Abstract
Objective
This study aimed to develop a simplified diagnostic tool for assessing sarcopenia and myosteatosis in gastrointestinal cancer patients, focusing on the creatinine to cystatin C ratio (CCR) as an evaluation marker.
Methods
955 patients were split into training (n = 671) and validation (n = 284) cohorts. Using logistic regression, risk factors for sarcopenia and myosteatosis were identified. The predictive capacity of the developed model was examined. The association between CCR and muscle imaging parameters, along with its impact on clinical outcomes, was analyzed.
Results
No significant differences were observed in baseline traits between cohorts. CCR emerged as a significant risk factor for both sarcopenia and myosteatosis. Nomograms for diagnosing these conditions demonstrated strong predictive ability, with AUC values indicating high accuracy (sarcopenia AUC: 0.865–0.872; myosteatosis AUC: 0.848–0.849). The clinical utility of the nomograms was confirmed through decision curve analysis. CCR showed significant association with muscle imaging parameters and was a reliable indicator for assessing the risk of sarcopenia, myosteatosis, and cachexia. Moreover, CCR was able to differentiate between patient survival and disease progression rates.
Conclusion
A diagnostic tool for sarcopenia and myosteatosis in gastrointestinal cancer patients was developed, with CCR being a pivotal biomarker for disease diagnosis and prognosis prediction.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Sarcopenia refers to the loss of muscle strength and mass, and its presence is associated with poorer health outcomes in all populations [1, 2]. Muscle fat infiltration, or myosteatosis, is a condition in which there is an increase in fat infiltration in the muscles, leading to a decrease in overall strength and function [3, 4]. Research has shown that sarcopenia and myosteatosis are key factors affecting patient treatment efficacy, functional status, and quality of life [5, 6]. Cachexia is a systemic wasting syndrome caused by chronic serious illness, characterized by muscle loss [7]. Muscle atrophy and myosteatosis occur before the onset of cancer cachexia and lead to its development [8, 9]. Early diagnosis and timely intervention of muscle atrophy and myosteatosis can improve the condition of patients and prevent complications.
Currently, various methods are used in clinical practice to detect sarcopenia and myosteatosis, such as CT, MRI, and DXA [10, 11]. However, due to their high cost and time-consuming nature, they are not universally applicable in clinical settings. Therefore, identifying serum biomarkers that can be used to assess skeletal muscle-related parameters in patients is crucial for clinical diagnosis and evaluation.
Serum creatinine and cystatin C are commonly used biomarkers for evaluating kidney function in clinical practice [12]. CRE varies with body composition, while cystatin C is widely present in nucleated cells and is less affected by muscle mass [13]. Therefore, the serum creatinine-to-cystatin C ratio (CCR) may be a promising alternative biomarker for sarcopenia.
Therefore, this study aims to thoroughly evaluate the CCR as a potential indicator for diagnosing and assessing muscle atrophy and myosteatosis. Additionally, we further seek to develop a diagnostic model based on CCR for skeletal muscle reduction syndrome and musculoskeletal disorders in gastrointestinal cancer patients.
Method
Study population
The study population of this research is patients with gastrointestinal tumors. The research data is sourced from our clinical database, covering the time period from January 2018 to December 2023. Inclusion criteria: age greater than 18 years. Exclusion criteria: patients with a pathological diagnosis of benign diseases, acute kidney injury, missing abdominal CT images, low-quality CT images or patients with any anatomical deformities (such as abdominal wall edema), and a history of previous abdominal surgery. This study has been approved by the hospital ethics committee and informed consent has been obtained from all patients.
Data collection
We collected demographic and clinical data, including the following variables: age, gender, height, weight, body mass index (BMI), tumor location, tumor necrosis factor (TNF), C-reactive protein (CRP), glucose (GLU), albumin (ALB), neutrophils (NEUT), lymphocytes (LYMPH), hemoglobin (HGB), white blood cells (WBC), platelets (PLT), serum creatinine (CRE), serum cystatin C (CYSC), creatinine to cystatin C ratio (CCR = CRE/CYSC), and blood urea nitrogen (BUN).
Imaging indicators include: skeletal muscle index (SMI), skeletal muscle density (SMD), skeletal muscle weight (SMG), skeletal muscle area (SMA), intermuscular adipose tissue percentage (IMAT%), and intermuscular adipose tissue (IMAT) cross-sectional area.
We also collected follow-up information, including patients' mortality, disease progression, time of death, and time of disease progression.
Blood collection standards require patients to fast for more than 8 h and collect blood from the patient’s cubital vein in the morning to measure biochemical indicators.The BMI calculation formula is: BMI = weight (kg)/height2 (m2).
Diagnostic criteria
This study used SMI to adjust for the impact of body size on evaluating skeletal muscle mass. SMI was calculated as SMI = SMA (cm2)/height squared (m2), with higher SMI indicating more skeletal muscle. Based on our previous research, the cut-off values for male muscle atrophy were 43.13 cm2/m2 and for female 37.81 cm2/m2 [14]. Higher area percent of intermuscular adipose tissue (IMAT) indicates worse muscle mass [15]. We used IMAT% as an indicator of muscle quality, calculated as IMAT% = IMAT/SMA × 100%. IMAT% > 7.51% for males or > 6.83% for females was considered as muscle fat infiltration [16]. The diagnostic criteria for cachexia were based on previous international consensus statements [7]: (1) Weight loss > 5% in the past 6 months without dieting; (2) BMI < 20 kg/m2 and weight loss > 2%; (3) decrease in skeletal muscle mass and sustained weight loss > 2%.
Construction of a forest plot and performance evaluation methods
We randomly divided the study subjects into a training group and a validation group in a 7:3 ratio. Independent prognostic factors were screened from the training set using univariate and multivariate logistic regression analysis and then used to construct a forest plot, calculate the hazard ratio (HR) and 95% confidence interval (CI). The predictive performance of the model was evaluated using the area under the curve (AUC). The similarity between the predicted and actual results was compared using calibration curves. Clinical utility and net benefit were evaluated using decision curve analysis [17].
Correlation analysis and risk survival analysis
The correlation between CCR and different radiological indicators was evaluated using a scatter plot with Spearman’s rank correlation coefficient. The ability of CCR to evaluate muscle atrophy and muscle fat infiltration was evaluated using AUC, and the optimal cut-off value was determined. The relationship between CCR and clinical outcomes was analyzed using Kaplan–Meier curves.
Statistical analysis
Frequency and mean ± standard deviation (SD) of baseline data were used to describe the characteristics of the two groups of patients. Continuous variables were analyzed using t-tests, and categorical variables were analyzed using Fisher’s exact test. All statistical analyses were performed using SPSS software version 26.0 (IBM SPSS Inc., New York, USA) and R version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria). P-values less than 0.05 were considered statistically significant.
Results
Baseline patient characteristics
Table 1 shows the baseline characteristics of the two patient groups, and randomization results indicated no significant differences between the groups. In assessing sarcopenia, single-factor and multi-factor logistic regression analyses found age, sex, BMI, CCR, and BUN to be significant variables and independent predictors of sarcopenia (Additional file 1: Table S1). In assessing myosteatosis, single-factor and multi-factor logistic regression analyses found age, sex, CCR, and CRE to be significant variables and independent predictors of myosteatosis (Additional file 2: Table S2).
Construction of the sarcopenia diagnostic nomogram model
Based on the results of logistic regression analysis, factors with minor impact were excluded. Age, sex, BMI, CCR, and BUN were used as sarcopenia prediction variables to construct the diagnostic nomogram model in the training set, and a calibration curve was plotted using the validation set. The calibration curve showed that the predicted results were highly similar to the actual results. By calculating the total score of the prediction factors in the nomogram, the risk level of sarcopenia in the target population can be predicted (Fig. 1).
Construction of the myosteatosis diagnostic nomogram model
Based on the results of logistic regression analysis, factors with minor impact were excluded. Age, sex, CCR, and CRE were used as myosteatosis prediction variables. The diagnostic nomogram model was constructed using the training set and validated using the validation set. The calibration curve showed that the predicted results were highly similar to the actual results. By calculating the total score of the prediction factors in the nomogram, the risk level of myosteatosis in the target population can be predicted (Fig. 2).
Evaluation of predictive ability and clinical efficacy
We evaluated the diagnostic ability of the models using the area under the ROC curve. The results showed that the sarcopenia prediction model (training group AUC: 0.865, validation group AUC: 0.872) had excellent predictive ability. The myosteatosis prediction model (training group AUC: 0.848, validation group AUC: 0.849) also had excellent predictive ability (Fig. 3). In addition, the decision curve showed that both the sarcopenia and myosteatosis diagnostic models had good clinical decision-making ability (Fig. 3).
Correlation between CCR and imaging parameters
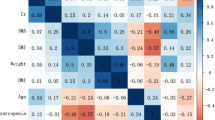
We found that CCR was a key predictive factor, and therefore we explored its relationship with relevant imaging indicators. Figure 4 shows the correlation between CCR and radiological measurements. The results indicated that CCR was positively correlated with SMI (r = 0.38, p < 0.001), SMD (r = 0.44, p < 0.001), SMA (r = 0.45, p < 0.001), and SMG (r = 0.50, p < 0.001), while it was negatively correlated with IMAT _CSA(r = − 0.29, p < 0.001) and IMAT% (r = − 0.43, p < 0.001) (Fig. 4).
Correlation analysis between CCR and imaging parameters of muscle adipose tissue. A Correlation between CCR and SMI (r = 0.38, p < 0.001). B Correlation between CCR and SMD (r = 0.44, p < 0.001). C Correlation between CCR and SMA (r = 0.45, p < 0.001). D Correlation between CCR and SMG (r = 0.50, p < 0.001). E Correlation between CCR and IMAT% (r = − 0.43, p < 0.001). F Correlation between CCR and IMAT (r = − 0.29, p < 0.001)
Evaluation of the therapeutic effect of sarcopenia and myosteatosis using CCR
The results indicated that CCR could be used to evaluate the reduction of skeletal muscle mass (training group AUC: 0.726, validation group AUC: 0.732). CCR showed good independent predictive ability in distinguishing muscle fat infiltration (training group AUC: 0.781, validation group AUC: 0.789). In the training group, the optimal threshold value of CCR for assessing the reduction of skeletal muscle mass was 75.898 (sensitivity = 0.651, specificity = 0.718), and the optimal threshold value of CCR for evaluating muscle fat infiltration was 76.142 (sensitivity = 0.711, specificity = 0.733). Similarly, good results were obtained in the validation group (Fig. 5).
Evaluation of cachexia using CCR
Sarcopenia and muscle fat infiltration are among the characteristics of cachexia patients. We further attempted to use CCR to evaluate cachexia. We evaluated cachexia in the training and validation groups using CCR (training group AUC: 0.651, validation group AUC: 0.626). Cachexia was evaluated when patients were divided into two groups using the cut-off value of CCR (training group AUC: 0.603, validation group AUC: 0.616). When cachexia was evaluated in patients with both low CCR and weight loss greater than 0.02 (training group AUC: 0.769, validation group AUC: 0.790) (Fig. 6).
ROC curves for evaluating cachexia using CCR. A, B ROC curves and cut-off values for evaluating cachexia using CCR in the training and validation groups. C, D ROC curves and cut-off values for evaluating cachexia using low/high CCR in the training and validation groups. E, F ROC curves and cut-off values for evaluating cachexia using low CCR and weight loss greater than 0.02 in the training and validation groups
Evaluation of clinical prognosis of patients using CCR cut-off value
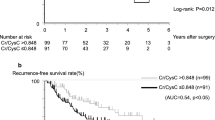
We used the CCR cut-off value to evaluate the clinical prognosis of the overall sample. The results showed that CCR effectively divided patients into high-risk and low-risk groups, assessing the risks of OS and DFS (P < 0.05) (Fig. 7).
Discussion
Clinical prediction models are used to predict disease risk based on easily obtainable predictors [18]. This study identified risk factors for sarcopenia and myosteatosis by analyzing clinical data and constructed two nomogram-based risk prediction models. We constructed the nomograms using simple clinical variables and successfully predicted disease risk in patients. Therefore, this prediction model has higher practicality and can be used not only in patients with gastrointestinal cancer but also in other populations.
As a non-invasive tool, the nomogram will be a convenient application for clinical doctors [19]. The nomogram prediction models demonstrate the impact of different predictors on the study population [20]. Age, sex, BMI, CCR, and BUN were used to evaluate sarcopenia in our constructed nomogram, while age, sex, CCR, and CRE were used to evaluate myosteatosis. The AUC indicated excellent predictive performance of our nomogram, and calibration curves showed that the predicted probabilities were close to actual outcomes. DCA analysis showed that the nomogram had excellent clinical decision-making ability, which needs to be further validated in clinical practice.
In this study, we found that CCR was a key factor in evaluating sarcopenia and myosteatosis. Further analysis revealed a significant positive correlation between CCR and SMI, and a negative correlation with IMAT%. Furthermore, we confirmed that CCR could be used to assess reductions in skeletal muscle mass and myosteatosis, and reported its diagnostic cutoff value. However, this result needs further validation in different populations. In clinical practice, CCR can be obtained through a simple blood test, without the need for complex imaging or bioelectrical impedance methods, making it more universally applicable.
Diagnosing cachexia in clinical practice is a challenge [21]. In this study, we attempted to use CCR to evaluate cachexia and found that CCR was an effective tool for its diagnosis. When CCR was combined with a weight loss of 2%, its diagnostic ability was significantly improved. Therefore, CCR may become one of the indicators for evaluating cachexia as a substitute for SMI. Meanwhile, the survival curve showed that CCR could effectively stratify patients into high-risk and low-risk groups, evaluating patients' OS and DFS risks, indicating its potential value in clinical prognostic assessment.
Imaging tests have multiple limitations in the routine diagnosis of malnutrition, including the analysis of images obtained in medical records, additional radiation exposure, and high costs [22]. CCR as a serum biomarker, is easily obtained and has a low cost in clinical settings. This study has demonstrated that CCR has the potential to function as a biomarker for diagnosing muscle mass and myosteatosis.This could aid in early disease detection and enable timely interventions, offering the advantages of being non-invasive and cost-effective. In addition, this study constructed a prediction model based on serum indicators and basic patient characteristics, without using difficult-to-obtain indicators. This model serves as a valuable tool for clinicians and nutritionists to conduct risk assessments, facilitating the timely identification of conditions that require early interventions and personalized treatment strategies, ultimately leading to improved patient outcomes. Additionally, our research findings are adaptable and practical for implementation in a variety of healthcare settings, including community hospitals.
However, this study still has several limitations. Firstly, further prospective collection and analysis are needed to investigate the relationship between CCR and other skeletal muscle mass indicators, such as walking speed and grip strength. Secondly, this research did not compare the data with other variables, including functional status, pathological outcomes, or other biomarker assessments, such as the Glasgow prognostic score and specific gut microbiota [23]. Thirdly, the findings are based on data from a single center, necessitating further validation to determine if the results can be generalized to other ethnic groups or patients with different diseases. Lastly, the evaluation effectiveness of the model developed in this study requires additional external validation.
Conclusion
In this study, we established a nomogram to evaluate the risk of sarcopenia and myosteatosis in clinical practice. The model demonstrated high accuracy and can assist clinicians and nutritionists in making assessments. Additionally, we found the potential of CCR in evaluating sarcopenia, myosteatosis, and cachexia. These findings may be helpful for the early diagnosis of muscle and fat depletion and could improve the management and prognosis of patients.
Availability of data and materials
All relevant data and materials have been involved in the article. Further inquiries can be directed to the corresponding authors.
References
Dhillon RJ, Hasni S. Pathogenesis and management of sarcopenia. Clin Geriatr Med. 2017;33(1):17–26.
Petermann-Rocha F, Balntzi V, Gray SR, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022;13(1):86–99.
Ebadi M, Tsien C, Bhanji RA, et al. Myosteatosis in cirrhosis: a review of diagnosis, pathophysiological mechanisms and potential interventions. Cells. 2022;11(7):1216.
Ahn H, Kim DW, Ko Y, et al. Updated systematic review and meta-analysis on diagnostic issues and the prognostic impact of myosteatosis: a new paradigm beyond sarcopenia. Ageing Res Rev. 2021;70:101398.
Cho MR, Lee S, Song SK. A review of sarcopenia pathophysiology, diagnosis, treatment and future direction. J Korean Med Sci. 2022;37(18): e146.
Li CW, Yu K, Shyh-Chang N, et al. Pathogenesis of sarcopenia and the relationship with fat mass: descriptive review. J Cachexia Sarcopenia Muscle. 2022;13(2):781–94.
Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–95.
Lobo DN, Gianotti L, Adiamah A, et al. Perioperative nutrition: recommendations from the ESPEN expert group. Clin Nutr. 2020;39(11):3211–27.
Meza-Valderrama D, Marco E, Davalos-Yerovi V, et al. Sarcopenia, malnutrition, and cachexia: adapting definitions and terminology of nutritional disorders in older people with cancer. Nutrients. 2021;13(3):761.
Albano D, Messina C, Vitale J, et al. Imaging of sarcopenia: old evidence and new insights. Eur Radiol. 2020;30(4):2199–208.
Amini B, Boyle SP, Boutin RD, et al. Approaches to assessment of muscle mass and myosteatosis on computed tomography: a systematic review. J Gerontol A Biol Sci Med Sci. 2019;74(10):1671–8.
Hyun YY, Lee KB, Kim H, et al. Serum creatinine to cystatin C ratio and clinical outcomes in adults with non-dialysis chronic kidney disease. Front Nutr. 2022;9:996674.
Onopiuk A, Tokarzewicz A, Gorodkiewicz E. Cystatin C: a kidney function biomarker. Adv Clin Chem. 2015;68:57–69.
Zhang S, Tan S, Jiang Y, et al. Sarcopenia as a predictor of poor surgical and oncologic outcomes after abdominal surgery for digestive tract cancer: a prospective cohort study. Clin Nutr. 2019;38(6):2881–8.
Jing X, Tan L, Fu H, et al. Associations of ADL disability with trunk muscle mass and muscle quality indicators measured by opportunistic chest computed tomography imaging among older inpatients. Front Med (Lausanne). 2021;8:743698.
Tan L, Ji G, Bao T, et al. Diagnosing sarcopenia and myosteatosis based on chest computed tomography images in healthy Chinese adults. Insights Imaging. 2021;12(1):163.
Hijazi Z, Oldgren J, Lindback J, et al. A biomarker-based risk score to predict death in patients with atrial fibrillation: the ABC (age, biomarkers, clinical history) death risk score. Eur Heart J. 2018;39(6):477–85.
Zhou ZR, Wang WW, Li Y, et al. In-depth mining of clinical data: the construction of clinical prediction model with R. Ann Transl Med. 2019;7(23):796.
Wu J, Zhang H, Li L, et al. A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: a population-based analysis. Cancer Commun (Lond). 2020;40(7):301–12.
Sui S, An X, Xu C, et al. An immune cell infiltration-based immune score model predicts prognosis and chemotherapy effects in breast cancer. Theranostics. 2020;10(26):11938–49.
Baracos VE, Martin L, Korc M, et al. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;4:17105.
Barazzoni R, Jensen GL, Correia M, et al. Guidance for assessment of the muscle mass phenotypic criterion for the global leadership initiative on malnutrition (GLIM) diagnosis of malnutrition. Clin Nutr. 2022;41(6):1425–33.
Agagunduz D, Cocozza E, Cemali O, et al. Understanding the role of the gut microbiome in gastrointestinal cancer: A review. Front Pharmacol. 2023;14:1130562.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization: HL, ST and GW. Data curation: HL and JW. Formal analysis: ZZ. Methodology: HL, ST and ZZ. Validation: GW and MY. Visualization: JW. Supervision: XS, JH and FY. Writing—original draft. HL: writing—review and editing: ST and GW.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethical committee in Zhongshan Hospital, Fudan University.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Logistic regression analysis results of risk factors for sarcopenia.
Additional file 2:
Table S2. Logistic regression analysis results of risk factors for myosteatosis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, H., Wang, J., Tan, S. et al. Sarcopenia and myosteatosis diagnostic tool for gastrointestinal cancer: creatinine to cystatin C ratio as evaluation marker. J Transl Med 21, 744 (2023). https://doi.org/10.1186/s12967-023-04628-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-023-04628-z