Abstract
Mitochondria determine the physiological status of most eukaryotes. Mitochondrial dynamics plays an important role in maintaining mitochondrial homeostasis, and the disorder in mitochondrial dynamics could affect cellular energy metabolism leading to tumorigenesis. In recent years, disrupted mitochondrial dynamics has been found to influence the biological behaviors of gastrointestinal cancer with the potential to be a novel target for its individualized therapy. This review systematically introduced the role of mitochondrial dynamics in maintaining mitochondrial homeostasis, and further elaborated the effects of disrupted mitochondrial dynamics on the cellular biological behaviors of gastrointestinal cancer as well as its association with cancer progression. We aim to provide clues for elucidating the etiology and pathogenesis of gastrointestinal cancer from the perspective of mitochondrial homeostasis and disorder.
Similar content being viewed by others
Introduction
Latest research indicates that gastrointestinal cancer is a malignant tumor of digestive system with the highest morbidity and mortality worldwide. Among them, gastric cancer has the fifth incidence and the fourth mortality rate. Colorectal cancer is a malignancy with the third incidence and the second mortality rate [1]. Exploration for the etiology and pathogenesis of gastrointestinal cancer would improve the individualized therapy and survival of cancer patients with great scientific significance and application value.
In recent years, a novel molecular perspective for studying tumorigenesis is the communication between cell organs and nucleus as well as the roles of cell organs in maintaining homeostasis [2]. Mitochondrion is a semi-autonomous organ in cells with its own DNA, making it a special organ co-regulated by nuclear DNA and mtDNA [3]. Mitochondria are organelles with double-layered membranes in eukaryotic cells taking the main place for cellular aerobic respiration. They are not only involved in energy metabolism but also generate reactive oxygen species (ROS) through electron transport controlling cell apoptosis and other functions [4, 5]. Mitochondria continuously fissure and fuse forming a homeostasis called mitochondrial dynamics [6,7,8]. As a highly dynamic organelle, mitochondrion maintains homeostasis by the aid of mitochondrial quality control (MQC) system. MQC system is in composed of mitochondrial dynamic balance (fusion and fission), biogenesis and mitophagy [9]. Mitochondrial fusion and fission can not only regulate mitochondria independently but also interact with other balances in MQC, forming a regulatory network to synergistically keep the normal function of mitochondria. When the dynamics is disrupted, MQC becomes abnormal and the homeostasis will be damaged resulting in structural damage, mitochondrial dysfunction and eventually tumorigenesis. It has been paid increased attention that mitochondrial dynamics functions in cancer genesis and progression.
This review briefly introduced the normal and aberrant status of mitochondrial dynamics, sorted out the role of mitochondrial dynamics in maintaining mitochondrial homeostasis and elaborated the effects of mitochondrial dysfunction on cellular biological behaviors and progression of gastrointestinal tumor. We aim to provide theoretical basis for elucidating the etiology and pathogenesis of gastrointestinal tumor from the perspective of mitochondrial homeostasis and disorder.
Roles of mitochondrial dynamics in maintaining mitochondrial homeostasis
Basis of mitochondrial homeostasis maintenance–mitochondrial quality control
Mitochondrial quality control (MQC) system acts as a “monitoring checker” and monitors dynamic balance to meet the energy demands and metabolic activity of cells maintaining mitochondrial homeostasis [10]. Mitochondria manage the process of fission and fusion, biogenesis and mitophagy through MQC to eliminate damaged or aging mitochondria and synthesize new mitochondria, ensuring the stability of mitochondrial quantity, morphology, quality and internal environment.
Substantial evidence suggested that disrupted mitochondrial homeostasis played a critical role in cancer genesis and progression. Across all the four parts of MQC including fusion, fission, biogenesis and mitophagy, disorder appears in any one of them that cannot be corrected could damage the homeostasis causing diseases related to mitochondrial dysfunction. The expression levels of relevant molecules are associated with disease progression. Detection for them has the potential to be diagnostic biomarkers and therapeutic targets of diseases.
Balance of mitochondrial dynamics (fusion and fission)
Mitochondrial dynamics keeps the balance and remodels mitochondrial network by fusion and fission to adapt to the needs of various cells and tissue, which is essential for the regulation of mitochondrial homeostasis.
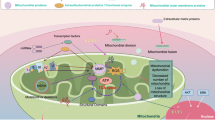
Fusion enables damaged and healthy mitochondrial contents to fully mix making metabolites exchanged. That may help to reduce the stress, prevent excessive fragment and maintain the morphology of mitochondria. Mitochondrial expansion in a network strengthens the oxidation capacity of metabolically active cells to increase ATP production catering to the high energy demands of cells [11, 12]. Mitochondrial fusion requires outer membrane (OMM) fusion mediated by mitofusion 1/2 (MFN1/2) and inner membrane (IMM) fusion mediated by optic atrophy 1 (OPA1), a GTPase. During OMM fusion, MFN1/2 can impel two close mitochondria to combine together by their interaction. Then mitochondrial phospholipase D (mitoPLD) alters the composition of membranes, forms smaller lipids as the second messenger to activate signaling pathways and hydrolyze allostery, and enables GTPases to mediate the fusion of mitochondrial membranes [13, 14]. In IMM fusion, OPA1 acts on mitochondrial IMM to affect its stability [15]. After OMM and IMM fusion, damaged mitochondria are replaced with newborn mitochondria to buffer the internal pressure (Fig. 1A).
Mitochondrial homeostasis is maintained by MQC system. Mitochondrial quality control system is composed of the balance between fusion (A) and fission (B), and between biogenesis (C) and mitophagy (D). A. Fusion. Mitochondria mediate fusion of IMM and OMM through OPA1 and MFN1/2. B. Fission. Mitochondria complete fission through fission-related factors such as DRP1 and dynein. C. Mitochondrial biogenesis. AMPK and SIRT1 pathways activate the PGC-1α/NRF1/2/TAFM axis and affect mitochondrial biogenesis. D. Mitophagy. Mitochondrial mitophagy is initiated to eliminate damaged mitochondria generated from disrupted fission and fusion
Mitochondrial fission also plays an indispensable role in maintaining mitochondrial morphology, which is a multi-step process coordinated by multiple factors [16]. DRP1 (dynamin-related protein 1) in cytoplasm is recruited to OMM, the self-assembled spherical oligomers slowly wrap the mitochondria and cut off the mitochondria to complete the fission. It requires the interaction among DRP1, mitochondrial adaptor proteins including mitochondrial fission factor (MFF), mitochondrial fission protein1 (FIS1), mitochondrial dynamic protein of 49kD (MiD49) and mitochondrial dynamic protein of 51kD (MiD51) as well as the organelle endoplasmic reticulum. First, mitochondria associate with endoplasmic reticulum (ER) to communicate information. Second, ER mediates DRP1 to bind to mitochondrial adaptor protein on OMM making DRP1 assembled into a spiral at the fission site. Finally, DRP1 hydrolyzes GTP to divide a mitochondrion into two sub-mitochondria (Fig. 1B).
Fusion and fission in mitochondria convert into each other under constant movement to form dynamic balance, which coordinately regulates mitochondrial morphology and enables mitochondria to respond accordingly to the changes of intracellular environment [17]. On the one hand, when cells are metabolically active with higher metabolic demands, mitochondria are elongated by fusion, the area of mitochondrial cristae increases and more ATP is generated for required energy [18]. On the other hand, aging cells produce excessive ROS and damaged mitochondria accumulate continuously. Mitochondrial fission is initiated to fragment damaged mitochondria into smaller pieces facilitating the clearance by mitophagy to prevent further damage of ROS [19]. The subtle regulation between fusion and fission ensures mitochondrial adaptability and keeps mitochondrial contents in a dynamic balance.
Balance of mitochondrial biogenesis and mitophagy
Mitochondrial biogenesis and mitophagy are suggested to be opposite processes constructing mitochondrial turnover together [20]. In mitochondrial biogenesis, proteins originated from nucleus enter into mitochondria to promote mitochondrial newborn and increase their number. While in mitophagy, damaged or aging mitochondria are self-selectively removed or degraded. Relative balance between the two courses maintains number stability and metabolic homeostasis in the mitochondrial pool [21].
Mitochondrial biogenesis supplies “fresh blood” to the mitochondrial pool and guarantees mitochondrial activity [22]. PGC-1α (PPARγ co-activator-1alpha)/NRF (nuclear respiratory factor)/TFAM (mitochondrial transcription factor A) is currently recognized as the key regulatory axis of biogenesis. AMPK-PGC-1α and SIRT1 (sirtuin1)-PGC-1α are the two major pathways regulating mitochondrial biogenesis [22]. In the AMPK-PGC-1α axis, AMPK can be activated along with increased AMP resulting in PGC-1α phosphorylation and the activation of whole pathway [23]. As for the SIRT1 (sirtuin1)-PGC-1α axis, SIRT1 (sirtuin1) is activated due to increased NAD/NADH and PGC-1α is further activated by SIRT1 deacetylation [24]. PGC-1α stimulated by AMPK and SIRT1 binds to NRF1/2 up-regulating TFAM. The elevation of transcription factor TFAM promotes mtDNA replication and transcription. Mounting mtDNA cooperates with mitochondrial protein encoded by nDNA (nucleus DNA) to trigger mitochondrial biogenesis [25] (Fig. 1C).
During mitochondrial mitophagy, damaged mitochondria are specifically and selectively degraded by autophagy in cells, which is a self-protective process [26, 27]. PINK1 (PTEN-induced putative kinase protein1) and PARK2 (cytosolic ubiquitin E3 ligase, Parkin) are the key proteins in the course. Mitophagy initiated by them could protect mitochondria from oxidative stress, prevent ROS overproduction and complete self-renewal in metabolically active tissue such as BAT (brown adipose tissue) [28] (Fig. 1D).
The balance between mitochondrial biogenesis and mitophagy is necessary for cells to recover from stressful and damaged status [29]. In mitochondrial biogenesis, PGC-1α and NRF1 were shown to up-regulate protein expression of mitophagy receptor FUNDC1, while knockout of FUNDC1 caused PGC-1α down-regulation. Therefore, mitochondrial biogenesis was accompanied with mitophagy and abnormal mitophagy could inhibit biogenesis as feedback. In some cells with activated mitophagy, the activation of biogenesis was also discovered [28, 30, 31]. These may illustrate that mitochondria require biogenesis to adapt to cellular changes in energy demands caused by mitophagy, which is regarded as an anabolic-catabolic balance [32].
Central roles of mitochondrial dynamics in mitochondrial homeostasis
Mitochondrial dynamics structured by fusion and fission has been found to have a certain regulatory effect on mitochondrial biogenesis and mitophagy, which constitute the core of mitochondrial homeostasis.
Numerous studies have shown that mitochondrial fusion and fission are closely associated with mitophagy. Disrupted mitochondrial dynamics affects the degradation of damaged organelles by mitophagy. When mitochondria undergo asymmetric fission, DRP1 segregates components of damaged mitochondria into a depolarized sub-organelle for mitophagy [33]. Hence, mitophagy is initiated immediately after mitochondrial fission [34]. Although DRP1 was suggested to be necessary for mitophagy [35], some studies demonstrated that mitophagy might be independent from it [36, 37]. Jonathan et al. proposed a new point that fission did not promote mitophagy directly but protect healthy mitochondrial subdomains from unexamined PINK1-Parkin feedback [38]. And mitochondrial fragmentation due to the loss of fusion resisted the turnover of mitophagy. MFN2 is an important regulator of the PINK-MFN2-Parkin mitophagy axis [39]. It drives mitophagy through ubiquitination by PINK1 and Parkin [40]. Therefore, the imbalance of fission and fusion can result in significant changes in mitochondrial mitophagy.
The importance of mitochondrial dynamics for biogenesis has also been emphasized by plenty of research [41, 42]. The reduction of DRP1 and FIS1 by an inhibitor of mitochondrial fission (Mdivi-1) increased the key regulators of biogenesis including PGC-1α, NRF1, NRF2 and TFAM [43]. A latest study showed that the activation of mitochondrial fission downregulated PGC-1α/PPARα signaling in hepatocellular carcinoma (HCC) cells and inhibited SIRT1 driving metabolic reprogramming in HCC [44]. Alteration in mitochondrial biogenesis can affect the dynamics in turn? In cardiomyocytes, the elevated expression of NRF2 could down-regulate DRP1 and up-regulate MFN2 leading to excessive mitochondrial fusion [45]. Ding et al. found that PGC-1α could bind to the transcriptional promoter of DRP1 to inhibit DRP1-mediated fission relieving diabetes-induced cardiac insufficiency [46]. Natia et al. also reported that PGC-1α inhibited DRP1 expression and improved myocardial ischemia–reperfusion injury [47]. In addition, PGC-1α inhibited mitophagy by attenuating MFN2 ubiquitination and degradation [48]. All these suggested that mitochondrial dynamic balance was closely associated with biogenesis making impacts on mitochondrial homeostasis.
Mitochondrial dysfunction and cancer based on big data analysis
Mitochondrial dysfunction is closely associated with tumorigenesis. It has been proven to affect oncogenic pathways and multiple cancer phenotypes (Table 1).
A great deal of big data analyses was conducted focusing on mitochondria-related biomarkers as therapeutic targets of cancer. In 72 non-small cell lung cancer (NSCLC) cases, overexpressed MFF formed complex with the key regulator of mitochondrial OMM permeability VDAC1 to regulate cell apoptosis [49]. Similar phenomenon was also observed in 192 patients of prostate cancer [50]. Among prognosis study, a pan-cancer analysis demonstrated that low expressed MFN2 due to mitochondrial dysfunction was associated with poor prognosis of renal clear cell carcinoma [51]. In 321 breast cancer cases, patients with high expression of Pink1 had shorter overall survival [52]. Relevant mechanisms have been preliminarily explored. Zamberlan M et al. revealed the close association of up-regulated OPA1 with poor prognosis of breast cancer based on bioinformatic databases. In breast cancer cells with OPA1 knock-down, the expression levels of miR-148/152 family increased inhibiting tumor growth and invasion. Therefore, mitochondrial dysfunction caused by OPA1 might regulate invasion and metastasis of breast cancer via the miR-148/152 family [53]. A total of 522 differential genes were identified by Li et al. [54] through RNA sequencing in HCC cells with OPA1 knock-down and 33 of them had most significant changes in metabolic pathways, suggesting that mitochondrial dysfunction resulted from aberrant fusion might promote HCC cell proliferation by cellular metabolism. Some lncRNAs were found to form complex with NRF1 to activate mitochondrial biogenesis in HCC [55] and colorectal cancer patients [56]. NRF1 was also found to activate E2F1 as a transcription factor to promote HCC proliferation by a ChIP-seq analysis for NRF1 target genes [57]. Similarly, the knock-down of TFAM could promote cancer progression according to bioinformatic databases of 18 head and neck cancer cases [58] as well as ovarian cancer [59]. All the big data analyses showed that mitochondrial dysfunction exerted roles in various cancer, which provided statistical clues for cancer therapy to some degree.
Disorder in mitochondrial dynamics and gastrointestinal cancer
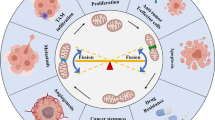
Mitochondrial dysfunction due to disrupted dynamics has been referred in many tumors, while its role in gastrointestinal tumor is rarely studied. Exploration for the mechanism of mitochondrial dysfunction is a promising field in gastrointestinal tumor. Recent research found that mitochondrial dysfunction was involved in cellular biological behaviors and progression of gastrointestinal tumor (Fig. 2).
Disorder in mitochondrial dynamics and cellular biological behaviors of gastrointestinal tumor
Disorder in mitochondrial dynamics and cell proliferation of gastrointestinal tumor
Mitochondrial over-fission enables tumor cells to proliferate in an unrestricted manner. Highly connected mitochondria could be observed in tumor cell cycle G1/S, which was thought to ensure a sufficient supply of ATP for tumor cell proliferation [115]. As a potential anticancer agent, PSII was found to inhibit colony formation and cell cycle arrest of G1 phase in HCT116 cell line, which might be caused by DRP1 knockdown inhibiting mitochondrial fission [116]. Therefore, disrupted mitochondrial dynamics may participate in cell proliferation of gastrointestinal tumor.
Disorder in mitochondrial dynamics and cell apoptosis of gastrointestinal tumor
Cellular apoptosis caused by disrupted mitochondrial dynamics has been extensively investigated. Yao et al. [117] believed that mitochondrial fission could increase ROS, activate caspase-9, induce cell apoptosis and decrease the viability of gastric cancer cells. Similarly, Somnath Mazumder et al. [118] suggested that indomethacin, a non-steroidal anti-inflammatory drug, might induce cell apoptosis of stomach cancer and impair mitochondrial dynamics by activating DRP1. Additionally, miR-148a-3p was identified as a miRNA with tumor suppressing effect. It was shown to enhance cell apoptosis induced by cisplatin through aggravating mitochondrial fission in gastric cancer cells intending to get better therapeutic effect, which was a first-line drug for treating locally advanced or metastatic gastric cancer [119]. Consistent result was also presented in mitochondrial membrane protein 18 (MTP18) for increasing DRP1 accumulation and promoting cell apoptosis of gastric cancer [120]. Moreover, under the stimulation of oxidative stress, the phosphorylation of atypical ERK sites in MFN1 increased the permeability of mitochondrial membrane and the oligomerization of apoptosis-related factor BAK (BCL-2 family member), promoted the release of cytochrome c and then cell apoptosis [121]. Therefore, any alteration in fusion and fission could disturb mitochondrial dynamics inducing cell apoptosis.
Disorder in mitochondrial dynamics and cell invasion & metastasis of gastrointestinal tumor
Mounting evidence suggested that disrupted mitochondrial dynamics was also involved in cell invasion and migration of gastrointestinal tumor. Mitochondrial fragmentation caused by over-fission increased the number of malignant cells and promoted the invasion of tumor cells in breast cancer [122]. FIS1 overexpression might be strongly associated with metastasis [81]. EBV virus was reported to induce mitochondrial fission by increasing DRP1 to promote Notch pathway-mediated migration of gastric cancer cells [123]. Besides, DRP1 knockout or Mfn1/2 overexpression was shown to increase fusion/fission ratio and significantly reduce migratory and invasive potentials of cancer cells [124]. Mfn1/2 overexpression in gastric cancer cells decreased their ability to migrate and invade, induced cell apoptosis via the PI3K-Akt pathway and impelled cell cycle to stagnate in G0/G1 phase [125].
All above-mentioned findings suggested that the disorder in mitochondrial dynamics affected malignant cellular biological behaviors of gastrointestinal tumor, and the key molecules could be targeted for tumor therapy.
Disorder in mitochondrial dynamics and the genesis, progression and prognosis of gastrointestinal tumor
Disorder in mitochondrial dynamics and the genesis of gastrointestinal tumor
Disrupted mitochondrial dynamics has been confirmed to participate in all aspects of tumorigenesis. It has considerable prospect in the early and non-invasive diagnosis of gastric cancer. Mfn2 expression in normal gastric mucosa was found to be low and negatively correlated with tumor size. Moreover, Mfn2 could inhibit cell proliferation, induce apoptosis and weaken the invasiveness of gastric cancer by arresting cell cycle. Therefore, aberrant Mfn2 was linked to gastric carcinogenesis [125]. Chen et al. reported that p65 with its target genes cyclin D1 and c-Myc were down-regulated by knocking down DRP1 in HCT116 cell line, indicating that mitochondrial fission might inhibit colorectal carcinogenesis by activating the NF-kB pathway [116]. The antiallergic drug azelastine inhibited the IQGAP1-ERK-DRP1 pathway by targeting ADP-ribosylation factor 1 (ARF1), suppressed mitochondrial fission and colon carcinogenesis [66]. The aberrant expression of characteristic proteins causes disrupted mitochondrial dynamics and cancer initiation with the potential to be applied to gastrointestinal tumor treatment.
Disorder in mitochondrial dynamics and the progression & prognosis of gastrointestinal tumor
DRP1 was shown to be up-regulated in gastric cancer patients with cachexia, suggesting that mitochondrial dysfunction might be involved in the progression of gastric cancer [126]. Recently, BRAF V600E was revealed to be a quite common mutation in colon cancer. And a higher DRP1 level was also presented in colon cancer cells with BRAF V600E than in BRAF WT cells. Therefore, DRP1 might promote the progression of colorectal cancer driven by BRAF V600E [68].
The poor prognosis of gastrointestinal tumor is closely associated with tumor cell invasion and migration. In advanced gastric cancer of infiltrative (Borrmann III) and diffuse infiltrative (Borrmann IV) types, FIS1 expression increases and promotes cancer metastasis, indicating that the poor prognosis of gastrointestinal tumor may be associated with mitochondrial over-fission [81]. OMA1 is the precursor of different isoforms of OPA1. It was found to be highly expressed in gastric cancer and associated with poor prognosis [127]. Decreased Mfn2 also manifested the association with an aggravation in the stage of gastric cancer and a poorer overall survival [122]. Therefore, disrupted mitochondrial dynamics influences the progression and prognosis of gastrointestinal tumor.
Summary and future direction
As the core of cellular metabolism, mitochondria appear to be vital in physiological process, pathological process and disease progression. The disorder in mitochondrial dynamics may regulate the cellular biological behaviors and progression of gastrointestinal tumor [83, 128, 129]. The mechanism about mitochondrial dysfunction with gastrointestinal cancer, however, remain poorly understood with the necessity to be further studied especially for the specific oncogenic pathways that mitochondrial dynamics participates in. Additionally, the clinical analysis of big data is also lacking for the expression of key molecules in mitochondrial dynamics during gastrointestinal cancer. Although the association of mitochondrial dynamics has been suggested with chemotherapy sensitivity of gastrointestinal cancer [119], more investigations are needed to support its roles in other aspects of cancer therapy such as drug resistance. The novel research has promising values in the diagnosis, treatment and prognosis of gastrointestinal cancer that cannot be neglected.
Availability of data and materials
Not applicable.
Abbreviations
- MQC:
-
Mitochondrial quality control
- mtDNA:
-
Mitochondrial DNA
- GTPase:
-
Guanosine triphosphatase
- GTP:
-
Guanosine triphosphate
- ATP:
-
Adenosine triphosphate
- ROS:
-
Reactive oxygen species
- DRP1:
-
Dynamin-related protein 1
- OMM:
-
Outer membranes
- IMM:
-
Inner membranes
- Cytc:
-
Cytochrome C
- MFN1/2:
-
Mitofusion 1/2
- OPA1:
-
Optic atrophy 1
- ER:
-
Endoplasmic reticulum
- MFF:
-
Mitochondrial fission factor
- Fis 1:
-
Mitochondrial fission protein1
- MiD49:
-
Mitochondrial dynamics protein of 49kD
- MiD51:
-
Mitochondrial dynamics protein of 51kD
- MTP18:
-
Mitochondrial membrane protein 18
- PGC-1α:
-
PPARγ co-activator-1alpha
- NRF:
-
Nuclear respiratory factor
- TFAM:
-
Mitochondrial transcription factor A
- SIRT1:
-
Sirtuin1
- PINK1:
-
PTEN-induced putative kinase protein1
- PARK2:
-
Cytosolic ubiquitin E3 ligase, Parkin
- BAT:
-
Brown adipose tissue
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49.
Hou XS, Wang HS, Mugaka BP, Yang GJ, Ding Y. Mitochondria: promising organelle targets for cancer diagnosis and treatment. Biomater Sci. 2018;6(11):2786–97.
Taylor DE, Kantrow SP, Piantadosi CA. Mitochondrial respiration after sepsis and prolonged hypoxia. Am J Physiol. 1998;275(1):L139–44.
Wallace DC, Fan W, Procaccio V. Mitochondrial energetics and therapeutics. Annu Rev Pathol. 2010;5:297–348.
Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol. 2012;13(12):780–8.
Kim JC, Park GD, Kim SH. Inhibition of oxidative stress by antioxidant supplementation does not limit muscle mitochondrial biogenesis or endurance capacity in rats. J Nutr Sci Vitaminol (Tokyo). 2017;63(5):277–83.
Cormio A, Musicco C, Gasparre G, Cormio G, Pesce V, Sardanelli AM, et al. Increase in proteins involved in mitochondrial fission, mitophagy, proteolysis and antioxidant response in type I endometrial cancer as an adaptive response to respiratory complex I deficiency. Biochem Biophys Res Commun. 2017;491(1):85–90.
Di Pietro V, Lazzarino G, Amorini AM, Signoretti S, Hill LJ, Porto E, et al. Fusion or fission: the destiny of mitochondria in traumatic brain injury of different severities. Sci Rep. 2017;7(1):9189.
Chang X, Lochner A, Wang HH, Wang S, Zhu H, Ren J, et al. Coronary microvascular injury in myocardial infarction: perception and knowledge for mitochondrial quality control. Theranostics. 2021;11(14):6766–85.
Simula L, Nazio F, Campello S. The mitochondrial dynamics in cancer and immune-surveillance. Semin Cancer Biol. 2017;47:29–42.
Arimura SI. Fission and fusion of plant mitochondria, and genome maintenance. Plant Physiol. 2018;176(1):152–61.
Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annu Rev Biochem. 2007;76:751–80.
Morita M, Prudent J, Basu K, Goyon V, Katsumura S, Hulea L, et al. mTOR controls mitochondrial dynamics and cell survival via MTFP1. Mol Cell. 2017;67(6):922–35.
Wong YC, Ysselstein D, Krainc D. Mitochondria-lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature. 2018;554(7692):382–6.
Li X, Li H, Xu Z, Ma C, Wang T, You W, et al. Ischemia-induced cleavage of OPA1 at S1 site aggravates mitochondrial fragmentation and reperfusion injury in neurons. Cell Death Dis. 2022;13(4):321.
Kraus F, Roy K, Pucadyil TJ, Ryan MT. Function and regulation of the divisome for mitochondrial fission. Nature. 2021;590(7844):57–66.
Wai T, Langer T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol Metab. 2016;27(2):105–17.
Khacho M, Tarabay M, Patten D, Khacho P, MacLaurin JG, Guadagno J, et al. Acidosis overrides oxygen deprivation to maintain mitochondrial function and cell survival. Nat Commun. 2014;5:3550.
Weir HJ, Yao P, Huynh FK, Escoubas CC, Goncalves RL, Burkewitz K, et al. Dietary restriction and AMPK increase lifespan via mitochondrial network and peroxisome remodeling. Cell Metab. 2017;26(6):884–96.
Palikaras K, Lionaki E, Tavernarakis N. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature. 2015;521(7553):525–8.
Palikaras K, Lionaki E, Tavernarakis N. Balancing mitochondrial biogenesis and mitophagy to maintain energy metabolism homeostasis. Cell Death Differ. 2015;22(9):1399–401.
Li PA, Hou X, Hao S. Mitochondrial biogenesis in neurodegeneration. J Neurosci Res. 2017;95(10):2025–9.
Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104(29):12017–22.
Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, et al. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007;26(7):1913–23.
Popov LD. Mitochondrial biogenesis: an update. J Cell Mol Med. 2020;24(9):4892–9.
Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A. 2010;107(1):378–83.
Lemasters JJ. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005;8(1):3–5.
Yau WW, Singh BK, Lesmana R, Zhou J, Sinha RA, Wong KA, et al. Thyroid hormone (T3) stimulates brown adipose tissue activation via mitochondrial biogenesis and MTOR-mediated mitophagy. Autophagy. 2019;15(1):131–50.
Zhu J, Wang KZ, Chu CT. After the banquet: mitochondrial biogenesis, mitophagy, and cell survival. Autophagy. 2013;9(11):1663–76.
Ivankovic D, Chau KY, Schapira AH, Gegg ME. Mitochondrial and lysosomal biogenesis are activated following PINK1/parkin-mediated mitophagy. J Neurochem. 2016;136(2):388–402.
Kang JW, Hong JM, Lee SM. Melatonin enhances mitophagy and mitochondrial biogenesis in rats with carbon tetrachloride-induced liver fibrosis. J Pineal Res. 2016;60(4):383–93.
Perez-Pinzon MA, Stetler RA, Fiskum G. Novel mitochondrial targets for neuroprotection. J Cereb Blood Flow Metab. 2012;32(7):1362–76.
Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27(2):433–46.
Park SJ, Shin JH, Kim ES, Jo YK, Kim JH, Hwang JJ, et al. Mitochondrial fragmentation caused by phenanthroline promotes mitophagy. FEBS Lett. 2012;586(24):4303–10.
Kageyama Y, Hoshijima M, Seo K, Bedja D, Sysa-Shah P, Andrabi SA, et al. Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J. 2014;33(23):2798–813.
Yamashita SI, Jin X, Furukawa K, Hamasaki M, Nezu A, Otera H, et al. Mitochondrial division occurs concurrently with autophagosome formation but independently of Drp1 during mitophagy. J Cell Biol. 2016;215(5):649–65.
Murakawa T, Yamaguchi O, Hashimoto A, Hikoso S, Takeda T, Oka T, et al. Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat Commun. 2015;6:7527.
Burman JL, Pickles S, Wang C, Sekine S, Vargas JNS, Zhang Z, et al. Mitochondrial fission facilitates the selective mitophagy of protein aggregates. J Cell Biol. 2017;216(10):3231–47.
Chen Y, Dorn GW 2nd. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340(6131):471–5.
McLelland GL, Goiran T, Yi W, Dorval G, Chen CX, Lauinger ND, et al. Mfn2 ubiquitination by PINK1/parkin gates the p97-dependent release of ER from mitochondria to drive mitophagy. Elife. 2018. https://doi.org/10.7554/eLife.32866.
Caffin F, Prola A, Piquereau J, Novotova M, David DJ, Garnier A, et al. Altered skeletal muscle mitochondrial biogenesis but improved endurance capacity in trained OPA1-deficient mice. J Physiol. 2013;591(23):6017–37.
Kim B, Kim JS, Yoon Y, Santiago MC, Brown MD, Park JY. Inhibition of Drp1-dependent mitochondrial division impairs myogenic differentiation. Am J Physiol Regul Integr Comp Physiol. 2013;305(8):R927–38.
Manczak M, Kandimalla R, Yin X, Reddy PH. Mitochondrial division inhibitor 1 reduces dynamin-related protein 1 and mitochondrial fission activity. Hum Mol Genet. 2019;28(2):177–99.
Wu D, Yang Y, Hou Y, Zhao Z, Liang N, Yuan P, et al. Increased mitochondrial fission drives the reprogramming of fatty acid metabolism in hepatocellular carcinoma cells through suppression of Sirtuin 1. Cancer Commun (Lond). 2022;42(1):37–55.
Chen QM. Nrf2 for protection against oxidant generation and mitochondrial damage in cardiac injury. Free Radic Biol Med. 2022;179:133–43.
Ding M, Feng N, Tang D, Feng J, Li Z, Jia M, et al. Melatonin prevents Drp1-mediated mitochondrial fission in diabetic hearts through SIRT1-PGC1alpha pathway. J Pineal Res. 2018;65(2):e12491.
Kelm NQ, Beare JE, Weber GJ, LeBlanc AJ. Thrombospondin-1 mediates Drp-1 signaling following ischemia reperfusion in the aging heart. FASEB Bioadv. 2020;2(5):304–14.
Kang C, Ji LL. PGC-1alpha overexpression via local transfection attenuates mitophagy pathway in muscle disuse atrophy. Free Radic Biol Med. 2016;93:32–40.
Seo JH, Chae YC, Kossenkov AV, Lee YG, Tang HY, Agarwal E, et al. MFF regulation of mitochondrial cell death is a therapeutic target in cancer. Cancer Res. 2019;79(24):6215–26.
Seo JH, Agarwal E, Chae YC, Lee YG, Garlick DS, Storaci AM, et al. Mitochondrial fission factor is a novel Myc-dependent regulator of mitochondrial permeability in cancer. EBioMedicine. 2019;48:353–63.
Cheng L, Wang Z, Nie L, Yang C, Huang H, Lin J, et al. Comprehensive analysis of MFN2 as a prognostic biomarker associated with immune cell infiltration in renal clear cell carcinoma. Int Immunopharmacol. 2022;111:109169.
Li Q, Chu Y, Li S, Yu L, Deng H, Liao C, et al. The oncoprotein MUC1 facilitates breast cancer progression by promoting Pink1-dependent mitophagy via ATAD3A destabilization. Cell Death Dis. 2022;13(10):899.
Zamberlan M, Boeckx A, Muller F, Vinelli F, Ek O, Vianello C, et al. Inhibition of the mitochondrial protein Opa1 curtails breast cancer growth. J Exp Clin Cancer Res. 2022;41(1):95.
Li M, Wang L, Wang Y, Zhang S, Zhou G, Lieshout R, et al. Mitochondrial fusion Via OPA1 and MFN1 supports liver tumor cell metabolism and growth. Cells. 2020;9(1):121.
Zhang J, Pan T, Zhou W, Zhang Y, Xu G, Xu Q, et al. Long noncoding RNA LINC01132 enhances immunosuppression and therapy resistance via NRF1/DPP4 axis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2022;41(1):270.
Liu X, Chen J, Zhang S, Liu X, Long X, Lan J, et al. LINC00839 promotes colorectal cancer progression by recruiting RUVBL1/Tip60 complexes to activate NRF1. EMBO Rep. 2022;23(9):e54128.
Wang D, Wan B, Zhang X, Sun P, Lu S, Liu C, et al. Nuclear respiratory factor 1 promotes the growth of liver hepatocellular carcinoma cells via E2F1 transcriptional activation. BMC Gastroenterol. 2022;22(1):198.
Hsieh YT, Tu HF, Yang MH, Chen YF, Lan XY, Huang CL, et al. Mitochondrial genome and its regulator TFAM modulates head and neck tumourigenesis through intracellular metabolic reprogramming and activation of oncogenic effectors. Cell Death Dis. 2021;12(11):961.
Baczewska M, Supruniuk E, Bojczuk K, Guzik P, Milewska P, Kononczuk K, et al. Energy substrate transporters in high-grade ovarian cancer: gene expression and clinical implications. Int J Mol Sci. 2022;23(16):8968.
Fan K, Ding X, Zang Z, Zhang Y, Tang X, Pei X, et al. Drp1-mediated mitochondrial metabolic dysfunction inhibits the tumor growth of pituitary adenomas. Oxid Med Cell Longev. 2022;2022:5652586.
Li Y, Chen H, Yang Q, Wan L, Zhao J, Wu Y, et al. Increased Drp1 promotes autophagy and ESCC progression by mtDNA stress mediated cGAS-STING pathway. J Exp Clin Cancer Res. 2022;41(1):76.
Xie C, Wang FY, Sang Y, Chen B, Huang JH, He FJ, et al. Mitochondrial micropeptide STMP1 enhances mitochondrial fission to promote tumor metastasis. Cancer Res. 2022;82(13):2431–43.
Xiong X, Hasani S, Young LEA, Rivas DR, Skaggs AT, Martinez R, et al. Activation of Drp1 promotes fatty acids-induced metabolic reprograming to potentiate Wnt signaling in colon cancer. Cell Death Differ. 2022;29(10):1913–27.
Huang TL, Chang CR, Chien CY, Huang GK, Chen YF, Su LJ, et al. DRP1 contributes to head and neck cancer progression and induces glycolysis through modulated FOXM1/MMP12 axis. Mol Oncol. 2022;16(13):2585–606.
Courtois S, de Luxan-Delgado B, Penin-Peyta L, Royo-Garcia A, Parejo-Alonso B, Jagust P, et al. Inhibition of mitochondrial dynamics preferentially targets pancreatic cancer cells with enhanced tumorigenic and invasive potential. Cancers (Basel). 2021;13(4):698.
Hu HF, Xu WW, Li YJ, He Y, Zhang WX, Liao L, et al. Anti-allergic drug azelastine suppresses colon tumorigenesis by directly targeting ARF1 to inhibit IQGAP1-ERK-Drp1-mediated mitochondrial fission. Theranostics. 2021;11(4):1828–44.
Prasad P, Ghosh S, Roy SS. Glutamine deficiency promotes stemness and chemoresistance in tumor cells through DRP1-induced mitochondrial fragmentation. Cell Mol Life Sci. 2021;78(10):4821–45.
Padder RA, Bhat ZI, Ahmad Z, Singh N, Husain M. DRP1 promotes BRAF(V600E)-driven tumor progression and metabolic reprogramming in colorectal cancer. Front Oncol. 2020;10:592130.
Zhao T, Guo BJ, Xiao CL, Chen JJ, Lu C, Fang FF, et al. Aerobic exercise suppresses hepatocellular carcinoma by downregulating dynamin-related protein 1 through PI3K/AKT pathway. J Integr Med. 2021;19(5):418–27.
Deng X, Liu J, Liu L, Sun X, Huang J, Dong J. Drp1-mediated mitochondrial fission contributes to baicalein-induced apoptosis and autophagy in lung cancer via activation of AMPK signaling pathway. Int J Biol Sci. 2020;16(8):1403–16.
Lee YG, Nam Y, Shin KJ, Yoon S, Park WS, Joung JY, et al. Androgen-induced expression of DRP1 regulates mitochondrial metabolic reprogramming in prostate cancer. Cancer Lett. 2020;471:72–87.
Gao T, Zhang X, Zhao J, Zhou F, Wang Y, Zhao Z, et al. SIK2 promotes reprogramming of glucose metabolism through PI3K/AKT/HIF-1alpha pathway and Drp1-mediated mitochondrial fission in ovarian cancer. Cancer Lett. 2020;469:89–101.
Liang J, Yang Y, Bai L, Li F, Li E. DRP1 upregulation promotes pancreatic cancer growth and metastasis through increased aerobic glycolysis. J Gastroenterol Hepatol. 2020;35(5):885–95.
Lin XH, Qiu BQ, Ma M, Zhang R, Hsu SJ, Liu HH, et al. Suppressing DRP1-mediated mitochondrial fission and mitophagy increases mitochondrial apoptosis of hepatocellular carcinoma cells in the setting of hypoxia. Oncogenesis. 2020;9(7):67.
Guo J, Ye F, Jiang X, Guo H, Xie W, Zhang Y, et al. Drp1 mediates high glucose-induced mitochondrial dysfunction and epithelial-mesenchymal transition in endometrial cancer cells. Exp Cell Res. 2020;389(1):111880.
Shi L, Liu J, Peng Y, Zhang J, Dai X, Zhang S, et al. Deubiquitinase OTUD6A promotes proliferation of cancer cells via regulating Drp1 stability and mitochondrial fission. Mol Oncol. 2020;14(12):3169–83.
Hu M, Zhao Y, Cao Y, Tang Q, Feng Z, Ni J, et al. DRP1 promotes lactate utilization in KRAS-mutant non-small-cell lung cancer cells. Cancer Sci. 2020;111(10):3588–99.
Wang B, Gan W, Han X, Li D. PRCC-TFE3 regulates migration and invasion of translocation renal cell carcinomas via activation of Drp1-dependent mitochondrial fission. Cell Biol Int. 2020;44(8):1727–33.
Liu B, Fan Y, Song Z, Han B, Meng Y, Cao P, et al. Identification of DRP1 as a prognostic factor correlated with immune infiltration in breast cancer. Int Immunopharmacol. 2020;89(Pt B):107078.
Tang Q, Liu W, Zhang Q, Huang J, Hu C, Liu Y, et al. Dynamin-related protein 1-mediated mitochondrial fission contributes to IR-783-induced apoptosis in human breast cancer cells. J Cell Mol Med. 2018;22(9):4474–85.
Karimi D, Pedram N, Kakaei F, Asadi M, Poursaei E, Kermani TA. FIS1 overexpression is correlated with tumor metastasis in gastric adenocarcinoma. J Gastrointest Cancer. 2022;53(2):466–71.
Phelan JJ, MacCarthy F, O’Toole D, Ravi N, Reynolds JV, O’Sullivan J. The mitochondrial genes BAK1, FIS1 and SFN are linked with alterations in mitochondrial membrane potential in Barrett’s esophagus. Int J Mol Sci. 2018;19(11):3483.
Li S, Han S, Zhang Q, Zhu Y, Zhang H, Wang J, et al. FUNDC2 promotes liver tumorigenesis by inhibiting MFN1-mediated mitochondrial fusion. Nat Commun. 2022;13(1):3486.
Wang D, Tian J, Yan Z, Yuan Q, Wu D, Liu X, et al. Mitochondrial fragmentation is crucial for c-Myc-driven hepatoblastoma-like liver tumors. Mol Ther. 2022;30(4):1645–60.
Zhang Z, Li TE, Chen M, Xu D, Zhu Y, Hu BY, et al. MFN1-dependent alteration of mitochondrial dynamics drives hepatocellular carcinoma metastasis by glucose metabolic reprogramming. Br J Cancer. 2020;122(2):209–20.
Ashraf R, Kumar S. Mfn2-mediated mitochondrial fusion promotes autophagy and suppresses ovarian cancer progression by reducing ROS through AMPK/mTOR/ERK signaling. Cell Mol Life Sci. 2022;79(11):573.
You MH, Jeon MJ, Kim SR, Lee WK, Cheng SY, Jang G, et al. Mitofusin-2 modulates the epithelial to mesenchymal transition in thyroid cancer progression. Sci Rep. 2021;11(1):2054.
Lin Z, Lin X, Chen J, Huang G, Chen T, Zheng L. Mitofusin-2 is a novel anti-angiogenic factor in pancreatic cancer. J Gastrointest Oncol. 2021;12(2):484–95.
Ahn SY, Song J, Kim YC, Kim MH, Hyun YM. Mitofusin-2 promotes the epithelial-mesenchymal transition-induced cervical cancer progression. Immune Netw. 2021;21(4):e30.
Pan L, Zhou L, Yin W, Bai J, Liu R. miR-125a induces apoptosis, metabolism disorder and migrationimpairment in pancreatic cancer cells by targeting Mfn2-related mitochondrial fission. Int J Oncol. 2018;53(1):124–36.
Dasgupta A, Chen KH, Lima PDA, Mewburn J, Wu D, Al-Qazazi R, et al. PINK1-induced phosphorylation of mitofusin 2 at serine 442 causes its proteasomal degradation and promotes cell proliferation in lung cancer and pulmonary arterial hypertension. FASEB J. 2021;35(8):e21771.
Zhuang W, Dong X, Wang B, Liu N, Guo H, Zhang C, et al. NRF-1 directly regulates TFE3 and promotes the proliferation of renal cancer cells. Oncol Lett. 2021;22(3):679.
Chen J, Wang M, Xiang Y, Ru X, Ren Y, Liu X, et al. Nrf1 is endowed with a dominant tumor-repressing effect onto the Wnt/beta-catenin-dependent and Wnt/beta-catenin-independent signaling networks in the human liver cancer. Oxid Med Cell Longev. 2020;2020:5138539.
Fu J, Zheng H, Cui Q, Chen C, Bao S, Sun J, et al. Nfe2l1-silenced insulinoma cells acquire aggressiveness and chemoresistance. Endocr Relat Cancer. 2018;25(3):185–200.
Vyas A, Harbison RA, Faden DL, Kubik M, Palmer D, Zhang Q, et al. Recurrent human papillomavirus-related head and neck cancer undergoes metabolic reprogramming and is driven by oxidative phosphorylation. Clin Cancer Res. 2021;27(22):6250–64.
Carmona-Carmona CA, Dalla Pozza E, Ambrosini G, Cisterna B, Palmieri M, Decimo I, et al. Mitochondrial elongation and opa1 play crucial roles during the stemness acquisition process in pancreatic ductal adenocarcinoma. Cancers (Basel). 2022;14(14):3432.
Nimmakayala RK, Rauth S, Chirravuri Venkata R, Marimuthu S, Nallasamy P, Vengoji R, et al. PGC1alpha-mediated metabolic reprogramming drives the stemness of pancreatic precursor lesions. Clin Cancer Res. 2021;27(19):5415–29.
Coazzoli M, Napoli A, Roux-Biejat P, Palma C, Moscheni C, Catalani E, et al. Acid sphingomyelinase downregulation enhances mitochondrial fusion and promotes oxidative metabolism in a mouse model of melanoma. Cells. 2020;9(4):848.
Wang C, Dong L, Li X, Li Y, Zhang B, Wu H, et al. The PGC1alpha/NRF1-MPC1 axis suppresses tumor progression and enhances the sensitivity to sorafenib/doxorubicin treatment in hepatocellular carcinoma. Free Radic Biol Med. 2021;163:141–52.
Huang X, Pan L, Zuo Z, Li M, Zeng L, Li R, et al. LINC00842 inactivates transcription co-regulator PGC-1alpha to promote pancreatic cancer malignancy through metabolic remodelling. Nat Commun. 2021;12(1):3830.
Zu Y, Chen XF, Li Q, Zhang ST, Si LN. PGC-1alpha activates SIRT3 to modulate cell proliferation and glycolytic metabolism in breast cancer. Neoplasma. 2021;68(2):352–61.
Mao L, Liu H, Zhang R, Deng Y, Hao Y, Liao W, et al. PINK1/Parkin-mediated mitophagy inhibits warangalone-induced mitochondrial apoptosis in breast cancer cells. Aging (Albany NY). 2021;13(9):12955–72.
Yin K, Lee J, Liu Z, Kim H, Martin DR, Wu D, et al. Mitophagy protein PINK1 suppresses colon tumor growth by metabolic reprogramming via p53 activation and reducing acetyl-CoA production. Cell Death Differ. 2021;28(8):2421–35.
Lou Y, Ma C, Liu Z, Shi J, Zheng G, Zhang C, et al. Antimony exposure promotes bladder tumor cell growth by inhibiting PINK1-Parkin-mediated mitophagy. Ecotoxicol Environ Saf. 2021;221:112420.
Dai K, Radin DP, Leonardi D. PINK1 depletion sensitizes non-small cell lung cancer to glycolytic inhibitor 3-bromopyruvate: involvement of ROS and mitophagy. Pharmacol Rep. 2019;71(6):1184–9.
Sriramkumar S, Sood R, Huntington TD, Ghobashi AH, Vuong TT, Metcalfe TX, et al. Platinum-induced mitochondrial OXPHOS contributes to cancer stem cell enrichment in ovarian cancer. J Transl Med. 2022;20(1):246.
Ippolito L, Morandi A, Taddei ML, Parri M, Comito G, Iscaro A, et al. Cancer-associated fibroblasts promote prostate cancer malignancy via metabolic rewiring and mitochondrial transfer. Oncogene. 2019;38(27):5339–55.
Wei Z, Jia J, Heng G, Xu H, Shan J, Wang G, et al. Sirtuin-1/mitochondrial ribosomal protein S5 axis enhances the metabolic flexibility of liver cancer stem cells. Hepatology. 2019;70(4):1197–213.
Xu YH, Song QQ, Li C, Hu YT, Song BB, Ye JM, et al. Bouchardatine suppresses rectal cancer in mice by disrupting its metabolic pathways via activating the SIRT1-PGC-1alpha-UCP2 axis. Eur J Pharmacol. 2019;854:328–37.
Wang W, Sun H, Ma X, Zhu T, Zhang H. Circ_0002476 regulates cell growth, invasion, and mtDNA damage in non-small cell lung cancer by targeting miR-1182/TFAM axis. Thorac Cancer. 2022;13(20):2867–78.
Yang S, He X, Zhao J, Wang D, Guo S, Gao T, et al. Mitochondrial transcription factor A plays opposite roles in the initiation and progression of colitis-associated cancer. Cancer Commun (Lond). 2021;41(8):695–714.
Huang Q, Wu D, Zhao J, Yan Z, Chen L, Guo S, et al. TFAM loss induces nuclear actin assembly upon mDia2 malonylation to promote liver cancer metastasis. EMBO J. 2022;41(11):e110324.
Zuo Y, Qu C, Tian Y, Wen Y, Xia S, Ma M. The HIF-1/SNHG1/miR-199a-3p/TFAM axis explains tumor angiogenesis and metastasis under hypoxic conditions in breast cancer. BioFactors. 2021;47(3):444–60.
Huangyang P, Li F, Lee P, Nissim I, Weljie AM, Mancuso A, et al. Fructose-1,6-bisphosphatase 2 inhibits sarcoma progression by restraining mitochondrial biogenesis. Cell Metab. 2020;31(1):174–88.
Mitra K, Wunder C, Roysam B, Lin G, Lippincott-Schwartz J. A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. Proc Natl Acad Sci U S A. 2009;106(29):11960–5.
Chen M, Ye K, Zhang B, Xin Q, Li P, Kong AN, et al. Paris Saponin II inhibits colorectal carcinogenesis by regulating mitochondrial fission and NF-kappaB pathway. Pharmacol Res. 2019;139:273–85.
Yao S, Yan W. Overexpression of Mst1 reduces gastric cancer cell viability by repressing the AMPK-Sirt3 pathway and activating mitochondrial fission. Onco Targets Ther. 2018;11:8465–79.
Mazumder S, De R, Debsharma S, Bindu S, Maity P, Sarkar S, et al. Indomethacin impairs mitochondrial dynamics by activating the PKCzeta-p38-DRP1 pathway and inducing apoptosis in gastric cancer and normal mucosal cells. J Biol Chem. 2019;294(20):8238–58.
Li B, Wang W, Li Z, Chen Z, Zhi X, Xu J, et al. MicroRNA-148a-3p enhances cisplatin cytotoxicity in gastric cancer through mitochondrial fission induction and cyto-protective autophagy suppression. Cancer Lett. 2017;410:212–27.
Aung LHH, Li R, Prabhakar BS, Maker AV, Li P. Mitochondrial protein 18 (MTP18) plays a pro-apoptotic role in chemotherapy-induced gastric cancer cell apoptosis. Oncotarget. 2017;8(34):56582–97.
Pyakurel A, Savoia C, Hess D, Scorrano L. Extracellular regulated kinase phosphorylates mitofusin 1 to control mitochondrial morphology and apoptosis. Mol Cell. 2015;58(2):244–54.
Zhao J, Zhang J, Yu M, Xie Y, Huang Y, Wolff DW, et al. Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene. 2013;32(40):4814–24.
Pal AD, Basak NP, Banerjee AS, Banerjee S. Epstein-Barr virus latent membrane protein-2A alters mitochondrial dynamics promoting cellular migration mediated by Notch signaling pathway. Carcinogenesis. 2014;35(7):1592–601.
Serasinghe MN, Wieder SY, Renault TT, Elkholi R, Asciolla JJ, Yao JL, et al. Mitochondrial division is requisite to RAS-induced transformation and targeted by oncogenic MAPK pathway inhibitors. Mol Cell. 2015;57(3):521–36.
Zhang GE, Jin HL, Lin XK, Chen C, Liu XS, Zhang Q, et al. Anti-tumor effects of Mfn2 in gastric cancer. Int J Mol Sci. 2013;14(7):13005–21.
Marzetti E, Lorenzi M, Landi F, Picca A, Rosa F, Tanganelli F, et al. Altered mitochondrial quality control signaling in muscle of old gastric cancer patients with cachexia. Exp Gerontol. 2017;87(Pt A):92–9.
Amini MA, Karimi J, Khodadadi I, Tavilani H, Talebi SS, Afshar B. Overexpression of ROMO1 and OMA1 are potentially biomarkers and predict unfavorable prognosis in gastric cancer. J Gastrointest Cancer. 2020;51(3):939–46.
Mallard J, Hucteau E, Charles AL, Bender L, Baeza C, Pelissie M, et al. Chemotherapy impairs skeletal muscle mitochondrial homeostasis in early breast cancer patients. J Cachexia Sarcopenia Muscle. 2022;13(3):1896–907.
Tang M, Yang M, Wu G, Mo S, Wu X, Zhang S, et al. Epigenetic induction of mitochondrial fission is required for maintenance of liver cancer-initiating cells. Cancer Res. 2021;81(14):3835–48.
Acknowledgements
Not applicable.
Funding
This work is supported by the National Key R&D Program of China (Grant #2018YFC1311600) and the Liaoning Provence Key R&D Program (Grant 2020JH2/10300063).
Author information
Authors and Affiliations
Contributions
QX, YY and LS conceived and designed this study. AL wrote the paper. AL, ZL, ZY, XW, LY, LS, QX and YY revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have read the journal’s authorship agreement and policy on disclosure of potential competing interest.
Competing interests
The authors declare that there is no competing interest regarding the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, Ar., Lv, Z., Yan, Zw. et al. Association of mitochondrial homeostasis and dynamic balance with malignant biological behaviors of gastrointestinal cancer. J Transl Med 21, 27 (2023). https://doi.org/10.1186/s12967-023-03878-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-023-03878-1