Abstract
Objective
Triglyceride glucose index (TyG index) has been recommended as an alternative indicator of insulin resistance. However, the association between TyG and regression from prediabetes to normoglycemia remains to be elucidated.
Methods
This retrospective cohort study involved 25,248 subjects with prediabetes at baseline conducted from 2010 to 2016. A Cox proportional hazard regression model was designed to evaluate the role of TyG in identifying people at converting from prediabetes to normoglycemia. Cox proportional hazards regression with cubic spline functions and smooth curve fitting was used to dig out the nonlinear relationship between them. Detailed evaluations for TyG were also performed using sensitivity and subgroup analyse.
Results
Among the included prediabetes subjects (n = 25,248), the mean age was 49.27 ± 13.84 years old, and 16,701 (66.15%) were male. The mean TyG was 8.83 ± 0.60. The median follow-up time was 2.96 ± 0.90 years. 11,499 (45.54%) individuals had a final diagnosis of normoglycemia.
After adjusting for covariates, TyG was negatively affecting the results of glucose status conversion in prediabetes people (HR 0.895, 95% CI 0.863, 0.928). There was a nonlinear connection between TyG and normoglycemia in prediabetes people, and the inflection point was 8.88. The effect sizes (HR) on the left and right sides of the inflection point were 0.99 (0.93, 1.05) and 0.79 (0.74, 0.85), respectively. Sensitivity analysis confirmed the robustness of these results. Subgroup analysis showed that TyG was more strongly associated with incident glucose status conversion in male, BMI ≥ 25. In contrast, there was a weaker relationship in those with female, BMI < 25.
Conclusion
Based on sample of subjects evaluated between 2010 and 2016, TyG index appears to be a promising marker for predicting normoglycemic conversion among prediabetes people in China. This study demonstrates a negative and non-linear association between TyG and glucose status conversion from prediabetes to normoglycemia. TyG is strongly related to glucose status conversion when TyG is above 8.88. From a therapeutic point of view, it is meaningful to maintain TyG levels within the inflection point to 8.88.
Similar content being viewed by others
Background
Diabetes is one of the most serious and common chronic diseases worldwide, which causes life threatening and disabling complications, and contributes to health burden especially in a developing country like China. It is deduced that 537 million people come down with diabetes in 2021, and this number will reach 643 million at the end of 2030, and 783 million at the end of 2045. In addition, 541 million people are deduced to have impaired glucose tolerance in 2021. It is also deduced that more than 6.7 million people aged 20–79 will die from diabetes-related causes in 2021 [1]. While prediabetes is a high-risk state for diabetes development. Prediabetes comprise two characteristics of impaired fasting glucose (IFG) and/or impaired glucose tolerance. The definition of IFG according to World Health Organization (WHO) using fasting plasma glucose (FPG) is in the range of 6.1–6.9 mmol/L, while according to the American Diabetes Association (ADA) FPG is in the range of 5.6–6.9 mmol/L with a lower threshold [2, 3]. Two recent studies have revealed that the prevalence of prediabetes is 35.2–35.7% [4, 5] in Chinese population with the ADA standard, which is greater than that with the WHO standard. Without any health intervention, approximately 30% of people with prediabetes will experience diabetes within 5 years [6]. Compared to those with normoglycemic people, even the progression of diabetes is prevented or delayed, both microvascular and macrovascular disease appears to be more prevalent in people with prediabetes [7]. Therefore, it is convincing that true prevention of diabetes and its complications is put down to the reversal of prediabetes and the restoration of normoglycemic regulation.
In a recent systematic review [8] which included 47 studies on prediabetes reversion, showed that glucose-regression rates in prediabetes were between 33 and 59% at 1–5 years of follow-up, and between 17 and 42% at 6–11 years of follow-up. In another study it showed that reversion rates ranged from 21 to 68% (Intervention and control/placebo arms) over a median follow up period of 2.7 years with or without interventions which includes medications, dietary or physical activity/exercise improvements, dietary supplements (vitamin D, magnesium, and Larginine), and Chinese medicine[9]. Recent literatures showed that female, as well as those with higher education were more likely to have normoglycemia reversion. While people with BMI ≥ 25 kg/m2, abdominal obesity, heavy drinking, hypertension, dyslipidemia, a family history of diabetes, higher baseline FPG, and higher baseline HbA1c lowered the probability of normoglycemia reversion [10,11,12]. Lifestyle modification and medications were also considered to be the modifiable factors of normoglycemia reversion with prediabetes status [13, 14].
Insulin resistance (IR) is a key feature of prediabetes and a precursor to the progression of type 2 diabetes mellitus (T2DM) [15], so a simple and effective method to assess IR status is important for implementing prevention and management strategies. The euglycemic-hyperinsulinemic clamp (EHC) has become the gold standard for direct assessing and identifying β cell sensitivity to insulin response. However, the EHC is invasive, time-consuming, expensive, and requires frequent blood drawing. The homeostatic model assessment for IR (HOMA-IR) and some other fasting insulin-based indexes are also inappropriate for screening IR in most health facilities because of their time-consuming nature, cost and requirement of skilled manpower. So indexes using routine physical examination indicators instead of insulin-based indicators have been supposed to be superior as alternatives for insulin measurements. Recently, triglyceride glucose index (TyG Index) has been identified as an novel and early indicator of IR [16] and can be easily applied in health facilities. Evidence showed that progression from prediabetes to diabetes was associated with severe deterioration of β-cell dysfunction. Improving insulin sensitivity and β-cell function has revealed to be beneficial for prediabetes stabilization and reversion from prediabetes to normoglycemia [17]. For TyG index, it is a compound index of fasting blood glucose and triglyceride, as a surrogate estimate of insulin sensitivity and β-cell function that could be applied constantly in large-scale observational and/or interventional cohorts [18], we hypothesized that TyG might also predict the reversion to normoglycemia from prediabetes. However, by consulting previous literature, the link between TyG and reversion to normoglycemia has not yet been widely explored in the prediabetes population.
To our best knowledge, there was only one study [19] has been conducted to assess the association between TyG and glucose status conversion among reproductive-aged women in Indonesia with 5 years of follow-up time and it only included reproductive-aged women in prediabetes state, which maybe limited when apply to the Chinese prediabetes population. Simultaneously, the effect of the intervention on TyG on the prevalence of glucose status conversion from prediabetes to normoglycemia also needs to be further explored. Hence we conducted a cohort study to investigate whether the TyG is independently associated with glucose status conversion in Chinese prediabetes people.
Materials and methods
Study design
This was a retrospective cohort study using records from a computerized database established by Chinese researchers (Chen et al.). The target independent variable was TyG at baseline. The outcome variable was normoglycemia (dichotomous variable: 0 = non-normoglycemia (pre-diabetes or diabetes or self-report diabetes), 1 = normoglycemia).
Data source
The raw data was downloaded freely from the DATADRYAD database (www.datadryad.org) provided by Chen et al. [20]. Data was from a published article: Association of body mass index and age with incident diabetes in Chinese adults: a population-based cohort study. This is an open-access article which is given in accordance with the Creative Commons Attribution Non-Commercial (CC BY-NC 4.0) license and enables people to share, remix, modify, and create a derivative work from this work for non-commercial purposes as long as the author and source are credited [20].
Study population
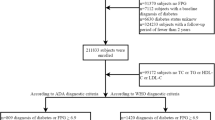
The source data were extracted from a computerised database established by the Rich Healthcare Group in China, which includes all medical records for participants who received a health check from 2010 to 2016. The present analysis included all study participants ≥ 20 years old and ≥ 2 visits between 2010 and 2016 (n = 685277). Exclusion criteria was as follows: (1) No available weight and height measurements (n = 103946), No available information on gender (n = 1), extreme BMI values (< 15 kg/m2 or > 55 kg/m2) (n = 152), or no available fasting plasma glucose value (n = 31370) at baseline; (2) Participants diagnosed with diabetes at baseline (2997 participants diagnosed by self-report, 4115 diagnosed by a FPG ≥ 7.0 mmol/L); (3)With visit intervals < 2 years (n = 324233); (4)With undefined diabetes status at follow-up (n = 6630). At last, the original study included a total of 211833 participants.
A total of 26,018 participants with baseline FPG of 5.6–6.9 mmol/l were enrolled in our study. Pre-diabetes is defined as an FPG level of 5.6–6.9 mmol/L according to the ADA criteria. After excluding participants due to TyG value missing (n = 618) and participants due to abnormal or extreme value of TyG (n = 152), In the end, 25248 eligible participants were left for data analysis (see flowchart for details in Fig. 1).
The original study was initially approved by the Rich Healthcare Group Review Board. The data was retrieved retroactively and the institutional ethics committee did not ask for informed consent or approval for the retrospective study.
Variables
Triglyceride glucose index (TyG index)
TyG Index is calculated by the following formula [21]. TyG Index = Ln (fasting glucose (mg/dL) × triglycerides (mg/dL)/2).
Outcome measures
The interesting outcome variable in our investigation was glucose status conversion from prediabetes to normoglycemia (dichotomous variable: 0 = non-normoglycemia, 1 = normoglycemia). FPG < 5.6 mmol/L without self-reported diabetes was defined as incident normoglycemia.
Covariates
We select the covariates according to our clinical experience and the previous literature. The covariates were as follows: (1) continuous variables: age, body mass index(BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipid cholesterol (LDL-C), alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen(BUN), serum creatinine (Scr), fasting plasma glucose (FPG). (2) categorical variables: sex, smoking status, drinking status, family history of diabetes.
Information on demographic characteristics (age, gender), living habit (cigarette smoking and alcohol consumption levels), medical history and family history of chronic disease diseases were collected through the detailed questionnaire in each visit to the health check center. Height, weight and blood pressure were measured by trained staff. BMI was calculated as weight in kilograms divided by height in meters square (kg/m2). Standard mercury sphygmomanometers were applied to measure blood pressure. Total cholesterol (TC), serum triglyceride (TG), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) were measured on an autoanalyzer (Beckman 5800). Fasting venous blood samples were collected after at least a 10 h fast at each visit. Plasma glucose levels were measured by the glucose oxidase method on an autoanalyzer (Beckman 5800).
Statistical analysis
Participants were stratified by TyG quartiles. Quantitative variables with normal distribution were expressed as the mean ± standard deviation, and variables with skewed distribution were expressed as the median (quartile), and categorical variables were expressed as the frequency (percentage). We used the One-Way ANOVA test (normal distribution), the χ2 (categorical variables), or the Kruskal–Wallis H test (skewed distribution) to test for differences among different TyG quartile groups.
To assess the impact of modifiable risk factors in blood glucose changes, we used univariate and multivariate Cox proportional risk regression models to construct three models, including unadjusted model (crude model: unadjusted covariate) and minimum adjusted model (Model I: only adjusted sociodemographic variables, Including age, sex, BMI, systolic blood pressure, diastolic blood pressure) and fully adjusted models (Model II: adjusted covariates including age, sex, BMI, systolic blood pressure, diastolic blood pressure, ALT, AST, BUN, LDL-C, HDL-c, Scr, family history of diabetes, drinking status, and smoking status).
Moreover, sensitivity analysis was conducted to test the robustness of our results by deleting participants with BMI ≥ 25 kg/m2, or deleting participants who had consumed alcohol, or deleting paticipants who had smoked, or deleting participants with a family history of diabetes, in order to evaluate the quality and consistency of the results. Besides, a generalized additive model (GAM) was used to insert the continuity covariate into the equation (model III) in curvy form to ensure the robustness of the results.
In order to investigate the nonlinear relationship between TyG and glucose state transition, Cox proportional hazards regression model with cubic spline functions and the smooth curve fitting was used to state the non-linear relationship. Besides, the two-piecewise Cox proportional-hazards regression model was used to further elaborate the non-linearity between TyG and glucose status conversion.
The subgroup analyses were conducted using a stratified Cox proportional-hazards regression model across various subgroups (sex, age, SBP, DBP, BMI). Continuous variables as age (< 30, ≥ 30 to < 40, ≥ 40 to < 50, ≥ 50 to < 60, ≥ 60 to < 70, ≥ 70 years), BMI (< 25, ≥ 25 kg/m2), SBP (< 140, ≥ 140 mmHg), DBP (< 90, ≥ 90 mmHg) [22,23,24] were transformed to a categorical variable based on the clinical cut point. Then we adjusted each stratification for all factors (Models were adjusted for age, sex, BMI, AST, ALT, SBP, DBP, HDL-c, LDL-C, BUN, Scr, smoking status, drinking status, and family history of diabetes, but not adjusted for stratification variables in each model). At last, tests for interaction were conducted with and without interaction terms.
The number of participants with missing data of BUN, Scr, SBP, DBP, HDL-C, LDL-C, AST, ALT, Drinking status and Smoking status were 7 (0.03%), 7 (0.03%), 9907 (39.24%), 9280 (36.76%), 210 (0.83%), 14098 (55.84%), 2435 (9.64%), 1119 (4.43%), 16710 (66.22%), 16710 (66.22%), respectively. Missing data of covariants was handled by multiple imputations method. The imputation model included age, sex, BMI, SBP, TC, HDL-C, LDL-C, ALT, AST, BUN, Scr, Drinking status, Smoking status, Family history of diabetes. Missing data analysis procedures use missing-at-random (MAR) assumptions.
All results were written according to the STROBE statement [25]. Both R statistical software packages (http://www.r-project.org, The R Foundation), SPSS 27.0 (SPSS Inc., Chicago, IL, USA) and Empower Stats (X&Y Solutions, Inc., Boston, MA, http://www.empowerstats.com) were used to conduct all analyses. Statistical significance was defined as P-values less than 0.05 (two-sided).
Results
Baseline characteristics of participants
The baseline characteristics of study participants were listed in Table 1. The mean age was 49.27 ± 13.84 years old, and 16,701 (66.15%) were male. The mean TyG was 8.83 ± 0.60. During a median follow-up time of 2.96 ± 0.90 years, 11,499 (45.54%) individuals had experienced a final diagnosis of normoglycemia. Participants were devided into subgroups according to TyG quartiles (< 8.41, ≥ 8.41 to < 8.81, ≥ 8.81 to < 9.22, ≥ 9.22). Compared with the Q1 (< 8.41) group, age, BMI, SBP, DBP, Cholosterol, LDL-C, ALT, AST, BUN, Scr increased significantly in the Q4 (≥ 9.22) group, while HDL-C decreased in the Q4 (≥ 9.22) group. Besides, Q4 (≥ 9.22) group had a higher proportion of male, current and ever smokers, current and ever drinkers when compared to Q1 (< 8.41) group.
Additional file 1: Fig S1 shows the distribution of TyG for the two groups. The results indicated that the distribution level of TyG was higher in the non-normoglycemia group. On the contrary, the group with normoglycemia had a lower distribution level of the TyG. Figure 2 showed the distribution of TyG levels. It showed a normal distribution within a range from 6.98 to 10.71 (with an average of 8.83).
The incidence rate of glucose status conversion from pre-diabetes to normoglycemia
Table 2 revealed that 11,499 (45.54%) participants had experienced glucose status conversion from pre-diabetes to normoglycemia during a median follow-up time of 2.96 years. The total cumulative incidence rate of all participants was 15.39 per 100 person-years. Respectively, the cumulative incidence of the four TyG quartile groups were 19.91, 16.24, 14.02, and 11.47 per 100 person-years.
The incidence rate (%) of each TyG group was 58.52 (95% CI 57.3–59.7), 47.75 (95% CI 46.5–49.0), 41.51 (95% CI 40.3–42.7), 34.41 (95% CI 33.2–35.6), respectively. Participants within the highest TyG group had lower incidence rates of glucose status conversion compared to the group with the lowest TyG (p < 0.0001 for trend) (Fig. 3).
When stratifying participants by 10 age intervals, female subjects had a higher incidence of normoglycemia than male subjects no matter what age group they were in Fig. 4. It also found that the incidence of normoglycemia decreased with age, both in males and females participants.
Normoglycemia incidence rate of age stratification by 10 intervals. This figure showed that in age stratification by 10 intervals, female subjects had a higher incidence of normoglycemia incidence rate than male subjects no matter what age group they were in. It also found that the incidence of normoglycemia decreased with age, both in males and females participants
The results of univariate analyses using cox proportional-hazards regression model
The univariate analyses showed that incidence of normoglycemia was positively related to female (HR = 1.259, 95% CI 1.212, 1.307), HDL-c (HR = 1.961, 95% CI 1.860, 2.067); and negatively related to Age (HR = 0.977, 95% CI 0.975, 0.978), BMI (HR = 0.937, 95% CI 0.931, 0.942), SBP (HR = 0.990, 95% CI 0.989, 0.991), DBP (HR = 0.985, 95% CI 0.983, 0.987), TC (HR = 0.877, 95% CI 0.860, 0.895), TyG (HR = 0.693, 95% CI 0.672, 0.715), LDL-C (HR = 0.930, 95% CI 0.906, 0.955), ALT (HR = 0.99, 95% CI 0.99, 0.99), AST (HR = 0.99, 95%CI 0.98, 0.99), BUN (HR = 0.957, 95% CI 0.943, 0.972), Scr (HR = 0.997, 95% CI 0.996, 0.998), family history of diabetes (HR = 0.767,95% CI 0.677, 0.868), history of smoking (HR = 0.873, 95% CI 0.838, 0.909) or drinking (HR = 0.858, 95% CI 0.822, 0.894) (Table 3).
Kaplan–Meier survival curves for normoglycemia-free survival probability stratified by the TyG group were shown in Fig. 5. There were significant differences in the probability of normoglycemia-free survival between the TyG quartile groups (log-rank test, p < 0.0001).The probability of normoglycemia-free survival gradually decreased with decreasing TyG, indicating that the group with the highest TyG had the lowest rate of normaglycemia converting from prediabetes.
Kaplan–Meier survival curve. The probability of normoglycemic conversion survival differed significantly between the TyG quartiles (log-rank test, p < 0.001).The probability of normoglycemia survival gradually decreased with increasing TyG, suggesting that the group with the highest TyG had the lowest probability of normoglycemic conversion
The results of multivariate analyses using cox proportional-hazards regression model
Three Cox proportional-hazards regression models were built to explore the relationship between TyG and incident normaglycemia (Table 4). In the unadjusted model (Crude model), an increase of 1 unit of TyG was connected with a 30.7% decrease in probability of normaglycemia converting from prediabetes. (HR = 0.693, 95% CI 0.672–0.715, P < 0.00001). In Model I, we adjusted for demographic variables (age, sex, BMI, SBP, DBP), each additional unit of TyG decreased by 15.4% in probability of normaglycemia converting from prediabetes. (HR = 0.846, 95% CI 0.817–0.875, P < 0.00001). While in fully adjusted model (Model II), unit of TyG decreased by 15.4% in probability of normaglycemia converting from prediabetes (HR = 0.895, 95% CI 0.863–0.928, P < 0.00001).
In addition, when we set the lowest quartile as a reference, the highest quintile of TyG was distinctly associated with decreased possibility of normaglycemia (Crude model, Q5: OR = 0.557, 95% CI 0.528–0.587, P < 0.001) (Model I, Q5: OR = 0.755, 95% CI 0.712–0.800, P < 0.001) (Model II, Q5: OR = 0.829, 95% CI 0.780–0.882, P < 0.001). The results also showed that the trends consistent with the result when TyG categorical values was continuous variables. (Table 4).
Sensitivity analysis
We used a GAM to insert the continuity covariate into the equation as a curve. The result remained consistent with the fully adjusted model (Table 4) (Model III, HR = 0.918, 95% CI 0.885–0.953, P < 0.00001). Besides, the authors excluded participants with BMI ≥ 25 mmol/L for sensitivity analyses, TyG was also negatively associated with normoglycemia converting possibility after adjusting the confounding factors (Model I, HR = 0.918, 95% CI 0.876–0.961, P = 0.00030) (Table 5).
Participants who had never consumed alcohol were also excluded for sensitivity analyses. The results remained consistent after adjusting the confounding factors (Model II, HR = 0.859, 95% CI 0.802, 0.920, P = 0.00001) (Table 5).
Model III on Table 5 was a sensitivity analysis performed on participants who had never smoked. We still got similar results (Model III, HR = 0.897, 95% CI 0.839–0.959, P = 0.00138).
participants without a family history of diabetes were also performed for sensitivity analyses, TyG also had negative association with normoglycemia converting from prediabetes after adjusting the confounding factors (Model IV, HR = 0.917, 95% CI 0.883–0.951, P < 0.00001) (Table 5).
The results obtained from all of the sensitivity analyses indicated the well-robustness of the relationship between TyG and normoglycemia convert.
The non-linearity addressed by Cox proportional hazards regression model with cubic spline functions
A non-linear relationship was detected between TyG and normoglycemia convert by the Cox proportional hazards regression model with cubic spline functions analyses (Fig. 6). To determine the best fit model using a log-likelihood ratio test, it turns out that the P for the log-likelihood ratio test was < 0.001. By recursive algorithm, we got the inflection point 8.88 (Table 6).
The non-linear relationship between eGFR and the normoglycemic conversion from prediabetes. We used a Cox proportional hazards regression model with cubic spline functions to evaluate the relationship between TyG and recovery of blood glucose incidence. The result showed that the relationship between TyG and recovery of blood glucose incidence was non-linear, with the inflection point of TyG index being 8.88
On the right side of the inflection point by two piecewise Cox proportional-hazards regression model, the HR and 95% CI were 0.79 (0.74, 0.85). That indicates when TyG value is greater than 8.88, the probability of normoglycemia convert events become smaller as TyG value increases.
While on the left side of the inflection point by two piecewise Cox proportional-hazards regression model, the relationship between TyG and normoglycemia convert was not statistically significant (HR = 0.99, 95% CI 0.93–1.05, P > 0.05). (Table 6).
The results of subgroup analyses
Subgroup analyses showed that there were no significant interaction between TyG and incident normoglycemia convert according to strata of age, SBP, DBP (Fig. 7).
Effect size of TyG on normoglycemia in prespecified and exploratory subgroups. The subgroup analyses were conducted using a stratified Cox proportional-hazards regression model across various subgroups. The model above is adjusted for age, sex, BMI, SBP, DBP, ALT, AST, BUN, LDL-C, HDL-c, Scr, family history of diabetes, drinking status, and smoking status, but not adjusted for stratification variables
The results showed that Sex and BMI could modify the relationship between TyG and incident normoglycemia convert (All P for interaction < 0.05). And a stronger association was observed in males (OR = 0.867, 95% CI 0.830–0.906) and the participants with BMI ≥ 25 kg/m2 (OR = 0.846, 95% CI 0.802–0.893), and with lower eGFR (OR = 0.84, 95% CI 0.78–0.89). In contrast, weaker association was observed in females (HR = 0.942, 95% CI 0.886–1.001), participants with BMI < 25 kg/m2 (OR = 0.922, 95% CI 0.882–0.964), and with higner eGFR (OR = 0.93, 95% CI 0.88–0.98) (Fig. 7).
Discussion
The purpose of this study was to investigate the relationship between TyG and blood glucose normalization in prediabetic patients. Our findings indicate a negative correlation between TyG levels and glucose normalization in prediabetic patients. Higher TyG levels were associated with a lower likelihood of glucose-normalizatio during follow-up, whereas lower TyG levels were associated with a greater tendency for glucose-normalization. Furthermore, we observed a non-linear relationship between TyG and glucose-normalization, with an inflection point at a value of 8.88. When TyG was greater than 8.88, the incidence of glucose-normalization decreased as TyG values increased. Additionally, subgroup analysis revealed that BMI and gender acted as influencing modifiers. Specifically, in male patients and patients with a BMI ≥ 25, TyG exhibited a stronger negative correlation with blood glucose normalization. In other words, lower TyG levels were associated with a higher likelihood of blood glucose normalization in those subgroup.
Prediabetes is a high risk factor for progression to diabetes. 5–10% of people with prediabetes developes diabetes each year. Prediabetes is closely related to eyes, kidneys and cardiovascular diseases. Therefore, it is important to pay attention to prediabetes and monitor risk indicators associated with prediabetes. Previous literatures have shown that prediabetes can be reversed to normoglycemia. In an Indian study, 32 (26.67%) of 120 patients with prediabetes had normalized blood glucose after 32 months of follow-up [26]. In a Swedish study of 918 elderly patients (age 60 years or older) with prediabetes, 204 (22%) reverted to normoglycemia, 119 (13%) developed diabetes, and 215 (23%) died after 12 years of follow-up. The incidence of blood glucose normalization, progression to diabetes, and death was higher during the first 6 years of follow-up than during the second 6 years of follow-up [27].In addition, it has reported that the normalization rate of blood glucose in middle-aged people is 23–30% [28]. In our study, after a median follow-up time of 2.96 years, 11,499 (45.54%) prediabetic patients were finally diagnosed with normalized blood glucose, which is higher than the conversion rate of 23–30% reported in the literature above. The observed variation in the relationship between TyG and blood glucose normalization in prediabetic patients might be attributed to several factors, including differences in ethnic groups, age groups, definitions of prediabetes, as well as different lifestyle and medications. Lifestyle interventions and medications have been reported to reduce the risk of progression to diabetes and increase the incidence of blood glucose normalization in patients with prediabetes. Therefore, in a certain range, the incidence of conversion of blood glucose normalization in prediabetes patients is affected by different lifestyle and drug therapies. However, further research is needed to confirm the main contributing factors in this regard.
In a cohort study based on a Chinese population, TyG was strongly associated with an increased risk of diabetes, with a HR of 3.34 (95% CI 3.11–3.60) for diabetes [29]. Another Korean study found that TyG could be used as a predictor of type 2 diabetes in non-obese adults. This study divided the population into four groups according to the TyG4 quantiles and when compared with group Q1 (HR = 1.0), Q2 (HR = 1.63, 95% CI 1.18 2.24), Q3 (HR = 2.3, 95% CI 1.68 3.14) and Q4 group (HR = 3.67, 95% CI 2.71 4.98) had higher rates of progressing to diabetes [30]. Studies have also found that increased TyG level is closely related to the risk of prediabetes, and the risk of progressing to prediabetes increased by 38% for every 1 SD increase of TyG (HR = 1.38, 95% CI 1.28–1.48) [31]. It was also found that TyG-BMI was an independent predictor of new-onset diabetes (HR 1.50 per SD increase, 95% CI 1.40–1.60, P-trend < 0.00001) [32]. In the Indonesia study, TyG index appears to be a promising marker for glucose status conversion among reproductive-aged women [19]. We have the similar results with the previous literatures. In our study, it was found that TyG was negatively correlated with the normalization of blood glucose, and there was a nonlinear correlation between TyG and conversion to normoglycemia in prediabetes people, with an infection point of 8.88. At the same time, sensitivity analysis was also conducted in our study. It was found that TyG was negatively correlated with blood glucose normalization regardless of family history of diabetes, smoking history, alcohol consuming history and obesity (BMI ≥ 25). The results of sensitivity analysis indicated the robustness between TyG and conversion to normoglycemia in prediabetes people. It is important to highlight that this study primarily investigated the impact of TyG on the reversal to normoglycemia in individuals with prediabetes. The findings of this study have clinical significance as they provide insights into early interventions for prediabetic individuals, facilitating their transition to normoglycemia and potentially preventing rapid progression to diabetes.
However, neither literature studying the effects on TyG predicting the conversion from prediabetes to normal blood glucose, nor the literature reporting the relationship between TyG and conversion from prediabetes to noroglycemia in Chinese people. The mechanism by which TyG predicts normoglycemia is uncertain, but it is known that IR plays an important role in the conversion from prediabetes to normal blood glucose. We speculated that the relationship between TyG and blood glucose reversal was mediated by IR. First, TyG has a strong ability to predict IR. In Mexican population, compared with hyperinsulinemic-euglycemic clamp, which is the gold standard of IR measurement, the sensitivity of TyG to predict IR is as high as 96.5% [21]. IR is the pathophysiological basis of abnormal blood glucose, which can explain why TyG has a strong predictive ability for prediabetes. TyG is a compound index of triglyceride and fasting blood glucose. Triglyceride can be degraded into glycerol and fatty acids, while the increased free fatty acids can be transferred from adipose tissue to non-adipose tissue, thus leading to insulin resistance. Hypertriglyceridemia leads to transport of high free fatty acids to the liver leading to high glucose output in the liver, that is, free fatty acids can increase gluconeogenesis in the liver [33], and high levels of triglycerides in the liver and muscle can interfere with glucose metabolism in each target organ. In addition, glucotoxicity and lipotoxicity can lead to increased production of reactive oxygen species, resulting in oxidative stress and β cell dysfunction. A reduction in the antioxidant defense of pancreatic beta cells can ultimately lead to IR. Long-term exposure to high concentrations of free fatty acids and long-term exposure to total cholesterol can lead to impaired beta cell function and reduced glucose-induced insulin secretion [34,35,36].
Subgroup analysis suggested that TyG was more strongly associated with glucose normalization in male patients (OR = 0.867, 95% CI 0.830–0.906). In female patients, the relationship was weaker (OR = 0.942, 95% CI 0.886, 1.001). Previous literature has reported that compared with women, men have a higher risk of developing type 2 diabetes, which is speculated to be due to higher insulin resistance in men [37]. That means women need to gain more weight and fat tissue to develop insulin resistance and then progress to type 2 diabetes. In addition, the distribution and quality of fat varies by gender, and in general, men have more visceral fat and liver fat than women. In men, ectopic fat accumulates in different organs including the liver, which determines the increase of liver enzymes and triglycerides, causing the organ to develop insulin resistance [38]. Toxic lipid derivatives (ceramides or diglycerides) or other molecules are involved in this process and regulate insulin pathway. Besides, HDL-C levels are generally lower in men than in women (accompanied by higher fasting glucose levels) [38]. Obesity and insulin resistance are important mediators in the development of type 2 diabetes in male, and also affect the outcome of blood glucose normalization. TyG is a complex index of blood glucose and lipid, and multiple studies have confirmed that TyG is closely related to IR, and TyG can reflect the function of IR and pancreatic β cells. Men have higher insulin resistance, and insulin resistance is highly correlated with TyG, so the reversion status of abnormal glycemia blood glucose can be better reflected by TyG in male rather than female.
By subgroup analysis, we also found that when BMI ≥ 25, TyG had a stronger relationship with blood glucose normalization (OR = 0.846, 95% CI 0.802, 0.893). When BMI < 25, the relationship was weaker (OR = 0.922, 95% CI 0.882, 0.964). BMI is an indicator of obesity. Higher visceral fat might enter and accumulate in the liver via the portal system, which increases liver TG levels, reduces insulin reabsorption, causes metabolic disturbances, and increases the risk of IR, thus finally leads to obesity. Excess visceral fat, in turn, promotes inflammation in the body by increasing certain cytokines. Meanwhile, macrophages in visceral adipose tissue secrete cytokines, including IL-6 and TNF-α, which can stimulate chronic inflammatory response, followed by the production of IR [39]. Whether prediabetes could reverse to normoglycemia is closely related to IR degree, while BMI is closely related to obesity, chronic inflammatory reaction and IR. Therefore, TyG can better reflect the outcome of reversion of normoglycemia in patients with higher BMI.
More importantly, our study is the first study to identify a non-linear relationship between TyG and reversion to normoglycemia in patients with prediabetes. After controlling a series of confounding factors, we found that the infection point value of TyG in predicting blood glucose normalization was 8.88. When TyG > 8.88, the possibility of reversion to normoglycemia was reduced by 21% for each additional unit of TyG. When TyG < 8.88, the increase or decrease of TyG had no statistical difference in the occurrence of reversion to normoglycemia with prediabetes. Compared with those with TyG < 8.88, those with TyG > 8.88 were older, had a greater proportion of BMI, SBP, DBP, LDL-c, ALT, AST, BUN, Scr, smoking history, drinking history, and had a lower proportion of HDL-c.
In our study, decreased TyG is significantly correlated with higher nomoglycemic conversion rate with prediabetes in a certain range. But when TyG < 8.88, the risk factors had no statistical influence on the outcome of reversion. Since TyG is a compound index of triglyceride and fasting blood glucose, we speculate that decreased FBG and/or decreased triglyceride might also increase the risk of IR and thus counteract the benefits. In fact, IR has been described in conditions not related to obesity or diabetes, such as starvation, malnutrition, chronic renal failure, where insulin levels may be normal or low. IR is also seen in conditions where body fat is often low, including congenital lipodystrophies, anorexia nervosa, and chronic stress conditions [40].
A previous study showed that in 35 healthy, sedentary, lean, collegeage subjects, 2.3% were IR by HOMA-IR > 2.1, 5.7% by ISI Matsuda < 5.9, and 22.9% had > one criteria for metabolic syndrome (MetS); 28.6% had some negative metabolic biomarker. Low body fat and hypoglycemia have been found to counteract the metabolic action of insulin and are considered to be a factor contributing to the development of IR and associated metabolic abnormalities [41]. In short, when TyG < 8.88, low TyG might increase the risk of IR, hence we could not discover significantly relationship between TyG and nomoglycemia conversion among prediabetes people. The nonlinear relationship between TyG and blood glucose normalization in patients with prediabetes had great clinical significance. It promotes clinical consultation and optimization of decision-making in the prevention of prediabetes/diabetes, and its inflection point provides clinicians with the prognosis of patients with prediabetes. From the perspective of treatment, controlling blood glucose and triglyceride levels through medications or lifestyle interventions to maintain TyG around 8.88 may effectively reduce the risk of patients progressing from prediabetes to diabetes.
The advantages of our study are as follows: (1) Our study is a large sample size study. (2) For the first time, our study described the relationship between TyG and the reversion to normoglycemia from prediabetes in Chinese people, and found the inflection point of TyG (TyG inflection point 8.88) through non-linear correlation statistic analysis, which provided prognostic prediction for screening prediabetes patients as well as provided strong data support for reducing the occurrence of prediabetes/diabetes. (3) Sensitivity analysis was used to evaluate the reliability and stability of our results. (4) We used subgroup analysis and found a stronger association between TyG and glycemic normalization outcomes in specific populations (males, BMI ≥ 25).
However, there are some shortcomings in our study: (1) Prediabetes includes two characteristics of impaired fasting glucose (IFG) and/or impaired glucose tolerance, but only fasting glucose data can be obtained in our database. In this study, we defined prediabetes by impaired fasting glucose, so we failed to identify those with impaired glucose tolerance, which may underestimate the prediabetes population at baseline. Despite its shortcomings, fasting glucose screening for prediabetes remains the most commonly used method because it is safe, convenient, inexpensive, and quick. (2) As all the other observational studies, although known potential confounders such as BMI, SBP, and age were controlled, there were still uncontrolled or unmeasured confounders. (3) The patient's medication regimen (hypoglycemic drugs, lipid-lowering drugs, uric-lowering drugs, antihypertensive drugs, etc. was not included in the follow-up, and the lifestyle which includes diet and exercise would also make influence on the outcome. In short, we still could not judge the effects on the reversion of normoglycemia with drugs and lifestyle. (4) There are some limiting conditions applying TyG to practice in all populations due to the Chinese population trending to have lower BMI, and in our study, obese people were excluded, which is an essential factor in developing insulin resistance and lipid disbalance. So the trend between TyG and glucose normalization or the threshold value of TyG might be changed when more obese people are included in the study. (5) Further studies are needed to improve the diagnosis and prediction of diabetes development in other populations in other countries where it is easy and chip the quantification of glucose and triglycerides instead of an insulin resistance test.
Conclusion
The incidenct rate of prediabetes progressing to T2DM is high. T2DM is associated with multiple complications, increasing the risk of cardiovascular events and all-cause mortality. However, the abnormal blood glucose in prediabetic people may be reversed, so early identification and intervention of high-risk prediabetic people may delay or even reverse the abnormal glycemia status. Our study showed that TyG index was a good and simple index to predict the blood glucose outcome of patients with prediabetes, so clinicians could consider widely applying TyG index to the clinical expected reversal of blood glucose normalization. When TyG > 8.88, patients with prediabetes are less likely to get reversion to normoglycemia. Therefore, instead of maintaining pre-diabetes or progressing to diabetes, people with prediabetes might benefit from limiting the TyG value to around 8.88.
Availability of data and materials
Data can be downloaded from the ‘DATADRYAD’ database (www.Datadryad.org). The original contributions presented in the study are included in the article/Additional file, further inquiries can be directed to the corresponding author.
Abbreviations
- FPG:
-
Fasting plasma glucose
- BMI:
-
Body mass index
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- TyG:
-
The triglyceride-glucose index
- BUN:
-
Blood urea nitrogen
- LDL-c:
-
Low-density lipid cholesterol
- Scr:
-
Serum creatinine
- HDL-c:
-
High-density lipoprotein cholesterol
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- IFG:
-
Impaired fasting glucose
- EHC:
-
The euglycemic-hyperinsulinemic clamp
- T2DM:
-
Type 2 diabetes mellitus
- HbA1c:
-
Hemoglobin A1c
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
- GAM:
-
Generalized additive model
- WHO:
-
World Health Organization
- ADA:
-
The American Diabetes Association
- IFG:
-
Impaired fasting glucose
- FPG:
-
Fasting plasma glucose
- IR:
-
Insulin resistance
- SD:
-
Standard deviation
- MAR:
-
Missing-at-random
References
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119.
Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–53.
Classification and Diagnosis of Diabetes. Standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S13-s27.
Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, Li Y, Zhao Z, Qin X, Jin D, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317(24):2515–23.
Li Y, Teng D, Shi X, Qin G, Qin Y, Quan H, Shi B, Sun H, Ba J, Chen B, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American diabetes association: national cross sectional study. BMJ. 2020;369:m997.
Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–50.
Cai X, Zhang Y, Li M, Wu JH, Mai L, Li J, Yang Y, Hu Y, Huang Y. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ. 2020;370:m2297.
Richter B, Hemmingsen B, Metzendorf MI, Takwoingi Y. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst Rev. 2018;10(10):012661.
Galaviz KI, Weber MB, Suvada KB, Gujral UP, Wei J, Merchant R, Dharanendra S, Haw JS, Narayan KMV, Ali MK. Interventions for reversing prediabetes: a systematic review and meta-analysis. Am J Prev Med. 2022;62(4):614–25.
Li N, Lu C, Ma Y, Wang X, Ling Y, Yin Y, Li S, Huang J, Yu L, Dong W, et al. Factors associated with progression of different prediabetic status to diabetes: a community-based cohort study. Diabetes Res Clin Pract. 2022;184:109193.
Jung JY, Oh CM, Ryoo JH, Choi JM, Choi YJ, Ham WT, Park SK. The influence of prehypertension, hypertension, and glycated hemoglobin on the development of type 2 diabetes mellitus in prediabetes: the Korean genome and epidemiology study (KoGES). Endocrine. 2018;59(3):593–601.
Nabila S, Kim JE, Choi J, Park J, Shin A, Lee SA, Lee JK, Kang D, Choi JY. Associations between modifiable risk factors and changes in glycemic status among individuals with prediabetes. Diabetes Care. 2023;46(3):535–43.
Cao Z, Li W, Wen CP, Li S, Chen C, Jia Q, Li W, Zhang W, Tu H, Wu X. Risk of death associated with reversion from prediabetes to normoglycemia and the role of modifiable risk factors. JAMA Netw Open. 2023;6(3):e234989.
Luo Y, Paul SK, Zhou X, Chang C, Chen W, Guo X, Yang J, Ji L, Wang H. Rationale, design, and baseline characteristics of Beijing prediabetes reversion program: a randomized controlled clinical trial to evaluate the efficacy of lifestyle intervention and/or pioglitazone in reversion to normal glucose tolerance in prediabetes. J Diabetes Res. 2017;2017:7602408.
Tsimihodimos V, Gonzalez-Villalpando C, Meigs JB, Ferrannini E. Hypertension and diabetes mellitus: coprediction and time trajectories. Hypertension. 2018;71(3):422–8.
Darshan An V, Rajput R, Meena Mohini, Garg R, Saini S. Comparison of triglyceride glucose index and HbA1C as a marker of prediabetes—a preliminary study. Diabetes Metab Syndr. 2022;16(9):102605.
Snehalatha C, Mary S, Selvam S, Sathish Kumar CK, Shetty SB, Nanditha A, Ramachandran A. Changes in insulin secretion and insulin sensitivity in relation to the glycemic outcomes in subjects with impaired glucose tolerance in the Indian Diabetes Prevention Programme-1 (IDPP-1). Diabetes Care. 2009;32(10):1796–801.
Mohd Nor NS, Lee S, Bacha F, Tfayli H, Arslanian S. Triglyceride glucose index as a surrogate measure of insulin sensitivity in obese adolescents with normoglycemia, prediabetes, and type 2 diabetes mellitus: comparison with the hyperinsulinemic-euglycemic clamp. Pediatr Diabetes. 2016;17(6):458–65.
Liberty IA, Kodim N, Sartika RAD, Trihandini I, Tjekyan RMS, Zulkarnain Pane M, Pratisthita LB, Tahapary DL, Soewondo P. Triglyceride/glucose index (TyG Index) as a marker of glucose status conversion among reproductive-aged women in Jakarta, Indonesia: the bogor cohort study (2011–2016). Diabetes Metab Syndr. 2021;15(6):102280.
Chen Y, Zhang XP, Yuan J, Cai B, Wang XL, Wu XL, Zhang YH, Zhang XY, Yin T, Zhu XH, et al. Association of body mass index and age with incident diabetes in Chinese adults: a population-based cohort study. BMJ Open. 2018;8(9):e021768.
Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, Jacques-Camarena O, Rodríguez-Morán M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–51.
Bhaskaran K, Dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol. 2018;6(12):944–53.
Tsimploulis A, Sheriff HM, Lam PH, Dooley DJ, Anker MS, Papademetriou V, Fletcher RD, Faselis C, Fonarow GC, Deedwania P, et al. Systolic-diastolic hypertension versus isolated systolic hypertension and incident heart failure in older adults: Insights from the cardiovascular health study. Int J Cardiol. 2017;235:11–6.
Cen J, Han Y, Liu Y, Hu H. Evaluated glomerular filtration rate is associated with non-alcoholic fatty liver disease: a 5-year longitudinal cohort study in Chinese non-obese people. Front Nutr. 2022;9:916704.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–9.
Dutta D, Mondal SA, Kumar M, Hasanoor Reza AH, Biswas D, Singh P, Chakrabarti S, Mukhopadhyay S. Serum fetuin-A concentration predicts glycaemic outcomes in people with prediabetes: a prospective study from eastern India. Diabet Med. 2014;31(12):1594–9.
Shang Y, Marseglia A, Fratiglioni L, Welmer AK, Wang R, Wang HX, Xu W. Natural history of prediabetes in older adults from a population-based longitudinal study. J Intern Med. 2019;286(3):326–40.
Song X, Qiu M, Zhang X, Wang H, Tong W, Ju L, Gu L, Sun S, Zhang H, Wang W, et al. Gender-related affecting factors of prediabetes on its 10-year outcome. BMJ Open Diabetes Res Care. 2016;4(1):e000169.
Li X, Li G, Cheng T, Liu J, Song G, Ma H. Association between triglyceride-glucose index and risk of incident diabetes: a secondary analysis based on a Chinese cohort study : TyG index and incident diabetes. Lipids Health Dis. 2020;19(1):236.
Park B, Lee HS, Lee YJ. Triglyceride glucose (TyG) index as a predictor of incident type 2 diabetes among nonobese adults: a 12-year longitudinal study of the Korean genome and epidemiology study cohort. Trans Res. 2021;228:42–51.
Wen J, Wang A, Liu G, Wang M, Zuo Y, Li W, Zhai Q, Mu Y, Gaisano HY, He Y, et al. Elevated triglyceride-glucose (TyG) index predicts incidence of prediabetes: a prospective cohort study in China. Lipids Health Dis. 2020;19(1):226.
Wang X, Liu J, Cheng Z, Zhong Y, Chen X, Song W. Triglyceride glucose-body mass index and the risk of diabetes: a general population-based cohort study. Lipids Health Dis. 2021;20(1):99.
Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Investig. 2016;126(1):12–22.
Lytrivi M, Castell AL, Poitout V, Cnop M. Recent insights into mechanisms of β-cell Lipo- and glucolipotoxicity in type 2 diabetes. J Mol Biol. 2020;432(5):1514–34.
Sasson S. Nutrient overload, lipid peroxidation and pancreatic beta cell function. Free Radical Biol Med. 2017;111:102–9.
Chen Z, Wen J. Elevated triglyceride-glucose (TyG) index predicts impaired islet β-cell function: a hospital-based cross-sectional study. Front Endocrinol. 2022;13:973655.
Paul S, Thomas G, Majeed A, Khunti K, Klein K. Women develop type 2 diabetes at a higher body mass index than men. Diabetologia. 2012;55(5):1556–7.
Sattar N. Gender aspects in type 2 diabetes mellitus and cardiometabolic risk. Best Pract Res Clin Endocrinol Metab. 2013;27(4):501–7.
Sung HH, Park CE, Gi MY, Cha JA, Moon AE, Kang JK, Seong JM, Lee JH, Yoon H. The association of the visceral adiposity index with insulin resistance and beta-cell function in Korean adults with and without type 2 diabetes mellitus. Endocr J. 2020;67(6):613–21.
Branth S, Ronquist G, Stridsberg M, Hambraeus L, Kindgren E, Olsson R, Carlander D, Arnetz B. Development of abdominal fat and incipient metabolic syndrome in young healthy men exposed to long-term stress. Nutr Metab Cardiovasc Dis. 2007;17(6):427–35.
Ghosh A, Gao L, Thakur A, Siu PM, Lai CWK. Role of free fatty acids in endothelial dysfunction. J Biomed Sci. 2017;24(1):50.
Acknowledgements
As this is a secondary analysis, the data and method description are mainly derived from the following research: Chen Y, Zhang XP, Yuan J, Cai B, Wang XL, Wu XL, Zhang YH, Zhang XY, Yin T, Zhu XH, Gu YJ, Cui SW, Lu ZQ, Li XY. Association of body mass index and age with incident diabetes in Chinese adults: a population-based cohort study. BMJ Open. 2018 Sep 28;8 (9): e021768. https://doi.org/10.1136/bmjopen-2018-021768. PMID: 30269064; PMCID: PMC6169758. We are grateful to all the authors of the study.
Funding
This work was supported by the grants from National Natural Science Foundation of China (NO. 81270816, NO. 81470974 & 82170731 to W.J.W), High-level Hospital Construction Project of Guangdong Province (DFJH201908 to W.J.W).
Author information
Authors and Affiliations
Contributions
XJ Chen conceived the research, drafted the manuscript, and did the statistical analysis, as well as interpretating the data. XJ Chen, HF Hu and WJ Wang revised the manuscript and designed the study. DF Liu and WT He revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Data was from a published article: Association of body mass index and age with incident diabetes in Chinese adults: a population-based cohort study. The original study was initially approved by the Rich Healthcare Group Review Board. The data was retrieved retroactively and the institutional ethics committee did not ask for informed consent or approval for the retrospective study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Distribution of TyG for non-normoglycemic conversion group and normoglycemic conversion group with prediabetes.(0,non-normoglycemia conversion group; 1,normoglycemia conversion group).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, X., Liu, D., He, W. et al. Predictive performance of triglyceride glucose index (TyG index) to identify glucose status conversion: a 5-year longitudinal cohort study in Chinese pre-diabetes people. J Transl Med 21, 624 (2023). https://doi.org/10.1186/s12967-023-04402-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-023-04402-1