Abstract
Background
Heart failure (HF) is a global leading cause of mortality despite implementation of guideline directed therapy which warrants a need for novel treatment strategies. Proof-of-concept clinical trials of anakinra, a recombinant human Interleukin-1 (IL-1) receptor antagonist, have shown promising results in patients with HF.
Method
We designed a single center, randomized, placebo controlled, double-blind phase II randomized clinical trial. One hundred and two adult patients hospitalized within 2 weeks of discharge due to acute decompensated HF with reduced ejection fraction (HFrEF) and systemic inflammation (high sensitivity of C-reactive protein > 2 mg/L) will be randomized in 2:1 ratio to receive anakinra or placebo for 24 weeks. The primary objective is to determine the effect of anakinra on peak oxygen consumption (VO2) measured at cardiopulmonary exercise testing (CPX) after 24 weeks of treatment, with placebo-corrected changes in peak VO2 at CPX after 24 weeks (or longest available follow up). Secondary exploratory endpoints will assess the effects of anakinra on additional CPX parameters, structural and functional echocardiographic data, noninvasive hemodynamic, quality of life questionnaires, biomarkers, and HF outcomes.
Discussion
The current trial will assess the effects of IL-1 blockade with anakinra for 24 weeks on cardiorespiratory fitness in patients with recent hospitalization due to acute decompensated HFrEF.
Trial registration: The trial was registered prospectively with ClinicalTrials.gov on Jan 8, 2019, identifier NCT03797001.
Similar content being viewed by others
Introduction
Heart failure (HF) represents a leading cause of morbidity and mortality worldwide, despite improvements in treatments and widespread efforts to implement guideline directed medical therapies [1, 2]. The high morbidity and mortality rates of patients with HF highlights the urgent need to identify new axes of disease pathogenesis for the development of novel therapies.
There is a mutual interplay between HF and inflammation suggesting that inflammation contributes to the pathogenesis and progression of HF [3,4,5,6,7,8]. Inflammation is highly prevalent in patients with HF, correlates with disease severity and appears to be more pronounced in patients with acute HF [9,10,11,12]. Comorbidities as well as acute stressors (i.e., ischemia, increased myocardial wall tension, hemodynamic overload) lead to the activation of the inflammatory response in the heart with activation of the NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome, subsequent maturation and release of proinflammatory cytokines such as Interleukin-1beta (IL-1β) and IL-18 [13]. IL-1β is one of two IL-1 isoforms shown to function as a cardio-depressant factor, and to promote adverse cardiac remodeling after myocardial injury [14,15,16,17]. IL-1 blockade with anakinra has been shown to prevent adverse cardiac remodeling and HF in animal models of acute myocardial infarction (AMI) and reduce the incidence of HF in patients with ST-segment elevation AMI [18, 19]. In the Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) trial, canakinumab, an IL-1β antibody, led to a significant reduction of the composite of HF hospitalization or HF–related mortality in a population of 10,061 patients with prior AMI and residual inflammatory burden [20]. In the REcently Decompensated Heart failure Anakinra Response Trial (REDHART) study, anakinra treatment led to an improvement in peak oxygen consumption (VO2), a primary measure of aerobic exercise capacity and cardiorespiratory fitness (CRF) [21, 22], in the group treated for 12 weeks, but not in the group treated for 2 weeks [23].
The REDHART2 study is designed to expand the previous findings, determine the effects of anakinra treatment administered for 24 weeks on peak VO2 and other measures of CRF (CRF is considered a vital sign) as well as derive an estimate for the potential effect size on HF outcomes[21].
Study objectives and hypothesis
The objective of the study is to determine the effects of IL-1 blockade with anakinra on peak VO2 (i.e., primary outcome) derived from cardiopulmonary exercise testing (CPX) after 24 weeks of treatment, as well as the effects of anakinra on cardiac biomarkers, echocardiographic data, noninvasive hemodynamic, body composition analysis, quality of life (QoL) questionnaires, perceived functional capacity and physical activity questionnaires, and HF-related clinical outcomes.
Methods
Study design
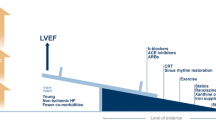
The REDHART2 study is a single-center, randomized, placebo-controlled, double-blinded, phase II clinical trial. Patients will be randomized in a 2:1 ratio to receive anakinra or placebo for 24 weeks. Figure 1 presents an overview of the study procedures.
Schematic protocol of the REDHART2 study. Schematic protocol of the REDHART2 study to investigate the potential effects of anakinra in a randomized double-blind placebo-controlled clinical trial. Patients will be followed for 24 weeks. Lab tests, questionnaires, bio-electrical impedance analysis, echocardiogram and cardiopulmonary exercise testing will be done at baseline, 6, 12, and 24 weeks. Visit 1.5 (after 2 weeks) is considered a safety follow-up assessment
Patients will undergo baseline clinical assessment, blood tests for biomarkers, transthoracic echocardiogram, body-composition analysis, QoL questionnaires, perceived functional capacity and physical activity questionnaires, and CPX. After completion of all baseline testing, patients that qualify for the study will be randomized and given a 14-day supply of anakinra or placebo. Clinical evaluations, blood tests, echocardiogram, body composition analysis, QoL questionnaires, perceived functional capacity and physical activity questionnaires, and CPX will be repeated at 6 ± 1 weeks, 12 ± 1 weeks, and 24 ± 2 weeks. A clinical evaluation will also be completed at 14 ± 3 days to evaluate preliminary tolerability of the treatment and measure a complete blood cell count with differential and comprehensive metabolic panels (Fig. 1). Additional supply of anakinra or placebo will be given at each visit.
Screening and enrollment
Adult patients with acute decompensated HFrEF (left ventricular EF ≤ 40%) will be eligible for this study within 14 days of hospital discharge if they have elevated levels of high sensitivity CRP (hsCRP > 2 mg/L) and meet the remaining inclusion criteria (Table 1). Patients will be excluded if the primary diagnosis for admission is not acute decompensated HF or if they have any contraindications against anakinra use (e.g., pregnancy, neutropenia, dialysis, acute or chronic infections), or have any concomitant medications or conditions that could affect the inflammatory response [e.g., recent infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), cancer, autoimmune diseases] (Table 1).
Randomization
Randomization will be handled by the investigational pharmacy using a dedicated randomization algorithm. Patients and physicians who involve in the study treatment will be blinded to the allocation status.
Investigational treatment
Anakinra (100 mg) or placebo (vehicle) dispensed in small syringes (0.67 mL) will be provided to the patient for daily subcutaneous injection. The syringes for anakinra or placebo have been purchased from the supplier (Swedish Orphan Biovitrum, Stockholm, Sweden) to ensure that they are indistinguishable from each other. Patients will also receive instruction from the investigators regarding self-injection technique, storage, and disposal.
Data collection
Cardiopulmonary exercise testing (CPX)
CPX will be performed at baseline, and after 6 ± 1, 12 ± 2, and 24 ± 2 weeks. A physician-supervised maximal exercise test will be administered using a metabolic cart (UltimaCardioO2, MGC Diagnostics, Saint Paul, MN) that is interfaced with a conservative ramping treadmill protocol [24,25,26]. The highest 10-s average value for VO2 during the final 30 s defines peak VO2 in mLO2 kg−1 min−1 [27]. Peak respiratory exchange ratio (RER) will be used for determining subject effort. A peak RER ≥ 1.10 is a well-accepted criterion for maximal effort and a peak RER ≥ 1.0 is considered a minimal acceptable threshold. Subjects with RER < 1.0, indicating submaximal effort, or with angina, abnormal blood pressure or heart rate response, or ECG changes suggestive of coronary ischemia, at time of initial CPX will be excluded.
Doppler echocardiography
Transthoracic Doppler echocardiography will be performed at baseline, 6 ± 1, 12 ± 2, and 24 ± 2 weeks. All measurements will be performed by 2 operators blinded to treatment allocations.
Bioelectrical impedance analysis
Bioelectrical Impedance Analysis (BIA) will be conducted at baseline, and after 6 ± 1, 12 ± 2, and 24 ± 2 weeks. Body composition (i.e., water, lean mass, and fat) will be determined using a Quantum IV Body Composition Analyzer (RJL Systems, Inc., Clinton Township, MI).
Questionnaires
Subjects will complete the Kansas City Cardiomyopathy Questionnaire (KCCQ),[28] the Duke Activity Status Index (DASI) questionnaire,[29] International Physical Activity Questionnaire—Short Form (IPAQ-SF) [30], and the Patient Health Questionnaire-9 (PHQ-9) [31] at baseline and after 6 ± 1, 12 ± 2, and 24 ± 2 weeks. The KCCQ is a 20-question graded questionnaire that has been extensively validated to measure impairment in QoL in patients with HF. The DASI is a twelve-item “yes/no” questionnaire that allows for the calculation of perceived functional capacity. The IPAQ-SF is a 7-item questionnaire that allows the estimation of daily physical activity. Lastly, subjects will also be asked to complete the PHQ-9, a nine-question standardized assessment of depression and anxiety which has been validated in patients with HF.
Biomarkers
Blood will be collected at each visit (baseline, 6 ± 1, 12 ± 2, and 24 ± 2 weeks) and analyzed for a panel of complete blood cell count with differential, basic metabolic panel, hsCRP, and N-terminal pro-brain natriuretic peptide (NTproBNP). To maintain allocation concealment, the investigators will also be blinded to hsCRP levels throughout follow-up, which may be affected by treatment.
Clinical events
Clinical events of interest will include death (both cardiac and non-cardiac), hospitalizations (for HF, for other cardiac causes not related to HF, or for non-cardiac reasons), worsening of HF as an outpatient, nonfatal myocardial infarction, unstable angina, urgent or unplanned revascularization, tachy- or brady-arrhythmias leading to a new hospitalization or prolongation of hospital stay, acute kidney injury, acute respiratory failure, sepsis or other serious infection, acute ischemic stroke, acute hemorrhagic stroke, or acute stroke (indeterminate) (Table 2).
Adjudication of these events will be performed by an ad hoc committee blinded to treatment allocation and the adjudication.
Primary outcomes
The primary analysis of REDHART2 will compare the effects of IL-1 blockade with anakinra versus placebo on peak VO2 (in mLO2 kg−1 min−1) during CPX after 24 weeks of treatment.
Secondary endpoints
A composite of cardiac death and re-hospitalization for HF within the first 6 months of hospitalization will serve as the clinical endpoint of interest for the proposed study. Cardiac death is defined as death in which a direct cause attributable to cardiac disease is present. Re-hospitalization for HF is defined as hospitalization in which the primary diagnosis is decompensated HF established as the finding at admission of both conditions listed: (1) dyspnea or respiratory distress or tachypnea at rest or with minimal exertion; and (2) evidence of elevated cardiac filling pressure or pulmonary congestion (defined as congestion/edema at physical exam or one of the following: congestion at chest X-ray, plasma BNP levels ≥ 200 pg/mL, invasive measurement of left ventricular end-diastolic pressure > 18 mmHg, or pulmonary artery occluding pressure (wedge) > 16 mmHg.
Exploratory endpoints include (1) additional CPX parameters: changes in peak VO2 at earlier endpoints (6 and 12 weeks), and changes in the VE/VCO2 slope, OUES, VO2 at VAT, and peak O2 pulse at 6, 12, and 24 weeks; (2) echocardiography parameters: changes in left ventricular volumes, diastolic function, right and left ventricular systolic function at 6, 12 at 24 weeks; (3) non-invasive hemodynamics: changes in ventriculo-arterial coupling at 6, 12 and at 24 weeks; (4) biomarkers: changes in hs-CRP and NTproBNP at 6, 12 and at 24 weeks; and (5) QoL assessment changes in the DASI and KCCQ, at 6, 12 and at 24 weeks.
Specification of safety parameters
Safety parameters will include data collected at baseline and each visit, including physical examination, laboratory results, and functional and imaging tests. Disease-related safety data (HF-related) will be assessed, including changes in symptoms (or new symptoms), functional capacity, vital signs (including weight), renal function, or any significant changes in medications.
A complete blood cell count will be measured at each visit to exclude unusual cases of anakinra-related neutropenia (absolute neutrophil count [ANC] < 1000/mm3), for which suspension of active treatment will be considered until return to a value of ANC > 1800/mm3 (or > 1000/mm3 if patient is African American).
Adverse events (AEs) are defined as untoward medical occurrence in a patient administered a pharmaceutical product considered to be causally related to the study treatment or research conduct. Serious AEs (SAEs) are defined as any adverse event/experience occurring between patient baseline assessment and the final study visit those results in life threatening events, requiring hospitalization, persistent or significant disability or incapacity and is unexpected or not consistent with the natural history of the disease. All unexpected SAEs will be promptly (within 24 h) reported to the Virginia Commonwealth University Institutional Review Board (IRB) and data and safety monitoring board (DSMB).
The data and safety monitoring board
The DSMB is composed of a coordinator and 5 voting members. The minutes from each meeting will be distributed to the board members, National Heart Lung Blood Institute (NHLBI) program officer, and to the IRB. A brief conclusive statement addressing whether the study should continue as planned will be provided to the investigators and the IRB every 6 months.
Alternate consent group
Any potential participants who are eligible to enroll in the study but decline to participate will be offered an alternate consent process that allows the research team to access the electronic health record for 6 months after the enrollment to ascertain vital status and any HF hospitalizations during follow-up. This alternate consent group will provide an estimate of the event rate in a group free of research interventions.
Statistical analysis
Demographics and baseline characteristics
Descriptive summaries of continuous measurements will be reported as median and interquartile ranges due to potential deviation from Gaussian distribution. Descriptive summaries of categorical measurements consist of frequencies, proportions, and 95% confidence intervals, when applicable.
Analysis
All analyses will be conducted after database locking once all data has been gathered and electronically captured. All analyses will be based on the intention-to-treat principle (i.e., analyzing groups as randomized and including all patients with outcome data available). The difference in interval changes in peak VO2 at 24 weeks between the anakinra versus placebo groups will be compared using random-effect analysis of variance for repeated measures to analyze the effects of treatment within each group and the effect of time x group allocation. Unadjusted p-values will be reported throughout, with statistical significance for the primary endpoint set at the 2-tailed alpha 0.05 level. To evaluate the group differences in the secondary endpoints, data will be compared across all groups using the random-effect analysis of variance for repeated measures as indicated above for paired analyses or using Chi-squared testing for event rates. The Statistical Package for Social Sciences software version 25 (IBM, New York, NY) will be used.
Sample size considerations
The sample size for this pilot study is calculated according to the primary endpoint of difference in interval change in peak VO2 at 24 weeks between anakinra and placebo. Given an expected average peak VO2 of 15 mLO2 kg−1 min−1 for HF patients, 68 subjects randomized to anakinra, and 34 subjects randomized to placebo (2:1 randomization) would provide approximately > 95% power to detect a difference of 1.6 mL O2 kg−1 min−1 (standard deviation of 1.7–2.0 mLO2 kg−1 min−1) in peak VO2 on top of placebo. A conservative estimate of 20% loss to follow-up or withdrawal would retain > 90% power (Table 3).
Discussion
The rationale for IL-1 blockade with anakinra in HF stems from the following evidence: (1) direct cardio-depressant effects of IL-1 in cardiac cells and animal models [4, 32, 33]; (2) reduced adverse cardiac remodeling HF with IL-1 blockers in animal models of AMI [15, 16, 34,35,36]; (3) reduced incidence of HF in patients with ST-segment elevation AMI [18, 19, 37]; 4) enhanced IL-1 activity in patients with HF [4]; (5) quenching of the acute inflammatory response in patients with acute decompensated HF [38]; and (6) favorable signals for improved cardiorespiratory fitness in pilot studies including patients with stable HFrEF, stable HF with preserved left ventricular ejection fraction (LVEF) (HFpEF), and in patients with recently decompensated systolic HF [23, 39, 40].
In the pilot REDHART trial, 60 patients with recently decompensated HFrEF were randomly assigned 1:1:1 to anakinra for 2 weeks, anakinra for 12 weeks, or placebo [23]. No significant differences in peak VO2 were seen with anakinra, or placebo, at 2 weeks. At 4 and 12 weeks, the patients treated with anakinra for 12 weeks showed an improvement in peak VO2 compared with baseline, whereas those treated with anakinra for 2 weeks or those treated with placebo did not improve peak VO2 [41]. In this trial, we expect that the increased sample size and prolonged treatment duration of the REHDART2 trial will provide sufficient power for an assessment of the treatment effect of IL-1 blockade on peak VO2. Furthermore, the REDHART2 trial will also employ a more stringent definition for “reduced” ejection fraction (LVEF ≤ 40%) as compared with the original pilot REDHART trial (LVEF ≤ 50%).
In the large multicenter CANTOS trial, treatment with canakinumab, IL-1β blocking antibody, reduced HF-related adverse events in patients with prior MI [20]. In a small single-center sub-study of the CANTOS trial in 15 patients with pre-existing HFrEF, canakinumab significantly increased peak VO2 at 3 months compared to placebo (median interval change of + 1.6 [from − 0.4 to + 3.4] mL kg−1 min−1; P = 0.026 between group changes) [40].
The limitations of the REDHART2 study should be discussed. This a single-center phase II clinical trial with a small sample size and relative short follow up period. While this is a limitation, the expertise required for the use of IL-1 blockers available at our center and the ability to standardize procedures related to peak VO2 measurement make this also a potential advantage. Furthermore, despite the small sample size, the study has a sufficient power for the primary endpoint. The study may not be powered to detect differences in clinical outcome, such as death or HF hospitalization, and while it may provide an estimate for the potential effect size on HF outcomes, these estimates need to be interpreted with caution. Moreover, sodium glucose cotransporter inhibitors received FDA approval during the conduct of this trial and have become part of guideline-directed medical therapy only more recently [42]. The resultant change in background therapy may increase the heterogeneity of the data. However, we hope to minimize the impact of this change—along with other potential evolutions in background therapy—through the ongoing process of randomized allocation. Finally, this study is being conducted during the COVID-19 pandemic, which has disrupted routine healthcare practices and may have altered the rates of hospitalization for many HF patients.
In conclusion, the REDHART2 study will determine whether IL-1 blockade with anakinra for 24 weeks will improve cardiorespiratory fitness measured with CPX, namely peak VO2, in patients with recently decompensated HFrEF.
Availability of data and materials
A simplified and fully de-identified database will be made available for sharing in accordance with requirements for NHLBI data repository datasets and associated documentation for submission to the Biological Specimen and Data Repository Information Coordinating Center (BioLINCC) and the NHLBI Policy for Data Sharing from Clinical Trials and Epidemiological Studies, and in accordance with the Guidelines for NHLBI Data Set Preparation, within 3 years of completion of the study.
Abbreviations
- AE:
-
Adverse event
- ANC:
-
Absolute neutrophil count
- AMI:
-
Acute myocardial infarction
- BIA:
-
Bio-electrical impedance analysis
- CANTOS:
-
Canakinumab anti-inflammatory thrombosis outcome study
- CPX:
-
Cardiopulmonary exercise test
- CRF:
-
Cardiorespiratory fitness
- DASI:
-
Duke activity status index
- DSMB:
-
Data safety and monitoring board
- Ea:
-
Estimates of arterial elastance
- Ees:
-
End-systolic elastance
- HF:
-
Heart failure
- HFpEF:
-
Heart failure with preserved ejection fraction
- HFrEF:
-
Heart failure with reduced ejection fraction
- hsCRP:
-
High sensitive C-reactive protein
- IL:
-
Interleukin
- IPAQ-SF:
-
International physical activity questionnaire—short form
- IRB:
-
Institutional review board
- KCCQ:
-
Kansas city cardiomyopathy questionnaire
- LVEF:
-
Left ventricular ejection fraction
- NHLBI:
-
National heart lung blood institute
- NLRP3:
-
NOD-, LRR- and pyrin domain-containing protein 3
- NT-proBNP:
-
N-terminal pro brain natriuretic peptide
- PHQ-9:
-
Patient health questionnaire-9
- OUES:
-
Oxygen uptake efficiency slope
- QoL:
-
Quality of life
- REDHART:
-
REcently decompensated heart failure anakinra response trial
- RER:
-
Respiratory exchange ratio
- SAE:
-
Severe adverse event
- VAT:
-
Ventilatory anaerobic threshold
References
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation. 2020;141(9):e139–596.
Wu JR, Moser DK. Health-related quality of life is a mediator of the relationship between medication adherence and cardiac event-free survival in patients with heart failure. J Card Fail. 2021;27(8):848–56.
Van Linthout S, Tschöpe C. Inflammation—cause or consequence of heart failure or both? Curr Heart Fail Rep. 2017;14(4):251–65.
Van Tassell BW, Arena RA, Toldo S, Mezzaroma E, Azam T, Seropian IM, et al. Enhanced interleukin-1 activity contributes to exercise intolerance in patients with systolic heart failure. PLoS ONE. 2012;7(3):e33438.
Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C, et al. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation. 2000;102(25):3060–7.
Dick SA, Epelman S. Chronic heart failure and inflammation: what do we really know? Circ Res. 2016;119(1):159–76.
Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11(5):255–65.
Zuurbier CJ, Abbate A, Cabrera-Fuentes HA, Cohen MV, Collino M, De Kleijn DPV, et al. Innate immunity as a target for acute cardioprotection. Cardiovasc Res. 2019;115(7):1131–42.
Pfisterer M, Buser P, Rickli H, Gutmann M, Erne P, Rickenbacher P, et al. BNP-guided vs symptom-guided heart failure therapy: the trial of intensified vs standard medical therapy in elderly patients with congestive heart failure (TIME-CHF) randomized trial. JAMA. 2009;301(4):383–92.
van Wezenbeek J, Canada JM, Ravindra K, Carbone S, Trankle CR, Kadariya D, et al. C-reactive protein and N-terminal pro-brain natriuretic peptide levels correlate with impaired cardiorespiratory fitness in patients with heart failure across a wide range of ejection fraction. Front Cardiovasc Med. 2018;5:178.
Murphy SP, Kakkar R, McCarthy CP, Januzzi JL Jr. Inflammation in heart failure: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(11):1324–40.
O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365(1):32–43.
Toldo S, Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol. 2018;15(4):203–14.
Toldo S, Mezzaroma E, O’Brien L, Marchetti C, Seropian IM, Voelkel NF, et al. Interleukin-18 mediates interleukin-1-induced cardiac dysfunction. Am J Physiol Heart Circ Physiol. 2014;306(7):H1025–31.
Abbate A, Van Tassell BW, Seropian IM, Toldo S, Robati R, Varma A, et al. Interleukin-1beta modulation using a genetically engineered antibody prevents adverse cardiac remodelling following acute myocardial infarction in the mouse. Eur J Heart Fail. 2010;12(4):319–22.
Van Tassell BW, Varma A, Salloum FN, Das A, Seropian IM, Toldo S, et al. Interleukin-1 trap attenuates cardiac remodeling after experimental acute myocardial infarction in mice. J Cardiovasc Pharmacol. 2010;55(2):117–22.
Abbate A, Toldo S, Marchetti C, Kron J, Van Tassell BW, Dinarello CA. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ Res. 2020;126(9):1260–80.
Abbate A, Trankle CR, Buckley LF, Lipinski MJ, Appleton D, Kadariya D, et al. Interleukin-1 blockade inhibits the acute inflammatory response in patients with ST-segment-elevation myocardial infarction. J Am Heart Assoc. 2020;9(5):e014941.
Abbate A, Wohlford GF, Del Buono MG, Chiabrando JG, Markley R, Turlington J, et al. Interleukin-1 blockade with anakinra and heart failure following ST-segment elevation myocardial infarction: results from a pooled analysis of the VCUART clinical trials. Eur Heart J Cardiovasc Pharmacother. 2021. https://doi.org/10.1093/ehjcvp/pvab075.
Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, et al. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. 2019;139(10):1289–99.
Ross R, Blair SN, Arena R, Church TS, Després JP, Franklin BA, et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American heart association. Circulation. 2016;134(24):e653–99.
Del Buono MG, Arena R, Borlaug BA, Carbone S, Canada JM, Kirkman DL, et al. Exercise intolerance in patients with heart failure: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(17):2209–25.
Van Tassell BW, Canada J, Carbone S, Trankle C, Buckley L, Oddi Erdle C, et al. Interleukin-1 blockade in recently decompensated systolic heart failure: results from REDHART (recently decompensated heart failure anakinra response trial). Circ Heart Fail. 2017;10(11):e004373.
Arena R, Myers J, Guazzi M. Cardiopulmonary exercise testing is a core assessment for patients with heart failure. Congest Heart Fail. 2011;17(3):115–9.
van Wezenbeek J, Canada JM, Ravindra K, Carbone S, Kadariya D, Trankle CR, et al. Determinants of cardiorespiratory fitness in patients with heart failure across a wide range of ejection fractions. Am J Cardiol. 2020;125(1):76–81.
Canada JM, Trankle CR, Buckley LF, Carbone S, Abouzaki NA, Kadariya D, et al. Severely impaired cardiorespiratory fitness in patients with recently decompensated systolic heart failure. Am J Cardiol. 2017;120(10):1854–7.
Arena R, Canada JM, Popovic D, Trankle CR, Del Buono MG, Lucas A, et al. Cardiopulmonary exercise testing—refining the clinical perspective by combining assessments. Expert Rev Cardiovasc Ther. 2020;18(9):563–76.
Spertus JA, Jones PG, Sandhu AT, Arnold SV. Interpreting the Kansas city cardiomyopathy questionnaire in clinical trials and clinical care. J Am Coll Cardiol. 2020;76(20):2379–90.
Grodin JL, Hammadah M, Fan Y, Hazen SL, Tang WHW. Prognostic value of estimating functional capacity with the use of the duke activity status index in stable patients with chronic heart failure. J Cardiac Fail. 2015;21(1):44–50.
Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95.
Hammash MH, Hall LA, Lennie TA, Heo S, Chung ML, Lee KS, et al. Psychometrics of the PHQ-9 as a measure of depressive symptoms in patients with heart failure. Eur J Cardiovasc Nurs. 2013;12(5):446–53.
Van Tassell BW, Seropian IM, Toldo S, Mezzaroma E, Abbate A. Interleukin-1β induces a reversible cardiomyopathy in the mouse. Inflamm Res. 2013;62(7):637–40.
Kumar A, Thota V, Dee L, Olson J, Uretz E, Parrillo JE. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med. 1996;183(3):949–58.
Abbate A, Salloum FN, Vecile E, Das A, Hoke NN, Straino S, et al. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117(20):2670–83.
Toldo S, Mezzaroma E, Van Tassell BW, Farkas D, Marchetti C, Voelkel NF, et al. Interleukin-1β blockade improves cardiac remodelling after myocardial infarction without interrupting the inflammasome in the mouse. Exp Physiol. 2013;98(3):734–45.
Abbate A, Salloum FN, Van Tassell BW, Vecile E, Toldo S, Seropian I, et al. Alterations in the interleukin-1/interleukin-1 receptor antagonist balance modulate cardiac remodeling following myocardial infarction in the mouse. PLoS ONE. 2011;6(11):e27923.
Abbate A, Kontos MC, Abouzaki NA, Melchior RD, Thomas C, Van Tassell BW, et al. Comparative safety of interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCU-ART2 pilot studies). Am J Cardiol. 2015;115(3):288–92.
Van Tassell BW, Abouzaki NA, Erdle CO, Carbone S, Trankle C, Melchior RD, et al. Interleukin-1 blockade in acute decompensated heart failure: a randomized, double-blinded, placebo-controlled pilot study. J Cardiovasc Pharmacol. 2016;67(6):544.
Van Tassell BW, Arena R, Biondi-Zoccai G, Canada JM, Oddi C, Abouzaki NA, et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am J Cardiol. 2014;113(2):321–7.
Trankle CR, Canada JM, Cei L, Abouzaki N, Oddi-Erdle C, Kadariya D, et al. Usefulness of canakinumab to improve exercise capacity in patients with long-term systolic heart failure and elevated C-reactive protein. Am J Cardiol. 2018;122(8):1366–70.
Van Tassell BW, Canada J, Carbone S, Trankle C, Buckley L, Oddi Erdle C, et al. Interleukin-1 blockade in recently decompensated systolic heart failure. Circ Heart Fail. 2017;10(11):e004373.
Maddox TM, Januzzi JL, Allen LA, Breathett K, Butler J, Davis LL, et al. 2021 Update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction. J Am Coll Cardiol. 2021;77(6):772–810.
Acknowledgements
Not applicable
Funding
The REDHART2 study is funded by the National Heart, Lung, and Blood Institute of the National Institute of Health (R61/R33HL133943). The study is also supported by a Clinical and Translational Science Award to Virginia Commonwealth University (UL1TR002649) from the National Center for Advancing Translational Sciences.
Author information
Authors and Affiliations
Contributions
All authors have contributed significantly to the manuscript, reviewed the manuscript, and approved it for submission. BVT made substantial contributions to the concept and design of the work. GT has made substantial contributions to acquisition of data. AM has made substantial contributions to acquisition of data. VM has made substantial contributions to acquisition of data. AHT made substantial contributions to acquisition of data and drafted the work. JL made substantial contributions to the concept and design of the work. LK has made substantial contributions to interpretation of data. AL has made substantial contributions to acquisition of data. JID has made substantial contributions to acquisition of data. DLD made substantial contributions to the concept and design of the work. RM has made substantial contributions to acquisition of data. JT has made substantial contributions to acquisition of data. EF has made substantial contributions to acquisition of data. MDB has made substantial contributions to acquisition of data. GB made substantial contributions to the concept and design of the work. JMC has made substantial contributions to acquisition of data. RA has made substantial contributions to the concept and design of the work. AA made substantial contributions to the concept and design of the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Institutional review board approval was obtained from VCU prior to the start of the trial. IRB HM20014686; initial approval date: 11/19/2018.
Consent for publication
Not applicable.
Competing interests
Dr. Abbate (AA) has served as consultant to Swedish Orphan Biovitrum in the past. Dr. Van Tassell (BVT) has served as consultant to Swedish Orphan Biovitrum in the past. No other authors have relevant disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Van Tassell, B., Mihalick, V., Thomas, G. et al. Rationale and design of interleukin-1 blockade in recently decompensated heart failure (REDHART2): a randomized, double blind, placebo controlled, single center, phase 2 study. J Transl Med 20, 270 (2022). https://doi.org/10.1186/s12967-022-03466-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-022-03466-9