Abstract
Objective
This systematic review and meta-analysis study aimed to evaluate the effectiveness of probiotics supplementation on glycaemic control in patients with type 2 diabetes mellitus (T2DM) based on the data from the randomised clinical trials (RCTs).
Methods
PubMed, Web of Sciences, Embase, and Cochrane Library were searched from the inception to October 2022, and RCTs about probiotics and T2DM were collected. The standardised mean difference (SMD) with 95% confidence interval (CI) was used to estimate the effects of probiotics supplementation on glycaemic control related parameters, e.g. fasting blood glucose (FBG), insulin, haemoglobin A1c (HbA1c), and homeostasis model of assessment of insulin resistance (HOMA-IR).
Results
Thirty RCTs including 1,827 T2MD patients were identified. Compared with the placebo group, the probiotics supplementation group had a significant decrease in the parameters of glycaemic control, including FBG (SMD = − 0.331, 95% CI − 0.424 to − 0.238, Peffect < 0.001), insulin (SMD = − 0.185, 95% CI − 0.313 to − 0.056, Peffect = 0.005), HbA1c (SMD = − 0.421, 95% CI − 0.584 to − 0.258, Peffect < 0.001), and HOMA-IR (SMD = − 0.224, 95% CI − 0.342 to − 0.105, Peffect < 0.001). Further subgroup analyses showed that the effect was larger in the subgroups of Caucasians, high baseline body mass index (BMI ≥ 30.0 kg/m2), Bifidobacterium and food-type probiotics (Psubgroup < 0.050).
Conclusion
This study supported that probiotics supplementation had favourable effects on glycaemic control in T2DM patients. It may be a promising adjuvant therapy for patients with T2DM.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus (T2DM), an endocrine and metabolic disease, is influenced by host physiology and environmental factors [1]. More than 500 million people are living with diabetes globally, and this number is expected to increase to 783 million by 2045 [2]. T2DM is a common disease that accounts for approximately 90% of all cases of diabetes [3], and it may cause reduced life expectancy and life-threatening and costly complications [4]. There is no radical cure for T2DM [5, 6], and its treatment relies on the long-term use of anti-diabetic drugs [7, 8]. Therefore, it is crucial to explore new methods that may effectively delay or even reverse the progression of T2DM.
Recent studies have shown that the gut microbiota plays a key role in the maintenance of host homeostasis and pathogenesis of T2DM [9, 10]. Probiotics are microbial dietary supplements that alter the gut microbiota. Some randomised controlled trials (RCTs) have investigated the effects of probiotic interventions on glycaemic control in T2DM patients. However, evidence from clinical trials on the effects of probiotic supplementation on glycaemic control remains inconsistent. Asemi et al. [11] conducted a randomised double-blind placebo-controlled clinical trial involving 54 T2DM patients, which revealed that multi-species probiotic (mixture of Lactobacillus and Bifidobacterium) supplementation prevented an increase in the fasting blood glucose (FBG) level from baseline in these patients. Meanwhile, Razmpoosh et al. [12] randomly assigned 60 T2DM patients into two groups to take either a probiotic (mixture of Lactobacillus and Bifidobacterium) or a placebo intervention, and the results showed no significant differences in insulin or insulin resistance levels between the two groups. In 2016, Li et al. performed a systematic review and meta-analysis of 12 RCTs with 714 individuals and reported that probiotic supplementation could alleviate FBG, but no significant differences were observed in the haemoglobin A1c (HbA1c) level or homeostatic model assessment of insulin resistance (HOMA-IR) score between the probiotic and control groups of T2DM patients [13]. In 2020, Tao et al. systematically summarised 15 RCTs with 902 individuals, and the results of the meta-analysis indicated that probiotic supplementation reduced HbA1c, FBG and insulin resistance levels in T2DM patients [14]. However, some related RCTs (n = 11, including 630 patients) were not included in their study. Since then, more RCTs (n = 6) of the effects of probiotic supplementation on glycaemic control, including a total of 511 T2DM patients, have been reported [15, 16]. Controversy still exists regarding the effects of probiotics on glycaemic control in T2DM patients. Variations in participant (e.g. race) and intervention characteristics (e.g. dose, probiotic genus, and duration) in different studies may have given rise to the contradictory results. No study has detected differences in the effects of probiotic supplementation on glycaemic control according to the participant and intervention characteristics.
In this systematic review and meta-analysis, we aimed to evaluate the effects of a probiotic intervention on glycaemic control in T2DM patients and to evaluate the variations in these effects due to participant characteristics, e.g. race and baseline body mass index (BMI), and intervention characteristics, e.g. the probiotic dose, the duration of the intervention, the probiotic genus, and the type of vehicle used to deliver the probiotics.
Methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [17] (Additional file 1: Table S1). The protocol for this study has been registered at the International Prospective Register of Systematic Reviews (registration number: CRD42022370226).
Search strategy
Two reviewers (Guang Li and Yan-Jun Deng) independently searched PubMed, Web of Science, Embase, and Cochrane Library databases from their inception until October 2022 using various probiotic-related words and Medical Subject Heading terms in combination with ‘T2DM’ (Additional file 2: Table S2). No language or other restrictions were applied during the search, and all relevant studies were found to be published in English. A manual search was also performed to identify relevant studies from the references of the included studies.
Inclusion and exclusion criteria
Studies were included in the analysis if: (1) the participants were T2DM patients aged ≥ 18 years; (2) the study design was an RCT; (3) the intervention was the intake of probiotics from supplements and/or food; (4) the control group received a placebo intervention; and (5) the main outcomes included the glycaemic profile, e.g. FBG, insulin, and HbA1c levels and the HOMA-IR score. Studies were excluded from the analysis if: (1) the participants had other types of diabetes, e.g. gestational diabetes or type 1 diabetes or (2) the participants were concurrently receiving other interventions, e.g. synbiotics, herbs, prebiotics, or micro- nutrients.
Data extraction and quality assessment
Two researchers (Guang Li and Yan-Jun Deng) independently performed the literature search and data extraction, and disagreements were resolved by a third senior researcher (Su-Mei Xiao). Basic information (e.g. first author, year, and country of the study and the age, sex, and BMI of the participants), the study design, intervention information (probiotic genus and dose and duration of the intervention), and outcomes were extracted from the included studies. Two reviewers (Xiao-Bao Wang and Qiong Zhang) evaluated the quality of the included studies using the Cochrane risk-of-bias assessment tool. The risk of bias in the included studies was classified as low, unclear, or high.
Data synthesis and statistical analysis
The change in glycaemic control parameters was the primary outcome in this study. It was calculated as the final measurement value minus the baseline measurement value in each group. The mean and standard deviation (SD) of the change in glycaemic control parameters for the control group and the intervention group were extracted, respectively. If the study provided the standard error (SE) of mean change, the SE was converted to SD based on the sample size. For studies that did not directly report SD of mean change, the SDs of the baseline and final measurement values and the correlation coefficient (Corr) were used to calculated SDEffect,change (SDE,change) according to the following formula [18]:
Corr is the correlation coefficient between the baseline and final measurement values. For the pretest–posttest design, presumably the correlation is at least 0.5. This was the Corr estimate value being used to impute the missing SDs of mean change in this study [18, 19]. If the study presented data in medians and quartiles, the mean and SD values were estimated [20, 21]. If the intervention included multiple time points, the longest intervention time was included in the analysis.
The standardised mean difference (SMD) with the 95% confidence interval (CI) was used to assess the effects of probiotic interventions on glycaemic control in T2DM patients. The boundary values of the SMD were set at 0.2, 0.5, and 0.8, corresponding to small, medium, and large effects, respectively [22]. Heterogeneity was assessed using Cochrane’s Q statistic (chi-square). The inverse variance (I2) was used to assess the size of the heterogeneity. A fixed-effects model was used for the meta-analysis when I2 ≤ 50%, and a random-effects model was used when I2 ≥ 50%. Subgroup analysis was used to explore the possible sources of heterogeneity. Subgroup analyses were performed for race (Asian vs. Caucasian), probiotic dose (≤ 1 × 1010 colony-forming units (CFU)/day vs. > 1 × 1010 CFU/day), the duration of the intervention (≤ 8 weeks vs. > 8 weeks), probiotic genus (Lactobacillus, Bifidobacterium, or Lactobacillus and Bifidobacterium), type of vehicle used to deliver the probiotics (food vs. non-food (powder/capsule/tablet), and baseline BMI (< 30 kg/m2 vs. ≥ 30 kg/m2). The leave-one-out approach was used in the sensitivity analysis. Funnel plots and Egger’s test were used to appraise the possible publication bias in this study.
Results
Study characteristics
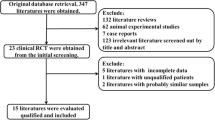
The database search yielded 4,048 records, and one additional record (a conference paper [23]) was obtained from the manual search of the references of the included RCTs. A total of 1,125 records were then excluded due to duplication, leaving 2,924 articles for screening. After the screening based on the titles and abstracts, 2,821 articles were further excluded (e.g. reviews, protocols, animal studies, etc.). The full texts of the remaining 103 potentially relevant studies were assessed according to the inclusion and exclusion criteria. Finally, thirty RCTs were included in this systematic review and meta-analysis (Fig. 1).
PRISMA flowchart for search strategy and study selection process. RCT, randomised controlled trial; T2DM, type 2 diabetes mellitus; PRISMA, preferred reporting items for systematic reviews and meta-analyses; FBG, fasting blood glucose; HbA1c, haemoglobin A1c; HOMA-IR, homeostasis model of assessment of insulin resistance
For the included 30 RCTs, all of them reported FBG, 17 RCTs reported HOMA-IR, 17 RCTs reported insulin, and 23 RCTs reported HbA1C (Fig. 1). Table 1 shows the basic information for the included 30 studies. Nine studies were conducted in Asian patients (three in China [15, 16, 24] and one each in India [25], Indonesia [26], Thailand [27], Japan [28], Malaysia [29], and Korea [30]), 19 studies were conducted in Caucasian patients (12 in Iran [11, 12, 31,32,33,34,35,36,37,38,39,40] and one each in Ukraine [41], Turkey [23], Sweden [42], Saudi Arabia40 [43], Egypt [44], Denmark [45], and Australia [46]) and two studies were conducted in other races (two in Brazil [47, 48]). In the 30 RCTs, there were a total of 1,827 subjects, with 922 in the probiotic group and 905 in the control group. The dose of probiotics used in the 30 studies ranged from 2 × 107 to 1 × 1012 CFU/day, the duration of the probiotic interventions ranged from 4 to 36 weeks, and the baseline BMI ranged from 23.1 to 35.9 kg/m2. The probiotics were consumed as food (n = 13) or non-food (powder/capsule/tablet; n = 15) forms, and the probiotic genera were mainly Lactobacillus (n = 11), Bifidobacterium (n = 2), and Lactobacillus and Bifidobacterium (n = 14; Table 1).
Risk of bias assessment of the included RCTs
The Cochrane risk-of-bias assessment tool was used to assess the bias of the 30 included studies. Approximately half of the studies (53%) were randomised, but 14 studies did not clearly report the randomisation process. The methods of allocation concealment were described in 43% of the included RCTs, and the majority of the studies (87%) described the blinding method. Approximately 40% of the studies provided information about the blinding outcome assessment. Most of the included studies had a low risk of attrition bias (73%), a low risk of reporting bias (93%), and a low risk of other types of bias (70%). Overall, four of the studies were classified as high quality (all terms were assessed as low risk), 19 studies were classified as moderate quality (no term was assessed as a high risk and one or more terms were assessed as unclear risks), and seven studies were classified as low quality (one or more terms were assessed as a high risk). The general and individual risks of bias are shown in Additional file 3: Fig. S1.
Effects of probiotic supplementation on glycaemic control
Effects on FBG
Thirty studies including a total of 1,827 T2DM patients were used to evaluate the effects of probiotic supplementation on FBG level. The pooled effects of probiotic supplementation indicated a significant decrease in FBG level in the probiotic group (SMD = − 0.331, 95% CI − 0.424 to − 0.238, Peffect < 0.001), and the heterogeneity was low (I2 = 29%, Pheterogeneity = 0.070; Fig. 2a). Leave-one-out sensitivity analysis confirmed that the pooled effects of probiotic supplementation on FBG level were stable and reliable (Additional file 4: Fig. S2a).
Subgroup analyses for FBG were performed according to race, probiotic intervention dose, probiotics genus, type of vehicle used to deliver the probiotics, and baseline BMI. As shown in Table 2, the significant subgroup differences (Psubgroup < 0.050) were observed for races (Asian vs. Caucasian), genus of probiotics (Lactobacillus vs. Bifidobacterium vs. Lactobacillus and Bifidobacterium), and baseline BMI (< 30 kg/m2 vs. ≥ 30 kg/m2). A stronger beneficial effect of the probiotic intervention was observed on FBG level in the Caucasian subgroup (SMD = − 0.448, 95% CI − 0.575 to − 0.322, Peffect < 0.001, Psubgroup = 0.020), in the Bifidobacterium subgroup (SMD = − 0.626, 95% CI − 1.221 to − 0.030, Peffect = 0.039, Psubgroup = 0.040), and in the high-baseline-BMI (≥ 30 kg/m2) subgroup (SMD = -0.490, 95% CI − 0.644 to − 0.336, Peffect < 0.001, Psubgroup = 0.007). No differences were observed between the subgroups of probiotic dose, intervention duration, or type of vehicle used to deliver the probiotics (Table 2, Psubgroup > 0.050).
Effects on insulin
Eight hundred and eighty-six patients in 17 RCTs were included in the meta-analysis of the effects of probiotic intake on insulin level. Probiotic supplementation in T2DM patients led to a significant reduction in insulin level (SMD = − 0.185, 95% CI − 0.313 to − 0.056, Peffect = 0.004) without heterogeneity (Fig. 2b, I2 = 0%, Pheterogeneity = 0.500). Sensitivity analysis also supported the robustness of the results for insulin level (Additional file 4: Fig. S2b).
As shown in Table 3, the magnitude of the reduction was significantly greater in the subgroup of patients taking food-type probiotics (SMD = − 0.386, 95% CI − 0.592 to − 0.180, Peffect < 0.001, Psubgroup = 0.014) than in the subgroup taking non-food (powder/capsule/tablet) types. In addition, no differences were observed between the subgroups of races, probiotic dose, intervention duration, probiotic genus, or baseline BMI (Table 3, Psubgroup > 0.050).
Effects on HbA1c
The effects of probiotic interventions on HbA1c level were evaluated in 23 RCTs including 1,466 T2DM patients. A significant decrease was observed in the HbA1c level in the probiotic group (Fig. 2c, SMD = − 0.421, 95% CI − 0.583 to − 0.258, Peffect < 0.001) with moderate heterogeneity (I2 = 56%, Pheterogeneity < 0.001). Sensitivity analysis showed that the results for HbA1 level were stable and reliable (Additional file 4: Fig. S2c).
The subgroup analysis was performed for HbA1c according to races (Asian vs. Caucasian), genera of probiotics (Lactobacillus vs. Bifidobacterium vs. Lactobacillus and Bifidobacterium), types of vehicle used to deliver the probiotics (food vs. non-food (powder/capsule/tablet)), and baseline BMI (< 30 kg/m2 vs. ≥ 30 kg/m2). As shown in Table 4, a significantly greater reduction was observed in the HbA1c level in the subgroups of Caucasians (SMD = − 0.465, 95% CI − 0.672 to − 0.257, Peffect < 0.001, Psubgroup = 0.032), Bifidobacterium probiotics (SMD = − 0.913, 95% CI − 1.387 to − 0.438, Peffect < 0.001, Psubgroup = 0.001), food-type probiotics (SMD = − 0.524, 95% CI − 0.800 to − 0.249, Peffect < 0.001, Psubgroup = 0.047), and baseline BMI ≥ 30 kg/m2 (SMD = − 0.485, 95% CI − 0.783 to − 0.188, Peffect = 0.001, Psubgroup = 0.018). No differences were observed between the subgroups of probiotic dose or intervention duration (Psubgroup > 0.050).
Effects on the HOMA-IR score
The results of the meta-analysis of 17 RCTs (n = 1,116) suggested significant effects of probiotic interventions on reducing the HOMA-IR scores in T2DM patients (SMD = − 0.224, 95% CI − 0.342 to − 0.105, Peffect < 0.001). The heterogeneity (I2 = 41%, Pheterogeneity = 0.040) of these RCTs was moderate (Fig. 2b). Sensitivity analysis showed that the pooled effects of probiotic supplementation on HOMA-IR scores did not significantly change, suggesting that the meta-analysis results were stable and reliable (Additional file 4: Fig. S2b).
No statistically significant differences were observed in the HOMA-IR score between subgroups (Table 5, Psubgroup > 0.050). However, an effective reduction in the HOMA-IR score was observed in the subgroups of Caucasians (SMD = − 0.308, 95% CI − 0.471 to − 0.146, Peffect < 0.001, Psubgroup = 0.173), high baseline BMI (≥ 30 kg/m2; SMD = − 0.320, 95% CI − 0.615 to − 0.026, Peffect = 0.033, Psubgroup = 0.144), and Bifidobacterium probiotics (SMD = − 0.248, 95% CI − 0.387 to − 0.109, Peffect = 0.004, Psubgroup = 0.345).
Publication bias analysis
Potential publication bias was assessed using funnel plots and Egger’s test. A visual inspection of the funnel plots revealed no publication bias for FBG, insulin, or HbA1c levels or the HOMA-IR score (Additional file 5: Fig. S3). Egger’s test results showed no publication bias for FBG (P = 0.349), insulin (P = 0.260) or HbA1c (P = 0.108) levels or the HOMA-IR score (P = 0.391).
Discussion
This systematic review and meta-analysis summarised data from 30 RCTs, including a total of 1,827 individuals, to evaluate the effects of probiotic supplementation on glycaemic control in T2DM patients. The results revealed that probiotic supplementation significantly decreased FBG, insulin, and HbA1c levels and HOMA-IR scores in T2DM patients. Further subgroup analyses showed that the effect was larger in the subgroups of Caucasians, high baseline BMI (≥ 30.0 kg/m2), Bifidobacterium probiotics, and food-type probiotics.
This study supported the notion that probiotics improve glycaemic control in T2DM patients. This is inconsistent with the results reported by the systematic review and meta-analysis of 12 RCTs in 2016 [13]. They found no significant differences in the HbA1c level and HOMA-IR score between the probiotic and control groups of T2DM patients. For their study, the meta-analysis of HbA1c and HOMA-IR were conducted with limited number of RCTs (n = 6), and five of them had the participants’ baseline BMI less than 30 kg/m2. In this study, the subgroup analysis found that the effect was larger in individuals with higher baseline BMI (≥ 30.0 kg/m2). These may partially explained the differences between the two studies. The gut microbiota is largely involved in the metabolic, nutritional, physiological, and immune functions of the host [49,50,51]. A previous study showed that T2DM patients are characterised by a decrease in the abundance of certain butyrate-producing bacteria and the enrichment of other microbial functions conferring sulphate reduction and oxidative stress resistance [52]. Changes in the gut microbial composition may be a mechanism whereby probiotic supplementation improves glycaemic control. Probiotic supplementation may modulate and increase the abundance of intestinal flora that are beneficial to glycaemic control [53, 54]. Moreover, the gut microbiota may regulate glucagon-like peptide 1, which promotes the secretion of insulin from islet β cells, and reduces the secretion of glucagon from islet α cells, resulting in a reduction in gastric emptying time, gastrointestinal peristalsis, and loss of appetite [55, 56]. Previous studies have found that probiotics may stimulate the production of short-chain fatty acids, especially butyrate, which increase insulin sensitivity and thus improve glycaemic control [57,58,59].
The subgroup analyses suggested that Bifidobacterium have greater effects than other probiotic genera. Probiotics that colonise the gut may change the host’s gut microbiota. According to a 5-year follow-up study, Bifidobacterium longum, a member of the core microbiota of the human gut, can stably colonise the gut [60]. Another study reported that oral supplementation with B. longum persists in the gut for 6 months in 30% of subjects [61]. Moreover, Xiao et al. (2020) found that Bifidobacterium appears to have a better ability to colonise the gut than Lactobacillus [62]. This may explain the finding that Bifidobacterium had a larger effect than other probiotic genera on glycaemic-control-related parameters (e.g. FBG and HbA1c levels) in T2DM patients, to some extent, in this study.
Food-type probiotics (e.g. yogurt and fermented milk) may have greater effects than other types of probiotics on glycaemic control in T2DM patients. Gastric acidity is thought to be one of the main obstacles to gut colonisation [63, 64]. Food-type probiotics (e.g. yogurt and fermented milk) may buffer the stomach acid, allowing the probiotics to better colonise the gut [65]. An in vitro study assessed the tolerance of probiotics in the human gastrointestinal tract by evaluating the effects of food addition on the viability of probiotics in simulated pH 2.0 gastric juices, revealing that adding soymilk or a liquid breakfast greatly enhanced the survival of the probiotics [66].
Compared to the baseline BMI < 30 kg/m2 subgroup, the stronger beneficial effects of a probiotic intervention were also observed on FBG and HbA1c levels in the baseline BMI ≥ 30.0 kg/m2 subgroup. This may be due to gut dysbiosis in obese individuals. In 2021, Liu et al. summarised the characteristics of the gut microbiota in obesity. Obese individuals were observed to have an increased Firmicutes/Bacteroidetes ratio at the phylum level and decreased abundances of the genera Lactobacillus and Bifidobacterium [67]. Probiotic supplementation may alleviate gut dysbiosis [68]. These findings indicate that obese individuals may be more sensitive to probiotic interventions. In addition, this may partly explain the observed racial differences, i.e. the effect was larger in Caucasians than in Asians. In this study, the average baseline BMI (30.3 kg/m2) was higher in Caucasians than in Asians (26.2 kg/m2).
In addition, no significant difference was observed between the longer-term intervention (> 8 weeks) and the shorter-term intervention (≤ 8 weeks) groups. In 2020, an RCT was conducted in 150 new-borns (38–40 weeks gestational age). In that study, the intervention group received probiotic supplementation containing 2 × 106 CFU/day of B. breve PB04 and L. rhamnosus KL53A. The stool samples from days 5, 6, and 30 were collected for an analysis of the gut microbiome. The results showed that L. rhamnosus and B. breve colonised rapidly, generally on days 5 and 6 [69]. This ability of the probiotics to rapidly colonise the gut may have resulted in the very small difference between the short and long intervention durations.
Furthermore, no significant differences were found between the higher-dose (> 1 × 1010 CFU/day) and lower-dose (≤ 1 × 1010 CFU/day) probiotic intervention groups. Several studies have reported similar results. Ibarra et al. (2018) performed a randomised double-blind, placebo-controlled trial to determine the effects of 4 weeks of supplementation with 1 × 109 or 1 × 1010 CFU of B. animalis subsp. lactis HN019 on adults diagnosed with functional constipation. The results showed no significant difference between the two groups with different doses of probiotics [70]. However, Whorwell et al. (2006) conducted a multi-centre clinical trial of 362 patients with irritable bowel syndrome (IBS) and found that 1 × 108 CFU of B. infantis 35,624 significantly alleviated the symptoms of IBS and that its effect was superior to that of the administration of 1 × 106 CFU/day and 1 × 1010 CFU/day of B. infantis 35624 [71]. In all of the included RCTs, the probiotic intervention doses were higher than 1 × 106 CFU/day, and only one RCT had a probiotic intervention dose lower than 1 × 108 CFU/day. Thus, these two doses were not used as the limits for subgroup analysis in this systematic review and meta-analysis. Further studies are warranted to determine the optimal dose of probiotics for glycaemic control in T2DM patients.
This study systematically and comprehensively evaluated the effects of probiotic supplementation on glycaemic control in T2DM patients. To the best of our knowledge, this is the first systematic review and meta-analysis study to investigate the differences in the effects of probiotic interventions on glycaemic control in T2DM patients according to participant characteristics (e.g. race, baseline BMI), and intervention characteristics, (e.g. probiotic doses, probiotic genus, treatment duration, and types of vehicles used to deliver the probiotics). However, this study also has some limitations. First, as 12 of the included studies (40%) were conducted in Iran, some racial and ethnic groups may be underrepresented. This may have resulted in a limited racial representation. Second, the number of RCTs in some subgroup analyses was low. For example, in the subgroup analysis of HbA1c level, the number of RCTs in the Bifidobacterium subgroup was only two. Third, the duration of most of the RCTs included in the analysis was from 4 to 24 weeks, and only one RCT was longer than 24 weeks (a 36-week intervention). Therefore, the long-term effects could not be explored in this study.
Conclusions
The findings of this study indicate that probiotic supplementation had favourable effects on glycaemic control in T2DM patients. Bifidobacterium and food-type probiotics had greater glucose-lowering effects than other probiotic genera and types of vehicle used to deliver the probiotics. Patients with a higher BMI may gain more glycaemic control benefits from a probiotic intervention. The administration of probiotics may be a promising adjuvant therapy for glycaemic control in T2DM patients.
Data availability
The data used in this study can be obtained by contacting the corresponding author.
Abbreviations
- T2DM:
-
Type 2 diabetes mellitus
- SMD:
-
Standardised mean difference
- FBG:
-
Fasting blood glucose
- HbA1c:
-
Haemoglobin A1c
- HOMA-IR:
-
Homeostasis model of assessment of insulin resistance
- RCTs:
-
Randomised controlled trials
- BMI:
-
Body mass index
- SD:
-
Standard deviation
- SE:
-
Standard error
- CI:
-
Confidence interval
- CFU:
-
Colony-forming units
- IBS:
-
Irritable bowel syndrome
References
Alvarez-Silva C, Kashani A, Hansen TH, et al. Trans-ethnic gut microbiota signatures of type 2 diabetes in Denmark and India. Genome Med. 2013;13(1):37.
Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183: 109119.
World Health Organization. Classification of diabetes mellitus. https://www.who.int/publications/i/item/classification-of-diabetes-mellitus. Accessed 21 Apr 2019.
Heald AH, Stedman M, Davies M, et al. Estimating life years lost to diabetes: outcomes from analysis of National Diabetes Audit and Office of National Statistics data. Cardiovasc Endocrinol Metab. 2020;9(4):183–5.
Ng SC, Xu Z, Mak JWY, et al. Microbiota engraftment after faecal microbiota transplantation in obese subjects with type 2 diabetes: a 24-week, double-blind, randomised controlled trial. Gut. 2022;71(4):716–23.
Umirah F, Neoh CF, Ramasamy K, Lim SM. Differential gut microbiota composition between type 2 diabetes mellitus patients and healthy controls: a systematic review. Diabetes Res Clin Pract. 2021;173: 108689.
Mcfarland LV. A review of the evidence of health claims for biotherapeutic agents. Microb Ecol Health Dis. 2009;12:65–76.
Salminen S. Human studies on probiotics: aspects of scientific documentation. Näringsforskning. 2001;45(1):8–12.
Yang K, Niu J, Zuo T, et al. Alterations in the gut virome in obesity and type 2 diabetes mellitus. Gastroenterology. 2021;161(4):1257-1269.e13.
Barrea L, Vetrani C, Verde L, et al. Comprehensive approach to medical nutrition therapy in patients with type 2 diabetes mellitus: from diet to bioactive compounds. Antioxidants (Basel). 2023;12(4):904.
Asemi Z, Zare Z, Shakeri H, Sabihi SS, Esmaillzadeh A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann Nutr Metab. 2013;63(1–2):1–9.
Razmpoosh E, Javadi A, Ejtahed HS, Mirmiran P, Javadi M, Yousefinejad A. The effect of probiotic supplementation on glycemic control and lipid profile in patients with type 2 diabetes: a randomized placebo controlled trial. Diabetes Metab Syndr. 2019;13(1):175–82.
Li C, Li X, Han H, et al. Effect of probiotics on metabolic profiles in type 2 diabetes mellitus: a meta-analysis of randomized, controlled trials. Medicine. 2017;95(26):e4088.
Tao YW, Gu YL, Mao XQ, Zhang L, Pei YF. Effects of probiotics on type II diabetes mellitus: a meta-analysis. J Transl Med. 2020;18(1):30.
Zhang Y, Gu Y, Ren H, et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat Commun. 2020;11(1):5015.
Jiang H, Zhang Y, Xu D, Wang Q. Probiotics ameliorates glycemic control of patients with diabetic nephropathy: a randomized clinical study. J Clin Lab Anal. 2021;35(4): e23650.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions. Chapter 16.1. Chichester: Wiley; 2019. p. 470–81.
Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45(7):769–73.
Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–805.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
Cohen J. Statistical power analysis for the behavioral sciences, chapter 10. Mahwah: Lawrence Erlbaum Associates; 1998. p. 467–81.
Tipici BE, Coskunpinar E, Altunkanat D, et al. Effects of Lactobacillus GG supplementation in type 2 diabetes: Are mucin genes expressions important? Diabetologia. 2020;1:63.
Hsieh MC, Tsai WH, Jheng YP, et al. The beneficial effects of Lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus: a randomized, double-blinded, placebo-controlled trial. Sci Rep. 2018;8(1):16791.
Madempudi RS, Ahire JJ, Neelamraju J, Tripathi A, Nanal S. Efficacy of UB0316, a multi-strain probiotic formulation in patients with type 2 diabetes mellitus: a double blind, randomized, placebo controlled study. PLoS ONE. 2019;14(11): e0225168.
Judiono J, Hadisaputro S, Indranila KS, et al. Effects of clear kefir on biomolecular aspects of glycemic status of type 2 diabetes mellitus (T2DM) patients in Bandung, West Java. Funct Foods Health Dis. 2014;4(8):340–8.
Toejing P, Khampithum N, Sirilun S, Chaiyasut C, Lailerd N. Influence of Lactobacillus paracasei HII01 supplementation on glycemia and inflammatory biomarkers in type 2 diabetes: a randomized clinical trial. Foods (Basel, Switzerland). 2021;10(7):1455.
Sato J, Kanazawa A, Azuma K, et al. Probiotic reduces bacterial translocation in type 2 diabetes mellitus: a randomised controlled study. Sci Rep. 2017;7(1):12115.
Firouzi S, Majid HA, Ismail A, Kamaruddin NA, Barakatun-Nisak MY. Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: a randomized controlled trial. Eur J Nutr. 2017;56(4):1535–50.
Jung SJ, Park SH, Choi EK, et al. Beneficial effects of Korean traditional diets in hypertensive and type 2 diabetic patients. J Med Food. 2014;17(1):161–71.
Bayat A, Azizi-Soleiman F, Heidari-Beni M, et al. Effect of Cucurbita ficifolia and probiotic yogurt consumption on blood glucose, lipid profile, and inflammatory marker in type 2 diabetes. Int J Prev Med. 2016;7:30.
Feizollahzadeh S, Ghiasvand R, Rezaei A, Khanahmad H, Sadeghi A, Hariri M. Effect of probiotic soy milk on serum levels of adiponectin, inflammatory mediators, lipid profile, and fasting blood glucose among patients with type II diabetes mellitus. Probiotics Antimicrob Proteins. 2017;9(1):41–7.
Khalili L, Alipour B, Asghari Jafar-Abadi M, et al. The effects of Lactobacillus casei on glycemic response, serum sirtuin1 and fetuin-a levels in patients with type 2 diabetes mellitus: a randomized controlled trial. Iran Biomed J. 2019;23(1):68–77.
Mohamadshahi M, Veissi M, Haidari F, Shahbazian H, Kaydani GA, Mohammadi F. Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. Bioimpacts. 2014;4(2):83–8.
Shakeri H, Hadaegh H, Abedi F, et al. Consumption of synbiotic bread decreases triacylglycerol and VLDL levels while increasing HDL levels in serum from patients with type-2 diabetes. Lipids. 2014;49(7):695–701.
Tajadadi-Ebrahimi M, Bahmani F, Shakeri H, et al. Effects of daily consumption of synbiotic bread on insulin metabolism and serum high-sensitivity C-reactive protein among diabetic patients: a double-blind, randomized, controlled clinical trial. Ann Nutr Metab. 2014;65(1):34–41.
Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28(5):539–43.
Mazloom Z, Yousefinejad A, Dabbaghmanesh MH. Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: a clinical trial. Iran J Med Sci. 2013;38(1):38–43.
Raygan F, Rezavandi Z, Bahmani F, et al. The effects of probiotic supplementation on metabolic status in type 2 diabetic patients with coronary heart disease. Diabetol Metab Syndr. 2018;10:51.
Hosseinzadeh P, Javanbakht MH, Mostafavi SA, et al. Brewer’s yeast improves glycemic indices in type 2 diabetes mellitus. Int J Prev Med. 2013;4(10):1131–8.
Kobyliak N, Falalyeyeva T, Mykhalchyshyn G, Kyriienko D, Komissarenko I. Effect of alive probiotic on insulin resistance in type 2 diabetes patients: randomized clinical trial. Diabetes Metab Syndr. 2018;12(5):617–24.
Mobini R, Tremaroli V, Ståhlman M, et al. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2017;19(4):579–89.
Sabico S, Al-Mashharawi A, Al-Daghri NM, et al. Effects of a 6-month multi-strain probiotics supplementation in endotoxemic, inflammatory and cardiometabolic status of T2DM patients: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38(4):1561–9.
Ismail A, Darwish O, Tayel D, Elneily D, Elshaarawy G. Impact of probiotic intake on the glycemic control, lipid profile and inflammatory markers among patients with type 2 diabetes mellitus. Clin Diabetol. 2021;10(6):468–75.
Hove KD, Brøns C, Færch K, Lund SS, Rossing P, Vaag A. Effects of 12 weeks of treatment with fermented milk on blood pressure, glucose metabolism and markers of cardiovascular risk in patients with type 2 diabetes: a randomised double-blind placebo-controlled study. Eur J Endocrinol. 2015;172(1):11–20.
Palacios T, Vitetta L, Coulson S, et al. Targeting the intestinal microbiota to prevent type 2 diabetes and enhance the effect of metformin on glycaemia: a randomised controlled pilot study. Nutrients. 2020;12(7):2041.
Tonucci LB, Olbrich Dos Santos KM, Licursi de Oliveira L, Rocha Ribeiro SM, Duarte Martino HS. Clinical application of probiotics in type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study. Clin Nutr (Edinburgh, Scotland). 2017;36(1):85–92.
Ostadrahimi A, Taghizadeh A, Mobasseri M, et al. Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Iran J Public Health. 2015;44(2):228–37.
Nicholson JK, Holmes E, Wilson ID. Gut microorganisms, mammalian metabolism and personalized health care. Nat Rev Microbiol. 2005;3(5):431–8.
Nicholson JK, Wilson ID. Opinion: understanding ‘global’ systems biology: metabonomics and the continuum of metabolism. Nat Rev Drug Discov. 2003;2(8):668–76.
Neves AL, Chilloux J, Sarafian MH, Rahim MB, Boulangé CL, Dumas ME. The microbiome and its pharmacological targets: therapeutic avenues in cardiometabolic diseases. Curr Opin Pharmacol. 2015;25:36–44.
Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60.
Johnson EL, Heaver SL, Walters WA, Ley RE. Microbiome and metabolic disease: revisiting the bacterial phylum Bacteroidetes. J Mol Med (Berl). 2017;95(1):1–8.
Jonsson AL, Bäckhed F. Role of gut microbiota in atherosclerosis. Nat Rev Cardiol. 2017;14(2):79–87.
Wang Y, Dilidaxi D, Wu Y, Sailike J, Sun X, Nabi XH. Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota and inducing GLP-1 secretion in db/db mice. Biomed Pharmacother. 2020;125: 109914.
Pizarroso NA, Fuciños P, Gonçalves C, Pastrana L, Amado IR. A Review on the Role of Food-Derived Bioactive Molecules and the Microbiota-Gut-Brain Axis in Satiety Regulation. Nutrients. 2021;13(2):632.
Martin-Gallausiaux C, Marinelli L, Blottière HM, Larraufie P, Lapaque N. SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc. 2021;80(1):37–49.
Mueller NT, Zhang M, Juraschek SP, Miller ER, Appel LJ. Effects of high-fiber diets enriched with carbohydrate, protein, or unsaturated fat on circulating short chain fatty acids: results from the OmniHeart randomized trial. Am J Clin Nutr. 2020;111(3):545–54.
Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16(8):461–78.
Faith JJ, Guruge JL, Charbonneau M, et al. The long-term stability of the human gut microbiota. Science. 2013;341(6141):1237439.
Maldonado-Gómez MX, Martínez I, Bottacini F, et al. Stable engraftment of Bifidobacterium longum AH1206 in the human gut depends on individualized features of the resident microbiome. Cell Host Microbe. 2016;20(4):515–26.
Xiao Y, Zhao J, Zhang H, Zhai Q, Chen W. Mining Lactobacillus and Bifidobacterium for organisms with long-term gut colonization potential. Clin Nutr (Edinburgh, Scotland). 2020;39(5):1315–23.
Fiocco D, Longo A, Arena MP, Russo P, Spano G, Capozzi V. How probiotics face food stress: they get by with a little help. Crit Rev Food Sci Nutr. 2020;60(9):1552–80.
Bove P, Gallone A, Russo P, et al. Probiotic features of Lactobacillus plantarum mutant strains. Appl Microbiol Biotechnol. 2012;96(2):431–41.
Charteris WP, Kelly PM, Morelli L, Collins JK. Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J Appl Microbiol. 1998;84(5):759–68.
Huang Y, Adams MC. In vitro assessment of the upper gastrointestinal tolerance of potential probiotic dairy propionibacteria. Int J Food Microbiol. 2004;91(3):253–60.
Liu BN, Liu XT, Liang ZH, Wang JH. Gut microbiota in obesity. World J Gastroenterol. 2021;27(25):3837–50.
Tiwari SK. Bacteriocin-producing probiotic lactic acid bacteria in controlling dysbiosis of the gut microbiota. Front Cell Infect Microbiol. 2022;12:851140.
Hurkala J, Lauterbach R, Radziszewska R, Strus M, Heczko P. Effect of a short-time probiotic supplementation on the abundance of the main constituents of the gut microbiota of term newborns delivered by cesarean section: a randomized, prospective, controlled clinical trial. Nutrients. 2020;12(10):3128.
Ibarra A, Latreille-Barbier M, Donazzolo Y, Pelletier X, Ouwehand AC. Effects of 28-day Bifidobacterium animalis subsp. lactis HN019 supplementation on colonic transit time and gastrointestinal symptoms in adults with functional constipation: a double-blind, randomized, placebo-controlled, and dose-ranging trial. Gut Microbes. 2018;9(3):236–51.
Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101(7):1581–90.
Acknowledgements
None.
Funding
This research was funded by the National Natural Science Foundation of China (No. 81400857), the Natural Science Foundation of Guangdong Province (No. 2021A1515011527), Pearl River S&T Nova Program of Guangzhou (No. 201610010093).
Author information
Authors and Affiliations
Contributions
GL designed and wrote the manuscript with SMX, HF, and XLM. GL, and YJD performed literature screening and data extraction. GL, HF, XLM, and SMX analyzed and interpreted the data. XBW, and QZ evaluated the quality of included studies. GL, SMX, HF, XLM, and YG proofread the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All the authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
PRISMA Checklist.
Additional file 2: Table S2.
MeSH and non-MeSH terms used in the systematic search.
Additional file 3: Figure S1.
(a) Risk of bias summary and (b) risk of bias graph.
Additional file 4: Figure S2.
Sensitivity analysis for studies included in this meta-analysis. (a) FBG, (b) Insulin, (c) HbAc1, and (d) HOMA-IR.
Additional file 5: Figure S3.
Funnel plot for studies included in this meta-analysis. (a) FBG, (b) Insulin, (c) HbAc1, and (d) HOMA-IR.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, G., Feng, H., Mao, XL. et al. The effects of probiotics supplementation on glycaemic control among adults with type 2 diabetes mellitus: a systematic review and meta-analysis of randomised clinical trials. J Transl Med 21, 442 (2023). https://doi.org/10.1186/s12967-023-04306-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-023-04306-0