Abstract
Background
Uterine clear cell carcinomas (CCC) represent less than 5% of uterine cancers. Their biological characteristics and clinical management remain uncertain. A multicenter study to explore both clinical and molecular features of these rare tumors was conducted.
Methods
This multicenter retrospective national study was performed within the French TMRG (Rare Gynecologic Malignant Tumors) network. Clinical data and, when available, FFPE blocks were collected. Clinical features, treatments, and outcome (progression-free survival (PFS) and overall survival (OS)) were analyzed and correlated to the protein (tissue micro-array), RNA (Nanostring nCounter® technology), and DNA (array-Comparative Genomic hybridization and target-next generation sequencing) levels using the tumor samples available.
Results
Sixty-eight patients with uterine CCC were enrolled, 61 from endometrial localization and 5 with cervix localization. Median age at diagnosis was 68.9 years old (range 19–89.7). Most tumors were diagnosed at an early stage (78% FIGO stage I–II). Hysterectomy (performed in 90%) and lymph node dissection (80%) were the most frequent surgical treatment. More than 70% of patients received external beam radiotherapy and 57% received brachytherapy. Nearly half (46%) of the patients received chemotherapy. After a median follow-up of 24.7 months, median PFS was 64.8 months (95 CI [5.3–124.4]) and median OS was 79.7 (IC95 [31.0–128.4]). Low hormone receptor expression (13% estrogen-receptor positive), frequent PI3K pathway alterations (58% PTEN loss, 50% PIK3CA mutations), and P53 abnormalities (41%) were observed. Mismatch repair deficiency was identified in 20%. P16 expression was associated with shorter PFS (HR = 5.88, 95 CI [1.56–25], p = 0.009). Transcriptomic analyzes revealed a specific transcriptomic profile notably with a high expression of immune response-associated genes in uterine CCC displaying a very good overall prognosis.
Conclusions
Uterine CCC reported to be potentially MSI high, hormone receptors negative, and sometimes TP53 mutated. However, some patients with immune response-associated features and better prognosis may be candidate to treatment de-escalation and immunotherapy.
Similar content being viewed by others
Background
Uterine clear cell carcinomas (CCC), arising both from the endometrium and the cervix are rare gynecological malignant tumors, representing less than 5% of all uterine epithelial cancers [1, 2]. Only few data were published concerning the clinical specificities of uterine CCC. Most of them are described in small subgroups from prospective studies focused on endometrial or cervix carcinoma taken as a whole, or in retrospective cohorts [3, 4]. Molecular descriptions of these rare diseases are also scarce [5]. Even though preliminary data have been published suggesting that endometrial CCC can be closer to ovarian CCC than to endometrial endometrioid tumors [6, 7], most of uterine CCC pathogenesis remains unknown. Therapeutics guidelines are also not specific for this pathological type and are based on guidelines designed for more frequent types: endometrial endometrioid carcinoma and cervical squamous cell carcinoma [8]. Description of more robust data related to these cancers is thus warranted to improve our knowledge and the clinical management. The TMRG (Tumeurs Malignes Rares Gynecologiques) network is a national French National Cancer Institute-accredited network dedicated to rare gynecological malignant tumors management including systematic second lecture for pathology and dedicated regional and national tumor boards. One of the main goal is also to develop multicenter research projects focused on rare gynecological cancers [9,10,11].
As first objective, we aimed to describe clinical presentation at diagnosis and treatment-related data of all patients with uterine clear cell cancer recorded in the TMRG network. Secondary objectives were to describe survival data (Progression-Free Survival and Overall Survival), as well as protein, transcriptomic, and genomic profiles and to evaluate their prognostic value.
Patients and methods
This work was a retrospective, multi-center, observational study. We retrieved all cases recorded in the TMRG database with sufficient pathological and clinical data available with a data cut-off on November 2017. Patients enrolled in this study should have been diagnosed with endometrium or cervix carcinoma with a clear cell component representing at least 10% of the tumor. For biological analyzes, they should have personally signed and dated informed consent (whether TRMG network or institutional consents). Patients with non-uterine CCC or with uterine carcinoma without CC component were excluded. All tumor samples were reviewed by expert pathologists as systematically performed within the TMRG network.
Our major endpoint was to describe patients (age, body mass index, hypertension, diabetes, in utero exposition to diethylstilbestrol, and personal and family history of cancer) and disease (FIGO stage, lymph node involvement, lymphovascular invasion, and pure clear cell or mixed histology) features. We have also collected treatment-related data: surgical procedure details (pathological margins status, lymph node dissection), external radiation therapy or brachytherapy data (dose (grays) and number of fractions), administration of chemotherapy (drugs identification, number of chemotherapy courses, sequential or concomitant to radiation therapy).
Immunohistochemistry
For cases with FFPE samples available, we analyzed the expression of proteins of interest by immunohistochemistry (IHC). Experiments were performed using a Tissue MicroArray (TMA) on which each case was deposited in duplicate with cores of 1 mm of diameter. We assessed the expression of ER (estrogen receptor, EP1 clone–Dako/Agilent), PR (progesterone receptor, PgR 636 clone–Dako/Agilent), P53 (DO7–Dako/Agilent), PTEN (6H2.1, Dako/Agilent), P16 (E6-H4, CINtec), PDL1 (22C3 pharmDx–Dako Omnis), mismatch repair proteins (MLH1 (ES05–Dako/Agilent), PMS2 (EP51–Dako/Agilent), MHS2 (FE11–Dako/Agilent), MSH6 (EP49–Dako/Agilent)), and EZH2 (D2C9–Cell Signaling). All stainings were performed using the Dako Link or Dako Omnis (for PDL1) autostainers (Agilent technologies™) with antibodies used at ready to use concentration, except for P53, PTEN, and EZH2 for which antibodies were diluted at 1/100, 1/50, and 1/2000, respectively. Antibodies staining were incubated for 20 to 40 min and revealed with the EnVision Flex kit (Agilent technologies™), according to manufacturer’s instructions. Mouse linkers were used for PgR, PTEN, PDL1, MLH1, and MSH2. A rabbit linker was used for PMS2. The threshold for positivity was set at 10% for hormone receptors, 10% for mismatch repair proteins, 10% for PTEN, and 80% for P53. As no specific data was available for clear cell uterine carcinoma, these thresholds were defined according to data validated in other disease localizations or other pathological subtypes [12,13,14,15,16,17]. Cases with no P53 staining, i.e. 0%, were also considered as mutant in comprehensive prognostic analyzes if the gene sequencing confirmed the mutation status. No a priori threshold was defined for the other markers. We used 28 high-grade endometrioid uterine tumors and 8 ovarian clear-cell tumors as controls to differentiate organ-related specificities to abnormalities associated with the clear-cell histology. These control samples were explored within the same TMA experiments beside clear cell cases.
Transcriptomic analyzes
For cases with sufficient tumor area, RNA was isolated and RNA templates were analyzed using the NanoString nCounter® Dx Analysis System [18]. A dedicated custom gene panel was developed including main genes implicated in gynecological cancers. This custom panel included 29 genes plus all genes from the Pan-Cancer pathway panel (770 genes from 13 canonical pathways). 200 to 500 ng of total RNA were used as input and sample hybridization was performed according to the manufacturer's instructions. Sample detection and analysis were completed on an nCounter® Digital Analyzer where genes were counted by scanning 555 Fields-of-view (FOV). The 410 endometrial endometrioid tumors from TCGA (out of 560 samples) and the 237 ovarian clear cell cancer (OvCC) from [19, 20] were used as controls, as well as eleven normal ovarian samples from the same external data set [19, 21].
Array-comparative genomic hybridization (aCGH)
Array-CGH was performed in order to study DNA copy number profile. Tumor DNA was extracted from FFPE blocks or hematoxylin–eosin–safran (HES) slides by automated methods using the EZ-1 tissue kit, QIAGEN™, according to the manufacturer’s instructions. The cases with DNA of sufficient quality (determined on Agilent Bioanalyzer (Agilent Technologies, Massy, France)) were analysed as previously described [22] using high-resolution 4 × 180 K CGH microarrays (SurePrint G3 Human CGH Microarray Kit, Agilent Technologies, Massy, France). Genomic data from the 393 endometrial endometrioid tumors from TCGA were used as control [21].
Target next-generation sequencing (t-NGS)
Panel-based next generation sequencing was conducted in cases with DNA that passed quality controls. Tumor DNA was sequenced using a home-made panel of genes as previously described [23]. For each tumor sample, a library of all coding exons and intron–exon boundaries of a panel of 794 target genes (Additional file 1: Table S1) was constructed using the SureSelect enrichment system (Agilent Technologies, Santa Clara, CA, USA). Sequencing was carried out using the Illumina NextSeq500 device (San Diego, CA, USA), according to the manufacturer’s instruction at a median depth of 162×.
Bioinformatics analysis
Single nucleotide mutations analyses
Sequence data were aligned to the human genome (UCSC hg19); alignment and variants calling and annotation were processed as previously described [24]. The Tumor mutational burden (TMB) and MSI (MicroSatellite Instability) score were defined as previously described [23]. Mutations were classified as driver or passenger alterations using the Cancer Genome Interpreter algorithm (https://www.cancergenomeinterpreter.org/home).
Array-CGH
All probes for aCGH were mapped according to the hg19/NCBI human genome mapping database. Log2ratio were segmented with Circular Binary segmentation (CBS) algorithm. We used two different threshold values (log2 ratio >|0.15| and |0.9|) to distinguish low (gain/loss) from high (amplification/deletion) level copy-number-alterations (CNA), respectively. Percentage of genome altered was calculated as the sum of altered probes divided by the total number of probes. To identify recurrent copy number alterations, we used the Genomic Identification of Significant Targets in Cancer (GISTIC) 2.0 algorithm calculated by multiple random iterations, with an amplification/deletion threshold > 0.9, confidence level 0.90, and a corrected threshold probability q < 0.25. A HRD (Homologous Recombination Deficiency) score, based on losses of heterozygosity, was calculated for each tumor sample from all tested aCGH genes [25].
Gene expression profiling (nCounter® platform, Nanostring™, Seattle, WA, USA)
Raw data processing, quality control, and normalization were performed using the nSolver™ 4.0 analysis software. Briefly, data processing of raw counts was done with background subtraction defined by the geometric mean of the eight negative control probes. Next, quality control of samples was checked according manufacturer requirement in the nSolver™ 4.0. Finally, normalization was done with the geometric mean algorithm using the 40 housekeeping and the six positive control probes. Processed data were then log2-transformed prior analysis. Messenger RNA analyzes were conducted with the Cancer Pathway panel plus 29 custom genes (Additional file 2: Table S2). Unsupervised analysis was done using hierarchical clustering using the Cluster program with data median-centered on genes [26], Pearson correlation as similarity metrics and centroid linkage clustering as parameters. Results were displayed using TreeView program [26]. In association with hierarchical classification, we used quality Threshold (qT) clustering to select clusters of gene by specifying minimum correlation and size values. Robustness of clusters was assessed using the R-package pvclust with uncentered Pearson correlation distance, the average agglomerative method and 100 bootstrap replications as parameters to assess the robustness of clusters [27]. The AU (Approximately Unbiased) p-values provided by multiscale bootstrap resampling indicate the robustness of tumor clusters, larger the p-values, more robust the clusters.
Gene expression profiling of public data
Our Nanostring UCCC data were completed by three public transcriptomic data sets including the Uterine Corpus Endometrial Carcinoma data set from TCGA, following Illumina RNA-seq processing, normalization, and publication through the UCSC Xena database [28], and the Winterhoff (GSE73614) and Bolton data sets [19, 20]. We merged these datasets by using COMBAT (empirical Bayes) as batch effects removal method [29], included in the inSilicoMerging R/Bioconductor package [30]. When multiple probes mapped to the same GeneID, we retained the one with the highest variance. Accurate normalization of the merged data sets including 786 commons genes was assessed by t-distributed Stochastic Neighbor Embedding (t-SNE). The 410 endometrial endometrioid tumors from TCGA (out of 560 samples) and the 237 OvCC from [19, 20] were selected for further analysis with our 47 UCCC. Eleven normal ovarian samples from TCGA ovarian cancer data set and Winterhoff’s set were also used as controls. Supervised analysis was done using a moderated t-test with empirical Bayes statistic [31] included in the limma R package (version 3.5.2; http://www.cran.r-project.org/). False discovery rate was applied to correct the multiple testing-hypothesis: the significant genes were defined by p < 0.05%, q < 1%, and fold change (FC) superior to |1.5×| [32]. Using the Gene Expression Signature (GES) obtained, each sample was classified using the nearest centroid algorithm. Robustness of classifier was done by tenfold cross validation using 1000 iterations using same parameters as supervised analysis and prediction accuracy was assessed using exact binomial test with the greater one-sided hypothesis. Ontology analysis of the differential gene list was based on the Reactome terms of the Database for Annotation, Visualization and Integrated Discovery (DAVID; david.abcc.ncifcrf.gov/). In order to assess the potential vulnerability or actionability of tumor samples to certain anti-cancer drugs used or in development in endometrial cancer [33], we applied to each dataset separately the following multigene signatures: two signatures predictive for response to immune checkpoint inhibitors (ICI), the T cell-inflamed signature (TIS) and immunologic constant of rejection signature (ICR) [34, 35], and the Rbsig signature predictive for resistance to CDK4/6 inhibitors [36].
All genomic data supporting our results can be found in Additional file 11: Materials S1, Additionnal file 12: Materials S2.
Statistical analyzes
The Pearson’s Chi2 test (categorical variables) and Wilcoxon test (continuous variables) were used to compare descriptive items. PFS (progression-free survival) was defined as the time from diagnosis to disease relapse, progression, or death from any cause. OS (overall survival) was defined as the time from diagnosis to death from any cause. Cause of death was collected to discriminate cancer-related events to death from other causes. Data concerning patients without disease progression or death at last follow-up were censored. Survival curves were estimated using the Kaplan–Meier method. Follow-up was estimated by the reverse Kaplan–Meier method. The prognostic impact of clinicopathological features was assessed by the Cox regression method in univariate and multivariate analyses and p-values estimated with the Wald test. All statistical tests were two-sided at the 5% level of significance. This work was done according to the Strengthening the Reporting of Observational Studies in Epidemiology criteria [37].
Results
Demographics (Table 1)
We retrospectively collected 68 cases of uterine cancer with a clear cell component in eight French cancer centers. Patients were diagnosed with uterine cancer from October 2000 until November 2017 (Fig. 1—Flow chart).
Most of them (N = 61, 90%) were diagnosed with endometrium cancer (Table 1). Median age was 68.9 years old (range 19–89.7). Median body mass index was 25.4 kg/m2 (range 16.6–50.8) and a minority reported previous history of diabetes (16%) or hypertension (27%). A quarter of them had a personal history of cancer, with breast cancer as the most frequent (88%) previous malignant disease. A third (30%) had a family history (parents, siblings, children) of cancer, with 16 cases of breast cancers, four of colo-rectal cancers, and one of ovarian cancer. We aimed to explore in utero exposition to diethylstilbestrol. Unfortunately, these data was unknown for most patients included in this study and could not be described. Thirty-eight (56%) were FIGO stage I at diagnosis, four (6%) were stage II, 15 (22%) stage III, and seven (10%) stage IV. Fifteen (22%) patients had loco-regional lymph node metastases. Lymphovascular invasion was observed in 29 (43%) cases.
Treatment
As endometrial cancer represented 90% of our set, we will describe below only treatments received by these patient (details for cervix tumors in Table 2). More than 90% of the patients underwent frontline surgery and forty-four (72%) received external beam radiotherapy, with a dose-equivalent of at least 45 grays in 25 fractions. Thirty-five patients (57%) also received high-dose brachytherapy. Twenty-eight patients (46%) received chemotherapy, mainly based on carboplatin-paclitaxel doublet. The median number of chemotherapy cycles was four (range 1–6).
Clinical outcome
Median follow-up was 24.7 months (95 CI [16.0–33.3]). Among the 68 patients included in this study, 39 (57%) were still disease-free at time of last news. Seventeen (25%) patients experienced disease progression with distant metastases. Ten had a loco-regional relapse prior to or concomitantly with distant metastases. Four other patients displayed pelvic relapse without metastasis. Metastases occurred in lymph nodes (N = 7), lungs or liver (N = 5 each), peritoneum (N = 3), pleura (N = 2), bones (N = 1), and brain (N = 1). Metastatic site details were not available in one patient.
At the date of the database locked, 17 patients were deceased, including 16 for whom death was related to cancer. Out of 51 alive patients, 39 were disease-free, one with disease in partial response to treatments, four with disease progression, and data was missing in seven cases. Five patients were diagnosed with a new primary malignancy during follow-up (three breast cancers, one colorectal cancer, and one kidney cancer).The 2-year PFS and OS rates were 73.1% and 84.5%, respectively (Additional file 6: Fig. S1).
Analysis of protein expression using immunohistochemistry
Immunohistochemistry (IHC) was performed in 48 patients (71%) for whom FFPE material was available and deposited onto a TMA. Clinical data were available in 42 of them. Clinical features and treatments of this subset were similar to that of the whole population (Table 1). Median follow-up was 20.4 months (95 CI [16.4–24.4]) in this subset, with similar 2y-PFS and OS rates than the whole cohort (65.6% and 78.4%, respectively). Out of the 48 cases, six (12%) were ER-positive and four (8%) were PR-positive, including two ER + /PR + cases (Table 3). By contrast, none of the eight ovarian clear cell controls (p = 0.65, Chi2-test for comparison versus uterine CCC) and 60% of 29 endometrioid uterine cancer controls (p = 1.2 E-05) were ER + . PR expression was also lower in uterine CCC versus endometrioid tumors (8% vs 58% p = 4.8 E−05, and 0% in OvCC, p = 0.9). Eleven cases (23%) were P53mut, including three of the five cervix cancers. Nineteen of the 48 cases (40%) presented a loss of at least one MMR (mismatch repair) protein and ten patients lost two markers (N = 7 for PMS2/MLH1, N = 2 for MSH6/MSH2, one tumor lost expression of all four proteins). PTEN was lost in 28 (58%) cases. Only four tumors were PDL1 + (8%), including one with PDL1 expression in more than 50% of tumor cells. We observed P16 expression in at least 10% of tumor cells in 38% of cases. Of the 19 cases with positive EZH2 expression, more than 50% of tumor cells were positive in 12 (25%) samples.
Except for hormone receptors expression, rates of positive tumors were similar between UCCC and endometrial endometrioid tumors. For instance, no significant difference was observed for MMR proteins taken alone (p = 0.69) or in dual combination (MLH1/PMS2 or MSH2/MSH6; p = 1). None of the ovarian CCC controls was negative for MMR proteins.
Analysis of gene expression profiles
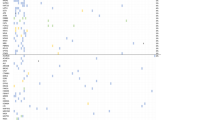
Unsupervised transcriptomic analysis of 47 tumors identified three subgroups with similar clinical features. Tumors from the cluster I were characterized by high expression of genes involved in epithelial-mesenchymal transition (EMT). Tumors from a second cluster (IIb) had high expression of immune response and cell cycle-related genes (Fig. 2A). Most tumors classified as MMRd by IHC, the POLE-mutated tumor, and all cases with PDL1 expression belonged to this cluster (Fig. 2B). The third tumor cluster (cluster IIa) was characterized by a lower expression of genes associated with EMT and immune response.
Gene expression profile of uterine CCC according to the 786-gene panel. A Hierarchical clustering (N = 47) with main three quality threshold clusters with Pearson correlation > 0.6 and including at least 20 genes defined by expression of genes related to epithelial-mesenchymal transition (EMT), immune response, and cell cycle. B Correlation with main clinical and pathological data. Pvclust R-package was used to explore clusters robustness, with approximately unbiased p-values. C Kaplan Meier curves of overall survival
We then included the control tumor samples. Unsupervised analysis showed that uterine CCC are closer to endometrioid endometrial cancers than to OvCC (Fig. 3A). Nevertheless, transcriptomic profiles of uterine CCC (N = 47) and endometrioid endometrial cancers (N = 410) were not fully similar and supervised analysis identified 154 genes differentially expressed between them (Fig. 3B). To remove the genes that could be associated to TCGA subtype obtained by ProMisE molecular classification [38], a multivariate logistic regression analysis with the glm function in R’s stastistical package was done on the 154 genes to compare uterine CCC to endometrioid endometrial tumors stratified with TCGA subtype. Off the 154 genes, nine were classified as dependent to the ProMisE classification and excluded from further analysis. Ontology analysis of the remaining 145-gene list showed that genes involved in immune response and cellular matrix regulation were more expressed in uterine CCC than in endometrioid tumors (Fig. 3C, Additional file 3: Table S3). At the opposite, uterine CCC had lower expression of genes associated with fatty acids metabolism and FGF-related pathways.
Analysis of gene expression profiles of uterine CCC, endometrioid tumors from TCGA, and ovarian clear cells (OvCC) tumors [19, 20]. A t-SNE (t-distributed Stochastic Neighbor Embedding) unsupervised analysis based on all 786 genes showing that centroid of the UCCC set is closer to all endometrial carcinoma than to ovarian clear cell carcinoma. B Volcano plot of differential mRNA expression between uterine CCC and the TCGA endometrioid endometrial carcinoma data set with 154 genes differentially expressed (moderated t-test: p < 5%, q < 10% and |FC|> 1.5x). C Ontology analysis based on the Reactome database. The best 20 pathways are represented
The number of genes tested (786 genes) did not allow to assess the potential vulnerability (based on gene expression signatures predictive for response or resistance) or actionability (based on gene expression level of the target protein) of tumor samples to most of anti-cancer drugs used or in development in endometrial cancer [33]. That was possible for a few drugs. HER2 expression, potential target of trastuzumab-based antibody–drug conjugates (ADCs), was more expressed in uterine CCC than in TCGA endometrioid endometrial carcinoma (p = 1.28E-04). Similarly, two signatures predictive for response to immune checkpoint inhibitors (ICI) showed higher score in uterine CCC: TIS (p = 3.90E−05) and ICR (p = 9.520E−03) [34, 35], suggesting higher potential vulnerability to ICI in uterine CCC. Conversely, the Rbsig signature showed higher score in uterine CCC (p = 0.082), suggesting lesser vulnerability to CDK4/6 inhibitors than in endometrioid endometrial carcinoma [36].
After filtering of organ-specific genes, a similar approach for uterine CCC versus OvCC comparison identified 207 differentially expressed genes (Additional file 7: Fig. S2, Additional file 4: Table S4). TRKA receptors and synaptic signals pathways were upregulated in uterine CCC, whereas extracellular matrix cell deaths associated genes were downregulated compared to OvCC.
Analysis of copy number alterations using aCGH
We performed aCGH on 47 uterine CCC samples. GISTIC-2.0 analysis identified numerous copy number alterations (CNAs). Notably, we observed frequent amplifications in 6p11.2 (PRIM2), 8p11.22 (IKBKB), 14q24.3 (SNW1), and 17q12 (ERBB2 and CDK12), Additional file 8: Fig. S3A). Deletions in regions of interest were also identified: 1p36.32, 6p21.32 (DAXX), 8p11.22 (ADAM family genes and TM2D2), 15q11.2 (UBE3A), and 19p13.3 (STK11).
Supervised analysis of CNAs identified in our uterine CCC cohort (N = 47) versus the TCGA data set (N = 393) showed that several regions were differentially altered (Additional file 8: Fig. S3B). 8p11.21, 10q22.2, 12q13.3, 14q24.3, 17q12, and 17q23.1 regions were statistically more frequently amplified in uterine CCC. Regions more frequently deleted were 1p31.1, 1q21.3–31.3, 4q13.2, 12p13.31, and 20p13.The HRD score was higher in uterine CCC than in TCGA endometrioid tumors (p = 2.39E−06, Student t-test; Additional file 8: Fig. S3B), suggesting potential higher vulnerability of CCC to PARP inhibitors.
Analysis of mutational profiles using t-NGS
After DNA quality control assessment, 19 of the 48 cases were available for t-NGS analysis covering 794 target genes. One hundred and ninety-one pathogenic driver alterations were identified in 118 genes (Fig. 4A and Additional file 5: Table S5). Most observed alterations (67%) were missense single nucleotide variations, followed by nonsense mutations (15%), indels 15%) and splicing mutations (3%). All analyzed tumors displayed at least one mutation of interest. Fourteen tumors (74%) had mutations in genes involved in the PI3K/AKT/mTOR pathway (Fig. 4B). Seven tumors had mutations in genes involved in DNA repair pathways. Genes coding for proteins of the SWI/SNF complex were also altered in five cases (26%). We observed mutations in genes coding for MMR proteins in two tumors (11%). POLE was mutated in one case. Fourteen (74%) of analyzed samples had alterations in cell cycle-related genes: TP53 (13 mutations), PPP2R1A (four mutations), and CDKN2A (one mutation).
ProMisE molecular classification
We combined results from IHC and t-NGS in order to classify tumors in one the four molecular subtypes identified in the TCGA cohort: POLE-mutated, MMR deficient by IHC, P53 mutant (P53 pos by IHC and/or TP53-mutated by t-NGS), and cases of non-specific molecular profile (NSMP). We identified one (2%) POLE-mutated tumor, 11 (23%) MMRd, 18 (38%) P53 mutant, and 18 (38%) NSMP cases (Additional file 9: Fig. S4A). As expected, we found correlations between these subtypes and genomic scores. The HRD score tended to be higher in the P53mut subtype (p = 0.072, Student t-test; Additional file 9: Fig. S4B), the MSI score tended to be higher in the MMRd subtype (p = 0.107, Student t-test; Additional file 9: Fig. S4C), and the TMB was higher in the POLE-mutated subtype (p = 0.048, Student t-test; Additional file 9: Fig. S4D), suggesting the coherence of data.
Correlation of clinical, pathological, and genomic alterations to survival (PFS)
Prognostic assessment of clinical features identified lymph node involvement as the only parameter tending to be associated with PFS (HR = 2.32; 95 CI [0.95–5.88]; p = 0.06, Wald’s test). No other baseline clinical feature was prognostic (Table 4). Patients who did not received radiotherapy or brachytherapy had a shorter PFS: HR = 3.07 (95 CI [1.26–7.48]) for radiotherapy, and HR = 3.08 (95 CI [1.21–7.86]) for brachytherapy. However, this was due to a lower rate of local treatment in patients with advanced stage disease. Forty-four percent of patients with FIGO stage III and IV tumors received radiotherapy versus 83% in FIGO stage I-II (p = 0.003, Pearson correlation). This was similar for brachytherapy: 29% versus 74%, p < 0.001).
Univariate analysis identified P16 as the only IHC marker significantly associated with PFS (Table 5). Cases without expression of P16 had a longer median PFS than cases with expression (64.8 months vs 17.3 months respectively; p = 0.003, log-rank test; Fig. 5A). Multivariate analysis for PFS including p16, FIGO stage (I–II vs. III–IV), and lymphovascular invasion showed that P16 prognostic value was independent from these clinicopathological features: HR = 6.67, 95 CI [1.37–33.33] for P16; HR = 0.70, 95 CI [0.19–2.52] for FIGO stage; HR = 0.99, 95 CI [0.23–4.29] for lymphovascular invasion. P16 Protein and CDKN2A (gene coding for P16) mRNA expression were correlated (p = 1.37 E-03, Student’s T-test), and CDKN2A expression tended to be correlated to PFS (HR = 1.35 [0.98–1.85], p = 0.06, Wald’s test, N = 36), reinforcing p16 prognostic value. Median PFS was 18.0 months in EZH2-positive cases versus 64.8 months in EZH2-negative tumors, but the small sample size could not lead to significance (p = 0.18, log-rank test, Fig. 5B).
The prognostic impact of the ProMisE classification in this clear cell tumors cohort was then explored. No survival difference between MMRd, P53mut, and NSMP subgroups (p = 0.97, log-rank test, Additional file 9: Fig. S4E) was observed. Nevertheless, patients in the “immune response subgroup” of transcriptomic analyzes experienced a very good prognosis, with a 3-y overall survival of 100% versus 33 to 68% in the two other clusters (Fig. 2C). Moreover, to reinforce the robustness of this classification in the absence of external validation set, we used the Pvclust algorithm and confirmed the low uncertainty of the hierarchical clustering with AU p-values of 95% to 98% [27].
Supervised analysis of aCGH data using GISTIC2.0 algorithm did not identify copy number alterations correlated to disease progression at the False Discovery Rate threshold of 0.25 (Additional file 10: Fig. S5). Alterations of the PI3K/AKT, MAPK, DNA repair, or SWI/SNF pathways, explored by TMA, NGS, and aCGH, were not predictive of poorer PFS: HR = 2.38 [0.62–9.10] for PI3K, HR = 1.09 [0.29–4.13] for MAPK, HR = 1.86 [0.50–6.92] for DNA repair, and HR = 0.52 [0.15–1.79] for SWI/SNF.
Discussion
This national retrospective study focused on a rare tumor type reports similar clinical features between uterine clear cell carcinoma and uterine endometrioid cancer. However, they have different biological profiles with specific molecular profiles. Comprehensive pathological, transcriptomic and genomic analyzes identified P16 and expression of immune response-related genes as potential prognostic markers in this subtype (Table 6).
If median age at diagnosis among the 68 patients enrolled in this cohort (69 years old) was similar to that of endometrioid uterine cancer [39], the frequencies of prior history of hypertension and diabetes were similar to general population, whereas they are more frequent in patients with endometrioid tumors. Consistently with literature, most patients were diagnosed with early stage disease (FIGO stage I in 56%, stage II in 22%). As supported by surgical guidelines in non-endometrioid tumors, most patients underwent surgery with lymph node dissection and received adjuvant radiotherapy. Chemotherapy administration was less frequent (< 50%) despite an aggressive histology classified as high-risk even when diagnosed at stage I [40].
Patients enrolled in this study have a good short-term outcome, with 2y-PFS and 2y-OS rates of 73.1% and 84.5%, respectively. Previous observations have already highlighted than stage I-II UCCC have a median survival similar to that of endometrioid uterine tumors and that UCCC have better prognosis than serous uterine tumors [1, 41]. We identified p16 loss of expression as the main feature associated with survival. Lower expression of p16 protein and under-expression of CDKN2A (gene coding for p16) were associated with increased PFS. To our knowledge, it is the first report of p16 prognostic value in uterine CCC. However, this has already been described in ovarian CCC with patients with no p16 expression displaying longer OS [42]. Endometrioid endometrial tumors with p16 overexpression also have a poorer prognosis [43].
Recent guidelines recommend that adjuvant treatments for endometrial cancer should be based on the ProMisE classification [38]. The cancer genome atlas classification has identified four molecular subgroups (p53-abnormal, POLE-mutated, MMRd/MSI-high, and tumor of non-specific molecular profile) [21]. This classification has a prognostic impact in endometrioid and serous subtypes but its correlation to outcome in rare pathological subtypes remains unclear. In a small-size single institution uterine CCC cohort, 2% of tumors were POLE-mut, 10% MMRd, 54% NSMP, and 35% p53abn [44]. This is close to what we observed in our cohort: 2% POLE-mut, 23% MMRd, 38% p53-abn, and 38% NSMP. We may have an increase rate of MMRd tumors as our set included mixed tumors that have been associated with higher rates of MMR alterations than pure CCC [45]. This is of interest as these patients may have a better outcome than MMR proficient CCC [46]. The limited sample size of our set may have precluded us to show a prognostic value of the ProMisE classification in uterine CCC. However, other small size cohorts also failed to demonstrate significant prognostic value of this classification, even though p53-abnormal cases seem to display the poorest outcome [44].
Nevertheless, transcriptomic analyzes showed that overexpression of genes associated with immune response was correlated to POLE-mut and MMRd subtypes, and associated with improved survival. Therefore, gene expression data may be a more efficient prognostic marker than the ProMisE classification for the UCCC population. However, due to our limited sample size, validation using an independent data set is warranted to confirm these results before suggesting that patients with an immune mRNA profile may benefit from treatment de-escalation. Gene expression analysis also showed that uterine CCC are closer to uterine endometrioid tumors than to ovarian clear cell carcinoma. This may limit the development of tumor agnostic clinical trials only based on pathological types.
Our genomic analyzes identified other altered pathways. Alterations of the PI3K/AKT pathway (PI3KCA, PTEN, or AKT1 mutations, STK11 deletions, PTEN loss) were frequent, as well as DNA repair (ATM, ATRX, ATR, BRCA1) and SWI/SNF complex (ARID1A, ARID1B, EZH2, SMARCA4) alterations. PI3K/AKT/mTOR pathway alterations are frequent in CCC, with more than one third with PI3KCA mutations and 40% with PTEN loss [47, 48]. PI3K, AKT, and mTOR inhibitors are under investigation in endometrial cancer with promising results in early phase studies [49,50,51]. DNA repair alterations (whether HRD-related genes mutations and/or high genomic instability score) we observed in our set may also be a target. PARP inhibitors are explored in the recurrent setting [52]. SWI/SNF pathway alterations are frequent in endometriosis-related tumors, including clear cell and endometrioid ovarian carcinoma [20, 53, 54]. Recent circulating tumor DNA data suggest that endometrial cancer is the tumor localization with the highest ARID1A mutation rate (21%). This may offer new opportunities for endometrial cancer treatment with the assessment of EZH2 inhibitors or ATR inhibitors in the setting of recurrent uterine CCC [55, 56]. Finally, we identified frequent 17q12 (ERBB2 amplicon) amplification. As some preliminary data suggest ERBB2-targeting efficacy in ERBB2-positive endometrial serous cancer and in a few case reports of uterine CCC [57, 58], use of trastuzumab or trastuzumab-based ADCs warrants to be further explored in ERBB2-amplified uterine CCC.
Despite these important findings, our study presents some limitations as sample size may have limited the power of our study and our capacity to identify some prognostic features, especially to perform multivariate analyses including genomic and transcriptomic classifications. However, as uterine CCC is a rare disease; comprehensive molecular studies in larger sets are difficult to conduct outside national and international networks such as the TMRG network. Moreover, the TMRG and the GINECO group have shown their expertise in rare gynecological tumors management by sponsoring randomized clinical trials in rare diseases such as sex cord-stromal tumors [59]. The BOUQUET study (NCT04931342), a basket trial dedicated to rare ovarian tumors is ongoing. A similar design may be developed for advanced endometrial cancer with specific molecular alterations. One of the potential limitation was to include both pure and mixed CCC, partially guided by real-world practice where CCC uterine tumors often display other components (mainly endometrioid or serous). For instance a recent cohort reported 60% of mixed uterine CCC [60]. Third, our protein analyzes were based on TMA results. Tumor heterogeneity may thus have been missed. Concerning transcriptomic analyzes, no external validation could be done as no independent public data is available in uterine CCC. Finally, the low amount of DNA of sufficient quality (old FFPE samples) did not allow us to explore mutation profile in all cases. Further NGS data would have been of interest.
In conclusions, we show with this comprehensive clinical, pathological and molecular study that such effort can be conducted in rare gynecological disease by national networks. This reinforces the need of collaborations and molecular profiling to identify potentially actionable alterations of such diseases and our capacity to propose individualized therapies based on ESCAT (ESMO Scale for Clinical Actionability of molecular Targets) evaluation [61]. Our data may help to propose new therapeutic alternatives to our patients and to develop new prospective trials in uterine clear cell carcinoma.
Availability of data and materials
All genomic and transcriptomic data analyzed in this study are available in supplementary materials.
Abbreviations
- 95 CI:
-
95% Confidence interval
- CCC:
-
Clear cell carcinomas
- CNA:
-
Copy-number-alterations
- ER:
-
Estrogen receptor
- ESCAT:
-
ESMO Scale for Clinical Actionability of molecular Targets
- GISTIC:
-
Genomic Identification of Significant Targets in Cancer
- HR:
-
Hazard ratio
- HRD:
-
Homologous recombination deficiency
- ICI:
-
Immune checkpoint inhibitors
- ICR:
-
Immunologic constant of rejection signature
- IHC:
-
Immunohistochemistry
- MMRd:
-
Mismatch repair deficiency
- MSI:
-
Micro-satellite instability
- NSMP:
-
Non-specific molecular profile
- OS:
-
Overall survival
- OvCC:
-
Ovarian clear cell cancer
- PFS:
-
Progression-free survival
- PR:
-
Progesterone receptor
- ProMisE:
-
Proactive Molecular Risk Classifier of Endometrial Cancer
- SD:
-
Standard deviation
- TCGA:
-
The Cancer Genome Atlas
- TIS:
-
T cell-inflamed signature
- TMA:
-
Tissue MicroArray
- TMB:
-
Tumor mutational burden
- TMRG:
-
Rare Gynecologic Malignant Tumors
- t-NGS:
-
Tumor next-generation sequencing
- t-SNE:
-
T-distributed Stochastic Neighbor Embedding
References
Olawaiye AB, Boruta DM. Management of women with clear cell endometrial cancer: a Society of Gynecologic Oncology (SGO) review. Gynecol Oncol. 2009;113:277–83.
Hanselaar A, van Loosbroek M, Schuurbiers O, Helmerhorst T, Bulten J, Bernhelm J. Clear cell adenocarcinoma of the vagina and cervix. An update of the central Netherlands registry showing twin age incidence peaks. Cancer. 1997;79:2229–36.
Bogani G, Ray-Coquard I, Concin N, Ngoi NYL, Morice P, Enomoto T, et al. Clear cell carcinoma of the endometrium. Gynecol Oncol. 2022;164:658–66.
Zhang Z, Gao P, Bao Z, Zeng L, Yao J, Chai D, et al. Clear cell carcinoma of the endometrium: evaluation of prognostic parameters in 27 cases. Front Oncol. 2021;11: 732782.
Zannoni GF, Santoro A, Angelico G, Spadola S, Arciuolo D, Valente M, et al. Clear cell carcinoma of the endometrium: an immunohistochemical and molecular analysis of 45 cases. Hum Pathol. 2019;92:10–7.
Lax SF, Pizer ES, Ronnett BM, Kurman RJ. Clear cell carcinoma of the endometrium is characterized by a distinctive profile of p53, Ki-67, estrogen, and progesterone receptor expression. Hum Pathol. 1998;29:551–8.
Fadare O, Zheng W. Endometrial glandular dysplasia (EmGD): morphologically and biologically distinctive putative precursor lesions of Type II endometrial cancers. Diagn Pathol. 2008;3:6.
Hasegawa K, Nagao S, Yasuda M, Millan D, Viswanathan AN, Glasspool RM, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for clear cell carcinoma of the uterine corpus and cervix. Int J Gynecol Cancer. 2014;24:S90-95.
Chevrot A, Pouget N, Bats A-S, Huchon C, Guyon F, Chopin N, et al. Fertility and prognosis of borderline ovarian tumor after conservative management: results of the multicentric OPTIBOT study by the GINECO & TMRG group. Gynecol Oncol. 2020;157:29–35.
Derquin F, Floquet A, Hardy-Bessard AC, Edeline J, Lotz JP, Alexandre J, et al. Need for risk-adapted therapy for malignant ovarian germ cell tumors: a large multicenter analysis of germ cell tumors’ patients from French TMRG network. Gynecol Oncol. 2020;158:666–72.
Romeo C, Le Saux O, Jacobs M, Joly F, Ferron G, Favier L, et al. Therapeutic challenges in patients with gynecologic carcinosarcomas: analysis of a multicenter national cohort study from the French prospective TMRG network. Cancers (Basel). 2022;14:354.
Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020;38:1346–66.
Ida N, Nakamura K, Saijo M, Nasu A, Yoshino T, Masuyama H, et al. DNA mismatch repair deficiency and p53 abnormality are age-related events in mixed endometrial carcinoma with a clear cell component. Pathol Res Pract. 2021;220: 153383.
Rescigno P, Lorente D, Dolling D, Ferraldeschi R, Rodrigues DN, Riisnaes R, et al. Docetaxel treatment in PTEN- and ERG-aberrant metastatic prostate cancers. Eur Urol Oncol. 2018;1:71–7.
Djordjevic B, Hennessy BT, Li J, Barkoh BA, Luthra R, Mills GB, et al. Clinical assessment of PTEN loss in endometrial carcinoma: immunohistochemistry outperforms gene sequencing. Mod Pathol. 2012;25:699–708.
Chung Y, Lee HW, Park JH, Yoo CH, Son BH, Kim K. Mutant pattern of p53 predicts local recurrence and poor survival rate in gastric cancer. Histol Histopathol. 2023;18596.
León-Castillo A, Gilvazquez E, Nout R, Smit VT, McAlpine JN, McConechy M, et al. Clinicopathological and molecular characterisation of “multiple-classifier” endometrial carcinomas. J Pathol. 2020;250:312–22.
Tsang HF, Xue VW, Koh SP, Chiu YM, Ng LPW, Wong SCC. NanoString, a novel digital color-coded barcode technology current and future applications in molecular diagnostics. Expert Rev Mol Diagn. 2017;17:95–103.
Winterhoff B, Hamidi H, Wang C, Kalli KR, Fridley BL, Dering J, et al. Molecular classification of high grade endometrioid and clear cell ovarian cancer using TCGA gene expression signatures. Gynecol Oncol. 2016;141:95–100.
Bolton KL, Chen D, Corona de la Fuente RI, Fu Z, Murali R, Köbel M, et al. Molecular subclasses of clear cell ovarian carcinoma and their impact on disease behavior and outcomes. Clin Cancer Res. 2022. https://doi.org/10.1158/1078-0432.CCR-21-3817.
Cancer Genome Atlas Research Network, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73.
Gonçalves A, Bertucci F, Guille A, Garnier S, Adelaide J, Carbuccia N, et al. Targeted NGS, array-CGH, and patient-derived tumor xenografts for precision medicine in advanced breast cancer: a single-center prospective study. Oncotarget. 2016;7:79428–41.
Bertucci F, Gonçalves A, Guille A, Adelaïde J, Garnier S, Carbuccia N, et al. Prospective high-throughput genome profiling of advanced cancers: results of the PERMED-01 clinical trial. Genome Med. 2021;13:87.
Bertucci F, Finetti P, Guille A, Adélaïde J, Garnier S, Carbuccia N, et al. Comparative genomic analysis of primary tumors and metastases in breast cancer. Oncotarget. 2016;7:27208–19.
Abkevich V, Timms KM, Hennessy BT, Potter J, Carey MS, Meyer LA, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer. 2012;107:1776–82.
Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–8.
Suzuki R, Shimodaira H. Pvclust: an R package for assessing the uncertainty in hierarchical clustering. Bioinformatics. 2006;22:1540–2.
Goldman MJ, Craft B, Hastie M, Repečka K, McDade F, Kamath A, et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol. 2020;38:675–8.
Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–27.
Taminau J, Steenhoff D, Coletta A, Meganck S, Lazar C, de Schaetzen V, et al. inSilicoDb: an R/Bioconductor package for accessing human Affymetrix expert-curated datasets from GEO. Bioinformatics. 2011;27:3204–5.
Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:1.
Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–8.
Lheureux S, McCourt C, Rimel BJ, Duska L, Fleming G, Mackay H, et al. Moving forward with actionable therapeutic targets and opportunities in endometrial cancer: a NCI clinical trials planning meeting report. Gynecol Oncol. 2018;S0090–8258(18):30124.
Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127:2930–40.
Bertucci F, Finetti P, Simeone I, Hendrickx W, Wang E, Marincola FM, et al. The immunologic constant of rejection classification refines the prognostic value of conventional prognostic signatures in breast cancer. Br J Cancer. 2018;119:1383–91.
Malorni L, Piazza S, Ciani Y, Guarducci C, Bonechi M, Biagioni C, et al. A gene expression signature of retinoblastoma loss-of-function is a predictive biomarker of resistance to palbociclib in breast cancer cell lines and is prognostic in patients with ER positive early breast cancer. Oncotarget. 2016;7:68012–22.
Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4: e297.
Concin N, Matias-Guiu X, Vergote I, Cibula D, Mirza MR, Marnitz S, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer. 2021;31:12–39.
Makker V, MacKay H, Ray-Coquard I, Levine DA, Westin SN, Aoki D, et al. Endometrial cancer. Nat Rev Dis Primers. 2021;7:88.
Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, et al (2016) ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer diagnosis, treatment and follow-up. Annals of Oncology. 27: 16–41.
Yuce Sari S, Guler OC, Oymak E, Gultekin M, Yigit E, Kahvecioglu A, et al. Uterine papillary serous and clear cell carcinomas: comparison of characteristics and clinical outcomes. J Obstet Gynaecol Res. 2022;48:1876–87.
Rambau PF, Vierkant RA, Intermaggio MP, Kelemen LE, Goodman MT, Herpel E, et al. Association of p16 expression with prognosis varies across ovarian carcinoma histotypes: an ovarian tumor tissue analysis consortium study. J Pathol Clin Res. 2018;4:250–61.
Pasanen A, Loukovaara M, Ahvenainen T, Vahteristo P, Bützow R. Differential impact of clinicopathological risk factors within the 2 largest ProMisE molecular subgroups of endometrial carcinoma. PLoS ONE. 2021;16: e0253472.
Kim SR, Cloutier BT, Leung S, Cochrane D, Britton H, Pina A, et al. Molecular subtypes of clear cell carcinoma of the endometrium: opportunities for prognostic and predictive stratification. Gynecol Oncol. 2020;158:3–11.
Köbel M, Tessier-Cloutier B, Leo J, Hoang LN, Gilks CB, Soslow RA, et al. Frequent mismatch repair protein deficiency in mixed endometrioid and clear cell carcinoma of the endometrium. Int J Gynecol Pathol. 2017;36:555–61.
Travaglino A, Raffone A, Santoro A, Raimondo D, Angelico G, Valente M, et al. Clear cell endometrial carcinomas with mismatch repair deficiency have a favorable prognosis: a systematic review and meta-analysis. Gynecol Oncol. 2021;162:804–8.
DeLair DF, Burke KA, Selenica P, Lim RS, Scott SN, Middha S, et al. The genetic landscape of endometrial clear cell carcinomas. J Pathol. 2017;243:230–41.
Murali R, Davidson B, Fadare O, Carlson JA, Crum CP, Gilks CB, et al. High-grade endometrial carcinomas: morphologic and immunohistochemical features, diagnostic challenges and recommendations. Int J Gynecol Pathol. 2019;38(Suppl 1):S40-63.
Kalinsky K, Hong F, McCourt CK, Sachdev JC, Mitchell EP, Zwiebel JA, et al. Effect of capivasertib in patients with an AKT1 E17K-mutated tumor: NCI-MATCH subprotocol EAY131-Y nonrandomized trial. JAMA Oncol. 2020. https://doi.org/10.1001/jamaoncol.2020.6741.
Westin SN, Labrie M, Litton JK, Blucher A, Fang Y, Vellano CP, et al. Phase 1b dose expansion and translational analyses of olaparib in combination with capivasertib in recurrent endometrial, triple negative breast, and ovarian cancer. Clin Cancer Res. 2021. https://doi.org/10.1101/2021.04.13.21255421.
Heudel P, Frenel J-S, Dalban C, Bazan F, Joly F, Arnaud A, et al. Safety and efficacy of the mTOR inhibitor, vistusertib, combined with anastrozole in patients with hormone receptor-positive recurrent or metastatic endometrial cancer: the VICTORIA multicenter, open-LABEL, phase 1/2 randomized clinical trial. JAMA Oncol. 2022. https://doi.org/10.1001/jamaoncol.2022.1047.
Konstantinopoulos PA, Gockley AA, Xiong N, Krasner C, Horowitz N, Campos S, et al. Evaluation of treatment with talazoparib and avelumab in patients with recurrent mismatch repair proficient endometrial cancer. JAMA Oncol. 2022;8:1317–22.
Wang Y, Hoang L, Ji JX, Huntsman DG. SWI/SNF complex mutations in gynecologic cancers: molecular mechanisms and models. Annu Rev Pathol. 2020;15:467–92.
Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–43.
Alldredge JK, Eskander RN. EZH2 inhibition in ARID1A mutated clear cell and endometrioid ovarian and endometrioid endometrial cancers. Gynecol Oncol Res Pract. 2017;4:17.
Banerjee S, Stewart J, Porta N, Toms C, Leary A, Lheureux S, et al. ATARI trial: ATR inhibitor in combination with olaparib in gynecological cancers with ARID1A loss or no loss (ENGOT/GYN1/NCRI). Int J Gynecol Cancer. 2021;31:1471–5.
Fader AN, Roque DM, Siegel E, Buza N, Hui P, Abdelghany O, et al. Randomized phase II trial of carboplatin-paclitaxel versus carboplatin-paclitaxel-trastuzumab in uterine serous carcinomas that overexpress human epidermal growth factor receptor 2/neu. J Clin Oncol. 2018;36:2044–51.
Gordeeva OO, Meshcheryakova LA, Karseladze AI, Meshcheryakova NA, Meshcheryakov AA. Combined anti-HER2 and hormonal treatment in a patient with HR+HER2+ clear-cell uterine carcinoma: a case report. Mol Clin Oncol. 2021;14:19.
Ray-Coquard I, Harter P, Lorusso D, Dalban C, Vergote I, Fujiwara K, et al. Effect of weekly paclitaxel with or without bevacizumab on progression-free rate among patients with relapsed ovarian sex cord-stromal tumors: the ALIENOR/ENGOT-ov7 randomized clinical trial. JAMA Oncol. 2020;6:1923–30.
Chang-Halpenny CN, Natarajan S, Hwang-Graziano JM. Early-stage uterine pure and mixed clear cell carcinoma: outcomes and recurrence with and without adjuvant therapy. Am J Clin Oncol. 2018;41:371–8.
Mateo J, Chakravarty D, Dienstmann R, Jezdic S, Gonzalez-Perez A, Lopez-Bigas N, et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO scale for clinical actionability of molecular targets (ESCAT). Ann Oncol. 2018;29:1895–902.
Acknowledgements
Authors would like to thank Pr Emmanuelle Charafe-Jauffret and the ICEP (IPC/CRCM experimental pathology) platform for their help in TMA analyses. We also want to thank all pathologists involved in the diagnostic process of the patients included in this work: Dr Marie-Aude Le Frere-Berda (APHP), Dr Isabelle Treilleux (Centre Leon Berard), Dr Laurent Arnould (Centre Georges-Francois Leclerc), Dr Eliane Mery (IUCT Oncopole), Dr Sebastien Henno (Centre Eugene Marquis), Pr Emmanuelle Charafe-Jauffret (Institut Paoli-Calmettes). We also would like to give special thanks to the clinical research associates, medical oncologists and medical interns who actively contributed to data collection/management or other organizational aspects of the TMRG network. We equally wish to thank all clinicians and pathologists of the TMRG network for their work and implication which is essential as it contributes to promote research studies such as this one, in the field of rare gynecological cancers. Finally, we would like to thank all the patients who have accepted to be registered on the TMRG network, thus allowing re- search studies in order to better treat, diagnose and establish the prognosis of patients with rare gynecological tumors.
Funding
This study was conducted with the help of ARCAGY-GINECO and TMRG is also supported by INCA.
Author information
Authors and Affiliations
Contributions
Design of the study: EN, VdC, RS. Molecular and statistical analyzes: PF, AG, JA. Patients monitoring and data collection: CL, AM, IRC, CC, AL, LF, ASB, VdC, MP, RS. Data interpretation: EN, VdC, FB, RS. Original draft: EN and RS. Validation of the final draft for submission: all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All patients have been informed that their data could be used for further research studies. For biological analyzes, they should have personally signed and dated informed consent (whether TRMG network or institutional consents).
Competing interests
The authors declare no competing interests that could have influenced this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
V11 NGS panel.
Additional file 2: Table S2.
Genes added to the Cancer Pathway panel for transcriptomic analyses.
Additional file 3: Table S3.
Supervised Analysis, 410 Endometrioid Endometrial Uterine Cancer (EEUC from TCGA) vs. 47 UCCC.
Additional file 4: Table S4.
Supervised analyses, 237 celar cell ovarian cancers (OvCC) vs. 47 UCCC.
Additional file 5: Table S5.
Deleterious DNA mutations identified by NGS (N=16 UCCC)
Additional file 6: Figure S1.
Kaplan Meier curves for progression-free survivaland overall survival.
Additional file 7: Figure S2.
Supervised analysis of gene expression profiles of UCCC and a set of ovarian clear cells tumors.Volcano plots of differential mRNA expression between UCCC and ovarian clear cell tumorsand normal ovarian tissue. Filtering of ovarian tissue related genes allow idenitification of 207 differentially expressed genes.
Additional file 8: Figure S3.
Copy number alterations profiles of uterine CCC tumors from the TMRGcohort.Frequency plot of recurrent copy number alterations identified in UCCC tumors using the GISTIC algorithm. Frequencies of gainsand lossesare plotted as a result of chromosome location. X-axis: top = log–scale ratio; bottom = q-values. Green lines represent the threshold for significance.Supervised analysis comparing uterine CCC samples to endometrial tumors from TCGA dataset. Dotted line: Threshold of significance associated with a False Discovery Rate < 0.25.Homologous recombination deficiencyscore between TMRG and TCGA cohorts.
Additional file 9: Figure S4.
ProMisE classification according to TMA and t-NGSdata.Distribution of molecular subtypes in our set.HRDMSIscore according to molecular subtype.TMBaccording to molecular subtype.Kaplan Meier curves for progression-free survival. MMRd: mismatch repair deficient; NSMP: non-specific molecular profile.
Additional file 10: Figure S5.
Supervised analysis of copy number alterations comparing cases with or without disease progression. Dotted line: Threshold of significance associated with a False Discovery Rate < 0.25.
Additional file 11: Materials S1.
aCGH data.
Additional file 12: Materials S2.
Nanostring nCounter mRNA data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nigon, E., Lefeuvre-Plesse, C., Martinez, A. et al. Clinical, pathological, and comprehensive molecular analysis of the uterine clear cell carcinoma: a retrospective national study from TMRG and GINECO network. J Transl Med 21, 408 (2023). https://doi.org/10.1186/s12967-023-04264-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-023-04264-7