q, Zhou Z, Wang S. Effects of propofol on macrophage activation and function in diseases. Front Pharmacol. 2022;13: 964771.
Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–6.
Gong S, Yan Z, Liu Z, Niu M, Fang H, Li N, Huang C, Li L, Chen G, Luo H, et al. Intestinal microbiota mediates the susceptibility to polymicrobial sepsis-induced liver injury by granisetron generation in mice. Hepatology. 2019;69:1751–67.
Yeh CH, Cho W, So EC, Chu CC, Lin MC, Wang JJ, Hsing CH. Propofol inhibits lipopolysaccharide-induced lung epithelial cell injury by reducing hypoxia-inducible factor-1alpha expression. Br J Anaesth. 2011;106:590–9.
Mei SH, Haitsma JJ, Dos Santos CC, Deng Y, Lai PF, Slutsky AS, Liles WC, Stewart DJ. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182:1047–57.
Zhang Y, Zheng D, Zhou T. Androgen deprivation promotes neuroendocrine differentiation and angiogenesis through CREB-EZH2-TSP1 pathway in prostate cancers. Nat Commun. 2018;9:4080.
Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, Zhou K, Liu X, Ren X, Wang F, et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. 2018;9:5395.
Hou J, Chen Q, Wu X, Zhao D, Reuveni H, Licht T, Xu M, Hu H, Hoeft A, Ben-Sasson SA, et al. S1PR3 signaling drives bacterial killing and is required for survival in bacterial sepsis. Am J Respir Crit Care Med. 2017;196:1559–70.
Hsiao CC, Chen PH, Cheng CI, Tsai MS, Chang CY, Lu SC, Hsieh MC, Lin YC, Lee PH, Kao YH. Toll-like receptor-4 is a target for suppression of proliferation and chemoresistance in HepG2 hepatoblastoma cells. Acta Neuropathol. 2015;368:144–52.
Hughes CD, Choi ML, Ryten M, Hopkins L, Drews A, Botia JA, Iljina M, Rodrigues M, Gagliano SA, Gandhi S, et al. Picomolar concentrations of oligomeric alpha-synuclein sensitizes TLR4 to play an initiating role in Parkinson’s disease pathogenesis. Acta Neuropathol. 2019;137:103–20.
Malinverno C, Corallino S, Giavazzi F, Bergert M, Li Q, Leoni M, Disanza A, Frittoli E, Oldani A, Martini E, et al. Endocytic reawakening of motility in jammed epithelia. Nat Mater. 2017;16:587–96.
Nguyen MK, Kim CY, Kim JM, Park BO, Lee S, Park H, Heo WD. Optogenetic oligomerization of Rab GTPases regulates intracellular membrane trafficking. Nat Chem Biol. 2016;12:431–6.
He K, Yan X, Li N, Dang S, Xu L, Zhao B, Li Z, Lv Z, Fang X, Zhang Y, Chen YG. Internalization of the TGF-beta type I receptor into caveolin-1 and EEA1 double-positive early endosomes. Cell Res. 2015;25:738–52.
Fuster JJ. TLR4 in atherogenesis: paying the toll for antimicrobial defense. J Am Coll Cardiol. 2018;71:1571–3.
Litvak V, Ramsey SA, Rust AG, Zak DE, Kennedy KA, Lampano AE, Nykter M, Shmulevich I, Aderem A. Function of C/EBPdelta in a regulatory circuit that discriminates between transient and persistent TLR4-induced signals. Nat Immunol. 2009;10:437–43.
Aksoy E, Taboubi S, Torres D, Delbauve S, Hachani A, Whitehead MA, Pearce WP, Berenjeno IM, Nock G, Filloux A, et al. The p110delta isoform of the kinase PI(3)K controls the subcellular compartmentalization of TLR4 signaling and protects from endotoxic shock. Nat Immunol. 2012;13:1045–54.
Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, Granucci F, Kagan JC. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell. 2011;147:868–80.
Taguchi T, Mukai K. Innate immunity signalling and membrane trafficking. Curr Opin Cell Biol. 2019;59:1–7.
Eun HS, Lee BS, Chun K, Kang SJ, Kim SC, Gao B, Kunos G, Kim HM, Jeong WI, Husebye H, et al. The Rab11a GTPase controls Toll-like receptor 4-induced activation of interferon regulatory factor-3 on phagosomes. Nat Commun. 2010;33:583–96.
Kim SY, Jeong JM, Kim SJ, Seo W, Kim MH, Choi WM, Yoo W, Lee JH, Shim YR, Yi HS, Lee YS. Pro-inflammatory hepatic macrophages generate ROS through NADPH oxidase 2 via endocytosis of monomeric TLR4-MD2 complex. Nat Commun. 2017;8:2247.
Wu KC, Condon ND, Hill TA, Reid RC, Fairlie D, Lim J. Ras related protein Rab5a regulates complement C5a receptor trafficking, chemotaxis and chemokine secretion in human macrophages. J Innate Immun. 2023;15:468–84.
Maganto-Garcia E, Punzon C, Terhorst C, Fresno M. Rab5 activation by Toll-like receptor 2 is required for Trypanosoma cruzi internalization and replication in macrophages. Traffic. 2008;9:1299–315.
Barbieri MA, Roberts RL, Gumusboga A, Highfield H, Alvarez-Dominguez C, Wells A, Stahl PD. Epidermal growth factor and membrane trafficking. EGF receptor activation of endocytosis requires Rab5a. J Cell Biol. 2000;151:539–50.
Zhang X, Chen C, Ling C, Luo S, Xiong Z, Liu X, Liao C, Xie P, Liu Y, Zhang L, et al. EGFR tyrosine kinase activity and Rab GTPases coordinate EGFR trafficking to regulate macrophage activation in sepsis. Cell Death Dis. 2022;13:934.
Chen Z, Wang S, Chen Y, Shao Z, Yu Z, Mei S, Li Q. Integrin β3 modulates TLR4-mediated inflammation by regulation of CD14 expression in macrophages in septic condition. Shock. 2020;53:335–43.
Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. 2018;6:223–30.
Schultz MJ, Dunser MW, Dondorp AM, Adhikari NK, Iyer S, Kwizera A, Lubell Y, Papali A, Pisani L, Riviello BD, et al. Current challenges in the management of sepsis in ICUs in resource-poor settings and suggestions for the future. Intensive Care Med. 2017;43:612–24.
Sinha M, Jupe J, Mack H, Coleman TP, Lawrence SM, Fraley SI. Emerging technologies for molecular diagnosis of sepsis. Clin Microbiol Rev. 2018;31:10–128.
Opal SM, Laterre PF, Francois B, LaRosa SP, Angus DC, Mira JP, Wittebole X, Dugernier T, Perrotin D, Tidswell M, et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA. 2013;309:1154–62.
Tse MT. Trial watch: sepsis study failure highlights need for trial design rethink. Nat Rev Drug Discov. 2013;12:334.
Prud’homme GJ, Glinka Y, Wang Q. Immunological GABAergic interactions and therapeutic applications in autoimmune diseases. Autoimmun Rev. 2015;14:1048–56.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
This study was supported by Grant No. 81671955 from the National Natural Science Foundation of China (to Dr. Ke-Xuan Liu); Grant No. 81730058 from the Key Program of the National Natural Science Foundation of China (to Dr. Ke-Xuan Liu) and Grant No. 2022B028 from the President Foundation of Nanfang Hospital (to Dr. Bo-Wei Zhou).
Author information
Authors and Affiliations
Contributions
BWZ and WJZ performed most experiments and contributed to writing the manuscript. FLZ, YX, YQD, ZWY reviewed and edited the manuscript. ZZY, BCZ, and XDC analyzed data. CL supervised the project. KXL conceived and designed the study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The clinical experimental design was reviewed by the Ethical Committee of Nanfang Hospital, Southern Medical University (approval number NFEC-202009-k2-01), and all patients provided written informed consent. All animal experiments were reviewed and approved by the Ethics Committee of Southern Medical University (approval number NFYY-2019-24).
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest in performing this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Cytokines and Chemokines PCR Array in BMDMs. (A) Heatmap showed differentially expressed genes in BMDMs by RT-RT-qPCR array. (B) The volcano plot analyzed the significantly up-(red) and down-regulated (green) based on fold-change (FC ≥ |2| and p < 0.05). The statistical model used for the volcano map data was negative binomial models, and the test method was generalized linear models, Benjamini and Hochberg for adjustments were made for multiple comparisons. (C) Differential expression analysis of DEGs.
Additional file 2: Figure S2.
Propofol does not affect the total expression of TLR4 in BMDMs. BMDMs were treated with LPS (1 μg/ml) for the indicated time with or without pretreatment of propofol (50 μM) for 30 min. (A) Western blotting of TLR4 in BMDMS. (B) The comparison of total TLR4 expression in BMDMs. Data are expressed as the mean ± SD, n = 3, NS: No Significant Difference, 1-way ANOVA with Tukey’s post hoc test. PPF: propofol.
Additional file 3: Figure S3.
Propofol reduces organ damage and improves survival in sepsis mice. C57BL mice that underwent CLP were pretreated with propofol (50 mg/kg) or an equal volume of fat emulsion for 30 min. (A) H&E staining and histological score of lung, liver, kidney, and intestinal of CLP mice. The mice were sacrificed 24 h after CLP (n = 8). (Scale bars: 50 μm). (B) Serum ALT, AST, and Scr levers of CLP mice. The mice were sacrificed 24 h after CLP (n = 8). (C) The survival curves of CLP mice. Kaplan Meier analysis was used to evaluate the survival rate of CLP mice (n = 20). Data are expressed as the mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001, unpaired t-test or 1-way ANOVA with Tukey’s post hoc test. PPF: propofol.
Additional file 4: Figure S4.
Propofol reduces membrane TLR4 expression on macrophages upon LPS treatment through downregulating Rab5a. BMDMs were transfected with GV141-mRab5a or empty vector and stimulated with LPS (1 μg/ml) for 24 h with or without pretreatment of propofol (50 μM) for 30 min. (A) The overexpression efficiency of Rab5a was confirmed by western blotting. (B) membrane TLR4 expression of BMDMs was analyzed by flow cytometry. Data are expressed as the mean ± SD, n = 3–5, *p < 0.05, **p < 0.01,***p < 0.001, 1-way ANOVA with Tukey’s post hoc test. PPF: propofol, EV: empty vector, OV: Overexpression.
Additional file 5: Figure S5.
Flow cytometric gating strategy.
Additional file 6: Table S1.
Cytokines and Chemokines PCR Array. Table S2. Characteristics of sepsis patients and health control. Table S3. Sequences of primers used for qRT-PCR. Table S4. Sequences for plasmids.
Additional file 7.
Raw data for RT-PCR array.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, BW., Zhang, WJ., Zhang, FL. et al. Propofol improves survival in a murine model of sepsis via inhibiting Rab5a-mediated intracellular trafficking of TLR4. J Transl Med 22, 316 (2024). https://doi.org/10.1186/s12967-024-05107-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-024-05107-9