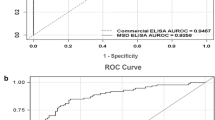
Abstract
Background
Due to insufficient accuracy, urine-based assays currently have a limited role in the management of patients with bladder cancer. The identification of multiplex molecular signatures associated with disease has the potential to address this deficiency and to assist with accurate, non-invasive diagnosis and monitoring.
Methods
To evaluate the performance of Oncuria™, a multiplex immunoassay for bladder detection in voided urine samples. The test was evaluated in a multi-institutional cohort of 362 prospectively collected subjects presenting for bladder cancer evaluation. The parallel measurement of 10 biomarkers (A1AT, APOE, ANG, CA9, IL8, MMP9, MMP10, PAI1, SDC1 and VEGFA) was performed in an independent clinical laboratory. The ability of the test to identify patients harboring bladder cancer was assessed. Bladder cancer status was confirmed by cystoscopy and tissue biopsy. The association of biomarkers and demographic factors was evaluated using linear discriminant analysis (LDA) and predictive models were derived using supervised learning and cross-validation analyses. Diagnostic performance was assessed using ROC curves.
Results
The combination of the 10 biomarkers provided an AUROC 0.93 [95% CI 0.87–0.98], outperforming any single biomarker. The addition of demographic data (age, sex, and race) into a hybrid signature improved the diagnostic performance AUROC 0.95 [95% CI 0.90–1.00]. The hybrid signature achieved an overall sensitivity of 0.93, specificity of 0.93, PPV of 0.65 and NPV of 0.99 for bladder cancer classification. Sensitivity values of the diagnostic panel for high-grade bladder cancer, low-grade bladder cancer, MIBC and NMIBC were 0.94, 0.89, 0.97 and 0.93, respectively.
Conclusions
Urinary levels of a biomarker panel enabled the accurate discrimination of bladder cancer patients and controls. The multiplex Oncuria™ test can achieve the efficient and accurate detection and monitoring of bladder cancer in a non-invasive patient setting.
Similar content being viewed by others
Background
Given the complexity of the molecular changes involved in the development of neoplastic disease, a necessary shift from the use of single diagnostic biomarkers to molecular signatures for patient evaluation has occurred. A multiplex diagnostic signature has the potential to perform accurately across the clinical and molecular spectrum of a disease, making individualized patient evaluation and care feasible. Coupled with advances in analytical instrument design, which enable the cost-effective, simultaneous measurement of molecular panels, multiplex tests are emerging as powerful tools. Several molecular signature assays have been incorporated into clinical practice for the management of prostate cancer [1, 2], breast cancer [3, 4] and colon cancer [5, 6]. However, no molecular signatures have been successfully incorporated into clinical practice for the management of bladder cancer. Bladder cancer is among the most common malignancies worldwide, and due to high rates of recurrence, one of the most prevalent.
The current primary diagnostic approach to bladder cancer is cystoscopy coupled with voided urine cytology (VUC). Cystoscopy is an uncomfortable, invasive procedure associated with significant cost and possible infection and trauma. VUC remains the method of choice for the noninvasive detection of bladder cancer. However, while the assay has good specificity, VUC sensitivity is suboptimal, especially for low-grade and low-stage tumors [7]. Consequently, the development of an accurate diagnostic bladder cancer assay that could be applied to non-invasively obtained urine samples would benefit both patients and health care systems.
In a series of previous studies, we have identified a panel of urine-based protein biomarkers that are significantly associated with bladder cancer [8,9,10,11]. The potential utility of the diagnostic panel was subsequently refined and validated in retrospective studies [12,13,14,15,16,17]. The optimal 10-biomarker panel; angiogenin, ANG; apolipoprotein E, APOE; alpha-1 antitrypsin, A1AT; carbonic anhydrase 9, CA9; interleukin 8, IL8; matrix metallopeptidase 9, MMP9; matrix metallopeptidase 10, MMP10; plasminogen activator inhibitor 1, PAI1; syndecan 1, SDC1 and vascular endothelial growth factor A, VEGFA [18]) was developed into a clinical grade, custom-designed multiplex immunoassay [19], and subsequently analytically validated, Fig. 1.
Flow diagram of phases project. Gene expression profiling (Affymetrix U133 Plus 2.0 arrays) followed by quantitative PCR verification, and glycoprotein profiling (dual-lectin affinity chromatography and liquid chromatography/tandem mass spectrometry) followed by Western blot analysis or ELISA verification were used to discover and validate RNA and protein expression profiles associated with bladder cancer. Data integration informed the selection of a 19-biomarker panel for testing which was narrowed to 10 protein biomarkers which has been validated in independent cohorts using commercial ELISA assays or custom-designed multiplex assay. The resulting Oncuria™assay used in this study has been analytically validated
In this study, we tested the potential clinical utility of the Oncuria™ multiplex immunoassay for the detection of bladder cancer in a prospectively recruited cohort of patients who presented for urological evaluation at three institutions. The Oncuria™ test achieved a strong overall diagnostic performance, achieving an overall AUC of 0.95, sensitivity and specificity values of 93% and 93%, respectively, and a negative predictive value (NPV) and positive predictive value (PPV) of 99% and 65%, respectively. The Oncuria™ test shows promise for clinical application in the non-invasive diagnosis and surveillance bladder cancer, and potentially for screening at-risk, asymptomatic individuals.
Methods
Patients and specimen processing
Ethical review of the study was performed by local institutional review boards. Patients visiting the Urology outpatient clinics at University of Hawaii Cancer Center and Cedars-Sinai Medical Center were consented. As this is a urine-based assay to detect a bladder cancer associated protein signature, subjects with a history of renal insufficiency, glomerular filtration rate (GFR) < 60 mL/min and/or reduced urinary creatinine (< 40 mg/dL) were excluded, since these patients are known to have large quantities of proteins in their urine. The study cohort (Table 1) was comprised of 362 subjects, 46 de-novo bladder cancer cases and 316 non-bladder cancer controls. No one had a history of bladder cancer. Control subjects were noted to have voiding symptoms (226), urinary tract infections (17), urolithiasis (11) and hematuria (37 gross hematuria and 25 microscopic hematuria) but no pathology. Midstream voided urine sample was collected prior to any instrumentation for cytology and multiplex testing. Urines were centrifuged at 1,000g for 10 min and supernatant decanted and immediately frozen. Each institute processed the urines similarly. All patients underwent cystoscopy and upper tract imaging. When an abnormality was present on cystoscopy the patient underwent a formal transurethral resection of bladder tumor (TURBT) for histological confirmation of urothelial carcinoma, including grade and stage. Data are reported according to International Consensus Panel on Bladder Tumor Markers [20] and PROBE criteria [21].
Multiplex immunoassay
The concentrations of the 10 proteins (A1AT, APOE, ANG, CA9, IL8, MMP9, MMP10, PAI1, SDC1 and VEGFA) were monitored using an analytically validated multiplex bead-based immunoassay (Oncuria™) from R&D Systems Inc. (Minneapolis, MN) for Luminex 200. Urine samples were passively thawed, centrifuged for 10 min × 1,000g. Urine samples were passively thawed and handled on ice prior to diluting twofold with R&D Assay Diluent 37. Samples, standards and controls (50 μl) were added to the 96 well plate in duplicate. The multiplex immunoassay was conducted according to the manufacturer’s instructions. A seven-point standard curve across the 4 log dynamic range of the assays was included in the current assay design. Plates were read on the Luminex® 100/200 (Luminex Corp, Austin, TX). Calibration curves were generated along with optimal fit in conjunction with Akaike’s information criteria (AIC) values [22].
Data analysis
A meta-cohort of 362 subjects (two missing clinical stage and one missing sex) was generated whose urine samples were analyzed in duplicate (n = 724 samples). Values were set to missing if the test–retest error was five standard deviations beyond the average test–retest error. Wald chi-square tests determined the association between each biomarker and bladder cancer. We investigated the diagnostic performance of individual biomarker for bladder cancer detection using the logistic regression analysis with bladder cancer status (yes vs. no) as the response variable and 10 biomarkers as the explanatory variables. Using cutoff values defined by a 50% predicted probability of disease, we defined each biomarker as either positive or negative when the biomarker was either ≥ or < the cutoff. Next, we analyzed the predictive power of the 10-biomarker molecular signature and a hybrid signature composed of the 10-biomarker molecular signature with three key demographic variables (age, sex and race) by constructing two models. For the molecular signature model, each sample is represented as a vector with 10 dimensions representing the 10 biomarkers. For the hybrid signature model, each sample is represented as 13-dimensional vectors with 10 dimensions representing the 10 biomarkers and the additional three dimensions representing the three demographic factors [23]. To compensate for the range variation between different biomarkers, we transformed the original biomarker data using log-transformation: \({\text{log}}_{10}(\text{Biomarker}+0.01)\). Then, we divided the cohort into a training (80%) and a test set. On the training set, we used the leave-one-out cross validation (LOOCV) method to estimate the parameters of a linear discriminant analysis (LDA) classifier [24, 25] and the performance of the classifier was evaluated on the test set. For performance evaluation, we calculated sensitivity, specificity, positive prediction value and negative prediction value, and a receiver operating characteristic (ROC) curve [26] was used to provide a direct view of how a prediction model functioned at different sensitivity and specificity levels. We evaluated the performance of the constructed classifiers on the test set. Statistical significance in this study was set at p < 0.05 and all reported p values were 2-sided. All analyses were performed using SAS software version 9.3 (SAS Institute Inc., Cary, NC).
Results
The study population was comprised of 362 subjects, 287 from the University of Hawaii Cancer Center and 75 subjects from Cedars-Sinai Medical Center. Clinical, pathologic and demographic characteristics of the 362 subjects (46 bladder cancer, 316 non-bladder cancer) comprising the study cohort are listed in Table 1. Median age of bladder cancer subjects was 69 years (range 38–87 years). Of the bladder cancer subjects, 76.1% were men and 67.4% were Caucasian. Of the 46 bladder cancer cases, 61.4% were classified as non-muscle invasive bladder cancer (NMIBC; stages Ta, Tis, T1), and 38.6% were muscle invasive bladder cancer (MIBC; stage ≥ T2), while 19.6% cases were reported as low-grade carcinoma and 80.4% cases as high-grade.
To reduce skewness when comparing results from different institutes, we used the log transformation, log10(data + 0.01) for each biomarker. There was limited variability observed in each biomarker concentration ranges between institutions (Supplemental Table). Urinary concentrations of all 10 biomarkers were elevated in patients with bladder cancer compared with non-bladder cancer (Table 2) with statistical significance being reached for MMP9, IL8, VEGFA, PAI1, ApoE, A1AT and ANG.
Table 3 provides AUC data for each individual biomarker and the combination of the ten biomarkers and the hybrid signature. The hybrid signature achieved superior AUC values. All ten biomarkers using optimal cutoff values defined by a 50% predicted probability of disease resulted in an AUC of 0.93 (95% confidence interval, 0.87–0.98), with a sensitivity of 87%, a specificity of 92%, a negative predictive value of 98% and a positive predictive value of 61% (Table 3). The AUC improved to 0.95 (95% confidence interval, 0.90–1.00) with the addition of the three demographic factors in the hybrid signature with corresponding sensitivity of 93%, specificity of 93%, negative predictive value of 99% and positive predictive value of 65% (Table 3). Univariate analysis indicated age, race, MMP9, IL8, VEGFA, CA9, PAI1, ApoE, A1AT, ANG and MMP10 were associated with bladder cancer (Table 4).
Urinary cytology was available in 35 of the cancer subjects with 8 being called positive (sensitivity of 22.8%). Table 5 denotes the overall sensitivity and specificity achieved using the Oncuria™ hybrid signature for low grade and high grade, and non-muscle invasive bladder cancers and muscle invasive bladder cancers.
Discussion
Cancer of the urinary bladder is a common neoplastic disease with high rates of recurrence and progression. The rate of recurrence makes it one of the most prevalent cancers worldwide [27]. Disease detection currently relies upon invasive cystoscopic examination of the bladder. The only urinary assay in routine use is voided urine cytology (VUC), but as it lacks sensitivity, it is typically deployed as an adjunct to cystoscopy rather than a stand-alone test. The development of accurate, non-invasive urinary tests would benefit both patients and health care systems. A robust test could avoid unnecessary invasive patient evaluation and improve patient compliance on clinical surveillance and follow-up regimes. The development of multiplex assays that reflect the complexity of molecular events involved in neoplasia can provide a more accurate assessment with broad clinical utility.
Multiplex assay advantages include reduced cost through lower labor needs and reagent consumption, and the generation of more data with less sample, but the major advantage is the potential to significantly improve clinical test sensitivity and specificity by a combination of multiple biomarkers. Many tissue-based analyses focus on multiplexing nucleic acid targets, but for liquid biopsy settings protein multiplexing may be more appropriate as the test is relatively straightforward with minimal sample processing, fast and economical throughput, and can achieve direct quantitation without requiring molecular target amplification. Notably, one multiplex protein cancer diagnostic test is FDA approved, OVA1, which is being employed for the early detection of ovarian cancer [28]. The test measures absolute serum levels of CA125, apolipoprotein A1, beta 2 microglobulin, prealbumin, and transferrin to determine the risk for malignancy. The test has a reported overall sensitivity of > 90% as a stand-alone test and can provide a valuable adjunct to ultrasound imaging and physical examination [28]. Coupling the advantages of a multiplex protein test with non-invasive urine sampling could provide a highly accurate bladder cancer diagnostic test as well as providing data for monitoring disease progression and response to therapy. The development of the Oncuria™ test has been reported from transcriptomic and proteomic profiling discovery [8,9,10,11], to refinement and validation of candidate biomarkers [12,13,14,15], to custom multiplex design and analytical validation [18, 19]. In this study, the test was applied to 348 naturally micturated urine samples prospectively obtained from patients visiting urology clinics at three institutions.
The 10 biomarkers associated with Oncuria™ were reliably detect in the 362 urine samples; MMP9 in 64.3%, IL8 in 84.4%, VEGFA in 88.6%, CA9 in 40.6%, SDC1 in 99.3%, PAI1 in 71.7%, ApoE in 95.7%, A1AT in 93.2%, ANG in 81.8% and MMP10 in 57.7%. Further, these 10 biomarkers were present at higher levels in voided urines from bladder cancer subjects compared to controls with significance being reached for IL8, VEGFA, PAI1, ApoE, A1AT and ANG. SDC1 had only slightly elevated mean levels in cancer compared to controls; 9,461 pg/mL vs. 8,707 pg/mL. Previously we reported that SDC1 levels are lower in controls compared to bladder cancer, but it hold prognostic significance in the high-grade and high stage tumors shed less SDC1 in voided urines than low-grade and low stage tumors [29]. Despite this, SDC1 adds value to the signature and thus is included in the combinatorial analysis of all ten biomarkers, obviously with a different trajectory in its weight compared to the other analtyes. Single biomarkers were noted to have lower sensitivity and/or specificity; best response PAI1 AUROC of 0.89 (95% confidence interval, 0.83–0.95) with a sensitivity of 78% and a specificity of 90% and ApoE AUROC of 0.89 (95% confidence interval, 0.84–0.94), with a sensitivity of 73% and a specificity of 90%. A combinatorial analysis of all ten biomarkers noted an AUROC of 0.93 (95% confidence interval, 0.87–0.98), with a sensitivity of 87% and a specificity of 92%. These parameters were noted to improve with the addition of the three demographic factors (age, sex and race) to the hybrid signature: AUROC of 0.95 (95% confidence interval, 0.90–1.00), with a sensitivity of 93% and a specificity of 93%. Lastly, we noted that urine samples from patients with history of renal cell carcinoma or renal cell carcinoma and urine samples from patients with history of prostate cancer or prostate cancer did not result in positive Oncuria™ test (data not shown). This finding confirms our previous report in that thus attesting to its specificity. We were able to confirm the clinical utility of monitoring a diagnostic biomarker signature for the detection of bladder cancer in non-invasively obtained urine samples. The Oncuria™ test achieved encouraging values of sensitivity and specificity and NPV.
Recently, several groups have begun to identify panels of diagnostic biomarkers for potential bladder cancer application. For example, through analysis of nine gene promoters, Hoque et al. found that 69% of bladder cancer patients had methylation in at least one of four genes (CDKN2A, ARF, MGMT, GSTP1), whereas the controls had no such methylation detectable [30]. By combining the data from all nine genes, a logistic prediction model was derived that achieved a sensitivity of 82% and specificity of 96%. Chung et al. selected 10 candidate hypermethylated genes from data collected from tumor tissue and tested these 10 genes in voided urine samples by quantitative methylation-specific RT-PCR and identified a multigene predictive model comprised of five target genes (MYO3A, CA10, NKX6-2, DBC1, and SOX11). Sensitivity and specificity of this model were 85% and 95%, respectively [31]. Further examples include RNA signatures proposed by Hanke et al. [32] and Mengual et al. [33] possessing sensitivities ranging from 80% to 92% and specificities ranging from 85% to 99%. To date, these studies have had small sample size, with limited populations analyzed (i.e., few benign confounding conditions included) and have not undergone extensive validation. Only Holyoake et al. from New Zealand have reported on the discovery [34] and validation of a multiplexed RNA signature comprised of CDC2, MDK, IGFBP5 and HOXA13 (Cxbladder™), with a reported sensitivity of 82% and specificity of 85% [35, 36].
We recognize that the study has several limitations. First, as tertiary-care facilities, we tend to see more high-grade, high-stage disease, which is reflected in our study cohort. To further confirm the robustness of the multiplex assay, subsequent studies must assess larger cohorts that include more subjects with low-grade, low-stage disease. Second, we did not have complete smoking data for all subjects in the cohort and, therefore, an association with smoking history was not possible. Third, processed, banked urines were analyzed. Urines were centrifuged and separated into cellular pellet and supernatant before storage at – 80 °C. It is feasible that freshly voided urine samples may provide different results. We are currently investigating the performance of the test in urines processed via a number of different protocols, including freshly voided urines. To address these issues, the Oncuria™ test is currently being evaluated in three large multicenter, international prospective clinical trials (NCT 03,193,515, 03,193,528, and 03,193,541). These trials will include first-event diagnosis and disease recurrence monitoring.
Conclusions
Bladder cancer is a common neoplastic disease encountered worldwide. The development of an accurate and robust urinary test for the detection of bladder cancer would benefit both patients and healthcare systems. In a multi-institutional cohort study, the multiplex Oncuria™ test achieved highly encouraging diagnostic performance. The test uses established technology enabling rapid uptake in clinical laboratories around the world. Additional studies are underway to evaluate the potential added value of the test in clinical decision making.
Availability of data and materials
Reasonable requests for data will be made available for review.
References
Knezevic D, Goddard AD, Natraj N, Cherbavaz DB, Clark-Langone KM, Snable J, Watson D, Falzarano SM, Magi-Galluzzi C, Klein EA, Quale C. Analytical validation of the Oncotype DX prostate cancer assay-a clinical RT-PCR assay optimized for prostate needle biopsies. BMC Genomics. 2013;8(14):690. https://doi.org/10.1186/1471-2164-14-690.
Boström PJ, Bjartell AS, Catto JW, Eggener SE, Lilja H, Loeb S, Schalken J, Schlomm T, Cooperberg MR. Genomic predictors of outcome in prostate cancer. Eur Urol. 2015;68(6):1033–44. https://doi.org/10.1016/j.eururo.2015.04.008 (Epub 2015 Apr 23).
van ’t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–6.
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–26.
Srivastava G, Renfro LA, Behrens RJ, Lopatin M, Chao C, Soori GS, Dakhil SR, Mowat RB, Kuebler JP, Kim G, Mazurczak M, Lee M, Alberts SR. Prospective multicenter study of the impact of oncotype DX colon cancer assay results on treatment recommendations in stage II colon cancer patients. Oncologist. 2014;19(5):492–7. https://doi.org/10.1634/theoncologist.2013-0401 (Epub 2014 Apr 7).
Olson JE, Kirsch EJ, Edwards VDK, Kirt CR, Kneedler B, Laffin JJ, Weaver AL, St Sauver JL, Yost KJ, Finney Rutten LJ. Colorectal cancer outcomes after screening with the multi-target stool DNA assay: protocol for a large-scale, prospective cohort study (the Voyage study). BMJ Open Gastroenterol. 2020;7(1):e000353. https://doi.org/10.1136/bmjgast-2019-000353.
Wiener HG, Vooijs GP, van’t Hof-Grootenboer B. Accuracy of urinary cytology in the diagnosis of primary and recurrent bladder cancer. ActaCytol. 1993. 37(2):163–9.
Yang N, Feng S, Shedden K, Xie X, Liu Y, Rosser CJ, Lubman DM, Goodison S. Urinary glycoprotein biomarker discovery for bladder cancer detection using LC/MS-MS and label-free quantification. Clin Cancer Res. 2011;17(10):3349–59.
Kreunin P, Zhao J, Rosser CJ, Urquidi V, Lubman DM, Goodison S. Bladder cancer associated glycoprotein signatures revealed by urinary proteomic profiling. J Proteome Res. 2007;6(7):2631–9.
Rosser CJ, Liu L, Sun Y, Villicana P, McCullers M, Porvasnik S, Young PR, Parker AS, Goodison S. Bladdercancer-associatedgeneexpressionsignaturesidentifiedbyprofilingofexfoliatedurothelia. Cancer Epidemiol Biomarkers Prev. 2009;18(2):444–53.
Urquidi V, Goodison S, Cai Y, Sun Y, Rosser CJ. A candidate molecular biomarker panel for the detection of bladder cancer. Cancer Epidemiol Biomarkers Prev. 2012;21(12):2149–58.
Goodison S, Chang M, Dai Y, Urquidi V, Rosser CJ. A multi-analyte assay for the non-invasive detection of bladder cancer. PLoS ONE. 2012;7(10):e47469.
Rosser CJ, Ross S, Chang M, Dai Y, Mengual L, Zhang G, Kim J, Urquidi V, Alcaraz A, Goodison S. Multiplex protein signature for the detection of bladder cancer in voided urine samples. J Urol. 2013;190(6):2257–62.
Chen LM, Chang M, Dai Y, Chai KX, Dyrskjot L, Sanchez-Carbayo M, Szarvas T, Zwarthoff EC, Lokeswhar V, Jeronimo C, Parker AS, Ross S, Borre M, Orntoft TF, Jaeger T, Beukers W, Lopez LE, Henrique R, Young PR, Urquidi V, Goodison S, Rosser CJ. External validation of a multiplex urinary protein panel for the detection of bladder cancer in a multicenter cohort. Cancer Epidemiol Biomarkers Prev. 2014;23(9):1804–12.
Rosser CJ, Chang M, Dai Y, Ross S, Mengual L, Alcaraz A, Goodison S. Urinary protein biomarker panel for the detection of recurrent bladder cancer. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1340–5.
Shimizu Y, Furuya H, Bryant Greenwood P, Chan O, Dai Y, Thornquist MD, Goodison S, Rosser CJ. A multiplex immunoassay for the non-invasive detection of bladder cancer. J Transl Med. 2016;14(1):31.
Goodison S, Ogawa O, Matsui Y, Kobayashi T, Miyake M, Ohnishi S, Fujimoto K, Dai Y, Shimizu Y, Tsukikawa K, Furuya H, Rosser CJ. A multiplex urinary immunoassay for bladder cancer detection: analysis of a Japanese cohort. J Transl Med. 2016;14(1):287.
Furuya H, Pagano I, Chee K, Kobayashi T, Wong RS, Lee R, Rosser CJ. Comparison of commercial ELISA Kits, a prototype multiplex electrochemoluminescent assay, and a multiplex bead-based immunoassay for detecting a urine-based bladder-cancer-associated diagnostic signature. Diagnostics (Basel). 2019;9(4):166. https://doi.org/10.3390/diagnostics9040166.
Furuya H, Tabula L, Lee R, Kralovec P, Ramsden M, Wong R, Rosser CJ. Analytical validation of ONCURIATM a multiplex bead-based immunoassay for the non-invasive bladder cancer detection. Pract Lab Med. 2020;13(22):e00189. https://doi.org/10.1016/j.plabm.2020.e00189.
Lokeshwar VB, Habuchi T, Grossman HB, Murphy WM, Hautmann SH, Hemstreet GP 3rd, Bono AV, Getzenberg RH, Goebell P, Schmitz-Dräger BJ, Schalken JA, Fradet Y, Marberger M, Messing E, Droller MJ. Bladder tumor markers beyond cytology: International Consensus Panel on bladder tumor markers. Urology. 2005;66(6 Suppl 1):35–63.
Pepe MS, Feng Z, Janes H, Bossuyt PM, Potter JD. Pivotal evaluation of the accuracy of a biomarker used for classification or prediction: standards for study design. J Natl Cancer Inst. 2008;100(20):1432–8.
Motulsky H, Christopoulos H. Fitting models to biological data using linear and nonlinear regression: a practical guide to curve fitting, vol. 351. Oxford: Oxford University Press; 2004. p. 17.
Huang S, Kou L, Furuya H, Yu C, Goodison S, Kattan MW, Garmire L, Rosser CJ. A Nomogram derived by combination of demographic and biomarker data improves the noninvasive evaluation of patients at risk for bladder cancer. Cancer Epidemiol Biomarkers Prev. 2016;25(9):1361–6. https://doi.org/10.1158/1055-9965. (Epub 2016 Jul 6).
Fisher RA. The use of multiple measurements in taxonomic problems. Annals Eugenics. 1936;7(2):179–88.
McLachlan, Geoffrey J. Discriminant analysis and statistical pattern recognition. John Wiley & Sons, 2004; 544.
Fawcett T. An introduction to ROC analysis. Pattern Recogn Lett. 2006;27(8):861–74.
Surveillance, Epidemiology and End Results Program (SEER). National Cancer institute, cancer incidence public-use database. CD-ROM. 2001. http://seer.cancer.gov
Grenache DG, Heichman KA, Werner TL, Vucetic Z. Clinical performance of two multi-marker blood tests for predicting malignancy in women with an adnexal mass. Clin Chim Acta. 2015;1(438):358–63. https://doi.org/10.1016/j.cca.2014.09.028 (Epub 2014 Oct 2).
Miyake M, Lawton A, Dai Y, Chang M, Mengual L, Alcaraz A, Goodison S, Rosser CJ. Clinical implications in the shift of syndecan-1 expression from the cell membrane to the cytoplasm in bladder cancer. BMC Cancer. 2014;13(14):86. https://doi.org/10.1186/1471-2407-14-86.
Hoque MO, Begum S, Topaloglu O, Chatterjee A, Rosenbaum E, Van Criekinge W, Westra WH, Schoenberg M, Zahurak M, Goodman SN, Sidransky D. Quantitation of promoter methylation of multiple genes in urine DNA and bladder cancer detection. J Natl Cancer Inst. 2006;98(14):996–1004.
Chung W, Bondaruk J, Jelinek J, Lotan Y, Liang S, Czerniak B, Issa JP. Detection of bladder cancer using novel DNA methylation biomarkers in urine sediments. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1483–91.
Hanke M, Kausch I, Dahmen G, Jocham D, Warnecke JM. Detailed technical analysis of urine RNA-based tumor diagnostics reveals ETS2/urokinase plasminogen activator to be a novel marker for bladder cancer. Clin Chem. 2007;53(12):2070–7.
Mengual L, Burset M, Ribal MJ, Ars E, Marin-Aguilera M, Fernandez M, et al. Gene expression signature in urine for diagnosing and assessing aggressiveness of bladder urothelial carcinoma. Clin Cancer Res. 2010;16(9):2624–33.
Holyoake A, O’Sullivan P, Pollock R, Best T, Watanabe J, Kajita Y, et al. Development of a multiplex RNA urine test for the detection and stratification of transitional cell carcinoma of the bladder. Clin Cancer Res. 2008;14(3):742–9.
O’Sullivan P, Sharples K, Dalphin M, Davidson P, Gilling P, Cambridge L, Harvey J, Toro T, Giles N, Luxmanan C, Alves CF, Yoon HS, Hinder V, Masters J, Kennedy-Smith A, Beaven T, Guilford PJ. A multigene urine test for the detection and stratification of bladder cancer in patients presenting with hematuria. J Urol. 2012;188(3):741–7. https://doi.org/10.1016/j.juro.2012.05.003 (Epub 2012 Jul 19).
Kavalieris L, O’Sullivan P, Frampton C, Guilford P, Darling D, Jacobson E, Suttie J, Raman JD, Shariat SF, Lotan Y. Performance characteristics of a multigene urine biomarker test for monitoring for recurrent urothelial carcinoma in a multicenter study. J Urol. 2017;197(6):1419–26. https://doi.org/10.1016/j.juro.2016.12.010 (Epub 2016 Dec 14).
Acknowledgements
The funding bodies did not have a role in the collection, analysis, and interpretation of data, or in the writing of the manuscript, or in the decision to submit the manuscript for publication.
Funding
This work was supported by research grants R01 CA206584 (SG) and R01 CA1988887 (CJR).
Author information
Authors and Affiliations
Contributions
HF–project oversight, manuscript drafting. YH–Sample collection and analysis. RC, IP, YD, YS–Statistical analysis. AG–Scientific input, manuscript drafting. SG–Provision of samples, scientific input and manuscript drafting. CJR–Project management, manuscript drafting. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
University of Hawaii and Cedars Sinai Local ethics review board approved. Subject gave written consent.
Consent for publication
Not applicable.
Competing interests
Dr. Charles Rosser is an officer of Nonagen Bioscience. No financial or commercial conflicts of interest were declared by other co-authors. CJR is an officer of Nonagen Bioscience Corporation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Boxplot of mean ± SD of urine concentrations of the 10 protein biomarkers between the bladder cancer and non-cancer groups from the participating institutes.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hirasawa, Y., Pagano, I., Chen, R. et al. Diagnostic performance of Oncuria™, a urinalysis test for bladder cancer. J Transl Med 19, 141 (2021). https://doi.org/10.1186/s12967-021-02796-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-021-02796-4