Abstract
Lung cancer is the second cancer and the leading cause of tumor-related mortality worldwide. Angiogenesis is a crucial hallmark of cancer development and a promising target in lung cancer. However, the anti-angiogenic drugs currently used in the clinic do not achieve long-term efficacy and are accompanied by severe adverse reactions. Therefore, the development of novel anti-angiogenic therapeutic approaches for lung cancer is urgently needed. Non-coding RNAs (ncRNAs) participate in multiple biological processes in cancers, including tumor angiogenesis. Many studies have demonstrated that ncRNAs play crucial roles in tumor angiogenesis. This review discusses the regulatory functions of different ncRNAs in lung cancer angiogenesis, focusing on the downstream targets and signaling pathways regulated by these ncRNAs. Additionally, given the recent trend towards utilizing ncRNAs as cancer therapeutics, we also discuss the tremendous potential applications of ncRNAs as biomarkers or novel anti-angiogenic tools in lung cancer.
Similar content being viewed by others
Background
Lung cancer ranks second in incidence among all tumor types worldwide and causes almost one-fourth of cancer deaths. Non-small cell lung cancer (NSCLC) accounts for more than 80% of lung cancers and is the most common histological type of lung cancer [1, 2]. Owing to the highly aggressive nature of NSCLC and the difficulty in diagnosing it at an early stage, the majority of NSCLC patients already have advanced-stage disease at diagnosis. The standard treatment for advanced NSCLC relies on systemic chemotherapy, targeted therapy, immunotherapy, and radiotherapy [3]. The overall survival of lung cancer patients has improved with advancements in treatment methods and earlier diagnosis in recent decades. However, due to distant metastasis and tumor recurrence after treatment, the outcomes of lung cancer patients are still poor [4].
Angiogenesis refers to the formation of new blood vessels from preexisting capillaries or postcapillary venules and is one of the hallmarks of cancer [5]. Unlike physiological conditions, such as wound healing, tumor angiogenesis is persistently aberrant in growing tumors, as they require oxygen and nutrients delivered through the blood circulation system to survive and proliferate [6]. In addition, cancer cells can enter the blood circulation through angiogenesis, resulting in hematogenous tumor metastasis [7, 8]. Over the past few decades, accumulating evidence has revealed that angiogenesis is a vital cancer hallmark related to poor prognosis in various solid tumors [9, 10]. Anti-angiogenic agents can affect the tumor microenvironment to regress existing tumor vessels while inhibiting tumor angiogenesis [11,12,13]. However, anti-angiogenic drugs targeting vascular endothelial growth factor (VEGF) or the vascular endothelial growth factor receptor (VEGFR) rarely result in durable responses and have had a limited effect on improving the overall survival of patients with lung cancer [14]. Consequently, to exploit new therapeutics to overcome this poor efficacy, further research is needed to discover the mechanisms of angiogenesis in lung cancer.
Angiogenesis is a complex process regulated by many pro-angiogenic and anti-angiogenic genes, and numerous studies have confirmed that angiogenesis is crucial for lung cancer cell proliferation and metastasis [15]. Accumulating evidence suggests that non-coding RNAs (ncRNAs), especially microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), can be involved in tumor angiogenesis by controlling different genes and pathways, resulting in distant metastasis and tumor recurrence [16]. Vascular homeostasis is managed by many different pro- and anti-angiogenic genes, and VEGF has been identified as a critical gene in inducing lung cancer angiogenesis [17,18,19]. The binding of VEGF and VEGFR initiates various intracellular signaling pathways and mediates the survival, proliferation, and migration of vascular endothelial cells (ECs), in turn promoting angiogenesis and enhancing vascular permeability in lung cancer [20]. Additionally, VEGF can also be produced by tumor immune cells and regulate the functions of innate and adaptive immune cells towards immunosuppression [21]. Hypoxia is the most crucial inducer of angiogenesis, and hypoxia-inducible factors (HIFs) have a crucial function in physiological adaptation to hypoxic states [22]. HIF proteins, especially HIF-1 alpha (HIF-1α), are closely associated with lung cancer occurrence, metastasis, and angiogenesis [23,24,25]. Multiple studies have indicated that the activation and expression levels of HIF-1α are closely correlated with the outcome of lung cancer [26, 27]. HIF-1α can target VEGFA and regulate its expression at the transcriptional level. Targeting the HIF-1α/VEGFA axis could be a promising strategy against tumor angiogenesis [28].
To date, the US Food and Drug Administration (FDA) has approved various drugs targeting VEGF/VEGFR for tumor anti-vascular therapy (Fig. 1) [29]. Although these drugs have demonstrated efficacy in NSCLC patients, the application of drugs is still affected by clinically significant bleeding events, such as major hemoptysis. On the other hand, long-term VEGF/VEGFR inhibitors probably lead cancer cells to exploit different angiogenic mechanisms and develop drug resistance [30]. Hence, full understanding of the mechanism of tumor angiogenesis will facilitate the discovery of novel therapeutic approaches to improve the prognosis of patients. NcRNAs have become promising biomarkers and potential therapeutic tools in many cancers because of their high stability and good biocompatibility [31,32,33]. Since ncRNAs act as pro- or anti-angiogenesis factors, their function in angiogenesis in lung cancer can be divided into enhancing or inhibiting, which is recapitulated in Table 1.
Hypoxia leads to activation of angiogenic signaling in lung cancer. Hypoxia in the tumor microenvironment can regulate angiogenesis in lung cancer by affecting HIF-1 protein expression and regulating the transcription of the downstream target gene VEGF. Created with www.BioRender.com
Angiogenesis related ncRNAs in lung cancer cells
Angiogenesis related miRNAs in lung cancer cells
MiRNAs are a class of short single-stranded ncRNA molecules with a length of approximately 22 nucleotides that can regulate gene expression by binding to the 3′ untranslated region (3′ UTR) of mRNAs [34, 35]. Abnormally expressed miRNAs were confirmed to affect various biological processes in cancer, including angiogenesis [36,37,38].
MiRNA directly targets angiogenesis related genes in lung cancer cells
MiRNAs can regulate tumor angiogenesis by directly binding to angiogenesis-related genes. For example, miR-29c has a binding site in the 3' UTR of VEGFA mRNA and can down-regulate the expression level of VEGFA, thereby promoting the capability of tumor cells to induce tube formation of human umbilical vein endothelial cells (HUVECs) in lung adenocarcinoma (LUAD) [39]. MiR-519c can target HIF-1α and reduce the expression of HIF-1α protein, thereby reducing tumor angiogenesis in NSCLC. In addition, hepatocyte growth factor (HGF), an inducer of HIF-1α, suppresses miR-519c maturation through an Akt-dependent pathway, indicating the important role of the HGF/miR-519c/HIF-1α axis in modulating angiogenesis in lung cancer [40]. The let-7 miRNA family is dysregulated in various tumors and participates in various biological processes, such as oncogenesis and development. The expression of let-7b and miR-126 decreases in NSCLC tissues, and their low expression is associated with poor prognosis. Regulation of these two miRNAs reduces tumor angiogenesis and thus inhibits tumor growth in lung cancer patients, which may be a potential treatment for lung cancer [41].
MiR-145-5p can directly target ceruloplasmin (CP), and loss of miR-145-5p in LUAD induces overexpression of CP. Overexpression of CP decreases Fe2+ and prolyl hydroxylase domain (PHD) 1/2 levels while inhibiting HIF-2α, leading to the activation of tumor angiogenesis [42]. The expression of miR-320b is down-regulated and related to improved overall survival in patients with lung cancer. The results of gain-of-function experiments suggested that miR-320b can target hepatocyte nuclear factor 4 gamma, thereby inhibiting tumor proliferation, invasion, and angiogenesis in xenografted nude mice [43]. MiR-497 expression has been reported to be significantly decreased in various cancers and acts as a tumor suppressor by targeting different oncogenes [44,45,46,47]. MiR-497 was also found to be considerably down-regulated in NSCLC and can suppress angiogenesis, cancer cell proliferation, and invasion by targeting HDGF and VEGFA [48, 49].
MiRNAs target angiogenesis related genes in lung cancer cells via exosomes
Microvesicles (MVs) and exosomes can transport ncRNAs and interact with other cells, thus influencing angiogenesis in lung cancer [50]. Jeong et al. demonstrated that exosomes loaded with miR-497 have a synergistic inhibitory effect on targeting the growth and angiogenesis of lung cancer cells and hold promise as a novel approach for tumor-targeted therapy [51]. The miR-200 family inhibits metastasis by regulating tumor angiogenesis in various tumors. Several studies have shown that miR-141, a member of the miR-200 family, exerts pro-angiogenic or anti-angiogenic effects in different cancers [52, 53]. Mao et al. reported that the miR-141 level was significantly increased in serum from patients with small cell lung cancer (SCLC) and that this increase was associated with advanced clinical characteristics. Mechanistically, miR-141 is packaged into exosomes released from SCLC cells and then targets KLF12 and GAX, leading to angiogenesis and malignant progression of lung cancer [54, 55]. A study by Kim et al. reported that NSCLC-derived exosomal miR-619-5p targets RCAN1.4, thereby inducing tumor angiogenesis and metastasis [56]. Fan et al. indicated that miR-210 regulates JAK2/STAT3 signaling and TET2 in recipient fibroblasts, thus initiating the pro-angiogenic switch of cancer-associated fibroblasts (CAFs). Moreover, miR-210 is up-regulated in exosomes released from lung cancer cells, indicating the key role of miR-210 in angiogenesis in lung cancer [57].
Angiogenesis related lncRNAs in lung cancer cells
LncRNAs are a class of single-stranded RNAs that are longer than 200 nucleotides and have no protein-coding function. LncRNAs can interact with proteins, DNA, and RNA to participate in the regulation of various biological processes. Numerous studies have indicated that dysregulated expression of lncRNAs is associated with the tumorigenesis and progression of multiple cancers, including lung cancer [58, 59].
LncRNAs regulate angiogenesis in lung cancer cells through ceRNA networks
LncRNAs can act as ceRNA to regulate gene expression by competitively binding miRNAs. For instance, Chen et al. reported that LINC00173.v1 binds to miR-511-5p to regulate VEGFA expression, thereby promoting angiogenesis and development in lung squamous cell carcinoma (LUSC). Animal experiments demonstrated that LINC00173.v1 increases the therapeutic sensitivity of LUSC cells to cisplatin and that targeting LINC00173.v1 could be a potential treatment for combating LUSC [60]. Li et al. found that the transcription factor YY1 mediates the transcription and expression of lncRNA MCM3AP-AS1 in lung cancer. In addition, MCM3AP-AS1 targets miR-340-5p to induce overexpression of KPNA4, thereby promoting angiogenesis and progression of lung cancer [61]. Linc00941 is a carcinogenic lncRNA and mediates the progression of gastric cancer, head and neck squamous cell carcinoma, and thyroid papillary carcinoma [62,63,64]. Ren et al. found that linc00941 expression is increased in NSCLC tissues and patient plasma. Additionally, linc00941 interacts with miR-877-3p to regulate VEGFA, accelerating NSCLC angiogenesis and tumor progression [65]. LncRNA F630028O10Rik is a novel lncRNA that can interact with miR‐223‐3p and results in VEGFA and VEGFR2 suppression, thereby regulating tumor angiogenesis and inhibiting tumor growth and progression [66]. Jiang et al. found that lncRNA FBXL19-AS1 expression is increased in lung cancer, and a high level of FBXL19-AS1 expression is related to poor prognosis. Exploration of the molecular mechanism suggested that FBXL19-AS1 participates in the development and angiogenesis in lung cancer by targeting the miR-431-5p/RAF1 axis [67].
LncRNAs regulate angiogenesis in lung cancer cells via exosomes
In addition to functioning as miRNA sponges, some lncRNAs can also regulate angiogenesis through exosome transport. LincRNA-p21 is a lncRNA activated by tumor protein p53 and the HIF-1α subunit. Hypoxia can guide lincRNA-p21 expression, which leads to the induction of angiogenesis and correlates with the poor prognosis in NSCLC patients. Meanwhile, NSCLC cell-derived exosomal lincRNA-p21 can promote tube formation of endothelial cells and enhance tumor cell adhesion to endothelial cells [68, 69]. These studies demonstrated the extensive influence of lncRNA-miRNA-mRNA systems in angiogenesis in lung cancer.
LncRNAs regulate angiogenesis in lung cancer cells by interacting with proteins
LncRNAs can also interact with proteins to affect the expression of genes. For example, linc00665 can directly interact with the YB-1 protein and accumulate in the nucleaus, thereby increasing ANGPT4, ANGPTL3, and VEGFA expression and promoting tumor-associated angiogenesis in lung cancer [70]. Wang et al. found that lncRNA TNK2-AS1 expression is increased in NSCLC and associated with poor prognosis. Molecular mechanistic studies revealed that the lncRNA TNK2-AS1 interacts with STAT3, increasing its protein stability, and that STAT3 can also bind to the TNK2-AS1 promoter to trigger its transcription. The positive feedback loop promotes the angiogenesis of NSCLC by enforcing STAT3/VEGFA signaling [71]. LncRNA colon cancer-associated transcript 1 (CCAT1) acts as a carcinogenic factor in various tumors and is significantly highly expressed in LUAD. Mechanistic studies revealed that CCAT1 interacts with fatty acid binding protein 5 (FABP5) and mediates the translocation of FABP5 into the nucleus. In addition, CCAT1 can stabilize the PI3K/Akt/mTOR signaling pathway, thus promoting tumor growth and angiogenesis in LUAD [72]. These studies further revealed the effects of lncRNAs on angiogenesis, suggesting that lncRNAs may be an effective target for the treatment of lung cancer.
Angiogenesis related circRNAs in lung cancer cells
Circular RNA (circRNA) is a novel type of circular ncRNA generated by back-splicing, and their primary function is to act as a sponge for miRNAs to regulate diverse functions of cells [50]. Circ_0006988 can competitively inhibit miR-491-5p, further regulating MAP3K3 and promoting proliferation, metastasis, and angiogenesis in NSCLC cells [73]. Circ_0016760 expression is significantly increased in both NSCLC tissues and cells and can promote the malignant phenotype of NSCLC. Mechanistically, up-regulation of circ_0016760 acts as a sponge of miR-29b and promotes the oncogenic effect of HIF-1α, further inducing malignancy and angiogenesis in NSCLC [74].
There are few studies on the function of circRNAs in lung cancer angiogenesis. However, considering the advantages of circRNAs, such as their low molecular weight and high stability, circRNA-based molecular therapy may be a potential treatment for lung cancer.
Angiogenesis related ncRNAs in the lung cancer tumor microenvironment
MiRNAs in the tumor microenvironment affect angiogenesis
Ample evidence indicates that the tumor microenvironment can influence tumor development from multiple aspects, including invasion, metastasis, and angiogenesis [75,76,77]. Shen et al. compared the miRNA expression profile in CAFs with that in matched NFs from lung cancer patients and found that miR-31 expression was increased in lung CAFs, whereas the expression of miR-1 and miR-206 was increased in the matched NFs. MiR-1, miR-206, and miR-31 can target VEGFA/CCL2 and FOXO3a to regulate their expression. Modulation of the expression of these miRNAs can markedly inhibit tumor angiogenesis, tumor-associated macrophages accumulation, tumor growth and lung metastasis, which may have potential clinical implications for therapy in lung cancer [78]. Zhang et al. indicated that the significant up-regulation of miR-224 in CAFs targeted SIRT3 and thus regulated the SIRT3/AMPK/mTOR/HIF-1 α axis. Furthermore, HIF-1α enhanced the expression of miR-224. This positive feedback loop induced endothelial cell angiogenesis and tumor progression in NSCLC [79].
Exosomal miRNAs in the tumor microenvironment regulate angiogenesis
Exosomes could perform a communication function between tumor microenvironmental components to regulate angiogenesis in lung cancer. Wei et al. found that M2 macrophage-derived exosomal miR-942 can regulate FOXO1 protein expression by binding to the 3′UTR of FOXO1, thereby promoting LUAD cell invasion and enhancing angiogenesis [80]. Hsu et al. reported that lung cancer-derived EV miR-103a targets phosphatase and tensin homologue deleted on chromosome 10 (PTEN) and enhances M2 polarization to up-regulate the stimulatory effect of macrophages on tumor development and angiogenesis. Mechanistically, miR-103a can directly target PTEN and lead to activation of the PI3K/Akt and STAT3 signaling pathways. Preventing the transfer of EVs from hypoxic cancer cells or suppressing miR-103a expression may be a new therapeutic option to activate the immune response [81].
Angiogenesis related ncRNAs in the tumor endothelium
Angiogenesis related miRNAs in the tumor endothelium
MiRNAs directly target angiogenesis related genes in tumor endothelial cells
NcRNAs in tumor endothelial cells can participate in lung tumor progression by regulating tumor angiogenesis. Hu et al. found that miR-128 can bind to VEGFC and inhibit tumor growth and invasiveness in vitro. Furthermore, up-regulation of miR-128 in NSCLC cells and HUVECs not only causes down-regulation of VEGFA, VEGFR2, and VEGFR3 but also inhibits angiogenesis and lymphangiogenesis in tumor xenografts [82]. Korde et al. indicated that the expression of miR-1 was decreased in NSCLC, which was related to overall survival. In endothelial cells, miR-1 can regulate the proliferation and angiogenesis of lung cancer cells by binding to the myeloproliferative leukemia virus oncogene [83]. As one of the members of the miR-200 family, miR-200b participates in multiple physiological functions of endothelial cells [84]. MiR-200b inhibits proliferation and sprouting angiogenesis through the QKI/CCND1 axis in endothelial cells and is expected to become a novel target for therapeutic inhibition of NSCLC metastasis [85].
miRNAs target angiogenesis related genes in tumor endothelial cells via exosomes
Tumor-derived exosomes can also transport miRNA to endothelial cells to inhibit angiogenesis in lung cancer. NSCLC-derived exosomal miR-192 can target endothelial cells and inhibit tumor angiogenesis and bone metastatic activity by suppressing proangiogenic IL-8, ICAM, and CXCL1 in lung cancer [86]. MiR-494 can be transfected from the lung cancer cell Line A549 into endothelial cells by MVs and promotes angiogenesis mediated by targeting PTEN and subsequently activating the Akt/eNOS pathway [87]. Liu et al. indicated that the miR-21 expression level in the serum of smokers was increased compared with that in non-smokers. In addition, miR-21 can be transferred among human bronchial epithelial (HBE) cells via exosomes, activating STAT3 and consequently up-regulating VEGF levels in recipient cells [88]. Hsu et al. found that miR-23a expression was significantly increased in lung cancer exosomes under hypoxic conditions. Hypoxic lung cancer cell-derived exosomal miR-23a directly binds to PHD1 and PHD2, resulting in HIF-1 accumulation in endothelial cells and enhancing angiogenesis [89].
Angiogenesis related lncRNAs in tumor endothelium
The lncRNA EPIC1, a MYC-interacting lncRNA, can promote cell-cycle progression in cancer [90]. The lncRNA EPIC1 was significantly up-regulated in NSCLC tissues and cell lines. Animal experiments demonstrated that overexpression of the lncRNA EPIC1 can activate HUVEC channel formation and proliferation by activating the Ang2/Tie2 axis in NSCLC [91]. Cheng et al. pointed out that the lung cancer cell-derived exosomal lncRNA growth arrest-specific transcript 5 (GAS5) competitively targets miRNA-29-3p with PTEN in HUVECs to affect their proliferation, apoptosis, and tube formation [92]. These outcomes demonstrate that regulation of lncRNAs in HUVECs can ameliorate the malignant phenotype of tumors and that lncRNAs could be a potential target in the anti-angiogenic treatment of lung cancer (see Fig. 2, 3).
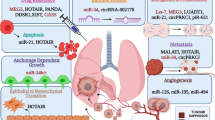
NcRNAs affecting angiogenesis in lung cancer cells and tumor microenvironment cells. NcRNAs can directly or indirectly interact with angiogenesis-related genes in lung cancer cells or the tumor microenvironment, thereby affecting lung cancer angiogenesis. Created with www.BioRender.com
NcRNAs affecting angiogenesis in tumor endothelium cells. In tumor endothelial cells, ncRNAs can act on angiogenic or proliferation-related genes to induce angiogenesis. Created with www.BioRender.com
Prospects and challenges of ncRNA-based anti-angiogenesis therapy
NcRNAs are crucial regulatory factors in tumorigenesis that activate or inhibit the oncogenic process. Therefore, advancing practical therapeutic approaches to suppress oncogenic ncRNAs or overexpress cancer-associated ncRNAs is becoming a hot research field [93]. Eleven ncRNA-based therapies, all of which are small interfering RNAs (siRNAs) or antisense oligonucleotides (ASOs) targeting specific genes, have been approved for clinical treatment. Moreover, many ncRNA-based therapeutics are in clinical development with potential applications in various tumors, including lung cancer [94]. The ncRNA-based anti-angiogenesis therapies in lung cancer presented in Table 2.
Nucleic acid aptamers are small, single-stranded DNA or RNA molecules chemically synthesized for binding to a specific target. As a therapeutic tool, aptamers have several advantages, such as their small physical size and lack of immunogenicity, and are thus an invaluable targeted delivery carrier for siRNAs, miRNAs, and chemotherapeutic agents [95]. Lai et al. constructed two nucleolin aptamer-siRNA chimeras targeting snail family zinc finger 2 (SLUG) and neuropilin 1 (NRP1). Combined treatment with these two aptamer siRNAs significantly silenced SLUG and NRP1 expression in lung cancer cells, leading to specific inhibition of tumor invasion and angiogenesis [96]. Liao et al. constructed a bivalent cyclic RGD–siVEGFR2 conjugate delivery system that can silence VEGFR2 expression by targeting neovascularization in endothelial cells. Furthermore, biRGD–siVEGFR2 exhibited synergistic antitumor activity with apatinib in NSCLC, which may represent a new strategy for clinical NSCLC treatment [97]. Activation of CXCR4 and STAT3 results in the initiation of multiple signaling pathways, leading to metastasis and angiogenesis in lung cancer; thus, CXCR4 and STAT3 are potential targets for anti-angiogenic therapy [98]. Li et al. developed a fluorinated polymeric CXCR4 antagonist-stabilized perfluorocarbon (PFC) to deliver therapeutic siSTAT3. These nanoemulsions suppressed tumor proliferation and angiogenesis by simultaneously down-regulating CXCR4 and STAT3 in lung metastases [99].
Dicer, a type III cytoplasmic endoribonuclease, is required for the maturation of the vast majority of miRNAs [100]. Chen et al. generated miRNA-deficient tumors by knocking out Dicer1 only and found that the depletion of all miRNAs notably suppressed NSCLC angiogenesis. Mechanistic studies suggested that miRNAs promote tumor responses to hypoxia and angiogenesis by repressing FIH1, an inhibitor of HIF-1α [101]. Previous research has shown that malaria infection could suppress Lewis lung cancer cell proliferation by inducing innate and adaptive antitumor immune responses [102]. Yang et al. found that intratumoral injection of exosomes derived from the plasma of Plasmodium-infected mice significantly suppressed Lewis lung cancer growth. High levels of miR-16/322/497/17 were detected in exosomes isolated from the plasma of mice infected with Plasmodium, and up-regulated expression of these miRNAs in ECs corresponded with down-regulated expression of VEGFR2 [103]. This study helped to advance potential exosome-based anti-angiogenic tumor therapeutics.
Muramatsu et al. found that miR-125b inhibits EC tube formation by inhibiting the translation of VE-cadherin. Then, they used non-viral vectors composed of the cationic polymer polyethylenimine to package miR-125b and directly injected it into subcutaneous tumors. By targeting VE-cadherin, miR-125b induced the formation of non-functional blood vessels and inhibited tumor growth in vivo, which suggested a therapeutic potential for tumor angiogenesis [104]. Li et al. designed a therapeutic strategy against orthotopic NSCLC tumors that can intelligently co-deliver siVEGF and the chemotherapeutic etoposide (ETO) through multi-functional nanoparticles (NPs). Compared with monotherapy, combination therapy in orthotopic NSCLC causes more significant tumor growth and metastasis by simultaneously inhibiting tumor proliferation and angiogenesis [105]. Therefore, ncRNA-based anti-angiogenic therapy may be a promising strategy.
Circulating miRNAs may potentially act as biomarkers for predicting the response to anti-angiogenic therapy. M. Joerger’s study revealed that circulating miRNAs could predict the efficacy of angiogenic drugs combined with targeted therapies. Pretreatment circulating miRNA profiling indicated that the expression of 12 miRNAs was significantly associated with tumor shrinkage after bevacizumab/erlotinib treatment, with miR-665 being the strongest predictive marker. Moreover, miR-223 was related to time to progression (TTP) after bevacizumab/erlotinib treatment andafter second-line chemotherapy [106].
Conclusion
Tumor metastasis and chemotherapeutic resistance lead to the poor prognosis of lung cancer. Current research suggests that ncRNAs are crucial players in tumorigenesis and tumor angiogenesis in lung cancer. Even though the function of ncRNAs has been determined in the tumor angiogenesis, less attention has been given to research on the clinical application of ncRNAs, suggesting that the requirement for ncRNA studies is unmet. Nucleic acid therapeutics targeting angiogenesis, such as modified siRNAs and miRNA mimics, are promising therapeutics for lung cancer. In conclusion, despite the emerging application of ncRNAs in tumor diagnosis and therapy and the challenges in this field, targeting ncRNAs is still an innovative and promising strategy to improve therapeutic outcomes for lung cancer patients.
Availability of data and materials
Not applicable.
Abbreviations
- NSCLC:
-
Non-small cell lung cancer
- VEGF:
-
Vascular endothelial growth factor
- VEGFR:
-
Vascular endothelial growth factor receptor
- ncRNAs:
-
Non-coding RNAs
- miRNAs:
-
MicroRNAs
- lncRNAs:
-
Long non-coding RNAs
- ECs:
-
Endothelial cells
- HIFs:
-
Hypoxia-inducible factors
- FDA:
-
Food and Drug Administration
- 3' UTR:
-
3' Untranslated region
- HUVECs:
-
Human umbilical vein endothelial cells
- LUAD:
-
Lung adenocarcinoma
- HGF:
-
Hepatocyte growth factor
- CP:
-
Ceruloplasmin
- PHD:
-
Prolyl hydroxylase domain
- MVs:
-
Microvesicles
- SCLC:
-
Small cell lung cancer
- CAFs:
-
Cancer-associated fibroblasts
- LUSC:
-
Lung squamous cell carcinoma
- CCAT1:
-
Colon cancer-associated transcript 1
- FABP5:
-
Fatty acid binding protein 5
- circRNA:
-
Circular RNA
- PTEN:
-
Phosphatase and tensin homologue deleted on chromosome 10
- HBE:
-
Human bronchial epithelial
- GAS5:
-
Growth arrest-specific transcript 5
- siRNAs:
-
Small interfering RNAs
- ASOs:
-
Antisense oligonucleotides
- SLUG:
-
Snail family zinc finger 2
- NRP1:
-
Neuropilin 1
- PFC:
-
Perfluorocarbon
- ETO:
-
Etoposide
- NPs:
-
Nanoparticles
- TTP:
-
Time to progression
References
Siegel RL, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Fitzmaurice C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3(4):524–48.
Perdrizet K, Leighl NB. The role of angiogenesis inhibitors in the era of immune checkpoint inhibitors and targeted therapy in metastatic non-small cell lung cancer. Curr Treat Options Oncol. 2019;20(3):21.
The L. Lung cancer: some progress, but still a lot more to do. Lancet. 2019;394(10212):1880.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: is there a role for combinations with immunotherapy? Angiogenesis. 2017;20(2):185–204.
Li S, et al. Angiogenesis in pancreatic cancer: current research status and clinical implications. Angiogenesis. 2019;22(1):15–36.
Popper HH. Progression and metastasis of lung cancer. Cancer Metastasis Rev. 2016;35(1):75–91.
Majidpoor J, Mortezaee K. Angiogenesis as a hallmark of solid tumors—clinical perspectives. Cell Oncol (Dordr). 2021;44(4):715–37.
Hilmi M, et al. Angiogenesis and immune checkpoint inhibitors as therapies for hepatocellular carcinoma: current knowledge and future research directions. J Immunother Cancer. 2019;7(1):333.
Huinen ZR, et al. Anti-angiogenic agents—overcoming tumour endothelial cell anergy and improving immunotherapy outcomes. Nat Rev Clin Oncol. 2021;18(8):527–40.
Itatani Y, et al. Resistance to anti-angiogenic therapy in cancer-alterations to anti-VEGF pathway. Int J Mol Sci. 2018;19(4):1232.
Song Y, et al. Anti-angiogenic agents in combination with immune checkpoint inhibitors: a promising strategy for cancer treatment. Front Immunol. 2020;11:1956.
Le X, et al. Dual EGFR-VEGF pathway inhibition: a promising strategy for patients with EGFR-mutant NSCLC. J Thorac Oncol. 2021;16(2):205–15.
Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020;77(9):1745–70.
Zhao Z, et al. Mechanisms of lncRNA/microRNA interactions in angiogenesis. Life Sci. 2020;254: 116900.
Frezzetti D, et al. VEGF as a potential target in lung cancer. Expert Opin Ther Targets. 2017;21(10):959–66.
Meder L, et al. Combined VEGF and PD-L1 blockade displays synergistic treatment effects in an autochthonous mouse model of small cell lung cancer. Cancer Res. 2018;78(15):4270–81.
Ohta Y, et al. Significance of vascular endothelial growth factor messenger RNA expression in primary lung cancer. Clin Cancer Res. 1996;2(8):1411–6.
Garcia J, et al. Bevacizumab (Avastin®) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86: 102017.
Fukumura D, et al. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325–40.
Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–32.
de Heer EC, Jalving M, Harris AL. HIFs, angiogenesis, and metabolism: elusive enemies in breast cancer. J Clin Invest. 2020;130(10):5074–87.
Theodoropoulos VE, et al. Hypoxia-inducible factor 1 alpha expression correlates with angiogenesis and unfavorable prognosis in bladder cancer. Eur Urol. 2004;46(2):200–8.
Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9(6):677–84.
Liu B, et al. Polymorphisms of HIF1A gene are associated with prognosis of early stage non-small-cell lung cancer patients after surgery. Med Oncol. 2014;31(4):877.
Ren W, et al. The expression of hypoxia-inducible factor-1α and its clinical significance in lung cancer: a systematic review and meta-analysis. Swiss Med Wkly. 2013;143: w13855.
Palazon A, et al. An HIF-1α/VEGF-A axis in cytotoxic T cells regulates tumor progression. Cancer Cell. 2017;32(5):669-683.e5.
Hack SP, Zhu AX, Wang Y. Augmenting anticancer immunity through combined targeting of angiogenic and PD-1/PD-L1 pathways: challenges and opportunities. Front Immunol. 2020;11: 598877.
Piperdi B, Merla A, Perez-Soler R. Targeting angiogenesis in squamous non-small cell lung cancer. Drugs. 2014;74(4):403–13.
Matsui M, Corey DR. Non-coding RNAs as drug targets. Nat Rev Drug Discov. 2017;16(3):167–79.
Anfossi S, et al. Clinical utility of circulating non-coding RNAs—an update. Nat Rev Clin Oncol. 2018;15(9):541–63.
Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861–74.
Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–22.
Berindan-Neagoe I, et al. MicroRNAome genome: a treasure for cancer diagnosis and therapy. CA Cancer J Clin. 2014;64(5):311–36.
Lai X, et al. Systems biology-based investigation of cooperating microRNAs as monotherapy or adjuvant therapy in cancer. Nucleic Acids Res. 2019;47(15):7753–66.
Pencheva N, et al. Convergent multi-miRNA targeting of ApoE drives LRP1/LRP8-dependent melanoma metastasis and angiogenesis. Cell. 2012;151(5):1068–82.
Wang Y, et al. New insights into the regulatory role of microRNA in tumor angiogenesis and clinical implications. Mol Cancer. 2018;17(1):22.
Liu L, et al. MicroRNA-29c functions as a tumor suppressor by targeting VEGFA in lung adenocarcinoma. Mol Cancer. 2017;16(1):50.
Cha ST, et al. MicroRNA-519c suppresses hypoxia-inducible factor-1alpha expression and tumor angiogenesis. Cancer Res. 2010;70(7):2675–85.
Jusufović E, et al. let-7b and miR-126 are down-regulated in tumor tissue and correlate with microvessel density and survival outcomes in non–small–cell lung cancer. PLoS ONE. 2012;7(9): e45577.
Tsai YM, et al. Loss of miR-145–5p causes ceruloplasmin interference with PHD-iron axis and HIF-2α stabilization in lung adenocarcinoma-mediated angiogenesis. Int J Mol Sci. 2020;21(14):5081.
Ma YS, et al. microRNA-320b suppresses HNF4G and IGF2BP2 expression to inhibit angiogenesis and tumor growth of lung cancer. Carcinogenesis. 2021;42(5):762–71.
Zhou TB, et al. Roles of miR-497 and its potential signaling pathway in diseases and with vascular endothelial growth factor. J Recept Signal Transduct Res. 2015;35(4):303–6.
Feng F, et al. Reduced expression of microRNA-497 is associated with greater angiogenesis and poor prognosis in human gliomas. Hum Pathol. 2016;58:47–53.
Qiu Y, et al. microRNA-497 inhibits invasion and metastasis of colorectal cancer cells by targeting vascular endothelial growth factor-A. Cell Prolif. 2016;49(1):69–78.
Wu Z, et al. miR-497 suppresses angiogenesis in breast carcinoma by targeting HIF-1α. Oncol Rep. 2016;35(3):1696–702.
Gu A, et al. Role of miR-497 in VEGF-A-mediated cancer cell growth and invasion in non-small cell lung cancer. Int J Biochem Cell Biol. 2016;70:118–25.
Zhao WY, et al. Downregulation of miR-497 promotes tumor growth and angiogenesis by targeting HDGF in non-small cell lung cancer. Biochem Biophys Res Commun. 2013;435(3):466–71.
Tang W, et al. Exosomes in triple negative breast cancer: From bench to bedside. Cancer Lett. 2021;527:1–9.
Jeong K, et al. Exosome-mediated microRNA-497 delivery for anti-cancer therapy in a microfluidic 3D lung cancer model. Lab Chip. 2020;20(3):548–57.
Pecot CV, et al. Tumour angiogenesis regulation by the miR-200 family. Nat Commun. 2013;4:2427.
Mateescu B, et al. miR-141 and miR-200a act on ovarian tumorigenesis by controlling oxidative stress response. Nat Med. 2011;17(12):1627–35.
Mao S, et al. Exosomal miR-141 promotes tumor angiogenesis via KLF12 in small cell lung cancer. J Exp Clin Cancer Res. 2020;39(1):193.
Wang W, et al. Tumor-derived exosomal miRNA-141 promote angiogenesis and malignant progression of lung cancer by targeting growth arrest-specific homeobox gene (GAX). Bioengineered. 2021;12(1):821–31.
Kim DH, et al. Tumor-derived exosomal miR-619–5p promotes tumor angiogenesis and metastasis through the inhibition of RCAN1.4. Cancer Lett. 2020;475:2–13.
Fan J, et al. miR-210 transferred by lung cancer cell-derived exosomes may act as proangiogenic factor in cancer-associated fibroblasts by modulating JAK2/STAT3 pathway. Clin Sci (Lond). 2020;134(7):807–25.
Huang Z, et al. The role of long noncoding RNAs in hepatocellular carcinoma. Mol Cancer. 2020;19(1):77.
Li Y, et al. The roles of exosomal miRNAs and lncRNAs in lung diseases. Signal Transduct Target Ther. 2019;4:47.
Chen J, et al. LINC00173.v1 promotes angiogenesis and progression of lung squamous cell carcinoma by sponging miR-511–5p to regulate VEGFA expression. Mol Cancer. 2020;19(1):98.
Li X, Yu M, Yang C. YY1-mediated overexpression of long noncoding RNA MCM3AP-AS1 accelerates angiogenesis and progression in lung cancer by targeting miR-340-5p/KPNA4 axis. J Cell Biochem. 2020;121(3):2258–67.
Luo C, et al. Regulatory network analysis of high expressed long non-coding RNA LINC00941 in gastric cancer. Gene. 2018;662:103–9.
Hu Y, et al. Screening key lncRNAs with diagnostic and prognostic value for head and neck squamous cell carcinoma based on machine learning and mRNA-lncRNA co-expression network analysis. Cancer Biomark. 2020;27(2):195–206.
Gugnoni M, et al. Linc00941 is a novel transforming growth factor β target that primes papillary thyroid cancer metastatic behavior by regulating the expression of cadherin 6. Thyroid. 2021;31(2):247–63.
Ren MH, et al. LINC00941 promotes progression of non-small cell lung cancer by sponging miR-877-3p to regulate VEGFA expression. Front Oncol. 2021;11: 650037.
Qin L, et al. A novel tumour suppressor lncRNA F630028O10Rik inhibits lung cancer angiogenesis by regulating miR-223-3p. J Cell Mol Med. 2020;24(6):3549–59.
Jiang Q, et al. FBXL19-AS1 exerts oncogenic function by sponging miR-431–5p to regulate RAF1 expression in lung cancer. Biosci Rep. 2019;39(1):BSR20181804.
Castellano JJ, et al. LincRNA-p21 impacts prognosis in resected non-small cell lung cancer patients through angiogenesis regulation. J Thorac Oncol. 2016;11(12):2173–82.
Castellano JJ, et al. Extracellular vesicle lincRNA-p21 expression in tumor-draining pulmonary vein defines prognosis in NSCLC and modulates endothelial cell behavior. Cancers (Basel). 2020;12(3):734.
Cong Z, et al. Long non-coding RNA linc00665 interacts with YB-1 and promotes angiogenesis in lung adenocarcinoma. Biochem Biophys Res Commun. 2020;527(2):545–52.
Wang Y, et al. The positive feedback between lncRNA TNK2-AS1 and STAT3 enhances angiogenesis in non-small cell lung cancer. Biochem Biophys Res Commun. 2018;507(1–4):185–92.
Chen J, et al. CCAT1/FABP5 promotes tumour progression through mediating fatty acid metabolism and stabilizing PI3K/AKT/mTOR signalling in lung adenocarcinoma. J Cell Mol Med. 2021;25(19):9199–213.
Yang C, et al. Circ_0006988 promotes the proliferation, metastasis and angiogenesis of non-small cell lung cancer cells by modulating miR-491-5p/MAP3K3 axis. Cell Cycle. 2021;20(13):1334–46.
Zhang H, et al. PESV represses non-small cell lung cancer cell malignancy through circ_0016760 under hypoxia. Cancer Cell Int. 2021;21(1):628.
Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–48.
Li J, et al. Small extracellular vesicle-bound vascular endothelial growth factor secreted by carcinoma-associated fibroblasts promotes angiogenesis in a bevacizumab-resistant manner. Cancer Lett. 2020;492:71–83.
She Q, et al. The effect of hepatocellular carcinoma-associated fibroblasts on hepatoma vasculogenic mimicry. Am J Cancer Res. 2020;10(12):4198–210.
Shen H, et al. Reprogramming of normal fibroblasts into cancer-associated fibroblasts by miRNAs-mediated CCL2/VEGFA signaling. PLoS Genet. 2016;12(8): e1006244.
Zhang J, et al. miR-224 aggravates cancer-associated fibroblast-induced progression of non-small cell lung cancer by modulating a positive loop of the SIRT3/AMPK/mTOR/HIF-1α axis. Aging (Albany NY). 2021;13(7):10431–49.
Wei K, et al. M2 macrophage-derived exosomes promote lung adenocarcinoma progression by delivering miR-942. Cancer Lett. 2022;526:205–16.
Hsu YL, et al. Hypoxic lung-cancer-derived extracellular vesicle microRNA-103a increases the oncogenic effects of macrophages by targeting PTEN. Mol Ther. 2018;26(2):568–81.
Hu J, et al. microRNA-128 plays a critical role in human non-small cell lung cancer tumourigenesis, angiogenesis and lymphangiogenesis by directly targeting vascular endothelial growth factor-C. Eur J Cancer. 2014;50(13):2336–50.
Korde A, et al. Lung endothelial microRNA-1 regulates tumor growth and angiogenesis. Am J Respir Crit Care Med. 2017;196(11):1443–55.
Cavallari I, et al. The miR-200 family of microRNAs: fine tuners of epithelial-mesenchymal transition and circulating cancer biomarkers. Cancers (Basel). 2021;13(23):5874.
Azam SH, et al. Quaking orchestrates a post-transcriptional regulatory network of endothelial cell cycle progression critical to angiogenesis and metastasis. Oncogene. 2019;38(26):5191–210.
Valencia K, et al. miRNA cargo within exosome-like vesicle transfer influences metastatic bone colonization. Mol Oncol. 2014;8(3):689–703.
Mao G, et al. Tumor-derived microRNA-494 promotes angiogenesis in non-small cell lung cancer. Angiogenesis. 2015;18(3):373–82.
Liu Y, et al. STAT3-regulated exosomal miR-21 promotes angiogenesis and is involved in neoplastic processes of transformed human bronchial epithelial cells. Cancer Lett. 2016;370(1):125–35.
Hsu YL, et al. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. 2017;36(34):4929–42.
Wang Z, et al. lncRNA epigenetic landscape analysis identifies EPIC1 as an oncogenic lncRNA that interacts with MYC and promotes cell-cycle progression in cancer. Cancer Cell. 2018;33(4):706-720.e9.
Hou Y, et al. LncRNA EPIC1 promotes tumor angiogenesis via activating the Ang2/Tie2 axis in non-small cell lung cancer. Life Sci. 2021;267: 118933.
Cheng Y, et al. Low long noncoding RNA growth arrest-specific transcript 5 expression in the exosomes of lung cancer cells promotes tumor angiogenesis. J Oncol. 2019;2019:2476175.
Levin AA. Treating disease at the RNA level with oligonucleotides. N Engl J Med. 2019;380(1):57–70.
Winkle M, et al. Noncoding RNA therapeutics - challenges and potential solutions. Nat Rev Drug Discov. 2021;20(8):629–51.
Zhou J, Rossi J. Aptamers as targeted therapeutics: current potential and challenges. Nat Rev Drug Discov. 2017;16(3):181–202.
Lai WY, et al. Synergistic inhibition of lung cancer cell invasion, tumor growth and angiogenesis using aptamer-siRNA chimeras. Biomaterials. 2014;35(9):2905–14.
Liao L, et al. A bivalent cyclic RGD-siRNA conjugate enhances the antitumor effect of apatinib via co-inhibiting VEGFR2 in non-small cell lung cancer xenografts. Drug Deliv. 2021;28(1):1432–42.
Kim JY, et al. CXCR4 uses STAT3-mediated slug expression to maintain radioresistance of non-small cell lung cancer cells: emerges as a potential prognostic biomarker for lung cancer. Cell Death Dis. 2021;12(1):48.
Li Z, et al. Increased survival by pulmonary treatment of established lung metastases with dual STAT3/CXCR4 inhibition by siRNA nanoemulsions. Mol Ther. 2019;27(12):2100–10.
Foulkes WD, Priest JR, Duchaine TF. DICER1: mutations, microRNAs and mechanisms. Nat Rev Cancer. 2014;14(10):662–72.
Chen S, et al. Global microRNA depletion suppresses tumor angiogenesis. Genes Dev. 2014;28(10):1054–67.
Chen L, et al. Antitumor effect of malaria parasite infection in a murine Lewis lung cancer model through induction of innate and adaptive immunity. PLoS ONE. 2011;6(9): e24407.
Yang Y, et al. Exosomes from Plasmodium-infected hosts inhibit tumor angiogenesis in a murine Lewis lung cancer model. Oncogenesis. 2017;6(6): e351.
Muramatsu F, et al. microRNA-125b inhibits tube formation of blood vessels through translational suppression of VE-cadherin. Oncogene. 2013;32(4):414–21.
Li F, et al. Co-delivery of VEGF siRNA and etoposide for enhanced anti-angiogenesis and anti-proliferation effect via multi-functional nanoparticles for orthotopic non-small cell lung cancer treatment. Theranostics. 2019;9(20):5886–98.
Joerger M, et al. Circulating microRNA profiling in patients with advanced non-squamous NSCLC receiving bevacizumab/erlotinib followed by platinum-based chemotherapy at progression (SAKK 19/05). Lung Cancer. 2014;85(2):306–13.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81202535), the Hunan Provincial Department of Education (nos. 19K084 and 19C1722), the Scientific Research Fund of the Education Department of Yunnan Province (2022Y199), the Hunan Provincial Science and Technology Department (2018JJ6097), the Science and Technology Bureau of Chenzhou, Hunan Province (zdyf201925), and the Research Center of Diagnosis and Treatment Technology for Lipid Metabolism Disorders of Chenzhou, Hunan Province (yfzx201907).
Author information
Authors and Affiliations
Contributions
YL and XW collected relevant literature and reviewed and drafted the manuscript. YF and MW helped to design the relevant pictures and tables. WT and JL reviewed the manuscript and revised it. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liao, Y., Wu, X., Wu, M. et al. Non-coding RNAs in lung cancer: emerging regulators of angiogenesis. J Transl Med 20, 349 (2022). https://doi.org/10.1186/s12967-022-03553-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-022-03553-x