Abstract
Background
Retinal degenerative disorders (RDDs) cause vision loss by damaging retinal neurons and photoreceptors, affecting individuals of all ages. Cell-based therapy has emerged as an effective approach for the treatment of RDDs with promising results. This meta-analysis aims to comprehensively evaluate the efficacy of cell therapy in treating age-related macular degeneration (AMD), retinitis pigmentosa (RP), and Stargardt macular degeneration (SMD) as the most prevalent RDDs.
Methods
PubMed, Scopus, Web of Science, and Embase were searched using keywords related to various retinal diseases and cell therapy treatments until November 25th, 2023. The studies’ quality was evaluated using the Joanna Briggs Institute’s (JBI) checklist for quasi-experimental studies. Visual acuity measured as LogMAR score was used as our main outcome. A three-level random-effect meta-analysis was used to explore the visual acuity in patients who received cell-based therapy. Heterogeneity among the included studies was evaluated using subgroup and sensitivity analyses. Moreover, meta-regression for the type of cells, year of publication, and mean age of participants were performed.
Results
Overall, 8345 studies were retrieved by the search, and 39 met the eligibility criteria, out of which 18 studies with a total of 224 eyes were included in the meta-analysis. There were 12 studies conducted on AMD, 7 on SMD, and 2 on RP. Cell therapy for AMD showed significant improvement in LogMAR (p < 0.05). Also, cell therapy decreased the LogMAR score in SMD and RP (p < 0.01 and p < 0.0001, respectively). Across all conditions, no substantial publication bias was detected (p < 0.05).
Conclusion
The findings of the study highlight that the application of cell therapy can enhance the visual acuity in AMD, SMD, and RP.
Similar content being viewed by others
Background
A considerable portion of the global population suffers from visual impairment and even permanent vision loss due to a group of heterogeneous diseases collectively known as retinal degenerative disorders (RDDs). Age-related macular degeneration (AMD) is the most common form of retinal degenerative problem, with an approximate prevalence of 20 million in the United States and 196 million globally. These diseases are characterized by the progressive deterioration of the retina, leading to the loss of photoreceptor cells and subsequent vision loss. The incidence of vision loss caused by Retinal Detachment (RD) is on the rise [1,2,3,4]. The characteristic pathological manifestations of RDD involve the degeneration and demise of photoreceptors (rods and cones), retinal ganglion cells (RGCs), and retinal pigment epithelium (RPE) cells, which exhibit an inability to regenerate. RDD can manifest in various forms, such as AMD, retinitis pigmentosa (RP), and less well-known inherited retinal dystrophies like Stargardt macular degeneration (SMD) [5, 6].
Age-related macular degeneration is a prevalent eye disease affecting millions of people worldwide and is widely recognized as the primary factor contributing to permanent vision loss among adults aged 60 and above in the developed world. Two primary categories of this disease exist: neovascular (wet) and non-neovascular (dry). Dry AMD, including approximately 80% to 85% of patients, exhibits a better visual prognosis. At the same time, neovascular AMD impacts the residual 15% to 20% of cases and is responsible for 80% of severe vision loss [7].
AMD is characterized by pathological alterations in the macula and its adjacent vasculature, resulting in the progressive impairment of central vision. Retinal deposits, known as drusen, are a significant clinical hallmark observed in individuals with age-related macular degeneration. Dry AMD is the prevailing morphological subtype and has the potential to advance into the neovascular type [8].
Another important external retinal disease is retinitis pigmentosa (RP), which is a commonly hereditary and severe degenerative retinal disease characterized by the gradual loss of photoreceptor cells and atrophy of the RPE. In the early stages, nyctalopia occurs, followed by a gradual deterioration of visual acuity, resulting in loss of vision. Globally, there is an observed increase in the prevalence of early-onset RP variants, likely due to advancements in genetic screening techniques. This visual impairment typically becomes more apparent in individuals between the ages of 40 and 50. Despite progress in the therapeutic methods, there is still no approved effective treatment for RP [2, 9, 10].
Stargardt macular degeneration is an inherited ocular disorder that leads to a gradual loss of visual acuity, mostly impacting the macula. In the majority of individuals diagnosed with SMD, there is a progressive accumulation of lipofuscin (a fatty yellow pigment) within the cells located beneath the macula, damaging cells critical for clear central vision. Additionally, this disease causes nocturnal visual impairment, and certain patients may experience compromised color vision. The manifestation of symptoms is commonly observed throughout the later stages of infancy, extending into early adulthood, and exhibits a progressive deterioration as time progresses [11, 12].
Generally, these conditions carry significant effects on quality of life, such as heightened susceptibility to falls, depression, and a greater reliance on long-term care services. Additionally, visual impairments caused by RDD can vary in severity and progression based on their type, and the onset of these diseases usually ranges from congenital to late adulthood, therefore making them complex and challenging conditions to treat effectively [10].
Stem cells, characterized by their ability to undergo self-renewal and differentiation into specialized cell types, have garnered attention as a potential treatment for a range of pathological conditions such as degenerative retinal diseases. The retina is a highly favorable candidate for stem cell therapies due to its accessibility, innovative surgical techniques, limited diversity of cell types, compact organ size, and immune-privileged characteristics [13].
On the contrary, conventional therapeutic approaches aimed at addressing retinal degeneration have been ineffective in terms of restoring and regenerating the impaired retina. The application of stem cell-based therapy has emerged as a promising approach in the treatment of retinal degeneration, owing to its remarkable attributes such as self-renewal, multi-directional differentiation, neuroprotection, and immuno-regulation. Additionally, they have the ability to act as inhibitors of neuronal cell apoptosis and promote the release of neurotrophins. Hence, the objective of stem cell replacement therapy in these diseases is to generate new retinal cells from stem cells to substitute injured photoreceptor cells and outer nuclear layers [14, 15].
Various types of stem cells, including induced pluripotent stem cells (iPSCs), mesenchymal stem cells (MSCs), and retinal progenitor cells (RPCs), are presently under investigation in phase 1 and 2 clinical trials for retinal degenerative diseases (RDDs) such as age-related macular degeneration (AMD), inherited retinal dystrophies, and retinal vascular disorders. These stem cells may be sourced from embryonic origins, known as embryonic stem cells (ESCs), or from adult sources, known as adult stem cells (ASCs) [16, 17].
In addition, the wide array of current methods for evaluating ocular structure and function enables continuous monitoring and surveillance of stem cell activity, positioning retinal conditions as a prominent focus in stem cell-oriented clinical investigations [13]. This review specifically aims to comprehensively synthesize and evaluate the effectiveness of cell therapies in addressing AMD, SMD, and RP. We conduct a meta-analysis of published clinical trial data, with a concentrated focus on assessing the outcomes and efficacy of these therapeutic interventions.
Methods
Study protocol and search strategy
This study followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) protocols for conducting a systematic review and meta-analysis. PubMed, Scopus, Web of Science, and Embase databases were searched using keywords of macular degeneration, retinal degeneration, stargardt's disease, macular dystrophy, retinitis pigmentosa, stem cell, regenerative medicine, cell therapy, extracellular matrix, and scaffold. A comprehensive list of keywords and search strategies for all databases is provided in Additional file 1. No restrictions were set on the search, and the search has been updated until November 25th, 2023. The protocol of the study has been registered in the PROSPERO (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=299200) with submission ID of CRD42022299200. A search for the gray literature was conducted using Google Scholar.
Eligibility criteria and screening
The studies that used cell therapies for retinal degeneration were considered for this review. The inclusion criteria were clinical trials that used cell therapy as an intervention on patients with AMD, SMD, and RP, provided enough information on the details of the procedure, and assessed the visual acuity of the patients in the follow-up visits. The exclusion criteria were: (1) review, letter articles, and studies with non-original data, (2) animal studies, (3) in vitro studies, (4) studies that do not have a cell therapy intervention, and (5) lack of baseline assessment. Two authors (NE and ZR) independently screened retrieved studies based on the criteria using title and abstract. Two authors (NE and ZR) performed the full-text assessment independently based on the same criteria. Conflicts were resolved by consulting with the third reviewer (ASK).
Data extraction
Two authors (NE and ZR) independently extracted data from all included studies. After extraction, the authors cross-checked their extracted data for any potential discrepancies. The extracted data was checked by the third author (ASK), and discrepancies were resolved. The following data were extracted from the studies: year of publication, first author’s name, type of study, type of retinal degeneration, inclusion and exclusion criteria of patients, sample size, demographic information (e.g., age and sex), source and type of applied cells, concentration of cells, procedure of application, and visual acuity. The missing data were retrieved by contacting the corresponding authors. In case data was presented in figures and plots, WebPlotDigitizer was used to extract it (https://apps.automeris.io/wpd/).
Quality assessment
Assessment of the studies' risk of bias was conducted utilizing the Joanna Briggs Institute’s (JBI) checklist for quasi-experimental studies [18]. In this scale, each study is evaluated based on nine items, including assessment for cause and effect, participant comparison, intervention, control, pre and post-intervention outcome measure, follow-up, outcome measure comparison, reliability of outcome measure, and statistical analysis. The detailed questions are provided in Table 2 and in Additional file 2. Each item was graded as 1 (yes), 0 (no), or NA (Not Applicable or Unclear). Two authors (ASK and NE) used the JBI Scale to independently assess quality, resulting in a score from 0 to 9. Discrepancies and uncertainness regarding questions were resolved by the third reviewer (MMJM).
Statistical analysis and data synthesis
Vision acuity in the LogMAR scale was collected as mean ± standard deviation (SD). In case median and interquartile range (IQR) were reported instead, they were converted to mean ± SD [19–21]. To homogenize the measures so they could be pooled, all visual acuity measures (ETDRS letter score and Snellen scale) were converted to LogMAR using the eye package in the R programming language [22]. Hedges’ g standardized mean difference and 95% confidence interval (CI) were used to calculate the effect size. Since there were multiple follow-ups, hence multiple effects from one study, a three-level meta-analysis using restricted maximum likelihood (REML) was used to prevent unit-of-analysis issues and handle the violation of independence assumption. To assess the goodness of the fit of the three-level model and compare it with the conventional model, we used log-likelihood-ratio tests. Funnel plot asymmetry and Egger’s test were used to assess the publication bias. If there is any publication bias, we will employ the Trim-and-fill method to address it. In the AMD meta-analysis, a subgroup analysis was performed based on whether the type of disease was wet or dry to investigate the source of heterogeneity. Moreover, meta-regression was performed for publication year, mean age of participants, type of cell (RPE, NSC, UMSC, and BMSC; ADSC was used as reference), and application of scaffold. Cochran’s Q test and I2 statistics were used to assess the heterogeneity, and a p-value < 0.1 was considered significant. Variances were evaluated at three levels: sampling variance (level 1), between effect sizes variance (level 2), and between-study variances (level 3). All meta-analyses were performed using ‘metafor’ and ‘meta’ packages (R programming language v 4.2.1).
Results
Study selection
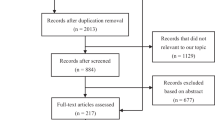
After searching the databases, a total of 8345 records were retrieved. After removing duplicates, 2874 results remained for screening, of which 79 articles remained following title/abstract screening [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101]. During the full-text screening process, 39 publications did not fulfill the eligibility requirements and were eliminated for various reasons, according to PRISMA guidelines [23,24,25,26,27,28,29,30, 34,35,36,37,38, 41, 47, 48, 50, 56, 59,60,61,62, 64, 71, 73, 74, 77, 79, 80, 83, 84, 86, 88, 89, 96,97,98,99, 102]. There were articles whose findings could not be included in this review due to being in vitro [59], in vivo [28], case reports [23, 74, 88], review article [79], conference/meeting abstracts [24,25,26,27, 29, 30, 34,35,36,37,38, 41, 47, 48, 50, 56, 60,61,62, 64, 71, 73, 77, 80, 83, 86, 96,97,98,99, 102], or full-text articles that could not be found [84, 89]. Based on the inclusion and exclusion criteria, 40 studies were considered for this study, of which 23 did not have enough data [40, 42, 45, 52,53,54,55, 58, 63, 67,68,69, 72, 75, 76, 78, 81, 91,92,93,94, 100, 101], and 17 had enough quantitative data to be included in the meta-analysis and presented in this study [33, 43, 46, 51, 65, 70, 85, 87, 103,104,105,106,107,108,109,110,111]. One article was added to the SMD meta-analysis by updating the search until November 25th [112]. The study selection process has been outlined in Fig. 1.
Study characteristics and quality assessment
The included studies’ publication times were between 2006 and 2023. Out of the 18 studies that were reviewed, 12 studies have focused on patients diagnosed with AMD [33, 43, 46, 51, 65, 70, 85, 105, 106, 108, 109, 111], 7 studies were focused on patients with SMD [70, 85, 87, 105, 107, 110, 112], and a mere 2 studies were related to RP patients [103, 104]. In a total of 18 investigations, ESC was employed as a therapeutic approach in 9 studies [33, 43, 85, 87, 106,107,108,109, 112], while adult stem cells (ASCs) were used as a treatment in the remaining 9 trials [46, 51, 65, 70, 103,104,105, 110, 111]. Among the aforementioned studies, three investigations employed scaffold structures for the purpose of cultivating stem cells and facilitating therapeutic applications. In the study conducted by Kashani et al., a parylene membrane was applied as the scaffold [43, 108]. In addition, Da Cruz et al. employed a scaffold composed of a human-vitronectin-coated polyester membrane [33]. The summary of the characteristics of the studies is available in Table 1. The total number of the included eyes in the LogMAR meta-analysis is 224. Table 2 presents the NOS scoring of the included studies. All of the clinical trial studies included in the analysis received a score of ≥ 6 out of 9.
Cell therapy for age-related macular degeneration
Overall, 12 studies were included in the meta-analysis, through which 140 eyes underwent the cell therapy intervention for AMD. The random-effect three-level meta-analysis demonstrated that cell therapy decreased the LogMAR score compared to baseline (g = − 0.47, 95% CI = − 0.91 to − 0.03, p = 0.04). The forest plot for meta-analysis has been shown in Fig. 2A. Heterogeneity was significant (Q = 52.51, p < 0.05) with the heterogeneity variance components of τlevel 3 = 0.40 and τlevel 2 = 0.00. Also, it was demonstrated that 58.63% of heterogeneity was attributable to level 3 (\({I}_{level 3}^{2}\); between-cluster variance), 0% was attributable to level2 (\({I}_{level 2}^{2}\); within-cluster variance), and 41.37% was due to sampling error variance (level 1). Variance components have been shown in Fig. 2B. The three-level model was shown to be superior to the two-level model, where within-study variance is disregarded based on the likelihood ratio test (X2 = 15.73, p < 0.0001).
Subgroup analysis was conducted to determine whether the type of AMD, wet or dry, has an impact on the results. It was observed that the decrease in the LogMAR was significant in the “wet” AMD (g = − 1.74, 95% CI = − 2.91 to − 0.58, p < 0.01), but not in “dry” AMD (g = − 0.26, 95% CI = − 0.73–0.22, p = 0.29). The heterogeneity for wet and dry subgroups was not significant (both p-values = 0.99), indicating that the heterogeneity was primarily due to the type of AMD. The chi-squared test for evaluating disparity between subgroups was significant (X2 = 5.35, p < 0.05).
Egger’s linear regression test for funnel plot asymmetry was insignificant, demonstrating no substantial publication bias (p = 0.09; Fig. 2C). Sensitivity analysis demonstrated that “A. Oner 2018” [105] analysis woutlier. Repeating the analysis without “A. Oner 2018” does not change the overall significance of the analysis (p = 0.10). Moreover, the significance of “wet” and “dry” subgroups and the difference between subgroups does not change (p < 0.01, p = 0.58, and p < 0.05, respectively). Meta-regression demonstrated no significant influence on bone marrow mesenchymal stem cell (BMSC) and umbilical cord MSC (UMSC), with p-values of 0.17 and 0.11. However, it was observed that NSC, RPE, publication year, the mean age of participants, and application of scaffold have significant effects on the overall LogMAR (p < 0.05, p < 0.001, p < 0.05, p < 0.0001, and p < 0.01, respectively).
Cell therapy for stargardt macular degeneration
Overall, 7 studies with 40 eyes entered the meta-analysis for cell therapy in SMD. Through random-effect Three-level meta-analysis, it was observed that cell therapy significantly reduced the LogMAR score (g = − 0.36, 95% CI = − 0.61–0.01, p < 0.01). The forest plot for meta-analysis has been shown in Fig. 3A. The heterogeneity was not significant (Q = 9.70, p = 1.00) with heterogeneity variance components of τlevel 3 = 0.02 and τlevel 2 = 0.00. Also, it was demonstrated that 5.13% of heterogeneity was attributable to level 3 (\({I}_{level 3}^{2}\)), 0% was attributable to level 2 (\({I}_{level 2}^{2}\)), and 94.87% was because of sampling error variance (level 1). Variance components have been shown in Fig. 3B. The likelihood ratio test demonstrated the three-level model does not provide a significantly better fit compared to the two-level model (X2 = 0.26, p = 0.61). The publication bias was not significant based on Egger’s linear regression (p = 0.96; Fig. 3C). No potential outlier was detected in the sensitivity analysis. The meta-regression demonstrated no significant effect for publication year, BMSC, and RPE with p-values of 0.74, 0.15, and 0.09. However, the mean age of participants has a significant effect on the overall LogMAR (p < 0.05).
Cell therapy for retinitis pigmentosa
Only 2 studies with 44 eyes were included in the meta-analysis. A significant improvement was achieved by cell therapy based on the three-level random-effect meta-analysis results (g = − 0.33, 95% CI = − 0.48 to − 0.17, p < 0.0001). The forest plot for meta-analysis has been shown in Fig. 4A. No significant heterogeneity was observed (Q = 4.53, p = 1.00). The variance components are τlevel 3 = 0.00 and τlevel 2 = 0.00. All heterogeneity was attributable to level 1 (\({I}_{level 1}^{2}\) = 100%; Fig. 4B). Also, the likelihood ratio test demonstrated that the three-level model was not superior to the two-level model (X2 = 0.00, p = 1.00). The Egger’s test showed no significant publication bias (p = 0.09; Fig. 4C). Sensitivity analysis revealed no outlier. The meta-regression was not performed since the effects were only extracted from two studies and the results may not be reliable.
Discussion
In this meta-analysis, we assessed the effect of cell-based therapies on patients with different types of retinal degeneration, with a specific focus on Age-related Macular Degeneration (AMD), Stargardt Macular Degeneration (SMD), and Retinitis Pigmentosa (RP). Our results were in line with prior studies that affirmed the efficacy of stem cell transplantation as an effective and safe therapeutic modality for individuals diagnosed with RDDs.
In this study, encompassing 18 studies and a total of 224 eyes, we quantitively assessed the improvement of best-corrected visual acuity among patients with AMD, SMD, or RP who have undergone stem cell therapy. Cell therapy resulted in decreased LogMAR scores for both types of AMD, whereas the improvement was only significant in wet-type AMD (p < 0.01) but not dry-type AMD (p = 0.29). Moreover, the utilization of cell therapy improved the visual acuity in SMD and RP (p < 0.01 and p < 0.0001, respectively). The study findings have been summarized in Table 3. Occurrence of severe adverse events following stem cell therapy was infrequent, and most of the participants experienced only a mild ocular side effect. To the best of our knowledge, this is the first available meta-analysis to examine the effect of cell therapy in all three prevalent retinal degenerative disorders concurrently. Most of the available previous studies have investigated cell-based therapy in one or two particular types of disorders [113,114,115]. Our study has included both types of AMD, RP, and SMD all together in one comprehensive study.
We presented queries such as “What impact does stem cell therapy have on visual performance in patients with retinal degenerative disorders?” and “Which factors can modify this impact?” across a range of clinical trial studies. Accordingly, it became imperative to carry out this meta-analysis for common retinal degenerative disorders to address these questions.
Cells used in the studies were either differentiated or undifferentiated stem cells. The stem cells that have been used are BMSC, UMSC, NSC, and ADSC. The stem cells underwent quality control, safety, and purity evaluation before application. RPEs are commonly obtained through spontaneous differentiation from specific human embryonic stem cell lines. Various methods were used to characterize RPE, confirm their purity, and avoid the inclusion of undifferentiated cells, including immunocytochemistry, fluorescence-activated cell sorting (FACS), electron microscopy, genetic analysis, and polymerase chain reaction (PCR) [33, 43, 65, 85, 103, 116].
In a study led by Takahashi et al., the effect of RPE cells derived from iPSC transplantation was assessed in right eye of a female patient with wet AMD. A four-year follow-up revealed that stem cells had survived and maintained a normal morphology. Although this study did not result in improved vision, the patient’s vision remained stable after the intervention despite a continuous reduction in the previous years [88]. In our study, A subgroup analysis based on AMD types revealed an increase in visual acuity following stem cell therapy in both types of AMD; however, LogMAR reduction was statistically significant only in patients with wet AMD. Da Cruz et al. developed an RPE patch comprised of hESC-derived RPE on an artificial basement membrane and implanted it into the subretinal space of two patients with severe exudative AMD. Based on their results, visual acuity was improved in both patients, gaining 29 and 21 letters over the course of 12 months of follow-up [33]. Additionally, a metanalysis has shown that stem cell transplantation would significantly improve visual acuity in dry-type AMD patients in 6 and 12-month follow-ups [113].
Our results align with prior studies providing evidence that cell transplantation is a potentially effective and safe treatment option for individuals diagnosed with RP or SMD [117,118,119]. Huang et al. reviewed 404 eyes with RP and 92 with SMD. BCVA improved significantly in both RP and SMD groups in 6-month follow-ups with a reduction of LogMAR score of − 0.12 and − 0.14 in each group, respectively. The outcome of cell therapy in RP patients in 12-month follow-ups showed a marginal yet significant improvement in visual acuity at the 12-month assessment point [118].
Stem cells are capable of renewing themselves through cell division and can differentiate into multi-lineage cells [120]. These cells are categorized as embryonic stem cells, induced pluripotent stem cells, and adult stem cells, particularly mesenchymal stem cells [13]. Several experimental studies have shown that transplanted stem cells can survive and enhance the functionality of damaged cells in degenerated retina [121,122,123]. Increased expression of retinal markers [124], prolongation of photoreceptors survival [125], reduction of retinal cell apoptosis [126], and improved visual outcomes [127, 128] have been detected following stem cell transplantation in animal models. Furthermore, intravitreal injection of stem cells has led to a reduction of inflammation markers and retinal damage [129]. A mitigating effect on oxygen-induced retinal damage in mouse models has also been seen following the utilization of endothelial cells derived from human-induced pluripotent stem cells, which has led to a reduction in pathological vaso-occlusion and neovascularization [130].
Finally, it should be noted that the number of studies included in the meta-analysis was limited, particularly with regard to RP and SMD. This could potentially impact the final results of the analysis and introduce bias. Additionally, the genetic characteristics and variability of the patients were only reported in a few studies and could not be taken into account in the meta-analysis as a possible factor influencing the results. Furthermore, due to inconsistencies in the reporting of outcome measures beyond visual acuity and the limited availability of studies providing data on these outcomes, it was not possible to include them in the meta-analysis. Therefore, further research should consider these factors for a more comprehensive understanding of the topic.
Conclusion
In summary, stem cell therapy has also been seen to be a potential treatment modality for retinal degeneration disorders, enhancing the visual acuity of those affected. Nevertheless, more studies and clear guidelines are needed to corroborate initial results. Future research should focus on acknowledging stem cell therapy mechanisms and comparing various stem cell types for efficacy. Our study had some limitations. First, we only used BCVA to compare the overall effect of stem cell therapy, and results from spectral domain-optical coherence tomography (SD-OCT), ERG, and fundus autofluorescence were not applied in this study. Second, we did not include post-therapeutic adverse effects, and hence, future studies are needed to compare cell therapy safety in various routes of stem cell transplantation. Third, we could not investigate the long-term impact given the data shortage.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Thapa R, Khanal S, Tan HS, Thapa SS, van Rens G. Prevalence, pattern and risk factors of retinal diseases among an elderly population in Nepal: the Bhaktapur retina study. Clin Ophthalmol. 2020;14:2109–18.
Chen TC, Huang DS, Lin CW, Yang CH, Yang CM, Wang VY, et al. Genetic characteristics and epidemiology of inherited retinal degeneration in Taiwan. NPJ Genom Med. 2021;6(1):16.
Fleckenstein M, Schmitz-Valckenberg S, Chakravarthy U. Age-related macular degeneration: a review. JAMA. 2024;331(2):147–57.
Tang Z, Zhang Y, Wang Y, Zhang D, Shen B, Luo M, et al. Progress of stem/progenitor cell-based therapy for retinal degeneration. J Transl Med. 2017;15(1):99.
Molday RS, Zhang K. Defective lipid transport and biosynthesis in recessive and dominant Stargardt macular degeneration. Prog Lipid Res. 2010;49(4):476–92.
Hamel C. Retinitis pigmentosa. Orphanet J Rare Dis. 2006;1:40.
Mody S, Joshi A. Age-related macular degeneration and its association with neurodegenerative disorders. Cureus. 2023;15(2): e34920.
Thomas CJ, Mirza RG, Gill MK. Age-related macular degeneration. Med Clin North Am. 2021;105(3):473–91.
Liu W, Liu S, Li P, Yao K. Retinitis pigmentosa: progress in molecular pathology and biotherapeutical strategies. Int J Mol Sci. 2022;23(9):4883.
Wu X, Yan N, Zhang M. Retinal degeneration: molecular mechanisms and therapeutic strategies. Curr Med Chem. 2022;29(40):6125–40.
Walia S, Fishman GA. Natural history of phenotypic changes in Stargardt macular dystrophy. Ophthalmic Genet. 2009;30(2):63–8.
Rotenstreich Y, Fishman GA, Anderson RJ. Visual acuity loss and clinical observations in a large series of patients with Stargardt disease. Ophthalmology. 2003;110(6):1151–8.
Sharma A, Jaganathan BG. Stem cell therapy for retinal degeneration: the evidence to date. Biologics. 2021;15:299–306.
Öner A. Stem cell treatment in retinal diseases: recent developments. Turk J Ophthalmol. 2018;48(1):33–8.
Huang X, Gao H, Xu H. Editorial: stem cell-based therapy in retinal degeneration. Front Neurosci. 2022. https://doi.org/10.3389/fnins.2022.879659.
Hinkle JW, Mahmoudzadeh R, Kuriyan AE. Cell-based therapies for retinal diseases: a review of clinical trials and direct to consumer “cell therapy” clinics. Stem Cell Res Ther. 2021;12(1):538.
Soltani Khaboushan A, Shakibaei M, Kajbafzadeh A-M, Majidi ZM. Prenatal neural tube anomalies: a decade of intrauterine stem cell transplantation using advanced tissue engineering methods. Stem Cell Rev Rep. 2022;18(2):752–67.
Barker TH, Stone JC, Sears K, Klugar M, Leonardi-Bee J, Tufanaru C, et al. Revising the JBI quantitative critical appraisal tools to improve their applicability: an overview of methods and the development process. JBI Evid Synth. 2023;21(3):494.
Shi J, Luo D, Weng H, Zeng X-T, Lin L, Chu H, et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods. 2020;11(5):641–54.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135.
Heeren TFC. The eye package for R: a tool to facilitate analysis of ophthalmic data. 2021.
Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;376(11):1038–46.
Arcieri R, Messias K, Castro V, Siqueira R, Jorge R, Messias A. Intravitreal autologous bone-marrow stem cells in retinitis pigmentosa patients: one-year results. Investig Ophthalmol Vis Sci. 2013;54(15):643.
Arturo J, Perez C, Segura O, Guerrero OS, Bastidas Y, Larios L. Endovascular retinal infusion of bone marrow hematopoietic stem cells for pigmentous retinitis. Cytotherapy. 2014;16:S64.
Banin E, Barak A, Boyer DS, Do DV, Ehrlich R, Jaouni T, et al. Phase I/IIa clinical trial of human embryonic stem Cell (hESC)-derived retinal pigmented epithelium (RPE, OpRegen) transplantation in advanced dry form age-related macular degeneration (AMD): interim results. Investig Ophthalmol Vis Sci. 2019;60(9):6402.
Beliakouski P, Pozniak N, Kovchel N. The influence of mesechymal stem cells in macular degeneration. Acta Ophthalmol. 2012;90:4.
Bharti K. Autologous iPSC-derived RPE transplantation for dry AMD. Investig Ophthalmol Vis Sci. 2018;59(9):3906.
Coffey P. Human embryonic stem cell derived retinal pigment epithelium transplantation in severe exudative age-related macular degeneration: So far so visual. Investig Ophthalmol Vis Sci. 2017;58(8):4770.
Cotrim CC, Jorge R, Messias A, De Sousa MV, Toscano L, Siqueira RC. Intravitreal autologous bone-marrow stem cells in nonexudative macular degeneration (dry AMD) patients: results after 3 months follow-up. Invest Ophthalmol Vis Sci. 2015;56(7):3783.
Cotrim CC, Messias AMV, Jorge R, Siqueira RC. Intravitreal use of a bone marrow mononuclear fraction (BMMF) containing cd34+cells in patients with Stargardt type macular dystrophy. Stem Cells Int. 2020. https://doi.org/10.1155/2020/8828256.
Cotrim CC, Toscano L, Messias A, Jorge R, Siqueira RC. Intravitreal use of bone marrow mononuclear fraction containing CD34+ stem cells in patients with atrophic age-related macular degeneration. Clin Ophthalmol. 2017;11:931–8.
da Cruz L, Fynes K, Georgiadis O, Kerby J, Luo YH, Ahmado A, et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol. 2018;36(4):328–37.
DaCruz L, Fynes K, Georgiadis O, Nommiste B, Carr AJF, Ramsden C, et al. Improvement and stabilization of vision for 18 months after Human Embryonic Stem-cell (hESC) derived, RPE-sheet transplantation on a synthetic basement membrane for trestment of severe, wet age-related macular degeneration. Investig Ophthalmol Visual Sci. 2018;59(9):2985.
De Sousa MV, Jorge R, Messias A, Cotrim CC, Rodrigues MW, Siqueira RC. Intravitreal autologous bone marrow derived stem cells in ischemic macular edema-results after 6 months follow-up. Invest Ophthalmol Vis Sci. 2015;56(7):4718.
Francis PJ, Birch DG, Davis JL, Lam BL, Spencer R, Stout JT, et al. A phase 1 open-label, non-comparative study evaluating the safety of a single, unilateral subretinal administration of CNTO2476 (human umbilical tissue-derived cells hUTC ) in advanced retinitis pigmentosa (RP). Investig Ophthalmol Vis Sci. 2010;51(13):4789.
García Inesta N, Iniesta F, García AV, Marín JM, García C, Rodríguez M, et al. Intravitreal injection of autologous bone marrow stem cells in retinitis pigmentosa patients. preliminary results of a phase I clinical trial. Bone Marrow Transplant. 2016;51:S317–8.
Georgiadis O, Fynes K, Luo Y, Nommiste B, Zhong J, Ramsden C, et al. Human embryonic stem Cell-derived retinal pigment epithelium sheet transplantation in severe neovascular age-related macular degeneration: 18-month survival and structural outcomes. Investig Ophthalmol Vis Sci. 2018;59(9):2984.
Heier JS, Ho AC, Samuel MA, Chang T, Riemann CD, Kitchens JW, et al. Safety and efficacy of subretinally administered palucorcel for geographic atrophy of age-related macular degeneration phase 2b study. Ophthalmol Retina. 2020;4(4):384–93.
Ho AC, Chang TS, Samuel M, Williamson P, Willenbucher RF, Malone T. Experience with a subretinal cell-based gossmark therapy in patients with geographic atrophy secondary to age-related macular degeneration. Am J Ophthalmol. 2017;179:67–80.
Jain V, Kadam S. Bestrophinopathies: fighting blindness with stem cells. Cytotherapy. 2018;20(5):S42.
Kahraman NS, Oner A. Umbilical cord derived mesenchymal stem cell implantation in retinitis pigmentosa: a 6-month follow-up results of a phase 3 trial. Int J Ophthalmol. 2020;13(9):1423–9.
Kashani AH, Lebkowski JS, Rahhal FM, Avery RL, Salehi-Had H, Chen S, et al. One-year follow-up in a phase 1/2a clinical trial of an allogeneic rpe cell bioengineered implant for advanced dry age-related macular degeneration. Transl Vis Sci Technol. 2021;10(10):13.
Kashani AH, Lebkowski JS, Rahhal FM, Avery RL, Salehi-Had H, Dang W, et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci Transl Med. 2018;10(435):eaao4097.
Kashani AH, Uang J, Mert M, Rahhal F, Chan C, Avery RL, et al. Surgical method for implantation of a biosynthetic retinal pigment epithelium monolayer for geographic atrophy: experience from a phase 1/2a study. Ophthalmol Retina. 2020;4(3):264–73.
Kumar A, Midha N, Mohanty S, Chohan A, Seth T, Gogia V, et al. Evaluating role of bone marrow-derived stem cells in dry age-related macular degeneration using multifocal electroretinogram and fundus autofluorescence imaging. Int J Ophthalmol. 2017;10(10):1552–8.
Kuppermann BD, Boyer DS, Mills B, Yang J, Klassen HJ. Safety and activity of a single, intravitreal injection of human retinal progenitor cells (jCell) for treatment of retinitis pigmentosa. Investig Ophthalmol Vis Sci. 2018;59(9):2987.
Kwon NJ, Song W, Choi J, Chung SY, Kim HJ, Lee JH. The embryonic stem cell derived retinal pigment epithelial cell trial for Stargardt macular dystrophy: preliminary phase 1 results in Asian. Acta Ophthalmol. 2014. https://doi.org/10.1111/j.1755-3768.2014.F017.
Li SY, Liu Y, Wang L, Wang F, Zhao TT, Li QY, et al. A phase I clinical trial of human embryonic stem cell-derived retinal pigment epithelial cells for early-stage Stargardt macular degeneration: 5-years’ follow-up. Cell Prolif. 2021. https://doi.org/10.1111/cpr.13100.
Liao D, Boyer DS, Kaiser P, Kuppermann BD, Heier J, Mehta M, et al. Intravitreal injection of allogeneic human retinal progenitor cells (hRPC) for treatment of retinitis pigmentosa: a prospective randomized controlled phase 2b trial. Investig Ophthalmol Vis Sci. 2021;62(8):3240.
Limoli PG, Limoli C, Vingolo EM, Scalinci SZ, Nebbioso M. Cell surgery and growth factors in dry age-related macular degeneration: visual prognosis and morphological study. Oncotarget. 2016;7(30):46913–23.
Limoli PG, Limoli CSS, Morales MU, Vingolo EM. Mesenchymal stem cell surgery, rescue and regeneration in retinitis pigmentosa: Clinical and rehabilitative prognostic aspects. Restor Neurol Neurosci. 2020;38(3):223–37.
Limoli PG, Vingolo EM, Limoli C, Nebbioso M. Stem cell surgery and growth factors in retinitis pigmentosa patients: pilot study after literature review. Biomedicines. 2019;7(4):94.
Limoli PG, Vingolo EM, Morales MU, Nebbioso M, Limoli C. Preliminary study on electrophysiological changes after cellular autograft in age-related macular degeneration. Medicine. 2014;93(29): e355.
Liu Y, Chen SJ, Li SY, Qu LH, Meng XH, Wang Y, et al. Long-Term safety of human retinal progenitor cell transplantation in retinitis pigmentosa patients. Stem Cell Res Ther. 2017. https://doi.org/10.1186/s13287-017-0661-8.
Liu Y, Liang QL, Yin ZQ. Safety of autologous bone marrow mesenchymal stem cells subretinal transplantation in diabetic retinopathy patients. Investig Ophthalmol Vis Sci. 2019;60(9):3931.
Liu Y, Xu HW, Wang L, Li SY, Zhao CJ, Hao J, et al. Human embryonic stem cell-derived retinal pigment epithelium transplants as a potential treatment for wet age-related macular degeneration. Cell Discov. 2018. https://doi.org/10.1038/s41421-018-0053-y.
Mangunsong CO, Wirohadidjojo YW, Djaja MS, Sartika CR, Wijaya A, Putera BW, et al. Multifocalelectroretinography result before and after peribulbar injection of allogeneic umbilical cord—mesenchymal stem cell secretome for late-stage retinitis pigmentosa. Indian JPublic Health Res Dev. 2021;12(3):322–9.
Matsuzaki M, Mandai M, Yamanari M, Totani K, Nishida M, Sugita S, et al. Polarization-sensitive optical coherence tomography for estimating relative melanin content of autologous induced stem-cell derived retinal pigment epithelium. Sci Rep. 2020. https://doi.org/10.1038/s41598-020-64601-.
Matsuzaki M, Takagi S, Mandai M, Sugiyama S, Yamanari M, Totani K. Observation of the transplanted autologous induced pluripotent stem cell-derived retinal pigment epithelial cell sheet using polarization-sensitive optical coherence tomography. Investig Ophthalmol Vis Sci. 2018; 59(9).
Mehat MS. Phase I/II clinical trial of human embryonic stem cell (hESC)-derived retinal pigmented epithelium (RPE) transplantation in Stargardt disease (STGD): One-year results. Investigative Ophthalmology & Visual Science. 57(12).
Mehat MS, Bainbridge JWB. Early phase clinical trial of human embryonic stem cell-derived retinal pigmented epithelium transplantation in Stargardt disease: 5-year results. Investig Ophthalmol Vis Sci. 2019;60(9):3940.
Mehat MS, Sundaram V, Ripamonti C, Robson AG, Smith AJ, Borooah S, et al. Transplantation of human embryonic stem cell-derived retinal pigment epithelial cells in macular degeneration. Ophthalmology. 2018;125(11):1765–75.
Nittala MG, Hariri AH, Uji A, Velaga SB, Naor J, Sadda SR. Effect of human central nervous system stem cells subretinal transplantation on progression of geographic atrophy secondary to non neovascular age-related macular degeneration. Investig Ophthalmol Vis Sci. 2017;58(8):29.
Nittala MG, Uji A, Velaga SB, Hariri AH, Naor J, Birch DG, et al. Effect of human central nervous system stem cell subretinal transplantation on progression of geographic atrophy secondary to nonneovascular age-related macular degeneration. Ophthalmol Retina. 2021;5(1):32–40.
Oner A, Gonen ZB, Sevim DG, Kahraman NS, Unlu M. Suprachoroidal adipose tissue-derived mesenchymal stem cell implantation in patients with dry-type age-related macular degeneration and Stargardt’s macular dystrophy: 6-month follow-up results of a phase 2 study. Cell Reprogramming. 2018;20(6):329–36.
Oner A, Gonen ZB, Sinim N, Cetin M, Ozkul Y. Subretinal adipose tissue-derived mesenchymal stem cell implantation in advanced stage retinitis pigmentosa: a phase I clinical safety study. Stem Cell Res Ther. 2016;7(1):1–12.
Özmert E, Arslan U. Management of retinitis pigmentosa by Wharton’s jelly derived mesenchymal stem cells: preliminary clinical results. Stem Cell Res Ther. 2020. https://doi.org/10.1186/s13287-020-1549-6.
Özmert E, Arslan U. Management of retinitis pigmentosa by Wharton’s jelly-derived mesenchymal stem cells: prospective analysis of 1-year results. Stem Cell Res Ther. 2020. https://doi.org/10.1186/s13287-020-01870-w.
Park SS, Bauer G, Abedi M, Pontow S, Panorgias A, Jonnal R, et al. Intravitreal autologous bone marrow CD34+ cell therapy for ischemic and degenerative retinal disorders: preliminary phase 1 clinical trial findings. Invest Ophthalmol Vis Sci. 2014;56(1):81–9.
Park SS, Bauer G, Panorgias A, Zawadzki RJ, Abedi M, Werner JS, et al. Intravitreal autologous bone marrow cd34+ stem cell therapy for macular degenerative disease-a pilot clinical trial. Invest Ophthalmol Vis Sci. 2014;55(13):2995.
Radtke ND, Aramant RB, Petry HM, Green PT, Pidwell DJ, Seiler MJ. Vision improvement in retinal degeneration patients by implantation of retina together with retinal pigment epithelium. Am J Ophthalmol. 2008;146(2):172-82.e1.
Sangkitporn S, Atchaneeyasakul L, Trinavarat A, Dumbua A, Khorchai A, Sangkitporn S. GMP-compliant bioprocess development of mesenchymal stem cells for clinical trial inretinitis pigmentosa patients. Cytotherapy. 2016;18(6):S152.
Saraf SS, Cunningham MA, Kuriyan AE, Read SP, Rosenfeld PJ, Flynn HW, et al. Bilateral retinal detachments after intravitreal injection of adipose-derived ‘stem cells’ in a patient with exudative macular degeneration. Ophthalmic Surg Lasers Imaging Retina. 2017;48(9):772–5.
Satarian L, Nourinia R, Safi S, Kanavi MR, Jarughi N, Daftarian N, et al. Intravitreal injection of bone marrow mesenchymal stem cells in patients with advanced retinitis pigmentosa; a safety study. J Ophthalmic Vis Res. 2017;12(1):58–64.
Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. The Lancet. 2012;379(9817):713–20.
Schwartz SD, Regillo C, Lam BL, Eliott D, Gregori N, Hubschman JP, et al. Human embryonic stem cell-derived retinal pigment epithelial transplantation for retinal degenerations: Three-year outcomes data. Investig Ophthalmol Vis Sci. 2018;59(9):5004.
Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385(9967):509–16.
Schwartz SD, Tan G, Hosseini H, Nagiel A. Subretinal transplantation of embryonic stem cell–derived retinal pigment epithelium for the treatment of macular degeneration: an assessment at 4 years. Investig Ophthalmol Vis Sci. 2016;57(5):ORSFc1–9.
Siqueira RC, Cotrim CC, Messias A, De Sousa MV, Toscano L, Jorge R. Intravitreal autologous bone marrow derived stem cells in dry age-related macular degeneration. Invest Ophthalmol Vis Sci. 2016;57(12):3704.
Siqueira RC, Messias A, Messias K, Arcieri RS, Ruiz MA, Souza NF, et al. Quality of life in patients with retinitis pigmentosa submitted to intravitreal use of bone marrow-derived stem cells (Reticell—clinical trial). Stem Cell Res Ther. 2015. https://doi.org/10.1186/s13287-015-0020-6.
Siqueira RC, Messias A, Voltarelli JC, Scott IU, Jorge R. Intravitreal injection of autologous bone marrow-derived mononuclear cells for hereditary retinal dystrophy: a phase I trial. Retina. 2011;31(6):1207–14.
Sobaci G, Ozmert E, Ovali E, Ozdek S, Yilmaz G, Gurelik G, et al. Submacular allogeneic ECTO-mesenchymal stem cell transplantation in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2014;55(13):5012.
Sobaci G, Sevinc K, Ovali E, Ozmert E, Ozdek S, Yilmaz G, et al. Submacular allogeneic ectomesenchymal stem cell transplantation in retinitis pigmentosa: One-year results. Invest Ophthalmol Vis Sci. 2015;56(7):2276.
Song WK, Park KM, Kim HJ, Lee JH, Choi J, Chong SY, et al. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients. Stem Cell Reports. 2015;4(5):860–72.
Sung Y, Lee DR, Shim SH, Chong SY, Choi SW, Song WK. Treatment of macular degeneration using human somatic cell nuclear transfer embryonic stem cell derived retinal pigment epithelium: 1-year results in an asian patient. Investig Ophthalmol Vis Sci. 2019;60(9):3938.
Sung Y, Lee MJ, Choi J, Jung SY, Chong SY, Sung JH, et al. Long-term safety and tolerability of subretinal transplantation of embryonic stem cell-derived retinal pigment epithelium in Asian stargardt disease patients. Br J Ophthalmol. 2021;105(6):829–37.
Takagi S, Mandai M, Gocho K, Hirami Y, Yamamoto M, Fujihara M, et al. Evaluation of transplanted autologous induced pluripotent stem cell-derived retinal pigment epithelium in exudative age-related macular degeneration. Ophthalmol Retina. 2019;3(10):850–9.
Thakkar A, Sarkar S, Hua F, Highfill S, Jha S, Bharti K, et al. GMP compliant manufacturing of induced pluripotent stem cells (iPSC) for a phase I clinical trial for “dry” age related macular degeneration. Mol Ther. 2018;27(4):146.
Tuekprakhon A, Sangkitporn S, Trinavarat A, Pawestri AR, Vamvanij V, Ruangchainikom M, et al. Intravitreal autologous mesenchymal stem cell transplantation: a non-randomized phase I clinical trial in patients with retinitis pigmentosa. Stem Cell Res Ther. 2021. https://doi.org/10.1186/s13287-020-02122-7.
Weiss JN, Levy S. Stem cell ophthalmology treatment study (SCOTS): bone marrow-derived stem cells in the treatment of age-related macular degeneration. Medicines. 2020;7(4):16.
Weiss JN, Levy S. Stem cell ophthalmology treatment study (SCOTS): bone marrow-derived stem cells in the treatment of stargardt disease. Medicines. 2021;8(2):10.
Weiss JN, Levy S. Stem cell ophthalmology treatment study: bone marrow derived stem cells in the treatment of retinitis pigmentosa. Stem Cell Investig. 2018;5:18.
Weiss JN, Levy S. Stem cell ophthalmology treatment study (SCOTS): bone marrow derived stem cells in the treatment of Usher syndrome. Stem Cell Investig. 2019;6:31.
Wiącek MP, Gosławski W, Grabowicz A, Sobuś A, Kawa MP, Baumert B, et al. Long-term effects of adjuvant intravitreal treatment with autologous bone marrow-derived lineage-negative cells in retinitis pigmentosa. Stem Cells Int. 2021. https://doi.org/10.1155/2021/6631921.
Yin ZQ. Transplantation of retinal progenitor cells into the subretinal space of retinitis pigmentosa patients: one year follow-up study. Doc Ophthalmol. 2013;127(1):3–4.
Yin ZQ, Liu Y, Li S, Xu HW, Wang Y, Qian C, et al. Clincal trial: subretinal transplantation of CTS hESC derived RPE in the treatment of wet age-related macular degeneration (wAMD). Invest Ophthalmol Vis Sci. 2016;57(12):3742.
Ying Li S, Liu Y, Wei XuH, Wang F, You Li Q, Zhao TT, et al. Functional change in different categories of stargardt disease: two years follow-up of human embryonic stem cell-derived retinal pigment epithelial cells transplantation. Doc Ophthalmol. 2019;139(SUPPL 1):S27–8.
Zarate J, Folgar M, Pelayes D, Piccone SC, Moviglia-Brandolino M, Moviglia GA. Study of coroideal tissue neovascularization after aMSC treatment through oct and digital biopsy analysis. Cytotherapy. 2016;18(6):S16–7.
Zhao TT, Liang QL, Meng XH, Duan P, Wang F, Li SY, et al. Intravenous infusion of umbilical cord mesenchymal stem cells maintains and partially improves visual function in patients with advanced retinitis pigmentosa. Stem Cells Dev. 2020;29(16):1029–37.
Zhao TT, Lie HX, Wang F, Liu Y, Meng XH, Yin ZQ, et al. Comparative study of a modified sub-tenon’s capsule injection of triamcinolone acetonide and the intravenous infusion of umbilical cord mesenchymal stem cells in retinitis pigmentosa combined with macular edema. Front Pharmacol. 2021. https://doi.org/10.3389/fphar.2021.694225.
Yin ZQ, Liu Y, Chen SJ, Ying Li S, Qu LH, Meng XH. Long-term safety of human retinal progenitor cell transplantation in retinitis pigmentosa patients. Doc Ophthalmol. 2018;136:4.
Wiącek MP, Gosławski W, Grabowicz A, Sobuś A, Kawa MP, Baumert B, et al. Long-term effects of adjuvant intravitreal treatment with autologous bone marrow-derived lineage-negative cells in retinitis pigmentosa. Stem Cells Int. 2021;2021:6631921.
Tuekprakhon A, Sangkitporn S, Trinavarat A, Pawestri AR, Vamvanij V, Ruangchainikom M, et al. Intravitreal autologous mesenchymal stem cell transplantation: a non-randomized phase I clinical trial in patients with retinitis pigmentosa. Stem Cell Res Ther. 2021;12(1):52.
Oner A, Gonen ZB, Sevim DG, Smim Kahraman N, Unlu M. Suprachoroidal adipose tissue-derived mesenchymal stem cell implantation in patients with dry-type age-related macular degeneration and Stargardt’s macular dystrophy: 6-month follow-up results of a phase 2 study. Cell Reprogram. 2018;20(6):329–36.
Liu Y, Xu HW, Wang L, Li SY, Zhao CJ, Hao J, et al. Human embryonic stem cell-derived retinal pigment epithelium transplants as a potential treatment for wet age-related macular degeneration. Cell Discov. 2018;4:50.
Li SY, Liu Y, Wang L, Wang F, Zhao TT, Li QY, et al. A phase I clinical trial of human embryonic stem cell-derived retinal pigment epithelial cells for early-stage Stargardt macular degeneration: 5-years’ follow-up. Cell Prolif. 2021;54(9): e13100.
Kashani AH, Lebkowski JS, Rahhal FM, Avery RL, Salehi-Had H, Dang W, et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci Transl Med. 2018. https://doi.org/10.1126/scitranslmed.aao4097.
Heier JS, Ho AC, Samuel MA, Chang T, Riemann CD, Kitchens JW, et al. Safety and efficacy of subretinally administered palucorcel for geographic atrophy of age-related macular degeneration: phase 2b study. Ophthalmol Retina. 2020;4(4):384–93.
Cotrim CC, Vieira Messias AM, Jorge R, Siqueira RC. Intravitreal use of a bone marrow mononuclear fraction (BMMF) containing CD34+ cells in patients with stargardt type macular dystrophy. Stem Cells Int. 2020;2020:8828256.
Cotrim CC, Toscano L, Messias A, Jorge R, Siqueira RC. Intravitreal use of bone marrow mononuclear fraction containing CD34(+) stem cells in patients with atrophic age-related macular degeneration. Clin Ophthalmol. 2017;11:931–8.
Brant Fernandes RA, Lojudice FH, Zago Ribeiro L, Santos da Cruz NF, Polizelli MU, Cristovam PC, et al. Transplantation of subretinal stem cell-derived retinal pigment epithelium for stargardt disease: a phase I clinical trial. Retina. 2023;43(2):263–74.
Li L, Yu Y, Lin S, Hu J. Changes in best-corrected visual acuity in patients with dry age-related macular degeneration after stem cell transplantation: systematic review and meta-analysis. Stem Cell Res Ther. 2022;13(1):237.
Moghadam Fard A, Mirshahi R, Naseripour M, Ghasemi FK. Stem cell therapy in stargardt disease: a systematic review. J Ophthalmic Vis Res. 2023;18(3):318–27.
Waugh N, Loveman E, Colquitt J, Royle P, Yeong JL, Hoad G, et al. Treatments for dry age-related macular degeneration and Stargardt disease: a systematic review. Health Technol Assess. 2018;22(27):1–168.
Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379(9817):713–20.
Bacci GM, Becherucci V, Marziali E, Sodi A, Bambi F, Caputo R. Treatment of inherited retinal dystrophies with somatic cell therapy medicinal product: a review. Life. 2022;12(5):708.
Chen X, Xu N, Li J, Zhao M, Huang L. Stem cell therapy for inherited retinal diseases: a systematic review and meta-analysis. Stem Cell Res Ther. 2023;14(1):286.
Ramsden CM, Powner MB, Carr AJ, Smart MJ, da Cruz L, Coffey PJ. Stem cells in retinal regeneration: past, present and future. Development. 2013;140(12):2576–85.
Nóbrega C, Mendonça L, Matos C. A handbook of gene and cell therapy. Cham: Springer; 2020.
Lako M, Armstrong L. Methods and advances in induced pluripotent stem cells-ophthalmology. Front Cell Dev Biol. 2023;11:1298956.
Lin TC, Hsu CC, Chien KH, Hung KH, Peng CH, Chen SJ. Retinal stem cells and potential cell transplantation treatments. J Chin Med Assoc. 2014;77(11):556–61.
Xiao-hong L, Ren-yi W. Current approaches and future prospects of stem cell transplantation for degenerative diseases of retina and optic nerve. Int Rev Ophthalmol. 2017;41(3):153.
Soleimannejad M, Ebrahimi-Barough S, Nadri S, Riazi-Esfahani M, Soleimani M, Tavangar SM, et al. Retina tissue engineering by conjunctiva mesenchymal stem cells encapsulated in fibrin gel: Hypotheses on novel approach to retinal diseases treatment. Med Hypotheses. 2017;101:75–7.
Arnhold S, Absenger Y, Klein H, Addicks K, Schraermeyer U. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefes Arch Clin Exp Ophthalmol. 2007;245:414–22.
Yu B, Shao H, Su C, Jiang Y, Chen X, Bai L, et al. Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci Rep. 2016;6(1):34562.
Tuekprakhon A, Sangkitporn S, Trinavarat A, Pawestri AR, Vamvanij V, Ruangchainikom M, et al. Intravitreal autologous mesenchymal stem cell transplantation: a non-randomized phase I clinical trial in patients with retinitis pigmentosa. Stem Cell Res Ther. 2021;12:1–15.
Wang S, Lu B, Girman S, Duan J, McFarland T, Zhang Q, et al. Non-invasive stem cell therapy in a rat model for retinal degeneration and vascular pathology. PLoS ONE. 2010;5(2):e9200.
Ji S, Xiao J, Liu J, Tang S. Human umbilical cord mesenchymal stem cells attenuate ocular hypertension-induced retinal neuroinflammation via toll-like receptor 4 pathway. Stem Cells Int. 2019. https://doi.org/10.1155/2019/9274585.
Cho H, Macklin BL, Lin YY, Zhou L, Lai MJ, Lee G, et al. iPSC-derived endothelial cell response to hypoxia via SDF1a/CXCR4 axis facilitates incorporation to revascularize ischemic retina. JCI Insight. 2020. https://doi.org/10.1172/jci.insight.131828.
Acknowledgements
This study is approved by the Tehran University of Medical Sciences under the reference number IR.TUMS.MEDICINE.REC.1402.308.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
ASK conceptualized the study, wrote the manuscript, performed formal analysis, and revision the final manuscript. NE, MMJM, and ZR performed data collection and wrote the final manuscript. AMK and MMZ conceptualized the study, supervised the project, and revised the final manuscript. All authors read and confirmed the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Khaboushan, A.S., Ebadpour, N., Moghadam, M.M.J. et al. Cell therapy for retinal degenerative disorders: a systematic review and three-level meta-analysis. J Transl Med 22, 227 (2024). https://doi.org/10.1186/s12967-024-05016-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-024-05016-x