Abstract
Background
Emerging research suggests that physical activity among children and adolescents decreased during the COVID-19 pandemic. However, a differentiated overview of European youth is lacking. In particular, no systematic analysis has been conducted to date on the impact of heterogeneous pandemic restrictions and school closures within European countries, and with regard to potentially vulnerable groups.
Methods
We searched seven databases and included studies for children and adolescents (≤ 19 years) of the WHO European Region that compared physical activity during the COVID-19 pandemic with a pre-pandemic baseline using validated measurement instruments. We used the Oxford Stringency Index and School Closure Index as indicators of restriction stringency. Screening for eligibility, data extraction, assessment of the study risk of bias (using the ‘Risk of Bias in Non-randomized Studies - of Exposure’ [ROBINS-E]) and certainty grading of evidence (using the GRADE approach), were all done in duplicate. Unpublished data was requested from study authors. Data were pooled in random effects models. An a priori protocol was published, reporting is carried out in accordance with the ‘Preferred Reporting Items for Systematic Review and Meta-Analyses’ (PRISMA) statement.
Results
Of 14,897 non-duplicate records, 26 publications (n = 15,038 pre-pandemic, n = 13,041 during pandemic) met full inclusion criteria. Comparison before and during the COVID-19 pandemic revealed a significant reduction in total physical activity (standardized mean difference [SMD], -0.57 [95%CI, -0.95; -0.20]) and moderate-to-vigorous physical activity (SMD, -0.43 [95% CI, -0.75; -0.10]), corresponding to a decrease of 12 min per day (a 20% reduction of the WHO recommendation). A decrease in sporting activity was also recorded. Subgroup analyses suggested that middle childhood (aged 8–12) and adolescents were particularly affected by the decline. School closures were associated with a reduction in physical activity. The certainty of evidence for all outcomes was low.
Conclusions
A sharp decline in all forms of physical activity was recorded among European children and adolescents during the COVID-19 pandemic. This decline was higher during periods of school closure and mainly affected younger schoolchildren and adolescents. Immediate action by policy-makers and practitioners, as well as evidence-based public health strategies, are imperative in reversing this trend.
Trial registration
PROSPERO: CRD42023395871
Similar content being viewed by others
Background
The positive effects of physical activity on the physical and mental health of children and adolescents have been outlined in numerous studies [1, 2]. In particular, cardiovascular diseases, metabolic diseases, obesity and also mental health and cognition in youth all benefit from physical activity [1,2,3,4,5]. Furthermore, regular physical activity at a young age forms healthy habits in later life [6] and helps to reduce risk factors and diseases over the long term [7]. However, experts are keen to stress that the lack of adequate physical activity levels in children and adolescents is a major health problem [8, 9] that brings with it an enormous global health and economic burden [9, 10].
During the COVID-19 pandemic, opportunities for continuous physical activity among children and adolescents were severely limited by various public health and social measures (PHSM), e.g. closures of educational institutions (kindergartens, schools, universities), the restriction of access to physical activity opportunities (swimming, outdoor play, sports clubs) and the limiting of social contacts [11]. The effects of these limitations may contribute to long-term behavioural change in children and adolescents and could accelerate the downward-trend in physical activity [8] that is already in evidence and thereby have a severe lasting impact on the health of the upcoming generation [12]. Meanwhile, summary analyses describe a global decline in physical activity in children and adolescents during the COVID-19 pandemic [13,14,15,16]. However, there are important research gaps concerning the impact of the restriction stringency, school closures, different measurement tools and different types of physical activity. For the WHO European Region, a systematic analysis of changes in youth’s physical activity is lacking at all, although the number of studies is constantly increasing and the results are partly heterogeneous. The consideration of the WHO European Region further enables the analysis of country-specific heterogeneous PHSM to infer possible links to a change in physical activity in children and adolescents, creating a quasi-experimental design. Our aim, therefore, is to assess the impact that the COVID-19 pandemic has had on physical activity among children and adolescents in the WHO European Region compared with a pre-pandemic baseline, taking particular account of the relevance of restriction stringency policies.
Methods
The systematic review and meta-analysis is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [17] statement (Additional file [AF1]: Table S1) and adheres to the Cochrane Handbook for Systematic Reviews [18]. It was registered on the International Prospective Register of Systematic Reviews (PROSPERO; CRD42023395871) [19] and an a priori protocol was published [20]. Deviations from the protocol are reported in AF1: Table S2.
Eligibility criteria
We defined the following criteria as being eligible for inclusion: (1) Children and adolescents from the WHO European Region [21] ≤ 19years; (2) physical activity measurement at least once during the COVID-19 pandemic; (3) reporting of a pre-pandemic baseline; (4) measuring of physical activity with validated instruments; and (5) primary studies (also including pre-prints and congress abstracts) or reports (grey literature). We placed no restrictions on language or effect measures.
Information sources and search strategy
We searched in seven electronic databases (PubMed, Embase, Sports Medicine & Education Index, PsycINFO, Web of Science, Cochrane Central Register of Controlled Trials [CENTRAL] and WHO COVID-19 Research Database [including pre-prints]) for eligible publications through to January 31, 2023. We tried to identify other potentially eligible publications by handsearching the reference lists of all included studies and related systematic reviews, and also searched for registered observational studies in clinicaltrials.gov. In addition, the data sources of the ‘Global Matrix 4.0 Physical Activity Report’ [16] and websites of key organizations (see AF1: Table S3) were checked.
We designed the search strategy by using validated or recommended search filters and conducted a peer-review process considering the evidence-based Peer Review of Electronic Search Strategies (PRESS) checklist [22] (see protocol [20] for further details). The search strategy for every database is presented in the Supplement (AF1: Table S4).
Selection process
We began by performing an automated deduplication process with assistance from the EPPI reviewer software [23]. This was followed by title/abstract screening, conducted independently in reviewer teams of two (HLW, ID, SH). We obtained the full text of all potentially relevant records. Disagreements were resolved through consensus. We prepared a PRISMA flow diagram for study selection (AF1: Fig. S1). Reasons for the exclusion of publications following full-text assessment were also provided (AF1: Fig. S1 and Table S5).
Data extraction
For studies meeting our inclusion criteria, reviewer teams of two (HLW, ID, SH) independently extracted key study characteristics in Table 1 ‘Characteristics of included studies’; disagreements regarding data extraction were resolved through discussion. For several publications, we requested further data via email from the authors and sent a reminder after 2 weeks if no response was received; eight authors provided us with additional, unpublished data. For three publications, the corresponding author could not be reached by email [24,25,26]. In the case of duplicate publications or multiple reports of a study, we compared and considered all available relevant data. We expanded our study characterization by adding the Oxford Stringency Index and the School Closure Index [11] for the measurement period of every study as policy indices for the classification of PHSM. The Oxford Stringency Index consists of nine variables; one of these variables represents school closures in the respective country. In compliance with the COVIDSurg Collaborative [27], we defined three cut-off points for the Oxford Stringency Index: light restrictions (index < 20), moderate lockdowns (index 20–60) and full lockdowns (index > 60). For the School Closure Index, we specified two cut-off points: no or few alterations compared with a pre-COVID-19 situation (index < 2) and partial or full school closure (index ≥ 2) [28]. More details on these indices are contained in the protocol [20].
We defined total physical activity (TPA), moderate-to-vigorous physical activity (MVPA) and sporting activity (SA) as primary outcomes. Validation of the measurement instrument used, including both self-reported and device-based measurements, was defined as a prerequisite. No limitations were set as regards effect measures.
Risk of bias assessment
All studies were independently assessed by two reviewers (HLW, SH), using the ‘Risk of Bias (RoB) in Non-randomized Studies - of Exposure’ (ROBINS-E) instrument. This tool comprises seven assessment criteria, with the RoB judgements expressed as ‘low RoB’, ‘some concerns RoB’, ‘high RoB’ or ‘very high RoB’ [57]; more details are provided in the protocol [20]. The studies were subsequently grouped into ‘some concerns RoB’ and ‘high RoB’ (including the categories ‘high RoB’ and ‘very high RoB’); no study received the rating ‘low RoB’. Interpretation of studies with ‘some concerns RoB’ was given preference in meta-analyses to deal with methodological heterogeneity and potential confounding.
Synthesis methods
For all of the studies that were included, we provide both the effect estimates at pre-pandemic and pandemic measurement and the change effect as standardized mean difference (SMD) or risk ratio with the corresponding 95% confidence interval (CI). We performed meta-analysis when data from at least three studies with different study populations could be pooled. First, we distinguished between TPA, MVPA and SA and pooled available data sets using SMD (95% CI) to summarize change estimates.
Second, we differentiated according to the measurement instrument used (accelerometer measurement versus self-reported scores). Device-based measurements (via accelerometer) were summarized as ‘minutes/day’ (details for data conversion are presented in AF1: Table S6). Self-reported measurements for TPA were summarized within the Physical Activity Questionnaire for Children/Adolescents (PAQ-C/A) since the majority of measurements used this instrument. Due to the heterogeneity in self-reported MVPA measurements, we summarized these measurements as SMD and subsequently re-expressed them using a familiar instrument (WHO Health Behaviour in School-aged Children [HBSC survey]), to ensure practical interpretability of the results.
Third, we analyzed change effect estimates for the subgroups: gender (female/male), age (age categories are based on those laid down by the Centers for Disease Control and Prevention [58]: ‘preschoolers/middle childhood’: 3 to 8 years; ‘middle childhood’: 9–11 years; and ‘young teens/teenagers’: 12 to 18 years, studies with overlapping age intervals were assigned based on the age structure that was most appropriate and studies in which there was a wide age interval were excluded from these analyses), measurement time point (spring/summer 2020, winter 2020/2021, spring 2021), Oxford Stringency Index (≤ 60 versus > 60), School Closure Index (< 2 versus ≥ 2) and length of pandemic-related restrictions before measurement (Oxford Stringency Index > 60 before measurement for 30/60/90 days).
We performed some data conversion before conducting meta-analyses (AF1: Table S6). If the studies that were included did not report sufficient data for inclusion in the meta-analysis (e.g. reporting percentage change) and we had not received the information we had requested from the authors, the results were reported in narrative tables. Where possible, we included adjusted effect estimates. If both self-reported and parent-reported data were available, we included the self-reported data.
We assessed heterogeneity by visual inspection of the forest plots, the I2 statistic [59] and with 95% prediction intervals when > 3 studies were included in meta-analyses [60,61,62]. We considered I2 values of greater than 50% as substantial. We tried to explain heterogeneity by conducting subgroup analyses and meta-regression (if ≥ 10 studies per examined variable) [60] with the potential categorical moderators: RoB, age, symptom reporter, country, Oxford Stringency Index (≤ 60 versus > 60), School Closure Index (< 2 versus ≥ 2) and study design. In addition, the following potential continuous moderators were considered: time of measurement during pandemic, publication year, Stringency Index, School Closure Index and sample size. We considered potential publication bias by conducting a visual inspection of (contour-enhanced) funnel plots [63, 64] and we applied the Egger’s test when a meta-analysis included ≥ 10 studies [65].
We conducted meta-analysis calculations with the package ‘meta’ [66] in R Studio 4.2.1 [67] using the random effects model with a restricted maximum likelihood approach [68] and the Hartung-Knapp method for calculating the 95% CI. All statistical analyses were performed based on the statistical guidelines presented in the Cochrane Handbook for Systematic Reviews of Interventions [69].
Certainty of evidence assessment
We applied the ‘Grading of Recommendations Assessment, Development and Evaluation’ (GRADE) approach, adapted to the use of non-randomized studies [70], to assess overall certainty of evidence for each of the primary outcomes; more information is provided in the protocol [20]. Certainty of evidence for each outcome was evaluated independently by two review authors (HLW, WS); differences were resolved through discussion. The ‘Summary of findings’ table summarizes the results regarding certainty of evidence. Details of the criteria used to grade the evidence are reported in AF1: Table S7; evidence profiles containing more detailed explanations can be found in AF1: Table S8.
Results
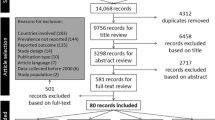
Our systematic literature search identified 14,891 non-duplicate records and six grey literature publications. Of these, 135 studies and six grey literature reports were assessed for eligibility (full-text screening) and 25 [24, 25, 29,30,31, 33,34,35, 37, 39,40,41,42,43, 45, 48,49,50,51,52,53,54,55, 71, 72] studies and one report [32] were deemed to meet the criteria for inclusion in the review (AF1: Fig. S1). In total, data from 15,038 children and adolescents pre-pandemic and 13,041 children and adolescents during pandemic were included in this review. The most relevant reasons for exclusion after full-text screening were ‘no validation of the measurement instrument’ (n = 80, 59.3%); and ‘no data reporting on physical activity’ (n = 19, 14.1%); details are described in AF1: Table S5.
Study characteristics
A detailed description of the included publications is presented in Table 1 and AF1: Table S9. The included 26 publications are scattered across 14 WHO European Region countries: four from Spain [43, 45, 48, 49], four from the United Kingdom [52,53,54,55], three from Germany [30, 31, 73], two from Croatia [25, 71], two from Italy [34, 35], two from Poland [39, 72], two from Slovenia [41, 42], and one each from Bosnia and Herzegovina [24], Czech Republic [29], Ireland [33], Netherlands [37], Portugal [40], Sweden [50] and Switzerland [51]. A graphical overview of how these studies are distributed is provided in AF1: Fig. S2. TPA and MVPA were analyzed in 15 publications (TPA: [24, 25, 29, 30, 34, 35, 40, 41, 43, 45, 48, 49, 52, 55, 71], MVPA: [31, 33, 35, 37, 39, 42, 43, 45, 48, 50, 53,54,55, 72, 73]) respectively, and SA in three publications [31, 51, 73]. Self-reported data were collected in 20 analyses [24, 25, 29,30,31, 33,34,35, 39,40,41,42, 49,50,51,52,53, 71,72,73] and accelerometer data in six analyses [35, 37, 43, 45, 48, 55].
The publications were conducted as cohort (n = 15, [24, 25, 31, 34, 35, 37, 41, 43, 45, 48, 50, 52, 55, 71, 73]), cross-sectional (n = 7, [29, 30, 39, 42, 49, 53, 54]) or retrospective studies (n = 4, [33, 40, 51, 72]). The majority were carried out in spring/summer 2020 (n = 18, [24, 25, 30, 31, 33, 34, 37, 40,41,42,43, 48, 51,52,53, 55, 71, 72]) or winter 2020/2021 (n = 6, [29, 35, 39, 45, 49, 73]). In 24 publications, the period during the pandemic was classified as ‘full lockdown’ (Oxford COVID-19 Stringency Index > 60, [24, 25, 29,30,31, 33,34,35, 37, 39,40,41,42,43, 45, 48,49,50,51,52,53, 55, 71, 73]). In 20 publications, pandemic-measurement occurred during partial or full school closures (School Closure Index ≥ 2, [24, 25, 29,30,31, 33,34,35, 39,40,41,42,43, 48, 52, 53, 55, 71,72,73]). The length of a ‘full lockdown’ (Oxford COVID-19 Stringency Index > 60) before pandemic measurement ranged from 0 to 405 days. The RoB assessment revealed ‘some concerns’ for 16 publications [25, 29, 31, 33,34,35, 37, 41, 42, 45, 49, 50, 53,54,55, 73], ‘high RoB’ for eight publications [24, 30, 38, 39, 48, 51, 52, 71] and ‘very high RoB’ for two publications [40, 43]. Details on RoB assessment are presented in AF1: Fig. S3 (traffic-light plots) und AF1: Fig. S4 (weighted bar plots).
Meta-analysis for total physical activity
For TPA, we performed a meta-analysis with 14 studies [24, 25, 29, 30, 34, 35, 40, 41, 43, 45, 48, 49, 55, 71] and certainty of evidence was graded as ‘low’ (Table 2). The pooled SMD estimate for change of TPA, including self-reported scores and accelerometer measurements, was -0.57 (95% CI, -0.95 to -0.20; I2 = 96%; Fig. 1) for all 14 studies, and a SMD of -0.47 (95% CI, -0.90 to -0.04; I2 = 96%; Fig. 1) for eight studies with a ‘some concerns RoB’ rating. The SMD for ‘high RoB’ studies had a wide 95% CI and crossed the null effect (-0.71, 95% CI -1.58 to 0.15; I2 = 96%).
Hereinafter, analyses were differentiated according to the outcome measurement instrument. Eight studies with the widely used PAQ-C/A instruments yielded a reduction of -0.29 score points (95% CI, -0.51 to -0.08; I2 = 96%; AF1: Fig. S5). A pooling of four studies with an accelerometer measurement revealed a reduction of -47.7 min (95% CI, -115.9 to 20.5; I2 = 96%; AF1: Fig. S6) per day.
Gender-stratified pooling yielded a SMD of -0.16 (95% CI, -0.46 to 0.15; I2 = 84%; AF1: Fig. S7) for female children and adolescents and a SMD of -0.37 (95% CI, -0.81 to 0.08; I2 = 86%; AF1: Fig. S7) for male CA. The age-group classification showed a significant decline for middle childhood (adapted to the age range of 8 to 12 years: SMD, -1.00; 95% CI, -1.86 to -0.13; I2 = 81%; Fig. 2) and young teens/teenagers (SMD, -0.30; 95% CI, -0.55 to -0.05; I2 = 96%; Fig. 2), but not for children younger than 7 years of age (SMD, -0.04; 95% CI, -1.00 to 0.91; I2 = 62%; Fig. 2).
Regarding the course of time, TPA decreased in spring/summer 2020 (10 studies: SMD, -0.60; 95% CI, -1.10 to -0.11; I2 = 97%; AF1: Fig. S8), in winter 2020/spring 2021 (3 studies: SMD, -0.59; 95% CI, -2.36 to 1.18; I2 = 94%; AF1: Fig. S8) and in spring 2021 (1 study: SMD, -0.29; 95% CI, -0.40 to -0.18; AF1: Fig. S8). A comparison regarding the Oxford Stringency Index was not possible because all studies had an index > 60 at the measurement time point. Comparisons of the School Closure Index revealed that full or partial school closures were associated with higher TPA reductions (SCI ≥ 2: SMD, -0.66; 95% CI, -1.08 to -0.24; I2 = 97%; Fig. 3), whereas no school closure or few alterations had no statistical association with TPA reductions (SMD, -0.10; 95% CI, -2.80 to 2.60; I2 = 85%; Fig. 3).
Although the analyses by restriction length (number of days before measurement in which the Oxford Stringency Index was > 60) revealed no significant associations, the trend indicated that TPA decreases more where the duration of the restriction is longer (Restriction before measurement ≥ 30 days: SMD, -0.48; 95% CI, -1.32 to 0.37; I2 = 97%; Fig. S9; Restriction before measurement ≥ 60 days: SMD, -0.63; 95% CI, -1.72 to 0.45; I2 = 95%; Fig. S10; Restriction before measurement ≥ 90 days: SMD, -0.77; 95% CI, -7.16 to 5.61; I2 = 95%; Fig. S11).
Meta-analysis for moderate-to-vigorous physical activity
In the meta-analysis for MVPA, we include 12 publications [31,32,33, 35, 37, 39, 42, 43, 45, 50, 54, 55] (data from two publications [31, 32] of the same study population with different measuring time points were aggregated) and certainty of evidence was rated as ‘low’ (AF1: Table S8). A SMD of -0.43 (95% CI, -0.75 to -0.10; I2 = 92%; AF1: Fig. S12) was calculated as the total change effect, while pooling of ‘some concerns RoB’ resulted in a SMD of -0.43 (95% CI, -0.84 to -0.02; I2 = 94%; AF1: Fig. S12).
Self-reported changes revealed a reduction of -0.55 score points when re-expressed with the WHO HBSC survey instrument based on the SD (= 1.9) from Chen et al. [50] (AF1: Fig. S13). Changes based on six accelerometer measurements resulted in a MVPA reduction of -12.0 min (95% CI, -27.1 to 3.1; I2 = 96%; AF1: Fig. S14) per day.
Subgroup analysis by gender revealed a SMD of -0.15 (95% CI, -0.48 to 0.18; I2 = 78%; AF1: Fig. S15) for female children and adolescents and a SMD of -0.33 (95% CI, -1.01 to 0.35; I2 = 90%; AF1: Fig. S15) for male children and adolescents regarding MVPA reduction. Stratification by age groups yielded a reduction, with a SMD of -0.74 (95% CI, -1.45 to -0.04; I2 = 95%; AF1: Fig. S16) for middle childhood, while change effect estimates for preschoolers and young teens/teenagers were imprecise and the 95% CI crossed the null effect (preschoolers: SMD, -0.08; 95% CI, -6.60 to 6.44; I2 = 88%; young teens/teenagers: SMD, -0.42; 95% CI, -1.56 to 0.72; I2 = 89%; Fig. S16). The analysis over time indicates a reduction in spring/summer 2020 (6 studies: SMD, -0.59; 95% CI, -1.14 to -0.04; I2 = 97%; AF1: Fig. S17) and winter 2020/spring 2021 (4 studies: SMD, -0.26; 95% CI, -1.09 to 0.57; I2 = 91%; AF1: Fig. S17); two studies were excluded from this analysis because measurement periods were too broad [50, 54]. Only comparisons regarding the School Closure Index were possible. In measurement periods with fully or partially closed schools, the reduction in MVPA was considerably higher than in periods with fewer school restrictions (SCI ≥ 2: SMD, -0.57; 95% CI, -0.96 to -0.17 versus SCI < 2: SMD, -0.19; 95% CI, -1.04 to 0.67; Fig. 4). Consideration of the restriction duration prior to measurement did not reveal any trend (Restriction before measurement ≥ 30 days: SMD, -0.36; 95% CI, -0.80 to 0.08; I2 = 93%; Fig. S18; Restriction before measurement ≥ 60 days: SMD, -0.30; 95% CI, -0.89 to 0.29; I2 = 94%; Fig. S19; Restriction before measurement ≥ 90 days: SMD, -0.36; 95% CI, -1.46 to 0.74; I2 = 90%; Fig. S20).
Sporting activity
Sporting activity was analyzed in three publications with different measurement instruments (self-reported score points [31, 32] and self-reported minutes/week [51]), two of them originating from the same study population with different measurement time points [31, 32]. As a result, no meta-analysis was performed. All of the studies described a statistically significant decline in sporting activity among children and adolescents both for spring 2020 [31, 51] and winter 2020/2021 [32]. Certainty of evidence when considering all three comparisons was rated as ‘low’.
Heterogeneity, sensitivity analysis and publication bias
The meta-analyses revealed substantial heterogeneity (I2 > 50% and wide prediction intervals) for the most part. We conducted meta-regression analyses using a range of different variables; however, none of these variables acted as moderator (AF1: Tables S10-S13).
We also performed sensitivity analyses by comparing the following: (1) cohort studies versus cross-sectional studies versus retrospective studies (if available); (2) converted versus unconverted effect estimates (e.g. summarizing weekday and weekend measures); and (3) adjusted versus unadjusted effect estimates (AF1: Tables S14-S15). We found no significant differences, except when comparing adjusted versus unadjusted effect estimates in TPA, although only one study with adjusted values was available.
To assess publication bias, we created (contour-enhanced) funnel plots for TPA, MVPA and SA (AF1: Figs. S21-S23). Visual inspection suggests some degree of reporting bias for both outcomes. In the application of Egger's test, a reporting bias for MVPA was confirmed (p = 0.02; AF1: Table S16) and also indicated for TPA (p = 0.052; AF1: Table S16).
Discussion
Our objective was to assess the impact of the COVID-19 pandemic and PHSM on physical activity among children and adolescents in Europe and to identify possible vulnerable subgroups. Overall, the results of this systematic review indicate a considerable decline in TPA, MVPA and SA in comparison with pre-pandemic values. Our analysis revealed that stringent school closures (partially or fully closed schools) are associated with a higher decline in TPA and MVPA versus schools with either no restrictions or only a small number of restrictions. Furthermore, the analyses emphasized a noticeable decrease in TPA and MVPA in middle childhood (8 to 12 years) and in TPA among adolescents. To our knowledge, this is the first systematic review on physical activity changes among youth from the WHO European Region considering pandemic-related restrictions and various subgroups.
Even before the pandemic, children and adolescents in Europe were not physically active enough [74]. Our study revealed that TPA in European children and adolescents declined further in a pre-during-comparison.. This corresponds to a reduction of approximately 48 min per day when considering accelerometer measurements only. MVPA also decreased in European youth, corresponding to a reduction of 12 min per day in accelerometer measurements. Moreover, SA showed a decline, even without effect pooling. Former reviews have also documented a decline in total PA during the COVID-19 pandemic ranging between 11 to 91 min per day [75, 76] respectively a reduction of 20% for TPA or 28% for MVPA [13]. Our results confirmed the general decline for European children and adolescents and further highlighted that this decline affects all types of physical activity. The decrease of 12 min per day in MVPA represents a 20% drop in what is recommended. However, we assume that there was a large variation in MVPA change as suggested by the wide prediction interval ranging even to more than 53 min decrease in MVPA regarding the lower limit. Also the reduction in TPA of 48 min per day represents a severe change in the daily routine of European youth.
Our analyses outline a possible association between stricter school closures (partial or full closure) and more significant reductions in both TPA and MVPA. This is consistent with two recent meta-analyses, which reported that during stringent PHSM and periods of school closure depression [28] and anxiety symptoms [77] among children and adolescents increased in particular. Thus, school closures seem to represent particularly sensitive periods for suboptimal health outcomes in children and adolescents. Our results must be placed in the context of the formation of research on health habits [6, 78], which proposes that (healthy) habits depend on stability mechanisms. This stability – based on family, social, and school support – was substantially disrupted for children and adolescents during strict lockdowns or school closures. From a public health perspective, it is imperative to note that perpetuation of inactive behaviors in young age contributes to tracking inactive patterns into adulthood, which in turn is associated with numerous suboptimal health consequences [7, 79]. Once restrictions had been lifted, a return to an active daily life seemed to pose a challenge for some children and adolescents [80]. A recent systematic review also points to an association between physical activity and youth’s mental health during the COVID-19 pandemic [81]. School closures also imply the elimination of physical activity in the school setting and for getting from one place to another, which contribute to the overall reduction. This highlights the importance of maintaining physical activity services and opportunities even during times of crisis, considering broader contextual and environmental conditions.
The pandemic-related reduction in physical activity varies between age groups. Our analyses revealed that children in middle childhood, aged approximately 8 to 12 years, recorded the strongest reductions in TPA and MVPA. Adolescents recorded a significant reduction in TPA. In contrast, there was no significant association for children aged 4 to 7 years, who were in pre-school or in the first year of elementary school, which is consistent with previous literature [82]. A decline in youth’s physical activity as they get older – particularly evident in early and late adolescence – was also documented even before the pandemic [7]. However, this trend in inactivity appears to have spread considerably into middle childhood (8 to 12 years of age) during the COVID-19 pandemic. This inactivity expansion in middle childhood could be a consequence of closing schools and restricting access during the COVID-19 pandemic to physical activity opportunities, which are more physically active in (un-) organized sports than younger children [83]. Further, a lack of adult activation and supervision during the COVID-19 pandemic was described as a main barrier for physical activity in middle childhood [84] and also parents’ attitudes towards risk, which have become more severe during the pandemic, correspond with children’s activity status [85]. Therefore, the group of middle childhood might represent a ‘new’ vulnerable group that should be addressed in further analyses.
Analyses based on the measurement time point revealed a significant reduction in TPA and MVPA at the beginning of the pandemic (spring/summer 2020). Taking into account an evident decline in physical activity among adolescents since 2001 [8], we can assume that the COVID-19 pandemic accelerated this process. Further closer monitoring and analyzing of physical activity among children and adolescents is essential to identify trends, specify vulnerable subgroups and ensure appropriate interventions implementation.
Stratification by gender revealed no significant differences. This result is in contrast to some primary studies [35, 42], although other reviews do not confirm a significant difference [13] and some reviews did not analyse a possible difference by gender [14, 76]. Considering all available data, significant decreases were revealed for both TPA and MVPA. When separated by measurement instrument (self-reported vs. accelerometer measurement), only self-reported TPA showed a significant decrease. Indeed, all measurement instruments were validated this could indicate an inaccuracy of the self-rating instruments as already reported in other studies [86, 87]. This emphasizes the need for stratifying results by measurement instrument in systematic reviews addressing physical activity.
The certainty of evidence assessment with the GRADE approach resulted in a low certainty for the analyzed outcomes meaning that the true effect might be markedly different from the estimated effect [88]. However, it must be noted, that GRADE was primarily developed for assessing the certainty of evidence of classical clinical questions according to the PICO-scheme (patient, intervention, comparison, outcome) and that a precise adaptation for public health questions is lacking [89]. Beyond the scope of this systematic review, the GRADE working Group suggested Evidence to Decision (EtD) criteria for making clinical recommendations, health system or public health recommendations. Although the EtD framework cannot be applied completely on our research question, important criteria from a population perspective (e.g. problem priority, desirable [un-]anticipated effects, certainty of evidence, equity, acceptability, and feasibility) allow a placement of our results [90,91,92]: It can be supposed that the consequences of decreasing physical activity levels during the COVID-19 pandemic among children and adolescents would be serious [1, 2]. Increasing physical activity is associated with a variety of short- and long-term health effects in children and adolescents (see explanations above). Adverse effects of increasing physical activity might be possible, but mainly in elite sports [93]. Thus, the desired effects outweigh the undesired effects. A positive cost-effectiveness rate [94, 95] and a reduction in social inequality [96, 97] can be assumed when interventions to increase physical activity are implemented. Implementation of interventions to increase physical activity is well feasible and should be based on the best available evidence [98].
It can be assumed that the opportunity costs in health terms will be high for the more than 156 million children and adolescents aged 0 to 19 years in Europe [99] due to the decline in physical activity (as outlined in our review), rise in mental health disorders [28, 77], increase in obesity [100] and screen time [101]. Additionally, financial and social constraints [102], and health impairments like immune function and viral and bacterial infections [103, 104] further affect the state of health of children and adolescents. No estimates are available on this yet, however.
Consequently, the downward spiral must be reversed. This is also underlined by the ‘strong recommendation’ of the WHO that ‘Children and adolescents should do at least an average of 60 min per day of moderate-to-vigorous-intensity […]’ [1]. Beyond the findings of this review and considering the scientific evidence, we suggest the following immediate short-term and long-term action by policy-makers and practitioners:
-
(I)
(Re-)increase physical activity through low-threshold, comprehensive, targeted, and evidence-based interventions [1, 105]. Special attention must be given here to vulnerable groups that are either already known or are to be identified. Schools and educational settings in particular are important locations for promoting physical activity as they reach children and adolescents on a broad basis, regardless of their socio-cultural background [1]. In contrast to previous – often unsuccessful – programs in school and educational settings [7], future programs should include multi-component interventions (e.g. comprehensive school physical activity programs [106, 107]). Physical education in the school environment should communicate physical activity as a positive element in an individual’s lifestyle, and one that should be integrated as a constant component in daily life [108, 109]. For this purpose, social support from family and friends as well as access to green places are important components in the implementation and stabilization of an active lifestyle among children and adolescents [7, 105, 110,111,112]. Moreover, the application of digital interventions to promote physical activity (eHealth) should be strengthened in the design of programs [113, 114]. These can also be applied in periods of crisis.
-
(II)
Implementation of a global and national monitoring and surveillance systems for the adversely impacted youth cohorts over a longer time period in order to assess medium-term and long-term health consequences and to be able to implement targeted health improvement interventions [115,116,117,118].
-
(III)
Restriction in youth’s social life and the closure of educational institutions should be carefully considered, taking into account children’s rights [119] the best scientific evidence.
Strengths and limitations
This systematic review adheres to the methodological recommendation of the Cochrane Handbook for Systematic Reviews [18]. The main strength is the broad number of studies that were able to be included, despite the restrictive inclusion criteria (only studies with a pre-pandemic baseline and instrument validation were incorporated); this improves the trustworthiness of the results. Furthermore, in an improvement over previous studies, outcomes could be separated into TPA, MVPA and SA. Authors of the studies were also contacted to provide further data, enabling to include unpublished data.
The evidence identified in this review also has several limitations. First, RoB was rated high or very high for over 38% of the studies included. Second, there was a high degree of heterogeneity for the most part in the meta-analyses and a publication bias was determined in MVPA. We addressed these by downgrading the certainty of evidence in GRADE and provided further analyses (meta-regression, sensitivity analyses). Third, the data available for young children (under 7 years) was limited. However, this age group appears to meet the TPA and MVPA recommendations [82]. Fourth, the analyses for school closures revealed a wide and overlapping subgroup CI and non-significance of the test for some subgroup analyses. The assumptions set out should therefore be interpreted with caution and further research is needed to confirm or refute these findings. Fifth, only a small number of studies from Eastern Europe were included and no appropriate pooling for single countries was possible. Sixth, subgroup analyses concerning social status were not possible due to a lack of data. Seventh, based on the literature search through to January 2023, analyses of the development of PA in the course of the pandemic and its aftermath are limited. It will take several more years to capture the longer-term trend in physical activity. Eight, the impact of the COVID-19 pandemic on the reduction in physical activity must be interpreted with caution. By performing a pre-during-comparison and stratifying by School Closure Index, we addressed this limitation and attempted to minimize it.
Conclusions
Among children and adolescents in Europe, TPA, MVPA and SA declined sharply during the COVID-19 pandemic. This was the case in particular for TPA and MVPA among the population groups of middle childhood (8 to 12 years) and for TPA among adolescents. There are indications that reductions were most pronounced during pandemic-related school closures. Our findings suggest that the decline in physical activity during the pandemic could accelerate the long-term trend in declining physical activity among CA. Rigorous strategies and ambitious (school) programs to increase physical activity are therefore required, along with long-term monitoring of further trends.
Availability of data and materials
All data are included in the manuscript and additional file.
Abbreviations
- AF:
-
Additional file
- CI:
-
Confidence interval
- COVID-19:
-
Coronavirus disease 2019
- EtD:
-
Evidence to Decision
- GRADE:
-
Grading of Recommendations Assessment, Development and Evaluation
- MVPA:
-
Moderate-to-vigorous physical activity
- PHSM:
-
Public health and social measures
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- PRESS:
-
Peer Review of Electronic Search Strategies
- PROSPERO:
-
International Prospective Register of Systematic Reviews
- RoB:
-
Risk of bias
- ROBINS-E:
-
Risk of Bias in Non-randomized Studies - of Exposure
- TPA:
-
Total physical activity
- SA:
-
Sporting activity
- SD:
-
Standard deviation
- SMD:
-
Standardized mean differences
References
World Health Organization. WHO guidelines on physical activity and sedentary behaviour. Geneva: World Health Organization; 2020.
Dale LP, Vanderloo L, Moore S, Faulkner G. Physical activity and depression, anxiety, and self-esteem in children and youth: an umbrella systematic review. Ment Health Phys Act. 2019;16:66–79. https://doi.org/10.1016/j.mhpa.2018.12.001.
Renninger M, Hansen BH, Steene-Johannessen J, Kriemler S, Froberg K, Northstone K, et al. Associations between accelerometry measured physical activity and sedentary time and the metabolic syndrome: a meta-analysis of more than 6000 children and adolescents. Pediatr Obes. 2020;15:e12578. https://doi.org/10.1111/ijpo.12578.
Skrede T, Steene-Johannessen J, Anderssen SA, Resaland GK, Ekelund U. The prospective association between objectively measured sedentary time, moderate-to-vigorous physical activity and cardiometabolic risk factors in youth: a systematic review and meta-analysis. Obes Rev. 2019;20:55–74. https://doi.org/10.1111/obr.12758.
Vazou S, Pesce C, Lakes K, Smiley-Oyen A. More than one road leads to Rome: a narrative review and meta-analysis of physical activity intervention effects on cognition in youth. Int J Sport Exerc Psychol. 2019;17:153–78. https://doi.org/10.1080/1612197X.2016.1223423.
Hawlader MDH, Mozid N-E, Sharmin S, Monju IH, Ahmed SB, Sarker W, et al. The art of forming habits: applying habit theory in changing physical activity behaviour. J Public Health (Berl). 2022. https://doi.org/10.1007/s10389-022-01766-4.
van Sluijs EMF, Ekelund U, Crochemore-Silva I, Guthold R, Ha A, Lubans D, et al. Physical activity behaviours in adolescence: current evidence and opportunities for intervention. Lancet. 2021;398:429–42. https://doi.org/10.1016/S0140-6736(21)01259-9.
Guthold R, Stevens GA, Riley LM, Bull FC. Global trends in insufficient physical activity among adolescents: a pooled analysis of 298 population-based surveys with 1·6 million participants. Lancet Child Adolesc Health. 2020;4:23–35. https://doi.org/10.1016/S2352-4642(19)30323-2.
Katzmarzyk PT, Friedenreich C, Shiroma EJ, Lee I-M. Physical inactivity and non-communicable disease burden in low-income, middle-income and high-income countries. Br J Sports Med. 2022;56:101–6. https://doi.org/10.1136/bjsports-2020-103640.
Ding D, Lawson KD, Kolbe-Alexander TL, Finkelstein EA, Katzmarzyk PT, van Mechelen W, Pratt M. The economic burden of physical inactivity: a global analysis of major non-communicable diseases. Lancet. 2016;388:1311–24. https://doi.org/10.1016/S0140-6736(16)30383-X.
Hale T, Angrist N, Goldszmidt R, Kira B, Petherick A, Phillips T, et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker). Nat Hum Behav. 2021;5:529–38. https://doi.org/10.1038/s41562-021-01079-8.
Maltagliati S, Rebar A, Fessler L, Forestier C, Sarrazin P, Chalabaev A, et al. Evolution of physical activity habits after a context change: the case of COVID-19 lockdown. Br J Health Psychol. 2021;26:1135–54. https://doi.org/10.1111/bjhp.12524.
Neville RD, Lakes KD, Hopkins WG, Tarantino G, Draper CE, Beck R, Madigan S. Global changes in child and adolescent physical activity during the COVID-19 pandemic: a systematic review and meta-analysis. JAMA Pediatr. 2022;176:886–94. https://doi.org/10.1001/jamapediatrics.2022.2313.
Stockwell S, Trott M, Tully M, Shin J, Barnett Y, Butler L, et al. Changes in physical activity and sedentary behaviours from before to during the COVID-19 pandemic lockdown: a systematic review. BMJ Open Sport Exerc Med. 2021;7:e000960. https://doi.org/10.1136/bmjsem-2020-000960.
Chaabna K, Chaabane S, Jithesh A, Doraiswamy S, Mamtani R, Cheema S. Effect of the COVID-19 pandemic on the proportion of physically active children and adults worldwide: a systematic review and meta-analysis. Front Public Health. 2022;10:1009703. https://doi.org/10.3389/fpubh.2022.1009703.
Aubert S, Barnes JD, Demchenko I, Hawthorne M, Abdeta C, Abi Nader P, et al. Global Matrix 4.0 physical activity report card grades for children and adolescents: results and analyses from 57 countries. J Phys Act Health. 2022;19:700–28. https://doi.org/10.1123/jpah.2022-0456.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions. 6th ed. 2022.
Ludwig-Walz H, Siemens W, Heinisch S, Dannheim I, Loss J, Bujard M. Physical activity and motor competence among children and adolescents after the onset of the COVID-19 pandemic in Europe: a systematic review protocol: CRD42023395871. 2023.
Ludwig-Walz H, Siemens W, Heinisch S, Dannheim I, Loss J, Bujard M. Physical activity and physical fitness among children and adolescents after the onset of the COVID-19 pandemic in the WHO European Region: a systematic review protocol. BMJ Open. 2023;13:e073397. https://doi.org/10.1136/bmjopen-2023-073397.
WHO Regional Office for Europe. Countries. 2022. https://www.euro.who.int/en/countries. Accessed 18 May 2022.
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. https://doi.org/10.1016/j.jclinepi.2016.01.021.
Thomas J, Graziosi S, Brunton J, Ghouze Z, O’Driscoll P, Bond M. EPPI-Reviewer: advanced software for systematic reviews, maps and evidence synthesis. EPPI-Centre Software. London: UCL Social Research Institute; 2020.
Geets Kesic M, Gilic B, Cerkez Zovko I, Drid P, Korovljev D, Sekulic D. Differential impact of COVID-19 lockdown on physical activity in younger and older adolescents - prospective study. Med Pr. 2021;72:633–43. https://doi.org/10.13075/mp.5893.01180.
Sekulic D, Blazevic M, Gilic B, Kvesic I, Zenic N. Prospective analysis of levels and correlates of physical activity during COVID-19 pandemic and imposed rules of social distancing; gender specific study among adolescents from Southern Croatia. Sustainability. 2020;12:4072. https://doi.org/10.3390/su12104072.
Sekulic D, Ostojic D, Decelis A, Castro-Piñero J, Jezdimirovic T, Drid P, et al. The impact of scholastic factors on physical activity levels during the COVID-19 lockdown: a prospective study on adolescents from Bosnia and Herzegovina. Children (Basel). 2021. https://doi.org/10.3390/children8100877.
COVIDSurg Collaborative. Effect of COVID-19 pandemic lockdowns on planned cancer surgery for 15 tumour types in 61 countries: an international, prospective, cohort study. Lancet Oncol. 2021;22:1507–17. https://doi.org/10.1016/S1470-2045(21)00493-9.
Ludwig-Walz H, Dannheim I, Pfadenhauer LM, Fegert JM, Bujard M. Increase of depression among children and adolescents after the onset of the COVID-19 pandemic in Europe: a systematic review and meta-analysis. Child Adolesc Psychiatry Ment Health. 2022;16:109. https://doi.org/10.1186/s13034-022-00546-y.
Štveráková T, Jačisko J, Busch A, Šafářová M, Kolář P, Kobesová A. The impact of COVID-19 on physical activity of Czech children. PLoS One. 2021;16:e0254244. https://doi.org/10.1371/journal.pone.0254244.
Kurz D, Braig S, Genuneit J, Rothenbacher D. Lifestyle changes, mental health, and health-related quality of life in children aged 6–7 years before and during the COVID-19 pandemic in South Germany. Child Adolesc Psychiatry Ment Health. 2022;16:20. https://doi.org/10.1186/s13034-022-00454-1.
Schmidt SCE, Anedda B, Burchartz A, Eichsteller A, Kolb S, Nigg C, et al. Physical activity and screen time of children and adolescents before and during the COVID-19 lockdown in Germany: a natural experiment. Sci Rep. 2020;10:21780. https://doi.org/10.1038/s41598-020-78438-4.
Schmidt SCE, Burchartz A, Kolb S, Niessner C, Oriwol D, Hanssen-Doose A, et al. Zur Situation der körperlich-sportlichen Aktivität von Kindern und Jugendlichen während der COVID-19 Pandemie in Deutschland: Die Motorik-Modul Studie (MoMo). 165th ed. 2021.
O’Kane SM, Lahart IM, Gallagher AM, Carlin A, Faulkner M, Jago R, Murphy MH. Changes in physical activity, sleep, mental health, and social media use during COVID-19 lockdown among adolescent girls: a mixed-methods study. J Phys Act Health. 2021;18:677–85. https://doi.org/10.1123/jpah.2020-0649.
Mastorci F, Piaggi P, Doveri C, Trivellini G, Casu A, Pozzi M, et al. Health-related quality of life in Italian adolescents during Covid-19 outbreak. Front Pediatr. 2021;9:611136. https://doi.org/10.3389/fped.2021.611136.
Dallolio L, Marini S, Masini A, Toselli S, Stagni R, Bisi MC, et al. The impact of COVID-19 on physical activity behaviour in Italian primary school children: a comparison before and during pandemic considering gender differences. BMC Public Health. 2022;22:52. https://doi.org/10.1186/s12889-021-12483-0.
Evenson KR, Catellier DJ, Gill K, Ondrak KS, McMurray RG. Calibration of two objective measures of physical activity for children. J Sports Sci. 2008;26:1557–65. https://doi.org/10.1080/02640410802334196.
ten Velde G, Lubrecht J, Arayess L, van Loo C, Hesselink M, Reijnders D, Vreugdenhil A. Physical activity behaviour and screen time in Dutch children during the COVID-19 pandemic: pre-, during- and post-school closures. Pediatr Obes. 2021;16:e12779. https://doi.org/10.1111/ijpo.12779.
Kołota A, Głąbska D. COVID-19 pandemic and remote education contributes to improved nutritional behaviors and increased screen time in a Polish population-based sample of primary school adolescents: diet and activity of youth during COVID-19 (DAY-19) study. Nutrients. 2021. https://doi.org/10.3390/nu13051596.
Łuszczki E, Bartosiewicz A, Pezdan-Śliż I, Kuchciak M, Jagielski P, Oleksy Ł, et al. Children’s eating habits, physical activity, sleep, and media usage before and during COVID-19 pandemic in Poland. Nutrients. 2021. https://doi.org/10.3390/nu13072447.
Mercê C, Cordeiro J, Romão C, Branco M, Catela D. Levels of physical activity in Portuguese children: the impact of the Covid-19 pandemic. Retos. 2022;47:174–80. https://doi.org/10.47197/retos.v47.94936.
Blazevic M, Gilic B, Peric I, Sekulic D. Physical activity before and during COVID-19 pandemic; analysis of changes and correlates in Croation adolescents. KINSI. 2021;27:5–17. https://doi.org/10.52165/kinsi.27.2.5-17.
Morrison SA, Meh K, Sember V, Starc G, Jurak G. The effect of pandemic movement restriction policies on children’s physical fitness, activity, screen time, and sleep. Front Public Health. 2021;9:785679. https://doi.org/10.3389/fpubh.2021.785679.
Alonso-Martínez AM, Ramírez-Vélez R, García-Alonso Y, Izquierdo M, García-Hermoso A. Physical activity, sedentary behavior, sleep and self-regulation in Spanish preschoolers during the COVID-19 lockdown. Int J Environ Res Public Health. 2021. https://doi.org/10.3390/ijerph18020693.
Crotti M, Foweather L, Rudd JR, Hurter L, Schwarz S, Boddy LM. Development of raw acceleration cut-points for wrist and hip accelerometers to assess sedentary behaviour and physical activity in 5–7-year-old children. J Sports Sci. 2020;38:1036–45. https://doi.org/10.1080/02640414.2020.1740469.
García-Alonso Y, García-Hermoso A, Izquierdo M, Legarra-Gorgoñon G, Ramírez-Vélez R, Alonso-Martínez AM. Relationship between parents’ and children’s objectively assessed movement behaviours prior to and during the COVID-19 pandemic. Pediatr Obes. 2022;17:e12923. https://doi.org/10.1111/ijpo.12923.
Hildebrand M, van Hees VT, Hansen BH, Ekelund U. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med Sci Sports Exerc. 2014;46:1816–24. https://doi.org/10.1249/MSS.0000000000000289.
Hildebrand M, Hansen BH, van Hees VT, Ekelund U. Evaluation of raw acceleration sedentary thresholds in children and adults. Scand J Med Sci Sports. 2017;27:1814–23. https://doi.org/10.1111/sms.12795.
Medrano M, Cadenas-Sanchez C, Oses M, Arenaza L, Amasene M, Labayen I. Changes in lifestyle behaviours during the COVID-19 confinement in Spanish children: a longitudinal analysis from the MUGI project. Pediatr Obes. 2021;16:e12731. https://doi.org/10.1111/ijpo.12731.
Tapia-Serrano MA, Sánchez-Oliva D, Sevil-Serrano J, Marques A, Sánchez-Miguel PA. 24-h movement behaviours in Spanish youth before and after 1-year into the covid-19 pandemic and its relationship to academic performance. Sci Rep. 2022;12:16660. https://doi.org/10.1038/s41598-022-21096-5.
Chen Y, Osika W, Henriksson G, Dahlstrand J, Friberg P. Impact of COVID-19 pandemic on mental health and health behaviors in Swedish adolescents. Scand J Public Health. 2022;50:26–32. https://doi.org/10.1177/14034948211021724.
Zehnder C, Nigg CR, Benzing V. COVID-19: sports activity and health-related quality of life of Swiss children and adolescents before and during the initial stay at home period. J Health Psychol. 2023;28:491–505. https://doi.org/10.1177/13591053221122722.
Bingham DD, Daly-Smith A, Hall J, Seims A, Dogra SA, Fairclough SJ, et al. Covid-19 lockdown: ethnic differences in children’s self-reported physical activity and the importance of leaving the home environment; a longitudinal and cross-sectional study from the Born in Bradford birth cohort study. Int J Behav Nutr Phys Act. 2021;18:117. https://doi.org/10.1186/s12966-021-01183-y.
James M, Marchant E, Defeyter MA, Woodside J, Brophy S. Impact of school closures on the health and well-being of primary school children in Wales UK: a routine data linkage study using the HAPPEN Survey (2018–2020). BMJ Open. 2021;11:e051574. https://doi.org/10.1136/bmjopen-2021-051574.
Salway R, Foster C, de Vocht F, Tibbitts B, Emm-Collison L, House D, et al. Accelerometer-measured physical activity and sedentary time among children and their parents in the UK before and after COVID-19 lockdowns: a natural experiment. Int J Behav Nutr Phys Act. 2022;19:51. https://doi.org/10.1186/s12966-022-01290-4.
Sheldrick MPR, Swindell NJ, Richards AB, Fairclough SJ, Stratton G. Homes became the “everything space” during COVID-19: impact of changes to the home environment on children’s physical activity and sitting. Int J Behav Nutr Phys Act. 2022;19:134. https://doi.org/10.1186/s12966-022-01346-5.
Chandler JL, Brazendale K, Beets MW, Mealing BA. Classification of physical activity intensities using a wrist-worn accelerometer in 8–12-year-old children. Pediatr Obes. 2016;11:120–7. https://doi.org/10.1111/ijpo.12033.
Higgins J, Morgan R, Rooney A, Taylor K, Thayer K, Silva R, Lemeris C, Akl A, Arroyave W, Bateson T, Berkman N, Demers P, Forastiere F, Glenn B, Hróbjartsson A, Kirrane E, LaKind J, Luben T, Lunn R, McAleenan A, McGuinness L, Meerpohl J, Mehta S, Nachman R, Obbagy J, O’Connor A, Radke E, Savović J, Schubauer-Berigan M, Schwingl P, Schunemann H, Shea B, Steenland K, Stewart T, Straif K, Tilling K, Verbeek V, Vermeulen R, Viswanathan M, Zahm S, Sterne J (ROBINS-E Development Group). Risk Of Bias In Non-randomized Studies - of Exposure (ROBINS-E): launch version. 2022. https://www.riskofbias.info/welcome/robins-e-tool.
Centers for Disease Control and Prevention. Child development. 2021. https://www.cdc.gov/ncbddd/childdevelopment/positiveparenting/index.html. Accessed 30 May 2023.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. https://doi.org/10.1136/bmj.327.7414.557.
Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions: version 6.3. 2022.
Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–59. https://doi.org/10.1111/j.1467-985X.2008.00552.x.
Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. https://doi.org/10.1136/bmj.d549.
Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions: version 6.3. 2022.
Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. https://doi.org/10.1136/bmj.d4002.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. https://doi.org/10.1136/bmj.315.7109.629.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–60.
RStudio Team. RStudio: Integrated Development for R. Boston: 2022.
Langan D, Higgins JPT, Jackson D, Bowden J, Veroniki AA, Kontopantelis E, et al. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Synth Methods. 2019;10:83–98. https://doi.org/10.1002/jrsm.1316.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions: version 6.3. 2022.
Schünemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K, et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J Clin Epidemiol. 2019;111:105–14. https://doi.org/10.1016/j.jclinepi.2018.01.012.
Zenic N, Taiar R, Gilic B, Blazevic M, Maric D, Pojskic H, Sekulic D. Levels and changes of physical activity in adolescents during the COVID-19 pandemic: contextualizing urban vs. rural living environment. Appl Sci. 2020;10:3997. https://doi.org/10.3390/app10113997.
Kołota A, Głąbska D. Analysis of association between adolescents’ food habits and body mass change in a population-based sample: diet and activity of youth during COVID-19 (DAY-19) study. Int J Environ Res Public Health. 2022. https://doi.org/10.3390/ijerph191811772.
Schmidt SC, Burchartz A, Kolb S, Niessner C, Oriwol D, Hanssen-Doose A, et al. Zur Situation der körperlich- sportlichen Aktivität von Kindern und Jugendlichen während der COVID-19 Pandemie in Deutschland: Die Motorik-Modul Studie (MoMo). KIT scientific working papers. 2021. p. 165.
World Health Organization. 2021 physical activity factsheets for the European Union Member States in the WHO European Region. Copenhagen: WHO Regional Office for Europe; 2021.
Rossi L, Behme N, Breuer C. Physical activity of children and adolescents during the COVID-19 pandemic-a scoping review. Int J Environ Res Public Health. 2021. https://doi.org/10.3390/ijerph182111440.
Pang JCY, Chan ELS, Lau HMC, Reeves KKL, Chung THY, Hui HWL, et al. The impacts of physical activity on psychological and behavioral problems, and changes in physical activity, sleep and quality of life during the COVID-19 pandemic in preschoolers, children, and adolescents: a systematic review and meta-analysis. Front Pediatr. 2023;11:1015943. https://doi.org/10.3389/fped.2023.1015943.
Ludwig-Walz H, Dannheim I, Pfadenhauer LM, Fegert JM, Bujard M. Anxiety increased among children and adolescents during pandemic-related school closures in Europe: a systematic review and meta-analysis. Child Adolesc Psychiatry Ment Health. 2023;17:109. https://doi.org/10.1186/s13034-022-00546-y.
Gardner B, Sheals K, Wardle J, McGowan L. Putting habit into practice, and practice into habit: a process evaluation and exploration of the acceptability of a habit-based dietary behaviour change intervention. Int J Behav Nutr Phys Act. 2014;11:135. https://doi.org/10.1186/s12966-014-0135-7.
Telama R. Tracking of physical activity from childhood to adulthood: a review. Obes Facts. 2009;2:187–95. https://doi.org/10.1159/000222244.
Walker R, House D, Emm-Collison L, Salway R, Tibbitts B, Sansum K, et al. A multi-perspective qualitative exploration of the reasons for changes in the physical activity among 10–11-year-old children following the easing of the COVID-19 lockdown in the UK in 2021. Int J Behav Nutr Phys Act. 2022;19:114. https://doi.org/10.1186/s12966-022-01356-3.
Marconcin P, Werneck AO, Peralta M, Ihle A, Gouveia ÉR, Ferrari G, et al. The association between physical activity and mental health during the first year of the COVID-19 pandemic: a systematic review. BMC Public Health. 2022;22:209. https://doi.org/10.1186/s12889-022-12590-6.
Bourke M, Haddara A, Loh A, Carson V, Breau B, Tucker P. Adherence to the World Health Organization’s physical activity recommendation in preschool-aged children: a systematic review and meta-analysis of accelerometer studies. Int J Behav Nutr Phys Act. 2023;20:52. https://doi.org/10.1186/s12966-023-01450-0.
Rittsteiger L, Hinz T, Oriwol D, Wäsche H, Santos-Hövener C, Woll A. Sports participation of children and adolescents in Germany: disentangling the influence of parental socioeconomic status. BMC Public Health. 2021;21:1446. https://doi.org/10.1186/s12889-021-11284-9.
Eyler AA, Schmidt L, Kepper M, Mazzucca S, Gilbert A, Beck A. Parent perceptions of changes in child physical activity during COVID-19 stay-at-home orders. Front Public Health. 2021;9:637151. https://doi.org/10.3389/fpubh.2021.637151.
Jerebine A, Mohebbi M, Lander N, Eyre ELJ, Duncan MJ, Barnett LM. Playing it safe: the relationship between parent attitudes to risk and injury, and children’s adventurous play and physical activity. Psychol Sport Exerc. 2023;70:102536. https://doi.org/10.1016/j.psychsport.2023.102536.
Burchartz A, Oriwol D, Kolb S, Schmidt SCE, Wunsch K, Manz K, et al. Comparison of self-reported & device-based, measured physical activity among children in Germany. BMC Public Health. 2021;21:1081. https://doi.org/10.1186/s12889-021-11114-y.
Colley RC, Butler G, Garriguet D, Prince SA, Roberts KC. Comparison of self-reported and accelerometer-measured physical activity among Canadian youth. Health Rep. 2019;30:3–12. https://doi.org/10.25318/82-003-x201900700001-eng.
Schünemann H, Brożek J, Guyatt G, Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated October 2013. https://gdt.gradepro.org/app/handbook/handbook.html. Accessed 25 May 2023.
Hilton Boon M, Thomson H, Shaw B, Akl EA, Lhachimi SK, López-Alcalde J, et al. Challenges in applying the GRADE approach in public health guidelines and systematic reviews: a concept article from the GRADE Public Health Group. J Clin Epidemiol. 2021;135:42–53. https://doi.org/10.1016/j.jclinepi.2021.01.001.
Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336:995–8. https://doi.org/10.1136/bmj.39490.551019.BE.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016;353:i2016. https://doi.org/10.1136/bmj.i2016.
Moberg J, Oxman AD, Rosenbaum S, Schünemann HJ, Guyatt G, Flottorp S, et al. The GRADE Evidence to Decision (EtD) framework for health system and public health decisions. Health Res Policy Syst. 2018;16:45. https://doi.org/10.1186/s12961-018-0320-2.
Runacres A, Mackintosh KA, McNarry MA. Health consequences of an elite sporting career: long-term detriment or long-term gain? A meta-analysis of 165,000 former athletes. Sports Med. 2021;51:289–301. https://doi.org/10.1007/s40279-020-01379-5.
Kuvaja-Köllner V, Lintu N, Lindi V, Rissanen E, Eloranta A-M, Kiiskinen S, et al. Cost-effectiveness of physical activity intervention in children - results based on the Physical Activity and Nutrition in Children (PANIC) study. Int J Behav Nutr Phys Act. 2021;18:116. https://doi.org/10.1186/s12966-021-01181-0.
Gc VS, Suhrcke M, Atkin AJ, van Sluijs E, Turner D. Cost-effectiveness of physical activity interventions in adolescents: model development and illustration using two exemplar interventions. BMJ Open. 2019;9:e027566. https://doi.org/10.1136/bmjopen-2018-027566.
Sher C, Wu C. Who stays physically active during COVID-19? Inequality and exercise patterns in the United States. Socius. 2021;7:2378023120987710. https://doi.org/10.1177/2378023120987710.
Humphreys DK, Ogilvie D. Social inequalities in physical activity: do environmental and policy interventions help to reduce the gap? A pilot systematic review. Lancet. 2012;380:S50. https://doi.org/10.1016/S0140-6736(13)60406-7.
Neil-Sztramko SE, Caldwell H, Dobbins M. School-based physical activity programs for promoting physical activity and fitness in children and adolescents aged 6 to 18. Cochrane Database Syst Rev. 2021;9:CD007651. https://doi.org/10.1002/14651858.CD007651.pub3.
United Nations, Department of Economic and Social Affairs, Population Division. World population prospects 2022, online edition. 2022.
Lange SJ, Kompaniyets L, Freedman DS, Kraus EM, Porter R, Blanck HM, Goodman AB. Longitudinal trends in body mass index before and during the COVID-19 pandemic among persons aged 2–19 years - United States, 2018–2020. MMWR Morb Mortal Wkly Rep. 2021;70:1278–83. https://doi.org/10.15585/mmwr.mm7037a3.
Madigan S, Eirich R, Pador P, McArthur BA, Neville RD. Assessment of changes in child and adolescent screen time during the COVID-19 pandemic: a systematic review and meta-analysis. JAMA Pediatr. 2022;176:1188–98. https://doi.org/10.1001/jamapediatrics.2022.4116.
Lange S, Altrock C-M, Gossmann E, Fegert JM, Jud A. COVID-19-what price do children pay? An analysis of economic and social policy factors. Int J Environ Res Public Health. 2022. https://doi.org/10.3390/ijerph19137604.
Shao T, Verma HK, Pande B, Costanzo V, Ye W, Cai Y, Bhaskar LVKS. Physical activity and nutritional influence on immune function: an important strategy to improve immunity and health status. Front Physiol. 2021;12:751374. https://doi.org/10.3389/fphys.2021.751374.
Nieman DC, Wentz LM. The compelling link between physical activity and the body’s defense system. J Sport Health Sci. 2019;8:201–17. https://doi.org/10.1016/j.jshs.2018.09.009.
World Health Organization. Global action plan on physical activity 2018–2030: more active people for a healthier world. Geneva: World Health Organization; 2019.
Lai SK, Costigan SA, Morgan PJ, Lubans DR, Stodden DF, Salmon J, Barnett LM. Do school-based interventions focusing on physical activity, fitness, or fundamental movement skill competency produce a sustained impact in these outcomes in children and adolescents? A systematic review of follow-up studies. Sports Med. 2014;44:67–79. https://doi.org/10.1007/s40279-013-0099-9.
Sutherland RL, Campbell EM, Lubans DR, Morgan PJ, Nathan NK, Wolfenden L, et al. The physical activity 4 everyone cluster randomized trial: 2-year outcomes of a school physical activity intervention among adolescents. Am J Prev Med. 2016;51:195–205. https://doi.org/10.1016/j.amepre.2016.02.020.
Woods CB, Volf K, Kelly L, Casey B, Gelius P, Messing S, et al. The evidence for the impact of policy on physical activity outcomes within the school setting: a systematic review. J Sport Health Sci. 2021;10:263–76. https://doi.org/10.1016/j.jshs.2021.01.006.
Gelius P, Messing S, Goodwin L, Schow D, Abu-Omar K. What are effective policies for promoting physical activity? A systematic review of reviews. Prev Med Rep. 2020;18:101095. https://doi.org/10.1016/j.pmedr.2020.101095.
Mendonça G, Cheng LA, Mélo EN, de Farias Júnior JC. Physical activity and social support in adolescents: a systematic review. Health Educ Res. 2014;29:822–39. https://doi.org/10.1093/her/cyu017.
Yao CA, Rhodes RE. Parental correlates in child and adolescent physical activity: a meta-analysis. Int J Behav Nutr Phys Act. 2015;12:10. https://doi.org/10.1186/s12966-015-0163-y.
Kärmeniemi M, Lankila T, Ikäheimo T, Koivumaa-Honkanen H, Korpelainen R. The built environment as a determinant of physical activity: a systematic review of longitudinal studies and natural experiments. Ann Behav Med. 2018;52:239–51. https://doi.org/10.1093/abm/kax043.
Shin Y, Kim SK, Lee M. Mobile phone interventions to improve adolescents’ physical health: a systematic review and meta-analysis. Public Health Nurs. 2019;36:787–99. https://doi.org/10.1111/phn.12655.
Champion KE, Parmenter B, McGowan C, Spring B, Wafford QE, Gardner LA, et al. Effectiveness of school-based eHealth interventions to prevent multiple lifestyle risk behaviours among adolescents: a systematic review and meta-analysis. Lancet Digit Health. 2019;1:e206–21. https://doi.org/10.1016/S2589-7500(19)30088-3.
World Health Organization. Monitoring and evaluation framework: COVID‑19 strategic preparedness and response. 2020.
Requejo J, Strong K, Agweyu A, Billah SM, Boschi-Pinto C, Horiuchi S, et al. Measuring and monitoring child health and wellbeing: recommendations for tracking progress with a core set of indicators in the Sustainable Development Goals era. Lancet Child Adolesc Health. 2022;6:345–52. https://doi.org/10.1016/S2352-4642(22)00039-6.
Marzi I, Tcymbal A, Gelius P, Abu-Omar K, Reimers AK, Whiting S, Wickramasinghe K. Monitoring of physical activity promotion in children and adolescents in the EU: current status and future perspectives. Eur J Public Health. 2022;32:95–104. https://doi.org/10.1093/eurpub/ckab193.
Popovic S, Sarmento H, Demetriou Y, Marques A. Editorial: Monitoring and promoting physical activity and physical fitness in children. Front Public Health. 2021;9:633457. https://doi.org/10.3389/fpubh.2021.633457.
Fegert JM, Ludwig-Walz H, Witt A, Bujard M. Children’s rights and restrictive measures during the COVID-19 pandemic: implications for politicians, mental health experts and society. Child Adolesc Psychiatry Ment Health. 2023;17:75. https://doi.org/10.1186/s13034-023-00617-8.
Cohen J. Statistical power analysis for the behavioral sciences. New York: Routledge; 2013.
Acknowledgements
We would like to acknowledge Dr Sabrina Schlesinger (Head of Research Group Systematic Reviews; German Diabetes Center) for her peer-review of the search strategy according to the Peer Review of Electronic Search Strategies (PRESS) Evidence-Based Checklist.
In addition, we would like to thank Salomé Aubert, PhD (Chair of the Active Healthy Kids Global Alliance Publications Committee) for providing us with information from the ‘Global Matrix 4.0 Physical Activity Report’.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
HLW and MB formulated the research question, with methodological feedback from WS and practical feedback from SH. All authors contributed to the study concept and design. HLW, ID and SH screened titles, abstracts, full text and extracted data. HLW and SH assessed risk of bias. HLW and WS conducted the GRADE assessment. HLW and MB prepared the first draft of the manuscript. The corresponding author had final responsibility for deciding to submit for publication. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Prof Dr Martin Bujard (last 5 years): Research funding from the European Union and BMBF (German Federal Ministry of Education and Research). Travel grants and honoraria from universities, federal and state parliaments, federal and state ministries, Evangelical-Lutheran Church, Federal Agency for Civic Education. Consultant for BMFSFJ (German Federal Ministry for Family Affairs, Senior Citizens, Women and Youth). All grants and honoraria were declared to the law office of the German Federal Institute for Population Research (BiB).
No competing interests are declared by any of the other authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
PRISMA item checklist for systematic reviews. Table S2. Deviations from the systematic review protocol. Table S3. Searched websites of key organizations. Table S4. Search strategy. Table S5. Reasons for exclusion of studies from the systematic literature search, after full-text screening. Table S6. Data conversion. Table S7. Criteria for grading evidence according to Grading of Recommendations, Assessment, Development and Evaluations (GRADE). Table S8. Evidence profile for grading evidence according to Grading of Recommendations, Assessment, Development and Evaluations (GRADE). Table S9. Summary of effect estimates. Table S10. Meta-regression for total physical activity with categorical moderators. Table S11. Meta-regression for total physical activity with continuous moderators. Table S12. Meta-regression for moderate-to-vigorous physical activity with categorical moderators. Table S13. Meta-regression for moderate-to-vigorous physical activity with continuous moderators. Table S14. Sensitivity analysis for total physical activity. Table S15. Sensitivity analysis for moderate-to-vigorous physical activity. Table S16. Eggers’ test. Figure S1. PRISMA Flow Chart. Figure S2. Graphical distribution of the studies included. Figure S3. Traffic-light plots of the domain-level judgements for each individual result. Figure S4. Weighted-bar plots of the distribution of risk of bias judgements within each bias domain. Figure S5. Forest plot of changes in total physical activity comparing before and during COVID-19 pandemic, using Physical Activity Questionnaire for Children and Adolescents. Figure S6. Forest plot of changes in total physical activity comparing before and during COVID-19 pandemic, using accelerometer measurements. Figure S7. Forest plot of changes in female and male total physical activity comparing before and during COVID-19 pandemic. Figure S8. Forest plot of changes according to time course in total physical activity comparing before and during COVID-19 pandemic. Figure S9. Forest plot of changes according to a restriction length > 30 days before measurement in total physical activity comparing before and during COVID-19 pandemic. Figure S10. Forest plot of changes according to a restriction length > 60 days before measurement in total physical activity comparing before and during COVID-19 pandemic. Figure S11. Forest plot of changes according to a restriction length > 90 days before measurement in total physical activity comparing before and during COVID-19 pandemic. Figure S12. Forest plot of changes in moderate-to-vigorous physical activity comparing before and during COVID-19 pandemic. Figure S13. Forest plot of changes in moderate-to-vigorous physical activity comparing before and during COVID-19 pandemic, using self-reported score measurements. Figure S14. Forest plot of changes in moderate-to-vigorous physical activity comparing before and during COVID-19 pandemic, using accelerometer measurements. Figure S15. Forest plot of changes in female and male moderate-to-vigorous physical activity comparing before and during COVID-19 pandemic. Figure S16. Forest plot of changes in moderate-to-vigorous physical activity comparing different age groups. Figure S17. Forest plot of changes according to time course in moderate-to-vigorous physical activity comparing before and during COVID-19 pandemic. Figure S18. Forest plot of changes according to a restriction length > 30 days before measurement in moderate-to-vigorous physical activity comparing before and during COVID-19 pandemic. Figure S19. Forest plot of changes according to a restriction length > 60 days before measurement in moderate-to-vigorous physical activity comparing before and during COVID-19 pandemic. Figure S20. Forest plot of changes according to a restriction length > 90 days before measurement in moderate-to-vigorous physical activity comparing before and during COVID-19 pandemic. Figure S21. Funnel plot of changes in total physical activity comparing before and during COVID-19 pandemic. Figure S22. Funnel plot of changes in moderate-to-vigorous physical activity comparing before and during COVID-19 pandemic. Figure S23. Funnel plot of changes in sporting activity comparing before and during COVID-19 pandemic.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ludwig-Walz, H., Siemens, W., Heinisch, S. et al. How the COVID-19 pandemic and related school closures reduce physical activity among children and adolescents in the WHO European Region: a systematic review and meta-analysis. Int J Behav Nutr Phys Act 20, 149 (2023). https://doi.org/10.1186/s12966-023-01542-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12966-023-01542-x