Abstract
Cancer, ranked as the second leading cause of mortality worldwide, leads to the death of approximately seven million people annually, establishing itself as one of the most significant health challenges globally. The discovery and identification of new anti-cancer drugs that kill or inactivate cancer cells without harming normal and healthy cells and reduce adverse effects on the immune system is a potential challenge in medicine and a fundamental goal in Many studies. Therapeutic bacteria and viruses have become a dual-faceted instrument in cancer therapy. They provide a promising avenue for cancer treatment, but at the same time, they also create significant obstacles and complications that contribute to cancer growth and development. This review article explores the role of bacteria and viruses in cancer treatment, examining their potential benefits and drawbacks. By amalgamating established knowledge and perspectives, this review offers an in-depth examination of the present research landscape within this domain and identifies avenues for future investigation.
Graphical Abstract
The double-edged sword role of bacteria and viruses in cancer therapy.

Similar content being viewed by others
Introduction
Cancer, being the second leading factor of mortality globally, claims the lives of approximately seven million individuals annually, thereby establishing itself as one of the most pernicious ailments worldwide [1, 2]. The unearthing and recognizing novel anti-cancer medications that eradicate or render cancerous cells inactive without inducing harm in sound and typical cells have reduced adverse impacts on the immune system. It has a potential challenge in medicine and medicine and is an essential goal in many studies. Cancer is a disease in which a cell or group of cells exhibits uncontrolled growth (i.e., division beyond normal), invasion (i.e., invasion and distortion of adjacent tissue), and metastasis (i.e., from one part of the body to another through the lymph or blood) these three characteristics distinguish cancer from benign tumors [2]. The survival rates for cancer have significantly risen since the beginning of the twenty-first century due to the development of more precise and enhanced treatment approaches. Approximately 609,820 individuals in the United States are projected to succumb to cancer in the year 2023, equating to an average of 1670 deaths per day. The highest mortality rates are attributed to prostate, lung, and colorectal cancers (CRC) in men and lung, breast, and CRC in women [1, 3]. The induction of apoptosis and the inhibition of tumor cell growth and proliferation have been the primary approaches employed in cancer therapy up until this point [4]. Alternative approaches to cancer treatment, including radiotherapy, chemotherapy, surgical intervention, and tumor extraction, have also proven to be beneficial in the management and recovery of patients. Nevertheless, these methods have shown limited efficacy in nearly 50% of cancer instances [5]. Chemotherapy medications have severe adverse effects and are toxic to all organs. Chemotherapy drugs are associated with severe side effects such as hair loss, bleeding, fatigue, sterility, cognitive defect, sensory anomalies, lung damage, Nervous Texture damage, liver damage, digestive system damage, etc. Emerging cancer medicine resistance is another serious problem with chemotherapy [6,7,8]. For numerous years, the quest for novel antitumor compounds with reduced side effects has been a matter of great importance. In this context, natural products derived from plants, marine Organisms, and microorganisms have attracted the attention of many scientists [9, 10]. Oncolytic virotherapy, an innovative technique in the field of cancer treatment, has exhibited encouraging outcomes over the past twenty years [11]. It was noted over one hundred years ago that individuals with cancer experienced a reversal of their disease when they contracted specific viral infections [12]. Most oncolytic viruses selected to treat cancer are either weakened strains or strains that can invade and reproduce within the human body without posing any significant risk of illness [13]. The investigation into the potential of bacteria as a viable method to combat and address cancer is a notable avenue being pursued within the field of immunotherapies [2, 14]. Streptococci and Clostridia were the initial types of bacteria employed as live agents in the fight against cancer. Currently, genetically engineered bacteria are predominantly used in anti-cancer therapies, wherein diverse anti-cancer mechanisms and strategies are utilized. These encompass live bacterial toxicity, the expression of distinct cancer-related factors, gene transfer, and RNA interference [2]. Tumor-targeting bacteria, such as Salmonella, Listeria, and Clostridium spp., have intrinsic properties that can target, penetrate, replicate, and shrink solid tumors through various mechanisms [15]. Once inside the tumor, S. typhimurium continues to multiply and directly kills and destroys cancer cells by inducing apoptosis, necrosis, and cell rupture [16,17,18]. Table 1 lists the cancers caused by Microorganism infections.
The study aims to investigate the role of bacteria and viruses in cancer treatment and the development and progression of cancer and to gain new insights for the future.
Bacteria and viruses in cancer therapy
Bacteria and viruses have emerged as novel therapeutic entities in the battle against cancer. Utilizing these living entities as curative agents has a lengthy historical background [64,65,66]. These biological agents can directly assail and remove malignant cells or serve as a strategy to enhance the efficacy of additional pharmaceuticals in cancer therapy [67, 68]. Oncolytic bacteria and viruses, such as Bifidobacteria, Clostridium, Listeria monocytogenes, Salmonella typhimurium, Bacillus, Vaccinia viruses, Adenoviruses, Reoviruses, Herpesviruses, and Coxsackieviruses, have arisen as remarkable therapeutic strategies in the quest for the treatment and potential eradication of malignant tumors [69]. Due to their inherent anti-cancer properties and ability to interact with tumor microenvironments (TME), these microbes are attractive options for cancer therapy [70, 71]. Living viruses were administered to cancer patients in the 1950s and 1970s, which improved their course of treatment or recovery [72, 73]. For almost a century, several organizations have promoted using microorganisms to cure cancer [74]. William Coley pioneered in the early 1900s when it came to using microorganisms to treat cancer [12]. Additionally, it has been shown that some bacteria and viruses have evolved defense systems that obstruct cellular pathways and lessen hosts' capacity to heal damage, resulting in cellular transformation and the advancement of cancer [75,76,77].
Virotherapy for cancer (oncolytic viruses and oncolytic viral vectors)
Virotherapy is defined as the use of viruses in the diagnosis and treatment of chronic diseases, including infectious and noninfectious diseases such as central nervous system (CNS) disorders, genetic diseases, and cancers [78,79,80]. These viruses, called oncolytic viruses (OVs), are in different research levels, from basic molecular studies to clinical trials, and some of these efforts led to approved drugs [81]. These viruses can target and lyse malignant cells and enhance tumor regression (Fig. 1) [82]. The OVs can be classified into at least two categories: wild-type viruses and oncolytic viral vectors [83]. OVs exert their effects by targeting malignant cells and inducing their demise. One prominent illustration in this particular domain is the agent "talimogene laherparepvec," which has exhibited favorable outcomes when managing melanoma [84, 85]. The immunosuppressive TME presents a formidable obstacle to virotherapy [86]. The presence of low oxygen levels in the TME can have contrasting effects on the replication of OVs, either enhancing or inhibiting their proliferation, depending on the specific type of virus being considered [87]. Genetic manipulation or molecular alterations aimed at decreasing hypoxia possess the potential to augment antitumor reactions. The integration of OVs that can induce tumor lysis within the hypoxic TME may present a compelling approach for surmounting the constraints encountered in therapy [88]. For almost a century, the idea of OVs has been discussed. It was reported in 1904 that a 42-year-old woman's leukemic condition improved after contracting influenza. Later, in 1912, Italian doctors found that rabies vaccination injections might cause cervical cancer [81, 89, 90]. OVs have been used to treat multiple myeloma (MM), an incurable hematological disease. Human viruses have been studied; however, pre-existing anti-virus immunity limits their efficacy. Bovine viruses have demonstrated the ability to destroy MM cells directly, including Bovine Viral Diarrhea Virus (BVDV) and Bovine Herpes Virus type 1 (BoHV-1) [83, 91,92,93,94]. Tumor cells are selectively attracted to OVs, which promotes oncolysis and increased replication [95]. To get over treatment roadblocks, combinatorial therapy which involves trans genes like GM-CSF expressed in T-VEC has been investigated in addition to conventional therapies [96]. Numerous OVs have been studied for their methods of action and impacts on immunogenic cell death, apoptosis, autophagy, and immune system modulation. These include oncolytic vaccinia virus (OVV), vesicular stomatitis virus (VSV), and herpes simplex virus (HSV) [97]. The therapeutic potential of self-replicating RNA viruses, including flaviviruses, alphaviruses, rhabdoviruses, measles viruses, and others, has also been studied in cancer treatment [98].
Bacteriotherapy for cancer
The potential of bacteria and their metabolites to destroy tumor cells as a novel anti-neoplastic technique has been investigated by scientists and researchers throughout the last ten years [14, 99]. They have discovered that on average, healthy cells, bacteria, and their products are not only less harmful but also have fewer adverse consequences [100, 101]. Patients with cancer have been treated with various bacterial species and their metabolites (peptides, bacteriocins, etc.) [102, 103]. The outcomes show that these substances can affect and selectively proliferate tumors while limiting their growth [104]. Almost all bacterial species emit cationic peptides called bacteriocins, produced in the ribosome [105, 106]. Certain bacteriocins exhibit a higher degree of toxicity towards cancerous cells than normal cells [107, 108]. Probiotic-derived probiotic-based medicines have demonstrated the potential to destroy cancer cells while sparing healthy cells from harm selectively [109, 110]. Certain bacteria can initiate infections within tumor tissue through biofilm formation. The infection triggers an immune response characterized by a swift influx of neutrophils to the site of infection [111,112,113]. Anaerobic bacteria spores possess the potential to be employed in the process of synthesizing, cultivating, and generating agents that exhibit anti-cancer properties. Gene and drug delivery to tumor tissues can also be facilitated by carriers [114,115,116,117]. These spores of bacteria can acquire hypoxic-necrotic tissues, in which they can sprout, reproduce, and demonstrate their antitumor role [118, 119].
Through enhancing immunity (activating inflammasome pathways- CD4/8):
Bacterial constituents can amplify the interplay between tumors and the immune system, functioning as adjuvants. Adjuvants serve to invigorate the antigen and trigger the innate immune system [120]. Cancer immunotherapy encompasses activating a precise immune response within the patient, thereby enabling various categories of indigenous immune cells to target and combat cancer cells [121, 122]. The immune system consists of CD4 + and CD8 + T lymphocytes, which are activated after antigen stimulation by antigen-presenting cells (APCs) following the production of specific antibodies against the antigen [123]. CD8 + T-lymphocytes, macrophages, NK cells, dendritic cells (DCs), and regulatory T cells (T-regs) are the most pivotal constituents of the immune system that exert a profound influence on the suppression of malignant and aberrant cells. These immune cells possess FOXP3 as a biomarker, emphasizing their significance [124, 125]. CD8 + T-lymphocytes have gained recognition as the most prominent constituents of the immune system in their ability to impede the proliferation of cancerous cells [126, 127]. Each person has a unique immune system component with a different capability to mount an immune response [128]. Tumor-associated antigens on the surface of cancer cells occur with rapid cell proliferation and escape from the TME [129, 130]. Once the host's immune cells are activated (primarily tumor antigen-specific CD8 + and CD4 + T lymphocytes that are activated and stimulated), they can recognize and destroy tumor cells [131]. DCs, antigen-presenting cells, are required to generate effective immune responses [132]. Pathogen-associated molecular patterns (PAMPs) upregulate proinflammatory cytokines (such as IL-12) and inflammatory molecules (such as CD40) [133]. These S. typhimurium flagellin enhances the antitumor response of CD8 + T and natural killer (NK) cells. It decreases the frequency of regulatory T cells (Tregs( mediators induce the production of interferon-gamma (IFN-γ) and initiate a Th1-dependent cellular response that is primarily mediated by CD8 + effector cells [131, 134]. Tumor tissues may be suppressed by certain microbial infections and their consequences, such as infections brought on by E. coli [135]. These infections enhance and quicken CD8 + killer T-cell development, leading to the production of IFN-γ and an uptick in the expression of primary histocompatibility complex subtype I (MHC-I) on malignant cells. Finally, by using this integrative process, CD8 + T-cell diapedesis into tumor tissue may be altered [136]. It should be mentioned that CD8 + T cells can target malignant tissues even in the absence of bacterial infections or activities since their function in fighting tumor cells is known to occur independently of bacterial activity [137, 138]. Moreover, other substances linked to microbes may potentially impact CD8 + T cells. As an illustration, research by Diwakar Davar and colleagues [139] revealed that responder-derived fecal microbiota transplant (R-FMT) combined with pembrolizumab could enhance the induction of CD8 + T cells, decrease IL-8 synthesis, and bolster the immune responses against tumor cells resistant to anti-PD-1 [139, 140].
The innate immune system ((TNF-α)) in bacteria
Tumor necrosis factor α (TNF-α) is a protumor factor in a chronic inflammatory environment, including tumors and many cancers [141, 142]. Phagocytic cells, including neutrophils, DCs, and macrophages, secrete TNF-α and are essential for limiting bacterial growth and triggering the production of more immune cells [143]. Through the NF-κB and AP-1 signaling pathways, TNF-α can cause inflammation by upregulating gene transcription [144, 145]. Additionally, involved in the pathophysiology of pulmonary illnesses, TNF-α plays a crucial role in host defense against intracellular pathogens such as Mycobacterium tuberculosis [146, 147]. TNF-α is also connected to neuroinflammation and the etiology of neurological disorders, such as infections and neurodegenerative illnesses [148, 149]. The multipurpose cytokine TNF-α is essential for controlling inflammation, cell death, and cell division [150, 151]. TNF-α is necessary for the development and spread of cancer. Research has revealed a strong correlation between TNF-α and lymphatic metastasis in cervical cancer. TNF-α activates vascular endothelial growth factor (VEGFC) mediated ERK and AKT pathways, promoting carcinogenesis, lymphangiogenesis, and lymphatic metastasis [141]. TNF-α activation improves the mesenchymality of breast cancer stem cells (BCSCs) in triple-negative breast cancer (TNBC), boosting their capacity for invasion, self-renewal, proliferation, and inducing intra-tumoral stromal invasion [152, 153]. Additionally, TNF-α stimulates stromal cells to produce matrix metalloprotease (MMP)-2, vascular endothelial growth factor (VEGF)-A, and colony-stimulating factor (CSF)-1, which promotes colon cancer carcinogenesis [154, 155]. Another treatment strategy to reduce angiogenic responses in the TME and stop secondary organ metastasis might be to target TNF-α [156, 157]. Knowledge of how TNF-α affects cancer growth and creating individualized treatment plans requires an understanding of how it interacts with other elements in the TME [158, 159]. The utilization of conventional TNF-α antibodies, which counteract the activity of TNF-α, produces a moderate antitumor outcome. In a syngeneic mouse melanoma experiment, the bacteria induced the upregulation of TNF-α, resulting in a synergistic effect with the secreted immunotoxin and significantly inhibiting tumor growth [160, 161]. This particular form of therapy restructured the TME in a manner that supported the presence of numerous immune cells with antitumor properties, such as M1 macrophages, N1 neutrophils, and activated CD4 + and CD8 + lymphocytes [162, 163].
Combination of bacteriotherapy and immunotherapy
One therapeutic strategy for cancer called immunotherapy is predicated on strengthening the host immune system against malignancy [164, 165]. A variety of techniques are employed to block immune cells, such as immune checkpoint inhibitors (ICIs), adoptive cell treatments (such as CAR-T cells), monoclonal antibodies targeting tumor antigens, and the injection of cytokines [166, 167]. Specific techniques, such as the use of chemokine receptor inhibitors (CXCR4 antagonist AMD3100) and monoclonal antibodies (anti-CCR4 mAb, Mogamulizumab), are already being employed in clinical practice for hematological malignancies [168,169,170]. Immunotherapy modifies the expression of chemokine receptors in cancers, which controls the angiogenesis, proliferation, and recruitment of leukocytes into the tumor [171]. Targeting cytotoxic T lymphocyte-associated molecule-4 (CTLA-4), programmed cell death receptor-1 (PD-1), and programmed cell death ligand-1 (PD-L1), immune checkpoints are recognized as a significant and successful kind of immunotherapy [172]. Additionally, the primary goal of the immunotherapy strategy is to use Toll-like receptor (TLR) agonists, which are related to innate immune activation, to target the tumor's microenvironment [173, 174].
In anti-cancer bacteria-based immunotherapy techniques, the utilized bacteria may exist in a state of being alive or weakened and potentially even manifest as genetically modified variants [99, 175]. A cancer therapy mediated by a safe bacterium should possess characteristics like cytotoxicity towards cancer cells or immunogenicity, thereby minimizing harm to healthy cells, exhibiting a preference for cancer cells, and maintaining stability within the physiological conditions of the human body [176, 177]. Dr. William B. Coley (1936–1862), a bone sarcoma surgeon, pioneered the treatment of his patients with both live bacteria and a mixture of heat-killed bacteria known as " Coley’s toxins" [178, 179]. Following his remarkable discovery, many investigations have demonstrated remarkable outcomes when employing diverse bacterial strains to eliminate distinct types of tumors [180]. Despite the incredible outcomes achieved, the evolution of alternative therapeutic strategies, including radiation therapy and chemotherapy, led to the gradual obsolescence of Coley's toxins. Recent immunological research indicates that the overarching principles of Coley's toxins hold validity, as certain types of cancer exhibit an increased susceptibility to bolstering and optimizing the patient's immune system [181, 182]. Numerous bacterial species have demonstrated a fantastic capacity to penetrate and colonize solid tumors, a phenomenon that frequently results in the growth retardation of neoplasms and the removal of tumors [183, 184]. The genera Bifidobacterium, Clostridium, Lactococcus, Shigella, Vibrio, Listeria, Escherichia, and Salmonella were used in animal cancer models [185]. Obligate anaerobes such as Bifidobacterium longumo (Clostridium novyi non-lethal toxin strain) have been shown to kill tumors in mice after systemic administration in hypoxic necrotic areas, which in some cases causes tumor regression [186]. Although the growth of viable tumor tissue was impeded by the presence of high oxygen tension, the anti-cancer properties of an attenuated facultative anaerobic auxotrophic mutant of Salmonella enterica serovar Typhimurium arise from the biological interactions between the bacteria and the host tumor, both directly and through immune-mediated mechanisms [187, 188]. Bacteria-mediated tumor treatment (BMTT) has been used for a long time to manage cancer despite its adverse effects. When using BMTT, it's important to balance its therapeutic benefits against potential adverse effects, including infection [189,190,191]. The only bacterial agent the FDA has licensed to treat non-muscle invasive bladder cancer (NMIBC) since the late 1970s is Bacillus Calmette-Guerin (BCG), an attenuated strain of Mycobacterium bovis. For high-risk NMIBC, BCG has been the accepted standard of care and is the most successful therapy [192,193,194].
Bacteria as anti-cancer agents through amplification Human immunity interacts with the host as one of the pathogenic factors or natural flora strengthens the host's immune system in interaction with pathogenic bacteria [195]. The Salmonella typhimurium strain ΔppGpp impedes the signals that are sent, which initiates inflammatory pathways [196]. The amount of inflammatory cytokine IL-1β, TNF-α, and Il- 18 in tumors leads to severe tumor growth suppression; IL-18 plays an essential role in immunity against pathogens [184]. Anaerobic bacteria such as Escherichia coli (E. coli) can are involved in solid tumors, indirectly Clearance of some tumor cells through the CT26) infectious defense mechanism; when these bacteria invade their host, they trigger the initiation of defense. This mechanism leads to the production of host T lymphocytes involved in antitumor activity [136, 197]. T cells are the only agents responsible for tumor clearance [136, 184]. Recombinant E. coli K12-producing TNF-α, an anti-cancer cytokine that may directly kill cancer cells and trigger antitumor immunity, was created by Murphy et al. [198,199,200,201,202]. Research revealed that the genetically modified E. Coli K12 gathered within tumors in a specific manner and significantly decreased tumor loads [203]. In a different investigation, Lee et al. [199] assessed the capacity of recombinant Salmonella with an endostatin expression vector to target tumors and deliver genes. Angiogenesis inhibitor endostatin can prevent vascularization and limit tumor development [204]. The recombinant Salmonella was found to produce tumor regression, decrease tumor microvessel density, and colonize tumors preferentially [17, 205] (Table 2).
Bacteria released substances (toxins or enzymes or bacteriocin) in cancer therapy
Numerous compounds generated from bacteria can selectively target cancer cells and provide a cytotoxic impact [215]. Enzymes, peptides, specific secondary bacterial metabolites, and bacterial toxins are examples of cytotoxic agents [216]. In this part, we look at how chemicals secreted by bacteria are used to treat cancer and discuss the significance and uses of these compounds in this area (Fig. 2).
Colorectal cancer: In the bacterial treatment of colorectal cancer, the whole bacterial cell and its metabolites can be used, including probiotics associated with the bacteria, peptides such as bacteriocins, or bacterial toxins. The anti-cancer mechanism of this type of treatment includes: 1) Creating pores in the cell membrane, 2) induction of apoptosis, 3) TNF-α production, 4) inhibition of metastasis. Sometimes molecular sites lead to apoptosis of cancer cells through intrinsic or extrinsic pathways. C. perfringens enterotoxin (bacterial toxin) CPE can directly interact with claudin-3 and claudin-4, which are overexpressed in colorectal cancer cell membranes. Another mechanism of the anticancer effect of bacterial toxins is cytotoxicity through the intrinsic pathway of apoptosis. Bacteriocins create membranous adhesions when attached to the membrane and a specific type of cell surface that induces cell lysis and cell death
Bacterial toxins
Bacterial toxins are one of the compounds produced by bacteria and employed in cancer therapy [217]. Certain toxins that are created and released by bacteria have the potential to be cytotoxic or, in less extreme cases, to change apoptosis, differentiation, and proliferation [218]. Through various methods, including cell-cycle arrest and disruption of tumor cell signal pathways, some of them can successfully prevent tumor development [219]. Cytolysin CylA is one of the most well-known bacterial toxins used as an anti-cancer agent [219, 220]. Pore-forming substances called cytolysins cause multimeric holes in cell membranes and aid in the death of cells. Salmonella typhimurium, E. coli, and Staphylococcus aureus are the usual sources of cytolysins [221,222,223,224]. Mice given strains of S. typhimurium or E. coli that produce the CylA toxin showed suppression of tumor development [225, 226]. The diphtheria toxin (DT), a primary virulence factor generated by Corynebacterium diphtheria, is another well-known toxin. The toxin exhibits fatal effects on mammalian cells at low doses. Several cancer types have shown improvement in response to therapy with modified DT-based toxins [227, 228]. The two kinds of cytotoxin and enterotoxin found in Clostridium difficile toxin can destroy cancer cells by attracting proinflammatory molecules and inducing an immune response [229,230,231]. Botulinum neurotoxin A, which Clostridium botulinum produces, causes apoptosis in BC cell lines T47D and decreases cell growth and proliferation in prostate cancer lines PC-3 and LNCaP [232,233,234,235]. The enterotoxin generated by Clostridium perfringens possesses anti-cancer properties as well. It binds to the overexpressed claudin-4 receptor on pancreatic cancer cells, causing dose-dependent acute toxicity [236, 237]. Produced by pathogenic E. coli, verotoxin 1 (VT-1), also known as Shiga toxin-1 (Stx1), can halt the cell cycle in the colon cancer HCT116 cell line [99, 238]. Pseudomonas aeruginosa produces exotoxin A (PE), which inhibits protein synthesis by ADP ribosylation and causes cancer cells to die [239, 240]. Cytotoxic necrotizing factor (CNF), a toxin produced by E. coli, stimulates DNA replication and results in the creation of multinucleated cells as a secondary effect of suppressing cell differentiation and inducing death [241, 242].
Bacterial enzymes
Numerous types of enzymes are produced by bacteria. A few can affect the vital amino acids needed for tumor development [243,244,245]. By increasing the effectiveness and specificity of treatment, bacterial enzymes have demonstrated promise in cancer therapy [246]. L-asparaginase, an enzyme generated by Bacillus subtilis, Streptomyces, Erwinia species, or E. coli, is one of the bacterial enzymes that is often studied [247]. This enzyme is responsible for catalyzing the hydrolysis of asparagine, which lowers its blood content and kills tumor cells [248]. Treatments for acute lymphoblastic leukemia, neoplasia, lymphosarcoma, and other cancers have demonstrated the efficacy of L-asparaginase [249, 250]. Two more bacterial enzymes that are important for the catabolism of arginine are arginine decarboxylase and arginine deiminase [251, 252]. It was shown that arginine in tumor cells may be consumed by arginine deaminase derived from Streptococcus pyogenes, which inhibits the growth of arginine-deficient tumor glioblastoma multiforme [253, 254].
Bacteriocins
Bacteriocins are primarily recognized as protein molecules produced by certain bacteria that inhibit the development of other bacterial species or maybe eradicate them [255, 256]. Bacteriocins have the occasional ability to stop tumor cell development. Because of its amphiphilic properties and cationic charge, bacteriocin can interact with negatively charged cell membranes to disrupt their integrity and cause cancer cells to undergo apoptosis [257, 258]. As an example, nisin, a well-researched and often utilized bacteriocin generated by Lactococcus lactis, has shown antibacterial action against most Gram-negative bacteria as well as being cytotoxic to MCF-7 cells (a cell line used to treat human BC) [259]. In vitro research on colicins, a well-known family of antimicrobial peptides, has also shown anti-cancer potential [184]. Important bacteriocins with antitumor qualities that can be used in cancer therapy include fermenticin HV6b, S2 pyocin, pediocins, nisin A, colicins, and bevacinocin HC5 [260, 261]. These bacteriocins' diverse properties have been examined from various angles and are included in Table 3.
Biosurfactant
The surface-active components of various structures made by microbes are known as biosurfactants. According to recent reports, biosurfactants can function as anti-cancer agents by blocking some critical signaling pathways, which interfere with the processes that lead to cancer development [279,280,281]. Furthermore, biosurfactants can activate NK cells, prevent angiogenesis, and cause cancer cells to undergo apoptosis through death receptors [282]. Compared to synthetic analogs, microbial biosurfactants are thought to be less harmful and biodegradable [283]. Among them is Bacillus safensis surfactin, which demonstrated antitumoral solid activity against B16F10 murine melanoma cells and T47D BC cells [284]. Another instance is the cyclic lipopeptide viscosin, which was shown to have significant anti-cancer activity and was derived from Pseudomonas libanensis. According to the MTT results, viscosin prevented MDA-MB-231 from proliferating in BC cells. Additionally, viscosin stopped the PC-3 M prostate cancer cell line from migrating [285, 286].
Bacteria can be anti-cancer agents through biofilms
In the extracellular polymeric matrix, bacteria assemble into thick, spatially ordered networks called biofilms that cling to biological and non-biological surfaces. The process of biofilm development is regulated by the quorum sensing phenomenon, which further helps the bacteria to survive in the host cells and evade the host defense immunological system [287, 288]. Furthermore, the development of biofilms may contribute to the advancement of colorectal and colon cancer [289]. Because biofilm can transport treatments and stop the spread of metastatic tumors, it can be used as a possible anti-cancer drug [290, 291]. When Pseudomonas aeruginosa was being treated for cancer with hydroxyurea and doxorubicin, anti-cancer drugs stimulated and encouraged the production of biofilms [239, 292]. An inducible DNA damage repair pathway known as the SOS response is activated by the bacteria growing on the cancer cells to evade the drug onslaught [293]. As a result, several distinct bacterial phenotypes emerge that assault or enter the cancer cells. Additionally, the bacteria that coat cancer cells release DNA and various proteins that prevent the tumor from spreading [294, 295]. To create naturally occurring nanowires that may be used as drug carriers for cancer treatment, Kumeria and colleagues [296] synthesized biofilms of the zetaproteobacterium Mariprofundus ferrooxydans. When exposed to an alternating magnetic field, biofilm-derived nanowires were discovered to be magnetic nanomaterials that could produce both passive and active trigger responses. Reduced cell viability was a result of the alternating magnetic field-induced hyperthermia. The doxorubicin-loaded biofilm-derived nanowires were examined for their carrier potential and were shown to be cytotoxic to human BC cells (MDA-MB231-TXSA) [296]. T cells play a crucial role in the initiation of biofilm formation, regardless of the underlying causes and the role they play in protecting bacteria from the host's immune response [297]. During cancer treatment, anti-cancer drugs induce biofilm formation, which leads to metastasis formation of bacterial biofilms on cancer cells during the SOS response, leading to the disorder of metastasis [184]. Bacterial biofilm can affect the growth of colon cancer; its progression can regulate cell proliferation by altering cancer metabolism [298]. Macromolecules, such as DNA and proteins, are essential for biofilm formation. These molecules form a protective layer around cancer cells. For instance, adhesion is inhibited by the polysaccharides that Streptococcus agalactia releases. Endothelial cells are produced by cancer cells, and this is crucial for the spread of cancer [296, 299]. Protein nanowires derived from bacteria called Mariprofundus ferroxydans (As a novel multipurpose medication carrier for the treatment of cancer and hyperthermia, it may be used [296].
Future perspectives: bacteriobots technique for cancer therapy
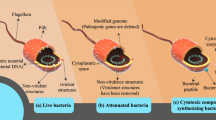
In certain publications, "bacteriobot" refers to a novel and inventive theranostic approach utilizing bacteria-based construction for tumor treatment [300, 301]. But, in a broader sense, it may be used for any bacteria that has undergone deliberate modification [302,303,304]. The effective delivery of drugs to tumor areas is one of the main obstacles in cancer research. Miniature gadgets that actively go towards the tumor, penetrate it, and accumulate there or in surrounding tissues might be an excellent solution to this issue [305, 306]. These devices can be entirely synthetic (chemically and, or physically actuated), comprising only materials, structures, and components that are manufactured by humans, or they can be biologically actuated (cellular microrobots), which are made entirely of human cells and have been carefully designed to have anti-cancer properties (Fig. 3) [307, 308]. Furthermore, hybrid versions that can be pushed by biological or artificial methods (typically biologically actuated) can be built, comprising both artificial and cell-made components [219]. Natural bacteria, especially human commensals, can be used as a vector to deliver a chemotherapeutic chemical directly into the tumor, which might greatly minimize the adverse effects of treatment often associated with conventional chemotherapy [309, 310]. Bacteria can enter the target actively—for example, by using their flagella—or passively—through blood flow. Bacterial flagella can swim up to 300 μm/s and revolve like propellers [311, 312].
Using magnetic fields to guide magnetic particles, irrespective of tumor location, from their application site to the malignancy seems like a promising approach. Bacteria in this situation need to be magnetic field sensitive. One intriguing example is magnetotactic bacteria, which can detect magnetic fields and align their swimming paths along them because they contain naturally occurring magnetic particles called magnetosomes [313,314,315,316]. Therefore, a Gram-negative coccus present in the Atlantic Ocean is Magnetococcus marinus (MC1). With two bundles of cilia organized at one pole, this microbe can move at a speed of 300 μm/s [317, 318]. This particular bacterium's magnetosomes are chains of magnetite (Fe3O4) particles encased in membranes that originate in the cytoplasm. The bacteria are oriented about the Earth's magnetic field by the presence of magnetite. It might be feasible to target bacteria carrying magnetosomes to the tumor by applying a strong magnetic field similar to that used in MRI (magnetic resonance imaging) procedures. A recent study found that, in comparison to nonguided MC1 bacteria, magnetic guidance significantly increased the tumor formation of MC1-based hybrid microrobots injected intraperitoneally in live mice [318,319,320]. Recently, it was possible to construct E. Coli to sense magnetic fields by forcing the bacterial cells to create iron-rich structures [321]. Enhanced tumor targeting is a noteworthy function that bacterial microrobots may assist with. Cancer cells and their microenvironments take on chemical and physical properties that set them apart from normal cells due to mutations and other genetic and epigenetic abnormalities [322,323,324].
The bacteria are shielded by the microbeads from opsonization and other potential physiological changes by the body [325]. Since these microbeads come into intimate contact with healthy organs, it is essential to use the right microbeads for creating practical bacterial robots that target tumors [326]. In the end, adding bacterial flagella to microbial granules would ease the agents' delivery to the target region and enable the movement of bacterial robots [327]. Salmonella typhimurium was used in one study to create a microbot that was based on bacteria. In this study, bacteria were encapsulated in biocompatible alginate granules and subsequently attached to microbeads as a motility section by S. typhimurium flagella. The tests showed that these robotic bacteria could successfully target the tumor [327]. Non-pathogenic E. Coli was used as living nanobots to cure cancer in another investigation by Al-Fandi and colleagues [328]. The nano-biosensor devices, spontaneously synthesized and attached to VEGF, were fitted to these living microrobots. The membranes of the tumor cells had an overexpression of the VEGF receptor, which the microbial nanobots were designed to recognize and adhere to [328]. Robotic bacteria are said to migrate to tumors at higher rates than healthy cells, possess better chemotactic motility, and target malignant tissue more effectively. Additionally, the latest breed of bacterial robots serves as micro-sensors and micro-stimuli. This implies they can be used as delivery systems to deliver medications and therapeutic nanoparticles (NPs) to tumors. Bacteriobots might be a novel therapeutic tool for identifying and combating solid tumors [329,330,331]. The perfect bacterial microrobot should resemble a type of "minicell"—a nanoscale, anucleated, nondividing, metabolically active cell that can translate and transcribe the desired gene. By simply modifying the surface of the minicell with particular antibodies directed against cancer cells that have receptors, minicells ought to encapsulate a broad spectrum of chemotherapeutic and molecular medicines, si/shRNA, antigens, and therapeutic toxins and transport them to cancer cells with precision [332].
Combination of virotherapy and immunotherapy
A range of viruses could be genetically modified to infect and lyse tumor cells as cloning technology advanced. Viral treatment has succeeded because of our growing understanding of viral mechanisms of action, which include regulating the TME and triggering both innate and adaptive antitumor immunity [333]. Currently being employed as OVs include several viruses, including vesicular stomatitis virus, coxsackievirus, adenovirus, measles virus, reovirus, and HSV [95, 334,335,336]. OVs elicit both anti-cancer and antiviral immunity. Tumor therapy benefits from antitumor immunity. Based on the idea that the antiviral immune response limits the growth and spread of OVs, host immunological responses have long been thought to be detrimental to the effectiveness of OVs [337,338,339]. However, it has recently been recognized that the antiviral immune response is advantageous in treating tumors for the first priming of antitumor immunity by OVs [340]. OVs increase innate immunity, turn "cold" tumors into "hot" tumors that impede tumor growth, draw immune cells, and activate systemic anti-cancer adaptive immunity [341, 342]. Reduced IFN activity in conjunction with elevated EGFR and downstream signaling pathways, including PI3K, Ras, and MAPK activation, may allow Ovs to evade the immune system and infect cancer cells while increasing and spreading to create offspring that may ultimately cause tumor cells to die [343,344,345]. A wide range of viruses thrive in the presence of cancer cells because these cells can evade the body's immune system to live. Because tumor cells do not undergo apoptosis and instead suppress interferon signaling, they have developed into suitable hosts for various viruses. Moreover, laminin, CAR, and CD155 overexpression increases the susceptibility of cancer cells to viral infection. Notably, many of the elements of the TME that give cancer stem cells (CSCs) resilience to conventional therapies are ineffective against OVs [346].
Newcastle disease virus (NDV) infects birds. It is non or less pathogenic to humans and is associated with Type I interferon signaling stimulation, and NDV/ HK84 strain can inhibit hepatocellular carcinoma (HCC) progression in cell lines and also in mice when injected intratumorally [347]. Some strains of reoviruses can replicate in cancer cells selectively and may induce tumor regression in subcutaneous tumor models in animals [348]. Additionally, parvovirus H1 showed partially successful results in metastatic pancreatic cancer in clinical trials [349]. Although it has been attempted to use viral strains that are not known to be associated with any human diseases, due to the potential toxicity and uncontrolled pathogenicity, researchers have turned to genetically modified viruses known as viral vectors [347]. Viral vectors can deliver therapeutic genes, in the case of cancers, tumor suppressors, and oncolytic genes, specifically to the tumor site [350]. Controlled and specified expression of genes and removal of virulent viral genes by genetic engineering techniques have made viral vectors attractive tools for targeting tumors [351, 352]. Several virus families that have been used as the viral vectors include retroviridae, adenoviridae, parvoviridae, herpesviridae, togaviridae, flaviviridae, rhabdoviridae, paramixoviridae, picornaviridae and poxviridae in preclinical and clinical trials [353].Viral vectors have many applications in infectious and non-infectious diseases, prevention, diagnosis, and treatment [354]. For example, one of the next-generation vaccine platforms of SARS-CoV-2 is viral vectors such as Oxford – AstraZeneca (based on chimpanzee adenovirus), Ad5-nCoV (based on adenovirus type 5) and Sputnik V (based on adenovirus types 5 and 26) which are expressed SARS-CoV-2 spike protein as the immunological dominant protein [21]. Human immunodeficiency virus (HIV)/AIDS, a chronic disease that spreads worldwide, is an excellent field of study about viral vectors, especially in preventive and therapeutic vaccines and cure strategies [353]. For example, designing a lentivector containing a mutant form of APOBEC3G and inhibiting infection spread in cell lines or targeting CNS latent reservoirs by a combination of Romidepsin as a latency reversal agent and Adeno-associated virus vector carrying thymidine kinase gene for inducing apoptosis in reactivated cells are just a few examples of the use of viral vectors in the field of HIV/AIDS [355]. As single agents, unarmed or armed OVs have shown excellent safety and potential therapeutic results in tumor therapy [356]. Monotherapies, on the other hand, are unlikely to entirely reverse T-cell function loss induced by tumor heterogeneity and an immunosuppressive microenvironment [334].
In several clinical studies, promising OVs genetically modified with other antitumor agents were able to eradicate tumors [357]. Armed OVs with ICIs plus adoptive T-cell therapy (ACT) have recently demonstrated exceptionally high efficacy by triggering several antitumor steps, such as promoting T-cell expansion and survival, boosting T-cell trafficking to tumors, improving APC function, and reversing T-cell exhaustion [334]. OVs designed to encode ICIs are potential therapeutics. However, the most prevalent approach to treating tumors with ICIs is to utilize ICI antibodies, such as the licensed medications ipilimumab (anti-CTLA-4), pembrolizumab (anti-PD-1), nivolumab (anti-PD-1), cemiplimab (anti-PD-1), avelumab (anti-PD-L1), and atezolizumab (anti-PD-L1) [358]. Despite the effectiveness of these ICIs, it is anticipated that only 12.5% of patients who undergo ICI treatment benefit [359]. The lack or low expression of PD-L1 on tumor cells is one of the most generally recognized explanations for initial resistance to ICI treatment [360]. OVs have been demonstrated to boost PD-L1 levels significantly, which is advantageous to ICI treatment [361]. Tumors become more receptive to ICI treatment when OVs armed with cytokines expand the altered form of the TME into a proinflammatory microenvironment [361]. Pembrolizumab treatment improved the 62% objective response rate with a 33% CR rate in patients with advanced melanoma who received IMLYGIC treatment. Patients also showed increased CD8 + T cells, elevated PD-L1 protein expression, and IFN-γ gene expression in several tumor cell subsets. In patients with advanced, incurable melanoma, similar outcomes were shown in a phase II trial assessing the safety and effectiveness of IMLYGIC with ipilimumab; combinatorial therapy produced a more significant objective response than ipilimumab alone [362, 363]. The findings show that combining ICIs and OVs can increase therapeutic outcomes in cancer patients who have become resistant to ICIs alone [364]. OVs, combined with CAR T-cell and TCR T-cell treatments, T-cell immunotherapies that have been genetically modified have lately demonstrated encouraging clinical results in treating hematologic malignancies [365].
Clinical trials
Numerous clinical trials have been undertaken to assess the effectiveness and safety of utilizing therapeutic bacteria and viruses in the treatment of cancer. These studies have explored different facets of using microbial agents for anticancer therapies, such as their efficacy in attacking and eradicating cancerous cells, their influence on tumor reduction, and their possible negative consequences on individuals undergoing treatment [366, 367]. A clinical trial in phase 1b investigated the impact of oncolytic virotherapy, Talimogene laherparepvec (T-VEC), in combination with pembrolizumab, an anti-PD-1 antibody, among individuals diagnosed with advanced melanoma. Patients who responded positively to the combined therapy showed a rise in CD8 + T cells, heightened levels of PD-L1 protein expression, and increased IFN-γ gene expression across various cell subsets within tumors following treatment with T-VEC. The response to combination therapy did not correlate with the initial levels of CD8 + T cell infiltration or the initial presence of an IFN-γ signature. Consequently, their research indicated that oncolytic virotherapy has the potential to enhance the effectiveness of anti-PD-1 therapy through modifications to the TME [368]. Prior research has shown that some patients exhibit resistance to PD-1 blockade as a result of a lack of CD8 + T cells in the tumor site [369, 370]. The phase 2 trial involving 692 participants in a similar environment demonstrated a satisfactory safety record; however, it did not achieve the primary progression-free survival (PFS) endpoint of 14.3 months (median; range = 10.3–22.1). In comparison, the PFS for the placebo and pembrolizumab group was 8.5 months (median; range = 5.7–13.5), with a hazard ratio of 0.86 (confidence interval = 0.71–1.04, p = 0.13) [371]. Research has demonstrated that JX-594, an engineered oncolytic poxvirus, when infused intravenously, spreads infection across tumors while sparing healthy tissues [372,373,374]. During phase I/II clinical trials, JX-594 demonstrated favorable tolerability following intravenous administration and did not elicit any dose-limiting toxicities, with the maximum tolerated dose not being achieved [372, 373, 375]. Nevertheless, the administration of JX-594 alongside sorafenib did not demonstrate a significant improvement in survival outcomes during a phase III clinical trial involving individuals with advanced HCC who had not received prior systemic treatment (NCT02562755). T3011 is a modified form of the HSV-1 that has been genetically engineered to encode IL-12 and an antibody targeting PD-1. The latest phase I clinical trial findings indicated that T3011 demonstrated favorable tolerability among patients diagnosed with advanced cutaneous or subcutaneous malignancies (NCT04370587) [376]. In a separate investigation, a new OVV containing a complete monoclonal antibody targeting TIGIT demonstrated enhanced effectiveness in combating tumors and elicited enduring tumor-specific immunological memory [377].
In recent decades, numerous research studies have been undertaken to investigate the utilization of various clostridium species as agents for targeting tumors. One of the most highly regarded options is a genetically engineered strain of Clostridium novyi-NT. The toxicity of this strain can be reduced by getting rid of a residential phage that carries the α-toxin. Thus far, phase I and II clinical studies including the attenuated C. novyi-NT strain have had favorable results [302, 378]. An example of complete tumor regression following intravenous injection of C. novyi-NT spores into a patient with advanced leiomyosarcoma was reported [379, 380]. Clinical trials investigating the potential therapeutic application of Salmonella enterica serovar Typhimurium (S. typhi) for the treatment of melanoma were initiated in 2002 and have progressed to phase I trials as of the present time. Furthermore, the VXM01 antitumor vaccine, derived from the weakened strain of S. typhi, has effectively completed phase I clinical trials [381,382,383]. A clinical study was carried out to assess the efficacy of live Bifidobacterium tetragenous bacteria tablets in managing functional constipation in cancer patients undergoing chemotherapy. The study revealed that administering live Bifidobacterium tetragenous bacteria tablets proved to be both efficacious and well-tolerated in managing functional constipation in cancer patients undergoing chemotherapy [384]. In a randomized controlled trial conducted in 2021, researchers sought to investigate the impact of synbiotics on bacterial translocation and consequent bacteremia in patients undergoing neoadjuvant chemotherapy for esophageal cancer. The researchers discovered that administering neoadjuvant chemotherapy to patients with esophageal cancer may lead to bacterial translocation and subsequent bacteremia. However, this adverse effect can be mitigated by using synbiotics [385]. In another research, a randomized controlled trial was conducted to assess the impact of inulin, a recognized prebiotic substance, on the advancement and evolution of colon cancer. The findings of the 28-week research study indicated that the consumption of inulin in the diet can effectively inhibit inflammatory processes that contribute to the progression of colon cancer [386].
Viruses and bacteria: a double-edged sword in cancer
Certain viruses and bacteria, alone or in conjunction with other cofactors, can cause cancer by disrupting critical cellular processes [387]. A growing body of research has demonstrated the tight relationship between the infection of various tumors and microbes such as bacteria and viruses [388, 389]. Every organ's carcinogenesis is also influenced differently by the microbiota, as the host's and the microbes' genotypes impact cancer susceptibility and promotion [390]. HPV, human T-cell leukemia virus type1 (HTLV-1), hepatitis B virus (HBV), hepatitis C virus (HCV), Epstein–Barr virus (EBV), and HHV-8 are among the DNA and RNA viruses that are known to be linked to cancer [77, 391, 392]. We can see that HTLV-1 is the most cancer-causing virus on the list, followed by HPV, HBV, and HCV. HHV-8 and EBV, for the most part, require a co-factor to exhibit their carcinogenic potential, but as global health issues, they have a far more significant impact due to their much higher prevalence. Their viral genomes are smaller (from a few Kb to around 200 Kb) and have less coding power. As a result, they rely on cellular proteins to complete their life cycles and promote viral particle production, affecting various cellular pathways such as DNA repair, proliferation, and apoptosis [393]. Consequently, these viruses induce oncogenesis via a multi-step process that involves tumor-initiating and, or later-stage, tumor-promoting and spreading, as well as apoptosis, the regulation of cell proliferation, and senescence [394, 395]. Although the process of tumor growth is not virus-specific, various viruses can alter different phases of the process [396, 397].
Up until now, only Helicobacter pylori (H. pylori) has been linked to carcinogenesis through epidemiological data; however, more and more bacteria have been linked to cancer in humans, and research on the human microbiome has revealed a variety of intricate interactions between prokaryotes and their hosts [398, 399]. While the specific molecular mechanisms by which these bacteria affect the cellular pathways that lead to transformation remain largely unknown, mounting data suggests that they may also inhibit p53 functions and impact DNA repair pathways, thereby increasing the accumulation of DNA damage and ultimately promoting cellular transformation as an antitumor progression [400,401,402]. Studies in animal models that demonstrate a decrease in tumor burden following antibiotic modification of gut microbiota have supported the idea that bacteria play a role in carcinogenesis [403]. According to this theory, in vivo tests using a variety of chemically generated or genetically deficient animal models show that the presence of germs inhibits the development of colonic tumors [404, 405]. More specifically, several patient studies have demonstrated correlations between non-Hodgkin's lymphoma (NHL) in HIV-positive individuals, Fusobacterium nucleatum (F. nucleatum) and CRC, Chlamydia trachomatis and cervical cancer, and mycoplasmas and prostate and CRC [77, 406]. Furthermore, although the bacterial protein responsible has not yet been identified, infections with several mycoplasmas (Mycoplasma fermentans, arginini, hominis, and arthritidis) inhibit p53 activity and collaborate with Ras to cause oncogenic transformation in vitro, firmly establishing them as top bacterial candidates with carcinogenic qualities [407]. Additionally, it has been demonstrated that p53 and p21 expression was decreased in gastric mucosal cells after a prolonged infection with M. penetrans in a model of chemically immunosuppressed mice, resulting in pathological alterations [408].
Limitations and potential drawbacks bacteria and viruses cancer therapy
Bacterial and viral therapies have demonstrated potential efficacy in cancer treatment; however, it is essential to acknowledge the existing limitations and potential disadvantages associated with these treatment modalities. One constraint involves safeguarding unaffected tissues while stimulating immune reactions [366]. Moreover, specific bacteria and viruses can potentially impede the mechanisms responsible for preserving genetic stability and cellular restoration, thereby diminishing the efficacy of therapeutic interventions [409,410,411]. Another problem encountered in managing cancer through the use of bacteria and viruses is their potential for toxicity [412, 413]. The dosage required to achieve a therapeutic outcome may result in toxicity and adverse effects, while lower dosages may impact the effectiveness of the treatment [412]. The equilibrium between the advantages and safety of the participants in the trial must be upheld. Suitable methodologies and strategies should be implemented to assess the immune response of the individual and the overall therapeutic efficacy [414]. Discrepancies in the tumor architecture between preclinical animal models and human subjects may influence the ability of bacteria to infiltrate and multiply within the tumor [409]. Therefore, it is crucial to optimize both the dosage and method of delivery carefully. Additionally, eliminating bacteria by the immune system before reaching the tumor location can lead to treatment failure [415]. Furthermore, bacterial mutations can potentially produce exacerbated infections and therapeutic loss. Recombinant DNA technology, however, has allayed chiefly the safety worries [412]. Influenza-like symptoms such as fevers and chills have been observed following the administration of OVs, whether locally or systemically, although they are typically mild [362, 416]. These responses are mitigated through the administration of acetaminophen before the commencement of the treatment [83]. Furthermore, specific therapeutic interventions involving the utilization of bacteria and viruses may result in adverse reactions such as elevated body temperature, emesis, and GI disturbances. These adverse reactions have the potential to impact the overall well-being of patients and may lead to the discontinuation or modification of their treatment regimen [184, 417].
Conclusion
Although bacteria and viruses are the causes of various cancers, they have recently played a significant role in the treatment and reduction of the side effects of cancer drugs. Many studies are conducted on the treatment of cancer using bacteria and viruses. Chemotherapy involves a lot of costs for patients. Therefore, using the derived compounds of microorganisms and viruses has attracted the attention of many people. Among the therapeutic methods of OVs, there has been significant progress in cancer treatment. Various are used in this field. Viral vectors show their effect in the treatment of malignancy that these vectors are immune modulators, influential factors on tumor suppressor genes and oncogenes Clinical trials of some of these treatment methods have been done. The anti-cancer potential of bacteria and viruses increases due to the anti-oncogene or immune antigen properties and the use of modified antitumor agents in combination with therapeutic processes. The role of bacteria in cancer treatment has grown a lot in the last few years, and the substances coming out of bacteria and secretions such as toxins, enzymes, bacteriocins, and biosurfactants have played a significant role in the treatment of cancer. The effective delivery of drugs to tumor areas is one of the main obstacles in cancer research. Miniature tools called "bacteriobot" that actively go to the tumor, penetrate it, and accumulate there or in the surrounding tissues, maybe a good solution to this problem. This type of treatment is promising, cost-effective, and without side effects. Promotion and Its development need more and more extensive studies.
Availability of data and materials
No datasets were generated or analysed during the current study.
References
Siegel RL, et al. Cancer statistics, 2023. Ca Cancer J Clin. 2023;73(1):17–48.
Baindara P, Mandal SMJB. Bacteria and bacterial anticancer agents as a promising alternative for cancer therapeutics. Biochimie. 2020;177:164–89.
Haroun R, Wood JN, Sikandar S. Mechanisms of cancer pain. Front Pain Res (Lausanne). 2023;3:1030899.
Baba AI, Câtoi C. Tumor cell morphology, in Comparative oncology. The Publishing House of the Romanian Academy; 2007. PMID: 20806453.
Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300.
Rizvi NA, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non–small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol. 2020;6(5):661–74.
Garon EB, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28.
Kantarjian H, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–47.
Paul S, Pal R, Kundu R. Antiproliferative activity of Phormidium valderianum and Phormidium tenue (Cyanobacteria) on human cervical cancer cells (HeLa) in vitro. Algal Biomass Utln. 2012;3(4):30–7.
Calvo GH, et al. Disaccharides obtained from carrageenans as potential antitumor agents. Sci Rep. 2019;9(1):6654.
Mondal M, et al. Recent advances of oncolytic virus in cancer therapy. Hum Vaccin Immunother. 2020;16(10):2389–402.
Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15(4):651–9.
Krutzke L, et al. Chorioallantoic membrane tumor model for evaluating oncolytic viruses. Hum Gene Ther. 2020;31(19–20):1100–13.
Magiorakos A-P, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–81.
Duong MT-Q, et al. Bacteria-cancer interactions: bacteria-based cancer therapy. Exp Mol Med. 2019;51(12):1–15.
Sarotra P, Medhi B. Use of bacteria in cancer therapy. Recent Results Cancer Res. 2016;209:111–21.
Carattoli A, et al. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Eurosurveillance. 2017;22(31):30589.
Helaine S, et al. Internalization of Salmonella by macrophages induces formation of nonreplicating persisters. Science. 2014;343(6167):204–8.
Barrett L, et al. Kaposi’s sarcoma-associated herpesvirus and extracellular vesicles. J Med Virol. 2021;93(6):3294–9.
Yoo S-M, Lee M-S. Kaposi’s Sarcoma-Associated Herpesvirus and Host Interaction by the Complement System. Pathogens. 2020;9(4):260.
Carabelli AM, et al. SARS-CoV-2 variant biology: immune escape, transmission and fitness. Nat Rev Microbiol. 2023;21(3):162–77.
Chang C, Worrell SG. Viruses and esophageal cancer. Dis Esophagus. 2020;33(12):doaa036.
Crosbie EJ, et al. Human papillomavirus and cervical cancer. Lancet. 2013;382(9895):889–99.
Tanaka TI, Alawi F. Human papillomavirus and oropharyngeal cancer. Dent Clin. 2018;62(1):111–20.
Berman TA, Schiller JT. Human papillomavirus in cervical cancer and oropharyngeal cancer: one cause, two diseases. Cancer. 2017;123(12):2219–29.
Bucchi D, et al. Human papillomavirus and gastrointestinal cancer: a review. World J Gastroenterol. 2016;22(33):7415.
Naseem M, et al. Outlooks on Epstein-Barr virus associated gastric cancer. Cancer Treat Rev. 2018;66:15–22.
Iizasa H, et al. Development of Epstein-Barr virus-associated gastric cancer: Infection, inflammation, and oncogenesis. World J Gastroenterol. 2022;28(44):6249.
Arias-Calvachi C, et al. Epstein-Barr virus association with breast cancer: evidence and perspectives. Biology. 2022;11(6):799.
Osorio JC, et al. Epstein-Barr virus infection in lung cancer: insights and perspectives. Pathogens. 2022;11(2):132.
Yang JF, You J. Merkel cell polyomavirus and associated Merkel cell carcinoma. Tumour Virus Res. 2022;13:200232.
Pietropaolo V, Prezioso C, Moens U. Merkel cell polyomavirus and Merkel cell carcinoma. Cancers. 2020;12(7):1774.
McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73:4–13.
Sausen DG, et al. Herpes Simplex virus, human papillomavirus, and cervical cancer: overview, relationship, and treatment implications. Cancers. 2023;15(14):3692.
Richardson A, et al. Breast cancer and cytomegalovirus. Clin Transl Oncol. 2020;22:585–602.
Haidar Ahmad S, El Baba R, Herbein G. Polyploid giant cancer cells, cytokines and cytomegalovirus in breast cancer progression. Cancer Cell Int. 2023;23(1):1–14.
Lv Y-L, et al. Cytomegalovirus infection is a risk factor in gastrointestinal cancer: a cross-sectional and meta-analysis study. Intervirology. 2020;63(1–6):10–6.
D’souza S, et al. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J Gastroenterol. 2020;26(38):5759.
Khatun M, Ray R, Ray RB. Hepatitis C virus associated hepatocellular carcinoma. Adv Cancer Res. 2021;149:103–42.
Ponzoni M, Ferreri AJ. Bacteria associated with marginal zone lymphomas. Best Pract Res Clin Haematol. 2017;30(1–2):32–40.
Travaglino A, et al. Prevalence of chlamydia psittaci, chlamydia pneumoniae, and chlamydia trachomatis determined by molecular testing in ocular adnexa lymphoma specimens: a systematic review and meta-analysis. Am J Clin Pathol. 2020;153(4):427–34.
Malik AA, et al. Can Mycobacterium tuberculosis infection lead to cancer? Call for a paradigm shift in understanding TB and cancer. Int J Med Microbiol. 2022;312(5):151558.
Alipour M. Molecular mechanism of Helicobacter pylori-induced gastric cancer. J Gastrointest Cancer. 2021;52:23–30.
Salvatori S, et al. Helicobacter pylori and Gastric Cancer: Pathogenetic Mechanisms. Int J Mol Sci. 2023;24(3):2895.
Uno Y. Prevention of gastric cancer by Helicobacter pylori eradication: a review from Japan. Cancer Med. 2019;8(8):3992–4000.
Shang F-M, Liu H-L. Fusobacterium nucleatum and colorectal cancer: a review. World J Gastrointestinal Oncol. 2018;10(3):71.
Hashemi Goradel N, et al. Fusobacterium nucleatum and colorectal cancer: a mechanistic overview. J Cell Physiol. 2019;234(3):2337–44.
Li R, Shen J, Xu Y. Fusobacterium nucleatum and colorectal cancer. Infect Drug Resist. 2022;15:1115–20.
Li S, et al. Tumorigenic bacteria in colorectal cancer: mechanisms and treatments. Cancer Biol Med. 2022;19(2):147.
Mármol I, et al. Colorectal carcinoma: a general overview and future perspectives in colorectal cancer. Int J Mol Sci. 2017;18(1):197.
Wassenaar TM. E. coli and colorectal cancer: a complex relationship that deserves a critical mindset. Crit Rev Microbiol. 2018;44(5):619–32.
Nouri R, et al. Escherichia coli and colorectal cancer: Unfolding the enigmatic relationship. Curr Pharm Biotechnol. 2022;23(10):1257–68.
Cheng Y, Ling Z, Li L. The intestinal microbiota and colorectal cancer. Front Immunol. 2020;11:615056.
Galdy S, Nastasi G. Unusual association of diseases/symptoms: Streptococcus bovis endocarditis and colon cancer: myth or reality? A case report and literature review. BMJ Case Rep. 2012;2012:bcr2012006961.
Koshiol J, et al. Salmonella enterica serovar Typhi and gallbladder cancer: a case–control study and meta-analysis. Cancer Med. 2016;5(11):3310–3235.
Khan AA, Bano Y. Salmonella enterica subsp. enterica host-pathogen interactions and their implications in gallbladder cancer. Microb Pathog. 2021;157:105011.
Di Domenico EG, et al. Biofilm producing Salmonella typhi: chronic colonization and development of gallbladder cancer. Int J Mol Sci. 2017;18(9):1887.
He Z, et al. Campylobacter jejuni promotes colorectal tumorigenesis through the action of cytolethal distending toxin. Gut. 2019;68(2):289–300.
Franchini APA, et al. The role of chlamydia trachomatis in the pathogenesis of cervical cancer. Cureus. 2022;14(1):e21331.
Paavonen J, et al. Chlamydia trachomatis, pelvic inflammatory disease, and epithelial ovarian cancer. J Infect Dis. 2021;224(Supplement_2):S121–7.
Lamont RJ, et al. Role of Porphyromonas gingivalis in oral and orodigestive squamous cell carcinoma. Periodontol 2000. 2022;89(1):154–65.
Irfan M, Delgado RZR, Frias-Lopez J. The oral microbiome and cancer. Front Immunol. 2020;11:591088.
Kerdreux M, et al. Porphyromonas gingivalis in colorectal cancer and its association to patient prognosis. J Cancer. 2023;14(9):1479.
Miller MB, Bassler BL. Quorum sensing in bacteria. Ann Rev Microbiol. 2001;55(1):165–99.
Bäumler AJ, Sperandio V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature. 2016;535(7610):85–93.
Delgado-Baquerizo M, et al. A global atlas of the dominant bacteria found in soil. Science. 2018;359(6373):320–5.
Thomaidou E, Ramot Y. Injection site reactions with the use of biological agents. Dermatol Ther. 2019;32(2):e12817.
Lacey L, et al. Insect pathogens as biological control agents: Back to the future. J Invertebr Pathol. 2015;132:1–41.
Ghalavand M, et al. Oncolytic bacterial and viral therapies as cancer prevention and treatment options: a comprehensive review. Iranian Red Crescent Med J. 2022:24(8):20220433156.
Divyashree M, et al. Bugs as drugs: neglected but a promising future therapeutic strategy in cancer. Future Oncol. 2022;18(13):1609–26.
Dhankhar R, et al. Microbial enzymes used in prodrug activation for cancer therapy: insights and future perspectives. Curr Protein Pept Sci. 2021;22(7):514–25.
Basch E, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197–8.
Duan R, Du W, Guo W. EZH2: a novel target for cancer treatment. J Hematol Oncol. 2020;13(1):1–12.
Yoo SM, Lee SY. Optical biosensors for the detection of pathogenic microorganisms. Trends Biotechnol. 2016;34(1):7–25.
Baptista PV, et al. Nano-strategies to fight multidrug resistant bacteria—“A Battle of the Titans.” Front Microbiol. 2018;9:1441.
Breijyeh Z, Jubeh B, Karaman R. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules. 2020;25(6):1340.
Zella D, Gallo RC. Viruses and bacteria associated with cancer: an overview. Viruses. 2021;13(6):1039.
Li L, et al. Delivery and biosafety of oncolytic virotherapy. Front Oncol. 2020;10:475.
Roy D, et al. Adjuvant oncolytic virotherapy for personalized anti-cancer vaccination. Nat Commun. 2021;12(1):2626.
Panagioti E, et al. Immunostimulatory bacterial antigen–armed oncolytic measles virotherapy significantly increases the potency of anti-PD1 checkpoint therapy. J Clin Invest. 2021;131(13):e141614.
Hemminki O, et al. Oncolytic viruses for cancer immunotherapy. J Hematol Oncol. 2020;13:1–15.
Hwang JK, Hong J, Yun CO. Oncolytic viruses and immune checkpoint inhibitors: preclinical developments to clinical trials. Int J Mol Sci. 2020;21(22):8627.
Lawler SE, et al. Oncolytic viruses in cancer treatment: a review. JAMA Oncol. 2017;3(6):841–9.
Koch MS, Lawler SE, Chiocca EA. HSV-1 oncolytic viruses from bench to bedside: an overview of current clinical trials. Cancers. 2020;12(12):3514.
De Matos AL, Franco LS, McFadden G. Oncolytic viruses and the immune system: the dynamic duo. Mol Ther Methods Clin Dev. 2020;17:349–58.
Schoot TS, et al. Immunosuppressive drugs and COVID-19: a review. Front Pharmacol. 2020;11:1333.
Howard F, Muthana M. Designer nanocarriers for navigating the systemic delivery of oncolytic viruses. Nanomedicine. 2020;15(1):93–110.
Sadri M, et al. Hypoxia effects on oncolytic virotherapy in Cancer: Friend or Foe? Int Immunopharmacol. 2023;122:110470.
Schirrmacher V. Cancer vaccines and oncolytic viruses exert profoundly lower side effects in cancer patients than other systemic therapies: a comparative analysis. Biomedicines. 2020;8(3):61.
Zhang B, Wang X, Cheng P. Remodeling of tumor immune microenvironment by oncolytic viruses. Front Oncol. 2021;10:561372.
Bayan C-AY, et al. The role of oncolytic viruses in the treatment of melanoma. Curr Oncol Rep. 2018;20:1–14.
Trager MH, Geskin LJ, Saenger YM. Oncolytic viruses for the treatment of metastatic melanoma. Curr Treat Options Oncol. 2020;21:1–16.
Heidary F, Gharebaghi R. Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen. J Antibiot. 2020;73(9):593–602.
Zhang Q, Liu F. Advances and potential pitfalls of oncolytic viruses expressing immunomodulatory transgene therapy for malignant gliomas. Cell Death Dis. 2020;11(6):485.
Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discovery. 2015;14(9):642–62.
Thangavelu S, Saikishore R, Palanivel V, Ranjithkumar D. Virotherapy. In: Viral Infections and Antiviral Therapies. Academic Press; 2023. p. 143–68. eBook.
Wu Y-Y, et al. Oncolytic viruses-modulated immunogenic cell death, apoptosis and autophagy linking to virotherapy and cancer immune response. Front Cell Infect Microbiol. 2023;13:1142172.
Lundstrom K. Therapeutic Applications for Oncolytic Self-Replicating RNA Viruses. Int J Mol Sci. 2022;23(24):15622.
Rommasi F. Bacterial-based methods for cancer treatment: What we know and where we are. Oncol Ther. 2022;10(1):23–54.
LeBlanc JG, et al. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb Cell Fact. 2017;16(1):1–10.
Stefka AT, et al. Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci. 2014;111(36):13145–50.
Blanco P, et al. Bacterial multidrug efflux pumps: much more than antibiotic resistance determinants. Microorganisms. 2016;4(1):14.
Kriventseva EV, et al. OrthoDB v10: sampling the diversity of animal, plant, fungal, protist, bacterial and viral genomes for evolutionary and functional annotations of orthologs. Nucleic Acids Res. 2019;47(D1):D807–11.
Roberts NJ, et al. Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci Transl Med. 2014;6(249):249ra111–249ra111.
Soltani S, et al. Bacteriocins as a new generation of antimicrobials: toxicity aspects and regulations. FEMS Microbiol Rev. 2021;45(1):fuaa039.
Heilbronner S, et al. The microbiome-shaping roles of bacteriocins. Nat Rev Microbiol. 2021;19(11):726–39.
Darbandi A, et al. Bacteriocins: Properties and potential use as antimicrobials. J Clin Lab Anal. 2022;36(1):e24093.
Simons A, Alhanout K, Duval RE. Bacteriocins, antimicrobial peptides from bacterial origin: Overview of their biology and their impact against multidrug-resistant bacteria. Microorganisms. 2020;8(5):639.
Maldonado Galdeano C, et al. Beneficial effects of probiotic consumption on the immune system. Ann Nutr Metab. 2019;74(2):115–24.
Dowarah R, et al. Selection and characterization of probiotic lactic acid bacteria and its impact on growth, nutrient digestibility, health and antioxidant status in weaned piglets. PLoS One. 2018;13(3):e0192978.
Zheng S, et al. Implication of surface properties, bacterial motility, and hydrodynamic conditions on bacterial surface sensing and their initial adhesion. Front Bioeng Biotechnol. 2021;9:643722.
Wang Y, et al. Antioxidant properties of probiotic bacteria. Nutrients. 2017;9(5):521.
Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14(8):e1002533.
Shaw DR, et al. Extracellular electron transfer-dependent anaerobic oxidation of ammonium by anammox bacteria. Nat Commun. 2020;11(1):2058.
Muhlebach MS, et al. Anaerobic bacteria cultured from cystic fibrosis airways correlate to milder disease: a multisite study. Eur Respir J. 2018;52(1):1800242.
Summers ZM, et al. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science. 2010;330(6009):1413–5.
Kelly D, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-γ and RelA. Nat Immunol. 2004;5(1):104–12.
Wang J, et al. Self-healing concrete by use of microencapsulated bacterial spores. Cem Concr Res. 2014;56:139–52.
Mathot AG, Postollec F, Leguerinel I. Bacterial spores in spices and dried herbs: the risks for processed food. Compr Rev Food Sci Food Saf. 2021;20(1):840–62.
Howell LM, Forbes NS. Bacteria-based immune therapies for cancer treatment. In: Seminars in Cancer Biology (Vol. 86). Academic Press Ltd- Elsevier Science Ltd; 2022. p. 1163–78.
Mpekris F, et al. Combining microenvironment normalization strategies to improve cancer immunotherapy. Proc Natl Acad Sci. 2020;117(7):3728–37.
Griffin ME, et al. Enterococcus peptidoglycan remodeling promotes checkpoint inhibitor cancer immunotherapy. Science. 2021;373(6558):1040–6.
Kruger S, et al. Advances in cancer immunotherapy 2019–latest trends. J Exp Clin Cancer Res. 2019;38(1):1–11.
Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32(19–20):1267–84.
Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol. 2015;15(6):335–49.
Turtle CJ, et al. CD19 CAR–T cells of defined CD4+: CD8+ composition in adult B cell ALL patients. J Clin Investig. 2016;126(6):2123–38.
McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol. 2019;37:457–95.
Kumar AR, et al. Harnessing the immune system against cancer: current immunotherapy approaches and therapeutic targets. Mol Biol Rep. 2021;48(12):8075–95. https://doi.org/10.1007/s11033-021-06752-9.
Wang S, et al. Antigen presentation and tumor immunogenicity in cancer immunotherapy response prediction. Elife. 2019;8:e49020.
Sato H, Okonogi N, Nakano T. Nakano, Rationale of combination of anti-PD-1/PD-L1 antibody therapy and radiotherapy for cancer treatment. Int J Clin Oncol. 2020;25:801–9.
Sedighi M, et al. Therapeutic bacteria to combat cancer; current advances, challenges, and opportunities. Cancer Med. 2019;8(6):3167–81.
Cabeza-Cabrerizo M, et al. Dendritic cells revisited. Annu Rev Immunol. 2021;39:131–66.
Reuter URM, Oettmeier R, Hobohm UJTO. Safety of therapeutic fever induction in cancer patients using approved PAMP drugs. Transl Oncol. 2018;11(2):330–7.
Zheng JH, et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci Transl Med. 2017;9(376):eaak9537.
Cluxton CD, et al. Suppression of Natural Killer cell NKG2D and CD226 anti-tumour cascades by platelet cloaked cancer cells: Implications for the metastatic cascade. PLoS One. 2019;14(3):e0211538.
Stern C, et al. Induction of CD 4+ and CD 8+ anti-tumor effector T cell responses by bacteria mediated tumor therapy. Int J Cancer. 2015;137(8):2019–28.
Raskov H, et al. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br J Cancer. 2021;124(2):359–67.
Dudek M, et al. Auto-aggressive CXCR6+ CD8 T cells cause liver immune pathology in NASH. Nature. 2021;592(7854):444–9.
Davar D, et al. Abstract LB062: Efficacy of Responder-derived Fecal Microbiota Transplant (R-FMT) and Pembrolizumab in Anti-PD-1 Refractory Patients with Advanced Melanoma. Cancer Res. 2021;81(13_Supplement):LB062–LB062.
Wargo JA. Modulating gut microbes. Science. 2020;369(6509):1302–3.
Chen X, et al. Tumor Necrosis Factor-α Promotes the Tumorigenesis, Lymphangiogenesis, and Lymphatic Metastasis in Cervical Cancer via Activating VEGFC-Mediated AKT and ERK Pathways. Mediators Inflamm. 2023;2023:5679966.
Lai W-Y, et al. A novel TNF-α-targeting aptamer for TNF-α-mediated acute lung injury and acute liver failure. Theranostics. 2019;9(6):1741.
Elliott MR, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461(7261):282–6.
Nieto-Vazquez I, et al. Insulin resistance associated to obesity: the link TNF-alpha. Arch Physiol Biochem. 2008;114(3):183–94.
Wilson A, et al. Single base polymorphism in the human tumour necrosis factor alpha (TNFα) gene detectable by Ncol restriction of PCR product. Hum Mol Genet. 1992;1(5):353–353.
Arnett HA, et al. TNFα promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4(11):1116–22.
Rui L, et al. Insulin/IGF-1 and TNF-α stimulate phosphorylation of IRS-1 at inhibitory Ser 307 via distinct pathways. J Clin Investig. 2001;107(2):181–9.
Feldmann M, Maini RN. Anti-TNFα therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol. 2001;19(1):163–96.
Jovanovic DV, et al. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-β and TNF-α, by human macrophages. J Immunol. 1998;160(7):3513–21.
Beg AA, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274(5288):782–4.
Dalvi PS, et al. High fat induces acute and chronic inflammation in the hypothalamus: effect of high-fat diet, palmitate and TNF-α on appetite-regulating NPY neurons. Int J Obes. 2017;41(1):149–58.
Johnson ZI, et al. Disc in flames: roles of TNF-α and IL-1β in intervertebral disc degeneration. Eur Cell Mater. 2015;30:104.
Narasimhan H, et al. Tumor Necrosis Factor-α (TNFα) stimulates triple-negative breast cancer stem cells to promote intratumoral invasion and neovasculogenesis in the liver of a xenograft model. Biology. 2022;11(10):1481.
Ben-Baruch A. Tumor necrosis factor α: Taking a personalized road in cancer therapy. Front Immunol. 2022;13:903679.
Jang D-I, et al. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int J Mol Sci. 2021;22(5):2719.
Morse MA, et al. The role of angiogenesis in hepatocellular carcinoma. Clin Cancer Res. 2019;25(3):912–20.
Zhao J, et al. LncRNA PVT1 promotes angiogenesis via activating the STAT3/VEGFA axis in gastric cancer. Oncogene. 2018;37(30):4094–109.
Marahleh A, et al. TNF-α directly enhances osteocyte RANKL expression and promotes osteoclast formation. Front Immunol. 2019;10:2925.
Liu Y, et al. Mesenchymal stem cell–based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat Med. 2011;17(12):1594–601.
Yoshimatsu Y, et al. TNF-α enhances TGF-β-induced endothelial-to-mesenchymal transition via TGF-β signal augmentation. Cancer Sci. 2020;111(7):2385–99.
Vos ACW, et al. Anti–tumor necrosis factor-α antibodies induce regulatory macrophages in an Fc region-dependent manner. Gastroenterology. 2011;140(1):221–230. e3.
Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Can Res. 2019;79(18):4557–66.
Zhou F, et al. Tumor microenvironment-activatable prodrug vesicles for nanoenabled cancer chemoimmunotherapy combining immunogenic cell death induction and CD47 blockade. Adv Mater. 2019;31(14):1805888.
Miao YR, et al. ImmuCellAI: a unique method for comprehensive T-cell subsets abundance prediction and its application in cancer immunotherapy. Advanced science. 2020;7(7):1902880.
Routy B, et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–7.
Sullivan RJ, Weber JS. Immune-related toxicities of checkpoint inhibitors: mechanisms and mitigation strategies. Nat Rev Drug Discovery. 2022;21(7):495–508.
Zhou Z, Li M. Evaluation of BRCA1 and BRCA2 as indicators of response to immune checkpoint inhibitors. JAMA Netw Open. 2021;4(5):e217728–e217728.
Wittamer V, et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198(7):977–85.
Micallef IN, et al. Plerixafor plus granulocyte colony-stimulating factor for patients with non-Hodgkin lymphoma and multiple myeloma: long-term follow-up report. Biol Blood Marrow Transplant. 2018;24(6):1187–95.
Perera LP, et al. Chimeric antigen receptor modified T cells that target chemokine receptor CCR4 as a therapeutic modality for T-cell malignancies. Am J Hematol. 2017;92(9):892–901.
Martin JD, et al. Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges. Nat Rev Clin Oncol. 2020;17(4):251–66.
Papalexi E, et al. Characterizing the molecular regulation of inhibitory immune checkpoints with multimodal single-cell screens. Nat Genet. 2021;53(3):322–31.
Gao W, et al. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct Target Ther. 2022;7(1):196.
Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity. 2020;52(1):17–35.
Ding Z, Zhou M, Zeng C. Recent advances in isatin hybrids as potential anticancer agents. Arch Pharm. 2020;353(3):1900367.
Gooding AJ, Schiemann WP. Epithelial–mesenchymal transition programs and cancer stem cell phenotypes: mediators of breast cancer therapy resistance. Mol Cancer Res. 2020;18(9):1257–70.
Jefremovas EM, et al. Nanoflowers versus magnetosomes: comparison between two promising candidates for magnetic hyperthermia therapy. IEEE Access. 2021;9:99552–61.
Bläsius F, et al. Surgical treatment of bone sarcoma. Cancers. 2022;14(11):2694.
Nauts HC, Swift WE, Coley BL. The treatment of malignant tumors by bacterial toxins as developed by the late William B. Coley, MD, reviewed in the light of modern research. Cancer Res. 1946;6(4):205–16.
Ebrahimzadeh S, et al. Colorectal cancer treatment using bacteria: focus on molecular mechanisms. BMC Microbiol. 2021;21(1):1–12.
Wiemann B, Starnes CO. Coley’s toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther. 1994;64(3):529–64.
McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J. 2006;26:154.
Sohlenkamp C, Geiger O. Bacterial membrane lipids: diversity in structures and pathways. FEMS Microbiol Rev. 2016;40(1):133–59.
Song S, Vuai MS, Zhong M. The role of bacteria in cancer therapy–enemies in the past, but allies at present. Infect Agent Cancer. 2018;13(1):1–7.
Cho S-Y, et al. An integrative approach to precision cancer medicine using patient-derived xenografts. Mol Cells. 2016;39(2):77.
Wishart DS. Emerging applications of metabolomics in drug discovery and precision medicine. Nat Rev Drug Discov. 2016;15(7):473–84.
Kramer MG, et al. Bacterial therapy of cancer: promises, limitations, and insights for future directions. Front Microbiol. 2018;9:16.
Yaghoubi A, et al. Bacteriotherapy in breast cancer. Int J Mol Sci. 2019;20(23):5880.
Antonelli AC, et al. Bacterial immunotherapy for cancer induces CD4-dependent tumor-specific immunity through tumor-intrinsic interferon-γ signaling. Proc Natl Acad Sci. 2020;117(31):18627–37.
Xu H, et al. IFN-γ enhances the antitumor activity of attenuated salmonella-mediated cancer immunotherapy by increasing M1 macrophage and CD4 and CD8 T cell counts and decreasing neutrophil counts. Front Bioeng Biotechnol. 2022;10:996055.
Liu Y, et al. Bacterial-mediated tumor therapy: old treatment in a new context. Advanced Sci. 2023;10(12):2205641.
Angelidou A, et al. BCG as a case study for precision vaccine development: lessons from vaccine heterogeneity, trained immunity, and immune ontogeny. Front Microbiol. 2020;11:332.
Xu J, et al. Can a reresection be avoided after initial en bloc resection for high-risk nonmuscle invasive bladder cancer? A systematic review and meta-analysis. Front Surg. 2022;9:849929.
Li Z, et al. Preliminary results from a phase II study of tislelizumab combined with radiotherapy as bladder-preserving treatment for patients with high-risk non-muscle-invasive bladder cancer (HR NMIBC) unresponsive to bacillus Calmette-Guerin (BCG). J Clin Oncol. 2023;41(6_suppl):510. https://doi.org/10.1200/JCO.2023.41.6_suppl.510.
Kucerova P, Cervinkova M. Spontaneous regression of tumour and the role of microbial infection–possibilities for cancer treatment. Anticancer Drugs. 2016;27(4):269.
Kröger C, et al. The transcriptional landscape and small RNAs of Salmonella enterica serovar Typhimurium. Proc Natl Acad Sci. 2012;109(20):E1277–86.
Phan TX, et al. Activation of inflammasome by attenuated Salmonella typhimurium in bacteria-mediated cancer therapy. Microbiol Immunol. 2015;59(11):664–75.
Murphy C, et al. Intratumoural production of TNFα by bacteria mediates cancer therapy. PLoS One. 2017;12(6):e0180034.
Lee CH, Wu CL, Shiau AL. Endostatin gene therapy delivered by Salmonella choleraesuis in murine tumor models. J Gene Med. 2004;6(12):1382–93.
Kim SH, et al. High efficacy of a Listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Can Res. 2009;69(14):5860–6.
Shinnoh M, et al. Clostridium butyricum MIYAIRI 588 shows antitumor effects by enhancing the release of TRAIL from neutrophils through MMP-8. Int J Oncol. 2013;42(3):903–11.
Josephs SF, et al. Unleashing endogenous TNF-alpha as a cancer immunotherapeutic. J Transl Med. 2018;16:1–8.
Castro-López DA, et al. A molecular dynamic model of tryptophan Overproduction in Escherichia coli. Fermentation. 2022;8(10):560.
O’Reilly MS, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88(2):277–85.
Zhang L, et al. Highly prevalent multidrug-resistant Salmonella from chicken and pork meat at retail markets in Guangdong. Front Microbiol. 2018;9:2104.
Parisa A, et al. Anti-cancer effects of Bifidobacterium species in colon cancer cells and a mouse model of carcinogenesis. PLoS One. 2020;15(5):e0232930.
Hoffman RM, Yano S. Salmonella typhimurium A1-R and cell-cycle decoy therapy of cancer. Methods Mol Biol. 2016;1409:165–75.
Olino K, et al. Tumor-associated antigen expressing Listeria monocytogenes induces effective primary and memory T-cell responses against hepatic colorectal cancer metastases. Ann Surg Oncol. 2012;19:597–607.
Cheng W, et al. Anti-tumor role of Bacillus subtilis fmbJ-derived fengycin on human colon cancer HT29 cell line. Neoplasma. 2016;63(2):215–22.
Yao Q, et al. Turn a diarrhoea toxin into a receptor-mediated therapy for a plethora of CLDN-4-overexpressing cancers. Biochem Biophys Res Commun. 2010;398(3):413–9.
Janku F, et al. Intratumoral injection of Clostridium novyi-NT spores in patients with treatment-refractory advanced solid tumors. Clin Cancer Res. 2021;27(1):96–106.
Nemunaitis J, et al. Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients. Cancer Gene Ther. 2003;10(10):737–44.
Le DT, et al. Safety and survival with GVAX pancreas prime and Listeria monocytogenes–expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;33(12):1325.
Cao W, et al. Clostridium butyricum potentially improves inflammation and immunity through alteration of the microbiota and metabolism of gastric cancer patients after gastrectomy. Front Immunol. 2022;13:1076245.
Ahmad F, Zhu D, Sun J. Bacterial chemotaxis: a way forward to aromatic compounds biodegradation. Environ Sci Eur. 2020;32:1–18.
Bracci L, et al. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21(1):15–25.
Minasyan H. Sepsis: mechanisms of bacterial injury to the patient. Scand J Trauma Resusc Emerg Med. 2019;27(1):1–22.
Jing W, et al. Activation mechanisms of inflammasomes by bacterial toxins. Cell Microbiol. 2021;23(4):e13309.
Ikryannikova LN, et al. Bacterial Therapy of Cancer: A Way to the Dustbin of History or to the Medicine of the Future? Int J Mol Sci. 2023;24(11):9726.
Pang X, et al. Sono-immunotherapeutic Nanocapturer to combat multidrug-resistant bacterial infections. Adv Mater. 2019;31(35):1902530.
Campos FV, et al. Fish cytolysins in all their complexity. Toxins. 2021;13(12):877.
Shewell LK, et al. All major cholesterol-dependent cytolysins use glycans as cellular receptors. Science Adv. 2020;6(21):eaaz4926.
McClelland M, et al. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature. 2001;413(6858):852–6.
Zhao B, et al. A review on metallic porous materials: pore formation, mechanical properties, and their applications. Int J Adv Manuf Technol. 2018;95:2641–59.
Liu X, et al. Radiotherapy combined with an engineered Salmonella typhimurium inhibits tumor growth in a mouse model of colon cancer. Exp Anim. 2016;65(4):413–8.
Jiang S-N, et al. Inhibition of tumor growth and metastasis by a combination of Escherichia coli–mediated cytolytic therapy and radiotherapy. Mol Ther. 2010;18(3):635–42.
Shafiee F, Aucoin MG, Jahanian-Najafabadi A. Targeted diphtheria toxin-based therapy: a review article. Front Microbiol. 2019;10:2340.
Golub JS, et al. Hair cell replacement in adult mouse utricles after targeted ablation of hair cells with diphtheria toxin. J Neurosci. 2012;32(43):15093–105.
Kelly CR, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109(7):1065.
Smits WK, et al. Clostridium difficile infection. Nat Rev Dis Primers. 2016;2(1):1–20.
Wei X, et al. Recent Advances in Bacteria-Based Cancer Treatment. Cancers. 2022;14(19):4945.
Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Yi KH, et al. Anatomical guide for botulinum neurotoxin injection: Application to cosmetic shoulder contouring, pain syndromes, and cervical dystonia. Clin Anat. 2021;34(6):822–8.
Karpiński TM, Adamczak A. Anticancer activity of bacterial proteins and peptides. Pharmaceutics. 2018;10(2):54.
Bandala C, et al. Effect of botulinum toxin A on proliferation and apoptosis in the T47D breast cancer cell line. Asian Pac J Cancer Prev. 2013;14(2):891–4.
Rood JI, et al. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe. 2018;53:5–10.
Lin ER, et al. Optimization of solid-state fermentation conditions of Bacillus licheniformis and its effects on Clostridium perfringens-induced necrotic enteritis in broilers. Revista Brasileira de Zootecnia. 2019;48:e20170298.
Debernardi J, et al. Verotoxin-1-Induced ER Stress Triggers Apoptotic or Survival Pathways in Burkitt Lymphoma Cells. Toxins. 2020;12(5):316.
Valentini M, Filloux A. Biofilms and cyclic di-GMP (c-di-GMP) signaling: lessons from Pseudomonas aeruginosa and other bacteria. J Biol Chem. 2016;291(24):12547–55.
DeLeon S, et al. Synergistic interactions of Pseudomonas aeruginosa and Staphylococcus aureus in an in vitro wound model. Infect Immun. 2014;82(11):4718–28.
Park SY, et al. Preparation of transparent and thick CNF/epoxy composites by controlling the properties of cellulose nanofibrils. Nanomaterials. 2020;10(4):625.
Sharma PC, Sharma D, Sharma A, Bhagat M, Ola M, Thakur VK, et al. Recent advances in microbial toxin-related strategies to combat cancer. In: Seminars in cancer biology (Vol. 86). Academic Press Ltd- Elsevier Science Ltd; 2022. p. 753–68.
Koskella B, Brockhurst MA. Bacteria–phage coevolution as a driver of ecological and evolutionary processes in microbial communities. FEMS Microbiol Rev. 2014;38(5):916–31.
Ramanan R, et al. Algae–bacteria interactions: evolution, ecology and emerging applications. Biotechnol Adv. 2016;34(1):14–29.
Arpaia N, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–5.
Egorov A, Ulyashova M, Rubtsova MY. Bacterial enzymes and antibiotic resistance. Acta Naturae. 2018;10(4 (39)):33–48.
Laliani G, et al. Bacteria and cancer: Different sides of the same coin. Life Sci. 2020;246:117398.
Nguyen HA, et al. A novel l-asparaginase with low l-glutaminase coactivity is highly efficacious against both T-and B-cell acute lymphoblastic leukemias in vivo. Can Res. 2018;78(6):1549–60.
Lubkowski J, et al. Mechanism of Catalysis by l-Asparaginase. Biochemistry. 2020;59(20):1927–45.
Sankaran H, et al. A comparison of asparaginase activity in generic formulations of E. coli derived L-asparaginase: In-vitro study and retrospective analysis of asparaginase monitoring in pediatric patients with leukemia. Br J Clin Pharmacol. 2020;86(6):1081–8.
Ramin KI, Allison SD. Bacterial tradeoffs in growth rate and extracellular enzymes. Front Microbiol. 2019;10:2956.
Zam W. Arginine enzymatic deprivation and diet restriction for cancer treatment. Brazilian J Pharm Sci. 2017;53:e00200.
Szlosarek PW, et al. Arginine deprivation with pegylated arginine deiminase in patients with argininosuccinate synthetase 1–deficient malignant pleural mesothelioma: a randomized clinical trial. JAMA Oncol. 2017;3(1):58–66.
Fiedler T, et al. Arginine deprivation by arginine deiminase of Streptococcus pyogenes controls primary glioblastoma growth in vitro and in vivo. Cancer Biol Ther. 2015;16(7):1047–55.
Alvarez-Sieiro P, et al. Bacteriocins of lactic acid bacteria: extending the family. Appl Microbiol Biotechnol. 2016;100:2939–51.
Hernández-González JC, et al. Bacteriocins from lactic acid bacteria. A powerful alternative as antimicrobials, probiotics, and immunomodulators in veterinary medicine. Animals. 2021;11(4):979.
O’Connor PM, et al. Antimicrobials for food and feed; a bacteriocin perspective. Curr Opin Biotechnol. 2020;61:160–7.
Kunda NK. Antimicrobial peptides as novel therapeutics for non-small cell lung cancer. Drug Discovery Today. 2020;25(1):238–47.
Kaur S, Kaur S. Bacteriocins as potential anticancer agents. Front Pharmacol. 2015;6:272.
Yang S-C, et al. Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Front Microbiol. 2014;5:241.
da Costa RJ, et al. Preservation of meat products with bacteriocins produced by lactic acid bacteria isolated from meat. J Food Qual. 2019;2019:1–12.
Paiva AD, et al. Toxicity of bovicin HC5 against mammalian cell lines and the role of cholesterol in bacteriocin activity. Microbiology. 2012;158(11):2851–8.
Paiva AD, et al. Interaction with lipid II induces conformational changes in bovicin HC5 structure. Antimicrob Agents Chemother. 2012;56(9):4586–93.
Soleimanpour S, et al. Bacteriotherapy in gastrointestinal cancer. Life Sci. 2020;254:117754.
Šmarda J, et al. The cytotoxic and cytocidal effect of colicin E3 on mammalian tissue cells. Folia Microbiol. 1978;23:272–7.
Gilbert R. Pore-forming toxins. Cell Mol Life Sci. 2002;59:832–44.
Goudarzi F, et al. In vitro characterization and evaluation of the cytotoxicity effects of nisin and nisin-loaded PLA-PEG-PLA nanoparticles on gastrointestinal (AGS and KYSE-30), hepatic (HepG2) and blood (K562) cancer cell lines. AAPS PharmSciTech. 2018;19:1554–66.
Zainodini N, et al. Nisin induces cytotoxicity and apoptosis in human asterocytoma cell line (SW1088). Asian Pac J Cancer Prev. 2018;19(8):2217.
Norouzi Z, et al. Nisin, a potent bacteriocin and anti-bacterial peptide, attenuates expression of metastatic genes in colorectal cancer cell lines. Microb Pathog. 2018;123:183–9.
Hosseini SS, Hajikhani B, Faghihloo E, Goudarzi H. Increased expression of caspase genes in colorectal cancer cell line by nisin. Arch Clin Infect Dis. 2020;15(2).
Raccach M. Pediococcus. In: Batt CA, Tortorello ML, editors. Encyclopedia of Food Microbiology. 2nd ed. London: Academic Press Inc.; 2014. p. 1–5. https://doi.org/10.1016/B978-0-12-384730-0.00247-0.
Villarante KI, et al. Purification, characterization and in vitro cytotoxicity of the bacteriocin from Pediococcus acidilactici K2a2-3 against human colon adenocarcinoma (HT29) and human cervical carcinoma (HeLa) cells. World J Microbiol Biotechnol. 2011;27:975–80.
Hoover DG, Steenson LR, editors. Bacteriocins of lactic acid bacteria. Academic Press; 2014.
Kaur B, et al. Isolation and In vitro characterization of anti-Gardnerella vaginalis bacteriocin producing Lactobacillus fermentum HV6b isolated from human vaginal ecosystem. Int J Fundam Appl Sci. 2012;1(3):41.
Kaur B, Balgir PP, Mittu B, Kumar B, Garg N. Biomedical applications of fermenticin HV6b isolated from Lactobacillus fermentum HV6b MTCC10770. Biomed Res Int. 2013;2013.
Sano Y, et al. Molecular structures and functions of pyocins S1 and S2 in Pseudomonas aeruginosa. J Bacteriol. 1993;175(10):2907–16.
Watanabe T, Saito H. Cytotoxicity of pyocin S2 to tumor and normal cells and its interaction with cell surfaces. Biochim Biophys Acta. 1980;633(1):77–86.
Abdi-Ali A, et al. Cytotoxic effects of pyocin S2 produced by Pseudomonas aeruginosa on the growth of three human cell lines. Can J Microbiol. 2004;50(5):375–81.
Płaza G, Achal V. Biosurfactants: Eco-friendly and innovative biocides against biocorrosion. Int J Mol Sci. 2020;21(6):2152.
Sanches MA, Luzeiro IG, Alves Cortez AC, Simplício de Souza É, Albuquerque PM, Chopra HK, et al. Production of biosurfactants by Ascomycetes. Int J Microbiol. 2021;2021.
Drakontis CE, Amin S. Biosurfactants: Formulations, properties, and applications. Curr Opin Colloid Interface Sci. 2020;48:77–90.
De Giani A, Zampolli J, Di Gennaro P. Recent trends on biosurfactants with antimicrobial activity produced by bacteria associated with human health: different perspectives on their properties, challenges, and potential applications. Front Microbiol. 2021;12:655150.
Marchant R, Banat IM. Microbial biosurfactants: challenges and opportunities for future exploitation. Trends Biotechnol. 2012;30(11):558–65.
Abdelli F, et al. Antibacterial, anti-adherent and cytotoxic activities of surfactin (s) from a lipolytic strain Bacillus safensis F4. Biodegradation. 2019;30:287–300.
Saini HS, et al. Efficient purification of the biosurfactant viscosin from Pseudomonas libanensis strain M9–3 and its physicochemical and biological properties. J Nat Prod. 2008;71(6):1011–5.
De Vleeschouwer M, et al. Identification of the molecular determinants involved in antimicrobial activity of pseudodesmin a, a cyclic lipopeptide from the viscosin group. Front Microbiol. 2020;11:646.
Rummel CD, et al. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ Sci Technol Lett. 2017;4(7):258–67.
Rizzato C, et al. Potential role of biofilm formation in the development of digestive tract cancer with special reference to Helicobacter pylori infection. Front Microbiol. 2019;10:846.
Raskov H, et al. Bacterial biofilm formation inside colonic crypts may accelerate colorectal carcinogenesis. Clin Transl Med. 2018;7(1):1–4.
Lu L, et al. Developing natural products as potential anti-biofilm agents. Chinese medicine. 2019;14(1):1–17.
Flemming H-C, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8(9):623–33.
Jennings LK, et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc Natl Acad Sci. 2015;112(36):11353–8.
Podlesek Z, Žgur Bertok D. The DNA damage inducible SOS response is a key player in the generation of bacterial persister cells and population wide tolerance. Fronti Microbiol. 2020;11:1785.
Weitao T. Bacteria form biofilms against cancer metastasis. Med Hypotheses. 2009;4(72):477–8.
Adnan M, et al. In pursuit of cancer metastasis therapy by bacteria and its biofilms: History or future. Med Hypotheses. 2017;100:78–81.
Kumeria T, Maher S, Wang Y, Kaur G, Wang L, Erkelens M, et al. Naturally derived iron oxide nanowires from bacteria for magnetically triggered drug release and cancer hyperthermia in 2D and 3D culture environments: bacteria biofilm to potent cancer therapeutic. Biomacromolecules. 2016;17(8):2726–36.
Kinnari TJ. The role of biofilm in chronic laryngitis and in head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2015;23(6):448–53.
Pham QN, et al. Magnetic enrichment of immuno-specific extracellular vesicles for mass spectrometry using biofilm-derived iron oxide nanowires. Nanoscale. 2023;15(3):1236–47.
Hasan I, et al. A N-acetyl-D-galactosamine-binding lectin from Amaranthus gangeticus seeds inhibits biofilm formation and Ehrlich ascites carcinoma cell growth in vivo in mice. Int J Biol Macromol. 2021;181:928–36.
Li D, et al. A hybrid actuated microrobot using an electromagnetic field and flagellated bacteria for tumor-targeting therapy. Biotechnol Bioeng. 2015;112(8):1623–31.
Han J-W, et al. Active tumor-therapeutic liposomal bacteriobot combining a drug (paclitaxel)-encapsulated liposome with targeting bacteria (Salmonella Typhimurium). Sens Actuators, B Chem. 2016;224:217–24.
Zhou S, et al. Tumour-targeting bacteria engineered to fight cancer. Nat Rev Cancer. 2018;18(12):727–43.
Riglar DT, Silver PA. Engineering bacteria for diagnostic and therapeutic applications. Nat Rev Microbiol. 2018;16(4):214–25.
Lim D, Song M. Development of bacteria as diagnostics and therapeutics by genetic engineering. J Microbiol. 2019;57:637–43.
Karthivashan G, et al. Therapeutic strategies and nano-drug delivery applications in management of ageing Alzheimer’s disease. Drug Delivery. 2018;25(1):307–20.
Patra JK, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):1–33.
Hosseinidoust Z, et al. Bioengineered and biohybrid bacteria-based systems for drug delivery. Adv Drug Deliv Rev. 2016;106:27–44.
Cao Z, Liu J. Bacteria and bacterial derivatives as drug carriers for cancer therapy. J Control Release. 2020;326:396–407.
Lin A, et al. Bacteria-responsive biomimetic selenium nanosystem for multidrug-resistant bacterial infection detection and inhibition. ACS Nano. 2019;13(12):13965–84.
Jin M, et al. Chlorine disinfection promotes the exchange of antibiotic resistance genes across bacterial genera by natural transformation. ISME J. 2020;14(7):1847–56.
Ozer E, et al. An inside look at a biofilm: Pseudomonas aeruginosa flagella biotracking. Sci Adv. 2021;7(24):eabg8581.
Zhuang XY, et al. Live-cell fluorescence imaging reveals dynamic production and loss of bacterial flagella. Mol Microbiol. 2020;114(2):279–91.
Makela AV, et al. Magnetic particle imaging of magnetotactic bacteria as living contrast agents is improved by altering magnetosome arrangement. Nano Lett. 2022;22(12):4630–9.
Schulz-Vogt HN, et al. Effect of large magnetotactic bacteria with polyphosphate inclusions on the phosphate profile of the suboxic zone in the Black Sea. ISME J. 2019;13(5):1198–208.
Łukasiewicz K, Fol M. Microorganisms in the treatment of cancer: advantages and limitations. J Immunol Res. 2018;2018.
Schmidt CK, et al. Engineering microrobots for targeted cancer therapies from a medical perspective. Nat Commun. 2020;11(1):5618.
Garcia Rubia G, et al. pH-dependent adsorption release of doxorubicin on MamC-biomimetic magnetite nanoparticles. Langmuir. 2018;34(45):13713–24.
Bazylinski DA, et al. Magnetococcus marinus gen. nov., sp. nov., a marine, magnetotactic bacterium that represents a novel lineage (Magnetococcaceae fam. nov., Magnetococcales ord. nov.) at the base of the Alphaproteobacteria. Int J Syst Evol Microbiol. 2013;63(Pt_3):801–8.
Price PM, et al. Magnetic drug delivery: where the field is going. Front Chem. 2018;6:619.
Felfoul O, et al. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat Nanotechnol. 2016;11(11):941–7.
Aubry M, et al. Engineering E coli for magnetic control and the spatial localization of functions. ACS Synth Biol. 2020;9(11):3030–41.
Wrede P, et al. Real-time 3D optoacoustic tracking of cell-sized magnetic microrobots circulating in the mouse brain vasculature. Sci Adv. 2022;8(19):eabm9132.
Urso M, Pumera M. Nano/microplastics capture and degradation by autonomous nano/microrobots: a perspective. Adv Func Mater. 2022;32(20):2112120.
Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–68.
Cheung PK, Fok L. Characterisation of plastic microbeads in facial scrubs and their estimated emissions in Mainland China. Water Res. 2017;122:53–61.
Li R, et al. Fluorometric lateral flow immunoassay for simultaneous determination of three mycotoxins (aflatoxin B 1, zearalenone and deoxynivalenol) using quantum dot microbeads. Microchim Acta. 2019;186:1–9.
Park SJ, et al. Effect of chitosan coating on a bacteria-based alginate microrobot. Biotechnol Bioeng. 2015;112(4):769–76.
Al-Fandi M, et al. Novel selective detection method of tumor angiogenesis factors using living nano-robots. Sensors. 2017;17(7):1580.
Gharaibeh RZ, Jobin C. Microbiota and cancer immunotherapy: in search of microbial signals. Gut. 2019;68(3):385–8.
Park D, et al. Motility analysis of bacteria-based microrobot (bacteriobot) using chemical gradient microchamber. Biotechnol Bioeng. 2014;111(1):134–43.
Park SJ, et al. New paradigm for tumor theranostic methodology using bacteria-based microrobot. Sci Rep. 2013;3(1):3394.
Ali MK, et al. Bacteria-derived minicells for cancer therapy. Cancer Lett. 2020;491:11–21.
Malfitano AM, et al. Virotherapy: From single agents to combinatorial treatments. Biochem Pharmacol. 2020;177:113986.
Tian Y, Xie D, Yang L. Engineering strategies to enhance oncolytic viruses in cancer immunotherapy. Signal Transduct Target Ther. 2022;7(1):117.
Zheng M, et al. Oncolytic viruses for cancer therapy: barriers and recent advances. Mol Ther Oncolyticss. 2019;15:234–47.
Shi T, et al. Combining oncolytic viruses with cancer immunotherapy: establishing a new generation of cancer treatment. Front Immunol. 2020;11:683.
Chaurasiya S, Fong Y, Warner SG. Optimizing oncolytic viral design to enhance antitumor efficacy: progress and challenges. Cancers. 2020;12(6):1699.
Filley AC, Dey M. Immune system, friend or foe of oncolytic virotherapy? Front Oncol. 2017;7:106.
Chaurasiya S, Chen NG, Fong Y. Oncolytic viruses and immunity. Curr Opin Immunol. 2018;51:83–90.
Gujar S, et al. Antitumor benefits of antiviral immunity: an underappreciated aspect of oncolytic virotherapies. Trends Immunol. 2018;39(3):209–21.
Sánchez D, et al. Oncolytic viruses for canine cancer treatment. Cancers. 2018;10(11):404.
Twumasi-Boateng K, et al. Oncolytic viruses as engineering platforms for combination immunotherapy. Nat Rev Cancer. 2018;18(7):419–32.
Pikor LA, Bell JC, Diallo J-S. Oncolytic viruses: exploiting cancer’s deal with the devil. Trends Cancer. 2015;1(4):266–77.
Rezaei R, et al. Combination therapy with CAR T cells and oncolytic viruses: a new era in cancer immunotherapy. Cancer Gene Ther. 2022;29(6):647–60.
Alvanegh AG, et al. Comparison of oncolytic virotherapy and nanotherapy as two new miRNA delivery approaches in lung cancer. Biomed Pharmacother. 2021;140:111755.
Chaurasiya S, Chen NG, Warner SG. Oncolytic virotherapy versus cancer stem cells: a review of approaches and mechanisms. Cancers. 2018;10(4):124.
Burman B, Pesci G, Zamarin D. Newcastle disease virus at the forefront of cancer immunotherapy. Cancers. 2020;12(12):3552.
Hermann LL, Coombs KM. Inhibition of reovirus by mycophenolic acid is associated with the M1 genome segment. J Virol. 2004;78(12):6171–9.
Geletneky K, et al. Double-faceted mechanism of parvoviral oncosuppression. Curr Opin Virol. 2015;13:17–24.
Belete TM. The current status of gene therapy for the treatment of cancer. Biol: Targets Ther. 2021:67–77. https://doi.org/10.2147/BTT.S302095.
Mody PH, et al. Herpes simplex virus: a versatile tool for insights into evolution, gene delivery, and tumor immunotherapy. Virology. 2020;11:1178122X20913274.
Kantor B, et al. Methods for gene transfer to the central nervous system. Adv Genet. 2014;87:125–97.
Zeng J, et al. Oncolytic viro-immunotherapy: an emerging option in the treatment of gliomas. Front Immunol. 2021;12:721830.
Fraser ME. Novel Anti-Viral Strategies for Lipid-Enveloped Viruses. University of Notre Dame; 2019. eBook.
Saeb S, et al. Brain HIV-1 latently-infected reservoirs targeted by the suicide gene strategy. Virol J. 2021;18(1):1–10.
Macedo N, Miller DM, Haq R, Kaufman HL. Clinical landscape of oncolytic virus research in 2020. J Immunother Cancer. 2020;8(2).
Guedan S, Alemany R. CAR-T cells and oncolytic viruses: joining forces to overcome the solid tumor challenge. Front Immunol. 2018;9:2460.
Vaddepally RK, et al. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers. 2020;12(3):738.
Da Mesquita S, et al. Meningeal lymphatics affect microglia responses and anti-Aβ immunotherapy. Nature. 2021;593(7858):255–60.
Daud AI, et al. Programmed death-ligand 1 expression and response to the anti–programmed death 1 antibody Pembrolizumab in melanoma. J Clin Oncol. 2016;34(34):4102.
Feist M, et al. Oncolytic virus promotes tumor-reactive infiltrating lymphocytes for adoptive cell therapy. Cancer Gene Ther. 2021;28(1–2):98–111.
Chesney J, et al. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol. 2018;36(17):1658.
Ribas A, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2018;174(4):1031–2.
Irvine DJ, Dane EL. Enhancing cancer immunotherapy with nanomedicine. Nat Rev Immunol. 2020;20(5):321–34.
Han D, et al. Current progress in CAR-T cell therapy for hematological malignancies. J Cancer. 2021;12(2):326.
Kaur T, Sharma D. Fundamentals of utilizing microbes in advanced cancer therapeutics: current understanding and potential applications. Adv Appl Microbiol. 2023;123:91–131.
Wang X, Maeng HM, Lee J, Xie C. Therapeutic implementation of oncolytic viruses for cancer immunotherapy: review of challenges and current clinical trials. Biomed J Sci Res. 2022;4(2):164. https://doi.org/10.36266/JBSR/164.
Ribas A, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170(6):1109–1119. e10.
Herbst RS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7.
Tumeh PC, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71.
Ribas A, et al. 1037O MASTERKEY-265: a phase III, randomized, placebo (Pbo)-controlled study of talimogene laherparepvec (T) plus pembrolizumab (P) for unresectable stage IIIB–IVM1c melanoma (MEL). Ann Oncol. 2021;32:S868–9.
Breitbach CJ, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477(7362):99–102.
Park SH, et al. Phase 1b trial of biweekly intravenous Pexa-Vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus in colorectal cancer. Mol Ther. 2015;23(9):1532–40.
Cripe TP, et al. Phase 1 study of intratumoral Pexa-Vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus, in pediatric cancer patients. Mol Ther. 2015;23(3):602–8.
Breitbach CJ, Moon A, Burke J, Hwang TH, Kirn DH. A phase 2, open-label, randomized study of Pexa-Vec (JX-594) administered by intratumoral injection in patients with unresectable primary hepatocellular carcinoma. Methods Mol Biol. 2015;1317:343–57. https://doi.org/10.1007/978-1-4939-2727-2_19.
Haydon AM et al. A phase 1, open-label, dose escalation study of the safety and tolerability of T3011 in advanced cutaneous or subcutaneous malignancies. Wolters Kluwer Health. 2021.
Zuo S, Wei M, He B, Chen A, Wang S, Kong L, et al. Enhanced antitumor efficacy of a novel oncolytic vaccinia virus encoding a fully monoclonal antibody against T-cell immunoglobulin and ITIM domain (TIGIT). EBioMedicine. 2021;64. https://doi.org/10.1016/j.ebiom.2021.103240.
Staedtke V, et al. Clostridium novyi-NT in cancer therapy. Genes Dis. 2016;3(2):144–52.
Roberts NJ, et al. Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci Transl Med. 2014;6(249):249ra111.
Feng X, et al. Novel insights into the role of Clostridium novyi-NT related combination bacteriolytic therapy in solid tumors. Oncol Lett. 2021;21(2):1.
Liang K, et al. New technologies in developing recombinant-attenuated bacteria for cancer therapy. Biotechnol Bioeng. 2021;118(2):513–30.
Schmitz-Winnenthal FH, et al. A phase 1 trial extension to assess immunologic efficacy and safety of prime-boost vaccination with VXM01, an oral T cell vaccine against VEGFR2, in patients with advanced pancreatic cancer. Oncoimmunology. 2018;7(4):e1303584.
Gupta KH, et al. Bacterial-based cancer therapy (BBCT): recent advances, current challenges, and future prospects for cancer immunotherapy. Vaccines. 2021;9(12):1497.
Liu J, Huang XE. Efficacy of Bifidobacterium tetragenous viable bacteria tablets for cancer patients with functional constipation. Asian Pac J Cancer Prev. 2015;15(23):10241–4.
Fukaya M, et al. Impact of synbiotics treatment on bacteremia induced during neoadjuvant chemotherapy for esophageal cancer: a randomised controlled trial. Clin Nutr. 2021;40(12):5781–91.
Hijová E, et al. Chemopreventive and metabolic effects of inulin on colon cancer development. J Vet Sci. 2013;14(4):387.
Smatti MK, et al. Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses. 2019;11(8):762.
Enamorado M, et al. Enhanced anti-tumour immunity requires the interplay between resident and circulating memory CD8+ T cells. Nat Commun. 2017;8(1):16073.
Moore PS, Chang Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat Rev Cancer. 2010;10(12):878–89.
Azevedo MM, Pina-Vaz C, Baltazar F. Microbes and cancer: friends or faux? Int J Mol Sci. 2020;21(9):3115.
Zuo X, et al. HTLV-1 persistent infection and ATLL oncogenesis. J Med Virol. 2023;95(1):e28424.
Maucort-Boulch D, et al. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer. 2018;142(12):2471–7.
Weitzman MD, Fradet-Turcotte A. Virus DNA replication and the host DNA damage response. Ann Rev Virol. 2018;5:141–64.
Carteaux G, et al. Zika virus associated with meningoencephalitis. N Engl J Med. 2016;374(16):1595–6.
Adams MJ, et al. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2017). Adv Virol. 2017;162(8):2505–38.
De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17(8):457–74.
Sullivan LB, et al. Aspartate is an endogenous metabolic limitation for tumour growth. Nat Cell Biol. 2018;20(7):782–8.
Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;321(8336):1273–5.
Goodrich JK, et al. The relationship between the human genome and microbiome comes into view. Annu Rev Genet. 2017;51:413–33.
Chang AH, Parsonnet J. Role of bacteria in oncogenesis. Clin Microbiol Rev. 2010;23(4):837–57.
Zou S, Fang L, Lee M-H. Dysbiosis of gut microbiota in promoting the development of colorectal cancer. Gastroenterol Rep. 2018;6(1):1–12.
Benedetti F, et al. Tampering of viruses and bacteria with host DNA repair: Implications for cellular transformation. Cancers. 2021;13(2):241.
Manfredo Vieira S, et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science. 2018;359(6380):1156–61.
Uronis JM, et al. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS One. 2009;4(6):e6026.
Li Y, et al. Gut microbiota accelerate tumor growth via c-jun and STAT3 phosphorylation in APC Min/+ mice. Carcinogenesis. 2012;33(6):1231–8.
Cohen MS, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505.
Logunov D, et al. Mycoplasma infection suppresses p53, activates NF-κB and cooperates with oncogenic Ras in rodent fibroblast transformation. Oncogene. 2008;27(33):4521–31.
Cao S, et al. Potential malignant transformation in the gastric mucosa of immunodeficient mice with persistent Mycoplasma penetrans infection. PLoS One. 2017;12(7):e0180514.
Nallar SC, Xu D-Q, Kalvakolanu DVJC. Bacteria and genetically modified bacteria as cancer therapeutics: current advances and challenges. Cytokine. 2017;89:160–72.
Figlerowicz M, et al. Genetic variability: the key problem in the prevention and therapy of RNA-based virus infections. Med Res Rev. 2003;23(4):488–518.
Hermiston TW, Kuhn I. Armed therapeutic viruses: strategies and challenges to arming oncolytic viruses with therapeutic genes. Cancer Gene Ther. 2002;9(12):1022–35.
Patyar S, et al. Bacteria in cancer therapy: a novel experimental strategy. 2010;17:1–9.
Chhabra N, Kennedy J. A review of cancer immunotherapy toxicity II: adoptive cellular therapies, kinase inhibitors, monoclonal antibodies, and oncolytic viruses. J Med Toxicol. 2022;18(1):43–55.
Curran CS, Rasooly A, He M, Prickril B, Thurin M, Sharon E. Report on the NCI microbial-based cancer therapy conference. Cancer Immunol Res. 2018;6(2):122–6. https://doi.org/10.1158/2326-6066.
Kramer MG, et al. Bacterial therapy of cancer: promises, limitations, and insights for future directions. Front Microbiol. 2018;9:297194.
Corrigan PA, et al. Talimogene laherparepvec: an oncolytic virus therapy for melanoma. Ann Pharmacother. 2017;51(8):675–81.
Klugar M, et al. Side effects of mRNA-based and viral vector-based COVID-19 vaccines among German healthcare workers. Biology (Basel). 2021;10(8):752.
Acknowledgements
None declared.
Funding
No Funding.
Author information
Authors and Affiliations
Contributions
AY-MZ: wrote the manuscript, MA: edited the manuscript, HA-GA.J: design and Supervision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yarahmadi, A., Zare, M., Aghayari, M. et al. Therapeutic bacteria and viruses to combat cancer: double-edged sword in cancer therapy: new insights for future. Cell Commun Signal 22, 239 (2024). https://doi.org/10.1186/s12964-024-01622-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12964-024-01622-w