Abstract
Cyclic bis-(3', 5')-dimeric guanosine monophosphate (c-di-GMP) is ubiquitous in many bacterial species, where it functions as a nucleotide-based secondary messenger and is a vital regulator of numerous biological processes. Due to its ubiquity, most bacterial species possess a wide range of downstream receptors that has a binding affinity to c-di-GMP and elicit output responses. In eukaryotes, several enzymes and riboswitches operate as receptors that interact with c-di-GMP and transduce cellular or environmental signals. This review examines the functional variety of receptors in prokaryotic and eukaryotic systems that exhibit distinct biological responses after interacting with c-di-GMP. Evolutionary relationships and similarities in distance among the c-di-GMP receptors in various bacterial species were evaluated to understand their specificities. Furthermore, residues of receptors involved in c-di-GMP binding are summarized. This review facilitates the understanding of how distinct receptors from different origins bind c-di-GMP equally well, yet fulfill diverse biological roles at the interspecies, intraspecies, and interkingdom levels. Furthermore, it also highlights c-di-GMP receptors as potential therapeutic targets, particularly those found in pathogenic microorganisms.
Video Abstract
Similar content being viewed by others
Introduction
Cyclic bis-(3', 5')-dimeric guanosine monophosphate (c-di-GMP) is a nucleotide-based signaling molecule discovered in 1987 as an effective activator of cellulose synthase in Gluconacetobacter xylinus [1]. Since then, c-di-GMP has been ubiquitously reported in bacterial species [2] as a universal secondary messenger that responds to various environmental and cellular cues [3]. c-di-GMP plays regulatory roles in numerous cellular activities, including cell motility, biofilm formation and dispersion, cell division, differentiation, quorum sensing, and virulence [4]. The intracellular concentration of c-di-GMP is controlled by diguanylate cyclases (DGCs) and phosphodiesterases (PDEs), respectively [5]. DGCs (contain GGDEF domain) catalyze c-di-GMP production by consuming two GTP molecules, whereas PDEs (have EAL or HD-GYP domains) catalyze the degradation of c-di-GMP into a pGpG linear nucleotide or two GTP molecules [6]. Signal transduction occurs through c-di-GMP binding to downstream receptors, triggered by sensing environmental or cellular cues [7]. Recent advances in bioinformatics, molecular biology, structural biology, and biochemistry have identified several types of receptors in bacterial species that bind to c-di-GMP signaling molecules and regulate several cellular processes. The PilZ protein, which regulates flagellar motility, was the first c-di-GMP receptor identified [8]. Moreover, PilZ proteins play key roles in cell aggregation by contributing to chemotaxis, biofilm formation, and colonization [9, 10].
Proteins with GGDEF and EAL domains have been discovered as c-di-GMP receptors [11], in addition to various transcription-factor families and riboswitches located in the 5'-untranslated region of mRNA [12]. Animals, humans, and other eukaryotic systems interact with bacteria in various ways, including symbiotic and non-symbiotic associations [13]. Bacterial c-di-GMP in these hosts can also serve as a nucleotide-based second messenger for various positive or negative cellular functions [14]. Studies have shown that the host receptor interacts with c-di-GMP, activating host immune systems to defend against intracellular bacterial infections [15, 16].
Understanding how the bacterial c-di-GMP functions as a universal signaling molecule at the interspecies, intraspecies, and interkingdom levels are essential due to its critical role in regulating various cellular processes. This review discusses several types of receptors that interact with c-di-GMP to perform various biological activities at the interspecies, intraspecies, and interkingdom levels. The evolutionary relationships between these receptors have been investigated to understand their c-di-GMP specificities. The residues of receptors implicated in c-di-GMP binding are also summarized.
Structural insight into the binding of c-di-GMP with their cognate receptors
The number of crystal structures of c-di-GMP receptors in alliance with c-di-GMP in various species is increasing [17]. Based on these structures, three types of domains containing several c-di-GMP binding motifs have been articulated [18]. Interestingly, the sorts of interactions between c-di-GMP and its receptors vary according to the type of c-di-GMP [19]. c-di-GMP interacts with its receptor as a mono, di, or tetramer. The c-di-GMP structure can also achieve a stacked or extended form as a result of variations in their torsional or glycosidic angles. In c-di-GMP receptor dimers, guanine bases may be entirely or partially stacked or de-stacked, enabling them to interact with the hydrophobic amino acid residues of a receptor. Various non-covalent interactions between c-di-GMP and its diverse receptors include hydrogen bonds, π–π, polar–π, hydrophobic–π, cation-π, anion–π, C-H bond–π, and lone pair–π interactions [17]. Each guanine base in c-di-GMP has Watson–Crick and Hoogsten edges that bind to glutamate or aspartate and arginine, respectively [20]. Specifically, the arginine of the receptor forms a hydrogen bond with the N7 and O6 atoms of the guanine Hoogsteen edge. Contrastingly, guanine's N1 and N2 atoms at the Watson–Crick edge bind to aspartic acid and glutamate. Examples of these interactions are presented below.
The interaction between c-di-GMP and the MshEN receptor of Vibrio cholerae is an example of a hydrophobic interaction [21]. MshEN contains a 53-residue long domain consisting of two 24-residue motifs [RLGXX(L)(V/I)XXG(I/F)(L/V)XXXXLXXXLXXQ] connected by a 5-residue spacer that accommodates c-di-GMP. The six well-conserved residues (in bold) play an important role in the c-di-GMP binding [21]. The arginine (R9) in RLG interacts with Gua1 of c-di-GMP through stacking, whereas the leucine forms a hydrophobic triangular cluster with the L54 and L58 in the second motif. This cluster also involves hydrophobic CH-π interactions with Gua2 that stabilize protein–ligand interactions [21, 22]. Glycine residues from the conserved motif and the remaining non-conserved residues establish two hydrogen bonds with N7 and O6 of Gua1 at the Hoogsteen edge (Fig. 1A). The four-helix MshEN_N also forms a bundle structure maintained by hydrophobic residues in its core (Fig. 1B).
Hydrophobic interaction between c-di-GMP and MshEN receptor (A and B). Reproduced with permission from reference [21]. Copyright, 2016, Springer Nature, (C) Interaction of c-di-GMP with the dimer of STING in the symmetry. Reproduced with permission from reference [23]. Copyright, 2012, Elsevier Inc. and Copyright, and (D) The multiple non-covalent interactions of c-di-GMP dimer with the σWhiG and two α helices of RsiG in Streptomyces venezuelae. Reproduced with permission from reference [20]. Copyright, 2020, Elsevier Inc
The stimulator of the interferon genes (STING) protein found in animals, mammals, and humans provides an example of arginine-guanine cation-π and guanine-guanine π-π interactions between c-di-GMP and its receptor [23]. A single c-di-GMP molecule is symmetrically linked to the STING dimer, with each stack formed by two guanines (Fig. 1C). STING dimers contain a c-di-GMP binding pocket formed by α1, α3, and the loop formed by β2 and β3 (Fig. 1C). The four-layer stack is formed by the stack of the guanidinium group of arginine with the guanine base and the interaction of the long side chain of arginine with Y167 [20]. It has been discovered that interactions between c-di-GMP and many residues, including P264, T263, E260, Y163, and S162, entail several Van der Waals interactions and hydrogen bonding [23]. This four-layer stack consists of Tyr/Gua/Arg/Tyr interactions. Additionally, because the guanine bases are partly stacked, a compact core ring is adopted when c-di-GMP is connected to the STING interface.
The interaction between c-di-GMP and the anti-sigma factor RsiG in combination with the sigma factor σWhiG at its interface involves lone pair–π interactions, polar–π interactions, and hydrophobic–π interactions [20]. These interactions have been reported in Streptomyces venezuelae, where it sequesters sporulation σWhiG, inhibiting the transcription of late sporulation genes [24]. Figure 1D shows interactions between the c-di-GMP dimer and σWhiG, in addition to two EXXXSXXRXXXQXXXD motifs from the α1 and α6 helices of RsiG. The two antiparallel helices of RsiG are aligned together, and residues such as glutamic acid, arginine, and aspartic acid present in the EXXXSXXRXXXQXXXD motif establish hydrogen bonds with the two c-di-GMP monomers. The two extended guanines and the Gln residues within this motif have been seen to interact non-covalently. Additionally, a water molecule also helps to stabilize the c-di-GMP dimer by creating two hydrogen bonds with Gua1's N7 and O6 atoms in each c-di-GMP monomer, one with the carboxyl oxygen of adjacent D105 and the other with Gua3 in the second c-di-GMP monomer. S. venezuelae BldD is another receptor that interacts with c-di-GMP through π–π interaction and helps to transition hypha-spore forms by suppressing BldD regulon genes during vegetative growth [25] (Fig. 2).
Interaction of tetrameric c-di-GMP with the two subunits of BldD via C-terminal domain with the involvement of bipartite RXD-X8-RXXD signature. (A) Structural details of BldD dimer-(c-di-GMP) complex, (B) Top and bottom layer of BldD C-terminal domain-(c-di-GMP) contact, (C) Middle layers of two c-di-GMP dimers that are intercalated, and (D) c-di-GMP layers in side view. Reprinted with permission from reference [25]. Copyright, 2014, Elsevier Inc
Biological roles of c-di-GMP receptors in prokaryotic systems
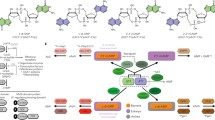
In the bacterial system, c-di-GMP helps with the transduction of external (e.g., temperature, energy, surfaces, light, redox potential, respiratory electron acceptors, and several natural and synthetic chemicals) [26] or internal (generated during developmental progression or cellular division) [27] signals by binding to the effector component or receptor. These c-di-GMP-bound receptors/effectors specifically interact with their targets, triggering a wide range of cellular processes. Moreover, c-di-GMP has demonstrated various biological functions, including motility, the formation and dispersion of biofilm, cell division, differentiation, quorum sensing, and virulence [4]. Here, we discuss the functional consequences of the interaction of c-di-GMP with its effectors or receptors (Fig. 3). Table 1 summarizes the types of c-di-GMP receptors found in prokaryotic and eukaryotic systems and the outcome of their interactions in terms of biological function. Table 1 also highlights the binding domains or motifs associated with interaction of c-di-GMP with its receptors. The outcome of this interaction is classified into the categories of control of transcription initiation and termination, control of translation, control of exopolysaccharide (EPS) synthesis and secretion, control of flagellar and type III secretion system assembly, control of cell cycle progression, proteolysis of target proteins, and involvement in the localization of target proteins [28]. Detailed descriptions of the biological functions of these outcome reactions are provided below.
Biofilm formation and bacterial colonization
Bacteria use c-di-GMP signaling to transition from a motile to a solitary existence in response to environmental cues [133]. Bacterial receptor proteins associate with c-di-GMP to create the extracellular cellulose matrix of biofilms [134]. For instance, Bcam1349 protein from Burkholderia cenocepacia, combined with c-di-GMP, promotes pellicle and biofilm architecture progression [39]. It has been shown that c-di-GMP increases the Bcam1349 protein's affinity for the promoter of a target gene, which results in the development of biofilms. In Pseudomonas aeruginosa, the binding of MapZ to c-di-GMP alters motility by controlling the switching frequency [91]. This interaction is also related to enhanced adhesion during biofilm formation. Additionally, HapZ interacts with SagS in P. aeruginosa in a c-di-GMP-dependent way to promote surface adhesion and biofilm formation [10]. This is consistent with the role of SagS in controlling surface adhesion during the early stages of biofilm formation. In Escherichia coli, c-di-GMP stimulates biofilm formation by directly binding to PgaC and PgaD [57]. The Pga machinery triggers the synthesis of poly-β-1, 6-N-acetylglucosamine, a biofilm component of E. coli. c-di-GMP also stimulates the expression of the gene encoding MrkH (a key factor in biofilm formation) in Klebsiella pneumoniae [60]. Here, the C-terminal- and N-terminal domains of MrkH promote biofilm development upon the mediation of dimer association by c-di-GMP. Similarly, V. cholerae biofilm development is aided by VpsT-sensing c-di-GMP via extracellular matrix synthesis regulation [108]. VpsT downregulates the motility genes of V. cholerae in a c-di-GMP-dependent fashion, leading to a sessile colony state. In addition, BrpT in V. vulnificus binds directly to c-di-GMP and promotes the expression of EPS, thereby improving cell surface adhesion [115]. BolA interacts with c-di-GMP and promotes motility and biofilm formation, allowing E. coli to transition between planktonic and sessile lifestyles [59]. Even in the presence of high c-di-GMP levels, the lack of BolA resulted in less robust biofilm development, emphasizing the importance of BolA in this regard. Alg44 in P. aeruginosa is involved in the biosynthesis of the EPS alginate via binding to c-di-GMP [75, 76, 135]. It has also been demonstrated that c-di-GMP regulates polysaccharide polymerization and transport via Alg44. In Sinorhizobium meliloti, CuxR stimulates EPS production at high doses of c-di-GMP [99]. TDE0214 protein produces a biofilm and exhibits virulence in the periodontal pathogen Treponema denticola via the c-di-GMP signaling cascade [105]. Here, TDE0214 plays a significant role in cell adhesion and colonization during the early stages of biofilm formation.
Bacterial survival and adaptation
Several bacterial species respond to environmental fluctuations via the c-di-GMP signaling system [136]. In addition, the presence of c-di-GMP in bacteria promotes host adaptation and persistence by regulating bacterial metabolism [137]. Tlp1 from Azospirillum brasilense promotes motility by binding to c-di-GMP through its C-terminal PilZ domain. This allows the microaerobic A. brasilense to reach a hypoxic environment [31]. ArgR from Mycobacterium bovis modulates c-di-GMP signaling to adapt to hypoxic environments [62]. Specifically, c-di-GMP mitigates the autorepression of ArgR expression and promotes nitrite respiration, thereby increasing the viability of M. bovis in hypoxic environments. c-di-GMP in M. smegmatis enhances their antioxidant resistance by binding to HpoR and LtmA [65, 66]. Here, c-di-GMP is upregulated by high levels of H2O2 and enhances antioxidant resistance by specifically binding to HpoR, which induces DNA-binding activity. In addition, LtmA enhances the growth of M. smegamatis under high antioxidant stress levels, in contrast to HpoR. Borrelia burgdorferi, a tick-borne pathogen, also enhances its host adaptability by binding PlzA to c-di-GMP [35]. These results are attributed to the effector mechanism that occurs through PlzA—c-di-GMP interaction [36].
Regulation of bacterial pathogenesis
c-di-GMP regulates factors that affect bacterial pathogenesis, including the expression of virulence genes, biofilm formation, and motility regulation [138]. The diversity of c-di-GMP receptors and effectors contributes to bacterial lifestyle transformation by regulating multiple cellular processes [139]. PlzC and PlzD in V. cholerae particularly cooperate with c-di-GMP to control the expression of the virulence genes and the production of biofilms [106]. PlzC and PlzD proteins regulate downstream processes by binding to c-di-GMP. Additionally, VpsR is connected to c-di-GMP and activates aphA and vpsT to govern biofilm development and virulence factor production [109]. FlrA controls the motility of V. cholerae by downregulating flagellar biosynthesis [110]. Furthermore, c-di-GMP inhibits V. cholerae motility by promoting the formation of extracellular Vibrio polysaccharides. WspR from P. aeruginosa regulates biofilm development by binding to c-di-GMP [79]. This is attributed to c-di-GMP, which restricts the mobility of the GGDEF domain of WspR. Furthermore, in the presence of c-di-GMP, P. aeruginosa reduces the expression of flagellar genes [86]. This effect is enhanced in the presence of FleN. Finally, E. coli YcgR also binds to c-di-GMP to regulate flagella-based motility [8].
Regulation of enzyme activity
c-di-GMP may also bind to enzymes and thus regulate their activities [27]. Cellulose production by pathogens is a key factor influencing virulence, since cellulose is a fundamental component of biofilm structures [140]. In B. cenocepacia, c-di-GMP controls cellulose synthesis by increasing the binding of Bcam1349 to the cellulose synthase gene [39]. BcsA controls cellulose synthesis in Erwinia amylovora in a c-di-GMP-dependent fashion [53]. Here, the allosteric binding of c-di-GMP to BcsA contributes to biofilm formation by activating cellulose biosynthesis. BcsE from E. coli maximizes cellulose production by binding to c-di-GMP through its DUF2819 domain [58]. Additionally, BcsA and BcsB form a complex to activate c-di-GMP, thereby upregulating cellulose synthesis in E. coli [97]. This occurs by activating the auto-inhibited enzyme by breaking the salt bridges that bind the gating loops controlling access to the active site and substrate coordination. Finally, GlgX from S. venezuelae interacts with c-di-GMP to stimulate the enzymatic hydrolyzation of glycogen [104]. This indicates that the GlgX-c-di-GMP interaction is vital for controlling glucose availability in Streptomyces.
Biological roles of c-di-GMP receptors in eukaryotic systems
Bacterial c-di-GMP can interact with eukaryotic cell systems as well [104]. c-di-GMP is used by eukaryotes as a pathogen presence indicator [27]. In this regard, c-di-GMP modulates the response of the eukaryotic immune system to microbial pathogens [14]. In mammals, STING acts as an immune sensor by inducing a type I interferon (IFN) response by binding to c-di-GMP [23, 128]. Furthermore, binding to c-di-GMP improves the interaction between the C-terminal domain of STING and TANK-binding kinase 1, resulting in IFN production [129] (Fig. 4). DDX41 (a c-di-GMP-detection pattern recognition receptor) encourages connections with STING, enhances STING's affinity for c-di-GMP, and activates IFNs [131]. STING from Larimichthys crocea (a large yellow croaker) enhances its immunity against the infection caused by the parasite Cryptocaryon irritans, by acting as a receptor for c-di-GMP [125]. During C. irritans infection, STING mediates the production of type I IFNs and inflammatory cytokines. In ItgaxCreTmem173Flox/Flox mice, c-di-GMP induces cytokine production by selectively activating cells expressing the mammalian c-di-GMP receptor MPYS (STING, MITA, and TMEM173) [126]. In Jurkat cells, c-di-GMP binds to the P21ras protein and stimulates CD4 receptor expression [127]. Specifically, c-di-GMP binding to P21ras maintains the protein in an active form following CD4 overexpression. In mammalian macrophages, coronin 1A and cyclophilin H bind to c-di-GMP and induce type I IFN expression, activating an inflammatory response [130]. These findings suggest that mammalian macrophages detect and respond to c-di-GMP-induced inflammation. Finally, the membrane polypeptide of cellulose synthase from cotton fibers (Gossypium hirsutum) was characterized using the same c-di-GMP receptor as the cellulose synthase from Acetobacter xylinum [124]. Plant cellulose synthase membrane polypeptides are identical to bacterial cellulose synthase subunits, allowing for the identification of genes responsible for cellulose synthesis in plants.
Downstream signaling induced by bacterial c-di-GMP binding to STING cognate receptors leads to the generation of type I IFNs through the IFN regulatory factor 3 and TANK-binding kinase 1 pathway. Reprinted with permission from the reference [129]. Copyright, 2012 Elsevier Inc
Evolutionary relationships between bacterial c-di-GMP receptors and identification of the bidirectional best hit
To investigate the evolutionary relationship of c-di-GMP receptors, a phylogenetic tree of receptors from several bacterial species was constructed (Fig. 5). Subsequently, the best bidirectional hit among these receptors was determined to demonstrate how these receptors from various bacterial species are clustered in the same branch of the tree. The bidirectional best hit helps to determine how the proteins share similar affinities for the same chemical (c-di-GMP). YcgR is recognized as a flagellar brake protein due to its role in controlling swarming and swimming in various flagellated bacterial species by interacting with c-di-GMP [141, 142]. YcgR from E. coli was found to be clustered with BrlR from P. aeruginosa in the same branch in the phylogenetic tree developed. In P. aeruginosa, BrlR regulates biofilm formation and multidrug efflux pump production, in addition to acting as a pyocyanin receptor [90]. However, a sequence similarity analysis revealed no similarities between the two proteins. Further, YcgR from E. coli was found to show 82.35% similarity to FlgZ from P. aeruginosa, indicating potentially similar binding specificities for c-di-GMPs and a role in flagellar motility (Table 2).
Phylogenetic tree of the c-di-GMP receptors from different species of bacteria. The red-colored proteins promote biofilm formation, the blue-colored proteins promote bacterial survival and adaptability, the yellow-colored proteins promote bacterial pathogenicity, and the green-colored proteins promote enzyme activity
PlzA is a protein that binds to c-di-GMP (29.6 kDa, 261 amino acids) found in many species of Lyme disease spirochetes, such as Borreliella spp. Multiple mechanisms were hypothesized to influence borrelial activity previously. PlzAs from all Borreliella species were grouped together in the same branch. Moreover, LapD is a c-di-GMP-binding receptor that regulates cell surface attachment and biofilm formation [92]. LapD from P. fluorescence was grouped in the same branch as CsrD from E. amylovora, CbrR from Campylobacter jejuni, and MrkH from K. pneumoniae. However, the sequence similarities indicated that CbrR and CsrD have 57.14% similarity, whereas MrkH has 66.67% similarity with BolA from E. coli and 50.00% similarity with PdtaS from M. tuberculosis.
Various proteins help regulate biofilm development by producing extracellular polymers such as alginate and cellulose [140]. Several bacterial polymer-synthesizing proteins, such as cellulose and alginate synthases, were grouped in the same branch as well (Fig. 5). BcsA from Dickeya oryzae, which is involved in cellulose synthesis, showed 91.95% similarity with BcsA from E. amylovora (Table 2), yet lower similarities were exhibited with BcsA from E. coli (69.68%) and S. fredii (52.73%). The YdaK protein from Bacillus subtilis, which is involved in the production of putative EPS facilitating biofilm formation, was grouped in the same branch as that of the cellulose-producing proteins (Fig. 5). Additionally, YdaK from B. subtilis showed high similarity (87.94%) with the YdaK protein from B. velezensis (Fig. 5). Tlp1 from A. brasilense exhibits sustained motility after interacting with c-di-GMP via the PilZ domain and was found to be clustered along YkuI from B. subtilis.
Although the precise role of Ykul is unknown [143], a high degree of similarity (100.00%) between Yku1 and Tlp1 from A. brasilense suggests that it may also influence motility. WspR from P. aeruginosa, which is involved in biofilm production, was grouped in the same branch as the WspR from Lysobacter enzymogenes with a sequence similarity of 72.22%. BalD (controls the expression of sporulation genes in S. coelicolor) is found in the same branch as CdbA, which is required for viability in Myxococcus xanthus and plays a role in its chromosomal organization and segregation [70]. Furthermore, a sequence similarity study showed 100.00% similarity between BalD and GlgX of S. venezuelae, which functions as a glycogen-debranching enzyme [104]. GlgX is thought to be important in sporulation since a mutation in the glgX gene results in a strain that produces less spores than the wild-type strain [104]. In the phylogenetic tree, GlgX from S. venezuelae was grouped in the same branch as PXO02374 from Xanthomonas oryzae and VpsT and GbpA from V. choloreae. CckA (cell cycle kinase) from Caulobacter vibrioides [48] is found in the same cluster as CdgR (controls cell size) from Synechocystis sp. and CuxR (regulates EPS synthesis) from S. meliloti [99]. Surprisingly, CckA has a strong resemblance (71.43%) to CbrR (which regulates flagellar motility) in C. jejuni [44].
Evolutionary relationship between eukaryotic c-di-GMP receptors and identification of the bidirectional best hit
A phylogenetic tree of receptors of eukaryotic origin was also constructed (Fig. 6). The results revealed that STING from Drosophila melanogaster shares a high degree of similarity with STING from Mus musculus (100.00%), Homo sapiens (100.00%), and L. crocea (60.00%). In contrast, STING from M. musculus had a similarity of 88.95% with STING from H. sapiens. Protein helicase DDX41 from H. sapiens shares a high degree of similarity with H. sapiens coronin 1A (91.13%) and helicase from M. musculus (99.52%). The cyclophilin H proteins from H. sapiens and M. musculus were found in the same branch and showed 100.00% similarity.
Methodology: phylogenetic analysis and bidirectional best hit analysis
The sequences of all receptors were retrieved from the National Center for Biotechnology Information database. After multiple sequence alignments of c-di-GMP receptors, the phylogenetic trees were constructed using MEGA version 11 software [144]. The Geneious® 11.0.2 program was used to perform the bidirectional best hit among c-di-GMP receptors and multiple sequence alignment using the BLOSUM-65 matrix.
Conclusion and future perspectives
With recent advances in bioinformatics, structural biology, molecular biology, and biochemistry, the discovery of c-di-GMP receptors has expanded from prokaryotic to eukaryotic systems. Bacterial species use c-di-GMP signaling molecules in their natural environment through interactions with receptors, which are either membrane-bound or cytoplasmic. Bacteria are ubiquitous and can have either beneficial or detrimental effects on host systems. c-di-GMP plays a crucial role in maintaining homeostasis in the cell life cycle. Eukaryotic systems also use c-di-GMP released by bacteria to control cellular processes. Control of intracellular processes at the post-translational, translational, and transcriptional stages is a significant ability of c-di-GMP receptors. Multiple non-covalent bonding interactions influence the interaction between c-di-GMP and its receptors in many biological systems. c-di-GMP receptors bind c-di-GMP via unique domains and motifs. Here, a phylogenetic tree of c-di-GMP receptors from bacterial and eukaryotic systems was created to understand distinct receptors that participate in diverse biological processes with similar c-di-GMP binding specificities. Several common receptors found in multiple bacterial species were found to be grouped in the same branch. A significant level of sequence similarity between the receptors in various bacterial genera implies shared specificities for c-di-GMP. The functionality of various bacterial species influenced upon binding receptor proteins with c-di-GMP is also clarified in this review. The following are the key aspects that must be addressed in future studies to better understand the mechanism of interaction between c-di-GMP and its receptors, as well as their diverse functional characteristics.
-
The receptor in pathogenic bacteria and its response upon interacting with c-di-GMP, which is also associated with cell division and virulence, may constitute viable therapeutic targets for controlling microbial infections.
-
It is difficult to anticipate how the selectivity of c-di-GMP will change when receptors from prokaryotes and eukaryotes are present simultaneously within the cell.
-
The conditions under which the host receptor protein detects c-di-GMP need to be elucidated.
-
Interactions between c-di-GMP and c-di-GMP receptors of various species have been studied in vitro using techniques such as differential radial capillary action of ligand assays, pull-down assays, and capture compound experiments [25]. These in vitro investigations should validate the binding interaction and affinity in the host system, followed by a study of the gene expression probing the response of the cell.
-
High-throughput screening of natural and synthesized compounds is required to antagonize the interaction between c-di-GMP and its receptor.
-
In vitro binding experiments have indicated that certain receptors also bind c-di-GMP. However, their biological functions remain unclear (Table 1) and require further in vivo studies.
Availability of data and materials
Not applicable.
Abbreviations
- c-di-GMP:
-
Cyclic bis-(3', 5')-dimeric guanosine monophosphate
- DGCs:
-
Diguanylate cyclases
- EPS:
-
Exopolysaccharides
- IFN:
-
Interferon
- PDEs:
-
Phosphodiesterases
- STING:
-
Stimulators of interferon gene
References
Schirmer T, Jenal U. Structural and mechanistic determinants of c-di-GMP signalling. Nat Rev Microbiol. 2009;7:724–35.
Liu Y, Lee C, Li F, Trček J, Bähre H, Guo R-T, et al. A cyclic di-GMP network is present in gram-positive Streptococcus and gram-negative Proteus species. ACS Infect Dis. 2020;6:2672–87.
Römling U, Galperin MY, Gomelsky M. Cyclic di-GMP: The first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev. 2013;77:1–52.
Jenal U, Reinders A, Lori C. Cyclic di-GMP: Second messenger extraordinaire. Nat Rev Microbiol. 2017;15:271–84.
Bordeleau E, Fortier LC, Malouin F, Burrus V. c-di-GMP turn-over in clostridium difficile is controlled by a plethora of diguanylate cyclases and phosphodiesterases. PLoS Genet. 2011;7:e1002039.
Stelitano V, Giardina G, Paiardini A, Castiglione N, Cutruzzola F, Rinaldo S. c-di-GMP hydrolysis by Pseudomonas aeruginosa HD-GYP phosphodiesterases: analysis of the reaction mechanism and novel roles for pGpG. PLoS ONE. 2013;8:e74920.
Mills E, Petersen E, Kulasekara BR, Miller SI. A direct screen for c-di-GMP modulators reveals a Salmonella Typhimurium periplasmic ʟ-arginine–sensing pathway. Sci Signal. 2015;8:ra57.
Ryjenkov DA, Simm R, Römling U, Gomelsky M. The PilZ domain is a receptor for the second messenger c-di-GMP: the PilZ domain protein YcgR controls motility in enterobacteria. J Biol Chem. 2006;281:30310–4.
Sun Y, Xie Z, Sui F, Liu X, Cheng W. Identification of Cbp1, a c-di-GMP binding chemoreceptor in Azorhizobium caulinodans ORS571 involved in chemotaxis and nodulation of the host plant. Front Microbiol. 2019;10:638.
Xu L, Venkataramani P, Ding Y, Liu Y, Deng Y, Yong GL, Xin L, Ye R, Zhang L, Yang L, Liang ZX. A cyclic di-GMP-binding adaptor protein interacts with histidine kinase to regulate two-component signaling. J Biol Chem. 2016;291:16112–23.
Römling U, Gomelsky M, Galperin MY. c-di-GMP: the dawning of a novel bacterial signalling system. Mol Microbiol. 2005;57:629–39.
Hengge R. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol. 2009;7:263–73.
Wiesmann CL, Wang NR, Zhang Y, Liu Z, Haney CH. Origins of symbiosis: Shared mechanisms underlying microbial pathogenesis, commensalism and mutualism of plants and animals. FEMS Microbiol Rev. 2022;15:fuac048.
Krasteva PV, Giglio KM, Sondermann H. Sensing the messenger: the diverse ways that bacteria signal through c-di-GMP. Protein Sci. 2012;21:929–48.
Karaolis DK, Means TK, Yang D, Takahashi M, Yoshimura T, Muraille E, Philpott D, Schroeder JT, Hyodo M, Hayakawa Y, Talbot BG, Brouillette E, Malouin F. Bacterial c-di-GMP is an immunostimulatory molecule. J Immunol. 2007;178:2171–81.
Karaolis DK, Newstead MW, Zeng X, Hyodo M, Hayakawa Y, Bhan U, Liang H, Standiford TJ. Cyclic di-GMP stimulates protective innate immunity in bacterial pneumonia. Infect Immun. 2007;75:4942–50.
He J, Yin W, Galperin MY, Chou SH. Cyclic di-AMP, a second messenger of primary importance: tertiary structures and binding mechanisms. Nucleic Acids Res. 2020;48:2807–29.
Navarro MVAS, De N, Bae N, Wang Q, Sondermann H. Structural analysis of the GGDEF-EAL domain-containing c-di-GMP receptor FimX. Structure. 2009;17:1104–16.
Ko J, Ryu KS, Kim H, Shin JS, Lee JO, Cheong C, Choi BS. Structure of PP4397 reveals the molecular basis for different c-di-GMP binding modes by Pilz domain proteins. J Mol Biol. 2010;398:97–110.
Chou SH, Galperin MY. Cyclic di-GMP in Streptomycetes: A new conformation, new binding mode, new receptor, and a new mechanism to control cell development. Mol Cell. 2020;77:443–5.
Wang YC, Chin KH, Tu ZL, He J, Jones CJ, Sanchez DZ, Yildiz FH, Galperin MY, Chou SH. Nucleotide binding by the widespread high-affinity cyclic di-GMP receptor MshEN domain. Nat Commun. 2016;7:12481.
Nishio M, Umezawa Y, Fantini J, Weiss MS, Chakrabarti P. CH–π hydrogen bonds in biological macromolecules. Phys Chem Chem Phys. 2014;16:12648–83.
Yin Q, Tian Y, Kabaleeswaran V, Jiang X, Tu D, Eck MJ, Chen ZJ, Wu H. Cyclic di-GMP sensing via the innate immune signaling protein STING. Mol Cell. 2012;46:735–45.
Gallagher KA, Schumacher MA, Bush MJ, Bibb MJ, Chandra G, Holmes NA, et al. C-di-GMP arms an anti-σ to control progression of multicellular differentiation in Streptomyces. Mol Cell. 2020;77:586–599.e586.
Tschowri N, Schumacher Maria A, Schlimpert S, Chinnam Naga B, Findlay Kim C, Brennan Richard G, et al. Tetrameric c-di-GMP mediates effective transcription factor dimerization to control Streptomyces development. Cell. 2014;158:1136–47.
Randall TE, Eckartt K, Kakumanu S, Price-Whelan A, Dietrich LEP, Harrison JJ. Sensory perception in bacterial cyclic diguanylate signal transduction. J Bacteriol. 2022;204:e0043321.
Mills E, Pultz IS, Kulasekara HD, Miller SI. The bacterial second messenger c-di-GMP: mechanisms of signalling. Cell Microbiol. 2011;13:1122–9.
Hengge R. Cyclic-di-GMP reaches out into the bacterial RNA world. Sci Signal. 2010;3:pe44–pe44.
Hengge R. Trigger phosphodiesterases as a novel class of c-di-GMP effector proteins. Philos Trans R Soc B Biol Sci. 2016;371:20150498.
Sondermann H, Shikuma NJ, Yildiz FH. You’ve come a long way: c-di-GMP signaling. Curr Opin Microbiol. 2012;15:140–6.
Russell MH, Bible AN, Fang X, Gooding JR, Campagna SR, Gomelsky M, Alexandre G. Integration of the second messenger c-di-GMP into the chemotactic signaling pathway. MBio. 2013;4:e00001-00013.
Minasov G, Padavattan S, Shuvalova L, Brunzelle JS, Miller DJ, Baslé A, Massa C, Collart FR, Schirmer T, Anderson WF. Crystal structures of YkuI and its complex with second messenger cyclic di-GMP suggest catalytic mechanism of phosphodiester bond cleavage by EAL Domains. J Biol Chem. 2009;284:13174–84.
Bedrunka P, Graumann PL. Subcellular clustering of a putative c-di-GMP-dependent exopolysaccharide machinery affecting macro colony architecture in Bacillus subtilis. Environ Microbiol Rep. 2017;9:211–22.
Gong H, Jiang W, Yang Y, Zhang Y, Chen X, Li W, et al. Cyclic di-GMP regulates bacterial colonization and further biocontrol efficacy of Bacillus velezensis against apple ring rot disease via its potential receptor YdaK. Front Microbiol. 2022;13:1034168.
Mallory KL, Miller DP, Oliver LD, Jr., Freedman JC, Kostick-Dunn JL, Carlyon JA, et al. Cyclic-di-GMP binding induces structural rearrangements in the PlzA and PlzC proteins of the Lyme disease and relapsing fever spirochetes: A possible switch mechanism for c-di-GMP-mediated effector functions. Pathog Dis. 2016;74.
Kostick-Dunn JL, Izac JR, Freedman JC, Szkotnicki LT, Oliver LD Jr, Marconi RT. The Borrelia burgdorferi c-di-GMP binding receptors, PlzA and PlzB, are functionally distinct. Front Cell Infect Microbiol. 2018;8:213.
Zhang JJ, Chen T, Yang Y, Du J, Li H, Troxell B, et al. Positive and negative regulation of glycerol utilization by the c-di-GMP binding protein PlzA in Borrelia burgdorferi. J Bacteriol. 2018;200:10–128.
Singh A, Izac JR, Schuler EJA, Patel DT, Davies C, Marconi RT. High-resolution crystal structure of the Borreliella burgdorferi PlzA protein in complex with c-di-GMP: New insights into the interaction of c-di-GMP with the novel xPilZ domain. Pathog Dis. 2021;79:ftab030.
Fazli M, O’Connell A, Nilsson M, Niehaus K, Dow JM, Givskov M, et al. The CRP/FNR family protein Bcam1349 is a c-di-GMP effector that regulates biofilm formation in the respiratory pathogen Burkholderia cenocepacia. Mol Microbiol. 2011;82:327–41.
Yang C, Cui C, Ye Q, Kan J, Fu S, Song S, et al. Burkholderia cenocepacia integrates cis-2-dodecenoic acid and cyclic dimeric guanosine monophosphate signals to control virulence. Proc Natl Acad Sci U S A. 2017;114:13006–11.
Shehata HR, Ettinger CL, Eisen JA, Raizada MN. Genes Required for the anti-fungal activity of a bacterial endophyte isolated from a corn landrace grown continuously by subsistence farmers since 1000 BC. Front Microbiol. 2016;7:1548.
Chan C, Paul R, Samoray D, Amiot NC, Giese B, Jenal U, Schirmer T. Structural basis of activity and allosteric control of diguanylate cyclase. Proc Natl Acad Sci U S A. 2004;101:17084–9.
Christen M, Christen B, Allan MG, Folcher M, Jenö P, Grzesiek S, Jenal U. DgrA is a member of a new family of cyclic diguanosine monophosphate receptors and controls flagellar motor function in Caulobacter crescentus. Proc Natl Acad Sci U S A. 2007;104:4112–7.
Cox CA, Bogacz M, El Abbar FM, Browning DD, Hsueh BY, Waters CM, et al. The Campylobacter jejuni response regulator and cyclic-di-GMP binding CbrR is a novel regulator of flagellar motility. Microorganisms. 2022;10:86.
Duerig A, Abel S, Folcher M, Nicollier M, Schwede T, Amiot N, Giese B, Jenal U. Second messenger-mediated spatiotemporal control of protein degradation regulates bacterial cell cycle progression. Genes Dev. 2009;23:93–104.
Kaczmarczyk A, Hempel AM, von Arx C, Böhm R, Dubey BN, Nesper J, et al. Precise timing of transcription by c-di-GMP coordinates cell cycle and morphogenesis in Caulobacter. Nat Commun. 2020;11:816.
Davis NJ, Cohen Y, Sanselicio S, Fumeaux C, Ozaki S, Luciano J, Guerrero-Ferreira RC, Wright ER, Jenal U, Viollier PH. De- and repolarization mechanism of flagellar morphogenesis during a bacterial cell cycle. Genes Dev. 2013;27:2049–62.
Lori C, Ozaki S, Steiner S, Böhm R, Abel S, Dubey BN, Schirmer T, Hiller S, Jenal U. Cyclic di-GMP acts as a cell cycle oscillator to drive chromosome replication. Nature. 2015;523:236–9.
Lee ER, Baker JL, Weinberg Z, Sudarsan N, Breaker RR. An allosteric self-splicing ribozyme triggered by a bacterial second messenger. Science. 2010;329:845–8.
Hendrick WA, Orr MW, Murray SR, Lee VT, Melville SB. Cyclic di-GMP binding by an assembly ATPase (PilB2) and control of type IV pilin polymerization in the gram-positive pathogen Clostridium perfringens. J Bacteriol. 2017;199:e00034–e17.
Chen Y, Lv M, Liang Z, Liu Z, Zhou J, Zhang LH. Cyclic di-GMP modulates sessile-motile phenotypes and virulence in Dickeya oryzae via two PilZ domain receptors. Mol Plant Pathol. 2022;23:870–84.
Gu W, Chen Y, Chen Z, Gao H, Xie C, Zhang LH, et al. Cyclic di-GMP interact with putrescine via a PilZ domain receptor YcgR. BioRxiv. 2023:2023.2003.2005.531214.
Castiblanco LF, Sundin GW. Cellulose production, activated by cyclic di-GMP through BcsA and BcsZ, is a virulence factor and an essential determinant of the three-dimensional architectures of biofilms formed by Erwinia amylovora Ea1189. Mol Plant Pathol. 2018;19:90–103.
Kharadi RR, Sundin GW. CsrD regulates amylovoran biosynthesis and virulence in Erwinia amylovora in a novel cyclic-di-GMP dependent manner. Mol Plant Pathol. 2022;23:1154–69.
Shi Z, Yang WZ, Lin-Chao S, Chak KF, Yuan HS. Crystal structure of Escherichia coli PNPase: central channel residues are involved in processive RNA degradation. RNA. 2008;14:2361–71.
Tuckerman JR, Gonzalez G, Gilles-Gonzalez MA. Cyclic di-GMP activation of polynucleotide phosphorylase signal-dependent RNA processing. J Mol Biol. 2011;407:633–9.
Steiner S, Lori C, Boehm A, Jenal U. Allosteric activation of exopolysaccharide synthesis through cyclic di-GMP-stimulated protein–protein interaction. EMBO J. 2013;32:354–68.
Fang X, Ahmad I, Blanka A, Schottkowski M, Cimdins A, Galperin MY, Römling U, Gomelsky M. GIL, a new c-di-GMP-binding protein domain involved in regulation of cellulose synthesis in enterobacteria. Mol Microbiol. 2014;93:439–52.
Moreira RN, Dressaire C, Barahona S, Galego L, Kaever V, Jenal U, et al. BolA is required for the accurate regulation of c-di-GMP, a central player in biofilm formation. MBio. 2017;8:10–128.
Schumacher MA, Zeng W. Structures of the activator of K. pneumonia biofilm formation, MrkH, indicates PilZ domains involved in c-di-GMP and DNA binding. Proc Natl Acad Sci U S A. 2016;113:10067–72.
Xu K, Wang L, Xiong D, Chen H, Tong X, Shao X, et al. The Wsp chemosensory system modulates c-di-GMP-dependent biofilm formation by integrating DSF quorum sensing through the WspR-RpfG complex in Lysobacter. npj Biofilms Microbiomes. 2022;8:97.
Zhang J, Hu L, Zhang H, He ZG. Cyclic di-GMP triggers the hypoxic adaptation of Mycobacterium bovis through a metabolic switching regulator ArgR. Environ Microbiol. 2022;24:4382–400.
Li W, He ZG. LtmA, a novel cyclic di-GMP-responsive activator, broadly regulates the expression of lipid transport and metabolism genes in Mycobacterium smegmatis. Nucleic Acids Res. 2012;40:11292–307.
Zhang L, Li W, He ZG. DarR, a TetR-like transcriptional factor, is a cyclic di-AMP-responsive repressor in Mycobacterium smegmatis. J Biol Chem. 2013;288:3085–96.
Li W, Li M, Hu L, Zhu J, Xie Z, Chen J, He ZG. HpoR, a novel c-di-GMP effective transcription factor, links the second messenger’s regulatory function to the mycobacterial antioxidant defense. Nucleic Acids Res. 2018;46:3595–611.
Li W, Hu L, Xie Z, Xu H, Li M, Cui T, He ZG. Cyclic di-GMP integrates functionally divergent transcription factors into a regulation pathway for antioxidant defense. Nucleic Acids Res. 2018;46:7270–83.
Hu Q, Zhang J, Chen Y, Hu L, Li W, He ZG. Cyclic di-GMP co-activates the two-component transcriptional regulator DevR in Mycobacterium smegmatis in response to oxidative stress. J Biol Chem. 2019;294:12729–42.
Hariharan VN, Yadav R, Thakur C, Singh A, Gopinathan R, Singh DP, Sankhe G, Malhotra V, Chandra N, Bhatt A, Saini DK. Cyclic di-GMP sensing histidine kinase PdtaS controls mycobacterial adaptation to carbon sources. FASEB J. 2021;35:e21475.
Petters T, Zhang X, Nesper J, Treuner-Lange A, Gomez-Santos N, Hoppert M, et al. The orphan histidine protein kinase SgmT is a c-di-GMP receptor and regulates composition of the extracellular matrix together with the orphan DNA binding response regulator DigR in Myxococcus xanthus. Mol Microbiol. 2012;84:147–65.
Skotnicka D, Steinchen W, Szadkowski D, Cadby IT, Lovering AL, Bange G, et al. CdbA is a DNA-binding protein and c-di-GMP receptor important for nucleoid organization and segregation in Myxococcus xanthus. Nat Commun. 2020;11:1791.
Seidel M, Skotnicka D, Glatter T, Søgaard-Andersen L. During heat stress in Myxococcus xanthus, the CdbS PilZ domain protein, in concert with two PilZ-DnaK chaperones, perturbs chromosome organization and accelerates cell death. PLoS Genet. 2023;19(6):e1010819.
Ramelot TA, Yee A, Cort JR, Semesi A, Arrowsmith CH, Kennedy MA. NMR structure and binding studies confirm that PA4608 from Pseudomonas aeruginosa is a PilZ domain and a c-di-GMP binding protein. Proteins. 2007;66:266–71.
Christen M, Kulasekara HD, Christen B, Kulasekara BR, Hoffman LR, Miller SI. Asymmetrical distribution of the second messenger c-di-GMP upon bacterial cell division. Science. 2010;328:1295–7.
Habazettl J, Allan MG, Jenal U, Grzesiek S. Solution structure of the PilZ domain protein PA4608 complex with cyclic di-GMP identifies charge clustering as molecular readout. J Biol Chem. 2011;286:14304–14.
Merighi M, Lee VT, Hyodo M, Hayakawa Y, Lory S. The second messenger bis-(3′-5′)-cyclic-GMP and its PilZ domain-containing receptor Alg44 are required for alginate biosynthesis in Pseudomonas aeruginosa. Mol Microbiol. 2007;65:876–95.
Whitney JC, Whitfield GB, Marmont LS, Yip P, Neculai AM, Lobsanov YD, et al. Dimeric c-di-GMP is required for post-translational regulation of alginate production in Pseudomonas aeruginosa. J Biol Chem. 2015;290:12451–62.
Roelofs KG, Wang J, Sintim HO, Lee VT. Differential radial capillary action of ligand assay for high-throughput detection of protein-metabolite interactions. Proc Natl Acad Sci U S A. 2011;108:15528–33.
Zhou E, Seminara AB, Kim SK, Hall CL, Wang Y, Lee VT. Thiol-benzo-triazolo-quinazolinone inhibits Alg44 binding to c-di-GMP and reduces alginate production by Pseudomonas aeruginosa. ACS Chem Biol. 2017;12:3076–85.
De N, Navarro MVAS, Raghavan RV, Sondermann H. Determinants for the activation and autoinhibition of the diguanylate cyclase response regulator WspR. J Mol Biol. 2009;393:619–33.
De N, Pirruccello M, Krasteva PV, Bae N, Raghavan RV, Sondermann H. Phosphorylation-independent regulation of the diguanylate cyclase WspR. PLoS Biol. 2008;6:e67.
Guzzo CR, Dunger G, Salinas RK, Farah CS. Structure of the PilZ–FimXEAL–c-di-GMP complex responsible for the regulation of bacterial type IV pilus biogenesis. J Mol Biol. 2013;425:2174–97.
Jain R, Behrens AJ, Kaever V, Kazmierczak BI. Type IV pilus assembly in Pseudomonas aeruginosa over a broad range of cyclic di-GMP concentrations. J Bacteriol. 2012;194:4285–94.
Qi Y, Chuah MLC, Dong X, Xie K, Luo Z, Tang K, Liang ZX. Binding of cyclic diguanylate in the non-catalytic EAL domain of FimX induces a long-range conformational change. J Biol Chem. 2011;286:2910–7.
Yang F, Tian F, Li X, Fan S, Chen H, Wu M, et al. The degenerate EAL-GGDEF domain protein filp functions as a cyclic di-GMP receptor and specifically interacts with the PilZ-domain protein PXO_02715 to regulate virulence in Xanthomonas oryzae pv. oryzae. Mol Plant Microbe Interact. 2014;27:578–89.
Li Z, Chen JH, Hao Y, Nair SK. Structures of the PelD cyclic diguanylate effector involved in pellicle formation in Pseudomonas aeruginosa PAO1. J Biol Chem. 2012;287:30191–204.
Baraquet C, Harwood CS. Cyclic diguanosine monophosphate represses bacterial flagella synthesis by interacting with the Walker A motif of the enhancer-binding protein FleQ. Proc Natl Acad Sci U S A. 2013;110:18478–83.
Chambers JR, Liao J, Schurr MJ, Sauer K. BrlR from Pseudomonas aeruginosa is a c-di-GMP-responsive transcription factor. Mol Microbiol. 2014;92:471–87.
Jain R, Sliusarenko O, Kazmierczak BI. Interaction of the cyclic-di-GMP binding protein FimX and the type 4 pilus assembly ATPase promotes pilus assembly. PLoS Pathog. 2017;13:e1006594.
Baker AE, Diepold A, Kuchma SL, Scott JE, Ha DG, Orazi G, et al. PilZ domain protein FlgZ mediates cyclic di-GMP-dependent swarming motility control in Pseudomonas aeruginosa. J Bacteriol. 2016;198:1837–46.
Wang F, He Q, Yin J, Xu S, Hu W, Gu L. BrlR from Pseudomonas aeruginosa is a receptor for both cyclic di-GMP and pyocyanin. Nat Commun. 2018;9:2563.
Xu L, Xin L, Zeng Y, Yam JKH, Ding Y, Venkataramani P, Cheang QW, Yang X, Tang X, Zhang LH, Chiam KH, Yang L, Liang ZX. A cyclic di-GMP–binding adaptor protein interacts with a chemotaxis methyltransferase to control flagellar motor switching. Sci Signal. 2016;9(450):ra102–ra102.
Newell PD, Monds RD, O’Toole GA. LapD is a bis-(3′, 5′)-cyclic dimeric GMP-binding protein that regulates surface attachment by Pseudomonas fluorescens Pf0–1. Proc Natl Acad Sci U S A. 2009;106:3461–6.
Navarro MVAS, Newell PD, Krasteva PV, Chatterjee D, Madden DR, O’Toole GA, Sondermann H. Structural basis for c-di-GMP-mediated inside-out signaling controlling periplasmic proteolysis. PLoS Biol. 2011;9:e1000588.
Giacalone D, Smith TJ, Collins AJ, Sondermann H, Koziol LJ, O’Toole GA. Ligand-mediated biofilm formation via enhanced physical interaction between a diguanylate cyclase and its receptor. MBio. 2018;9:01254–18.
Cooley RB, O'Donnell JP, Sondermann H. Coincidence detection and bi-directional transmembrane signaling control a bacterial second messenger receptor. Elife. 2016;5.
Pultz IS, Christen M, Kulasekara HD, Kennard A, Kulasekara B, Miller SI. The response threshold of Salmonella PilZ domain proteins is determined by their binding affinities for c-di-GMP. Mol Microbiol. 2012;86:1424–40.
Morgan JLW, McNamara JT, Zimmer J. Mechanism of activation of bacterial cellulose synthase by cyclic di-GMP. Nat Struct Mol. 2014;21:489–96.
Pérez-Mendoza D, Rodríguez-Carvajal MÁ, Romero-Jiménez L, Farias GdA, Lloret J, Gallegos MT, Sanjuán J. Novel mixed-linkage β-glucan activated by c-di-GMP in Sinorhizobium meliloti. Proc Natl Acad Sci U S A. 2015;112:E757–65.
Schäper S, Steinchen W, Krol E, Altegoer F, Skotnicka D, Søgaard-Andersen L, Bange G, Becker A. AraC-like transcriptional activator CuxR binds c-di-GMP by a PilZ-like mechanism to regulate extracellular polysaccharide production. Proc Natl Acad Sci U S A. 2017;114:e4822–31.
Chin KH, Liang JM, Yang JG, Shih MS, Tu ZL, Wang YC, Sun XH, Hu NJ, Liang ZX, Dow JM, et al. Structural insights into the distinct binding mode of cyclic di-AMP with SaCpaA_RCK. Biochem. 2015;54:4936–51.
Moscoso JA, Schramke H, Zhang Y, Tosi T, Dehbi A, Jung K, et al. Binding of cyclic di-AMP to the Staphylococcus aureus sensor kinase KdpD occurs via the universal stress protein domain and downregulates the expression of the Kdp potassium transporter. J Bacteriol. 2016;198:98–110.
Zhang X, Wang Y, Wu Y, Yuan ZH, Cai Z, Qian W, Ge X, Wang FF. Dual regulatory role exerted by cyclic dimeric GMP to control FsnR-mediated bacterial swimming. MBio. 2022;13:e01414-01422.
Makitrynskyy R, Tsypik O, Nuzzo D, Paululat T, Zechel DL, Bechthold A. Secondary nucleotide messenger c-di-GMP exerts a global control on natural product biosynthesis in streptomycetes. Nucleic Acids Res. 2020;48:1583–98.
Schumacher MA, Wörmann ME, Henderson M, Salinas R, Latoscha A, Al-Bassam MM, Singh KS, Barclay E, Gunka K, Tschowri N. Allosteric regulation of glycogen breakdown by the second messenger cyclic di-GMP. Nat Commun. 2022;13:5834.
Bian J, Liu X, Cheng YQ, Li C. Inactivation of cyclic di-GMP binding protein TDE0214 affects the motility, biofilm formation, and virulence of Treponema denticola. J Bacteriol. 2013;195:3897–905.
Pratt JT, Tamayo R, Tischler AD, Camilli A. PilZ domain proteins bind cyclic diguanylate and regulate diverse processes in Vibrio cholerae. J Biol Chem. 2007;282:12860–70.
Benach J, Swaminathan SS, Tamayo R, Handelman SK, Folta-Stogniew E, Ramos JE, Forouhar F, Neely H, Seetharaman J, Camilli A, Hunt JF. The structural basis of cyclic diguanylate signal transduction by PilZ domains. EMBO J. 2007;26:5153–66.
Krasteva PV, Fong JCN, Shikuma NJ, Beyhan S, Navarro MVAS, Yildiz FH, et al. Vibrio cholerae VpsT regulates matrix production and motility by directly sensing cyclic di-GMP. Science. 2010;327:866–8.
Srivastava D, Harris RC, Waters CM. Integration of cyclic di-GMP and quorum sensing in the control of vpsT and aphA in Vibrio cholerae. J Bacteriol. 2011;193:6331–41.
Srivastava D, Hsieh ML, Khataokar A, Neiditch MB, Waters CM. Cyclic di-GMP inhibits Vibrio cholerae motility by repressing induction of transcription and inducing extracellular polysaccharide production. Mol Microbiol. 2013;90:1262–76.
Roelofs KG, Jones CJ, Helman SR, Shang X, Orr MW, Goodson JR, et al. Systematic identification of cyclic-di-GMP binding proteins in Vibrio cholerae reveals a novel class of cyclic-di-GMP-binding ATPases associated with type II secretion systems. PLoS Pathog. 2015;11:e1005232.
Kariisa AT, Weeks K, Tamayo R. The RNA domain Vc1 regulates downstream gene expression in response to cyclic diguanylate in Vibrio cholerae. PLoS ONE. 2016;11:e0148478.
Joshi A, Mahmoud SA, Kim SK, Ogdahl JL, Lee VT, Chien P, et al. c-di-GMP inhibits LonA-dependent proteolysis of TfoY in Vibrio cholerae. PLoS Genet. 2020;16:e1008897.
Sudarsan N, Lee ER, Weinberg Z, Moy RH, Kim JN, Link KH, Breaker RR. Riboswitches in eubacteria sense the second messenger cyclic di-GMP. Science. 2008;321:411–3.
Chodur DM, Guo L, Pu M, Bruger E, Fernandez N, Waters C, Rowe-Magnus DA. The proline variant of the W[F/L/M][T/S]R cyclic di-GMP binding motif suppresses dependence on signal association for regulator function. J Bacteriol. 2017;199:e00344-e317.
Chin KH, Lee YC, Tu ZL, Chen CH, Tseng YH, Yang JM, et al. The cAMP receptor-like protein CLP is a novel c-di-GMP receptor linking cell–cell signaling to virulence gene expression in Xanthomonas campestris. J Mol Biol. 2010;396:646–62.
Zhao Z, Wu Z, Zhang J. Crystal structure of the YajQ-family protein XC_3703 from Xanthomonas campestris pv. campestris. Acta Crystallogr F Struct Biol Commun. 2016;72:720–5.
Yang F, Tian F, Chen H, Hutchins W, Yang CH, He C. The Xanthomonas oryzae pv. oryzae PilZ domain proteins function differentially in cyclic di-GMP binding and regulation of virulence and motility. Appl Environ Microbiol. 2015;81:4358–67.
Shahbaz MU, Qian S, Yun F, Zhang J, Yu C, Tian F, et al. Identification of the regulatory components mediated by the cyclic di-GMP receptor Filp and its interactor PilZX3 and functioning in virulence of Xanthomonas oryzae pv. oryzae. Mol Plant Microbe Interact. 2020;33:1196–208.
Yan W, Wei Y, Fan S, Yu C, Tian F, Wang Q, et al. Diguanylate cyclase GdpX6 with c-di-GMP binding activity involved in the regulation of virulence expression in Xanthomonas oryzae pv. oryzae. Microorganisms. 2021;9:495.
Zeng X, Huang M, Sun QX, Peng YJ, Xu X, Tang YB, Zhang JY, Yang Y, Zhang CC. A c-di-GMP binding effector controls cell size in a cyanobacterium. Proc Natl Acad Sci U S A. 2023;120:e2221874120.
Enomoto G, Okuda Y, Ikeuchi M. Tlr1612 is the major repressor of cell aggregation in the light-color-dependent c-di-GMP signaling network of Thermosynechococcus vulcanus. Sci Rep. 2018;8:5338.
Bridges D, Fraser ME, Moorhead GBG. Cyclic nucleotide binding proteins in the Arabidopsis thaliana and Oryza sativa genomes. BMC Bioinform. 2005;6:6.
Amor Y, Mayer R, Benziman M, Delmer D. Evidence for a cyclic diguanylic acid-dependent cellulose synthase in plants. Plant Cell. 1991;3:989–95.
Zheng LB, Wang J, Mao Y, Chen RN, Su YQ, Chen J, et al. A novel stimulator of interferon gene (STING) from Larimichthys crocea and their involvement in immune response to ectoparasite Cryptocaryon irritans infection. Fish Shellfish Immunol. 2017;71:239–45.
Blaauboer SM, Mansouri S, Tucker HR, Wang HL, Gabrielle VD, Jin L. The mucosal adjuvant cyclic di-GMP enhances antigen uptake and selectively activates pinocytosis-efficient cells in vivo. Elife. 2015;4:e06670.
Steinberger O, Lapidot Z, Ben-Ishai Z, Amikam D. Elevated expression of the CD4 receptor and cell cycle arrest are induced in Jurkat cells by treatment with the novel cyclic dinucleotide 3’,5’-cyclic diguanylic acid. FEBS Lett. 1999;444:125–9.
Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–8.
Ouyang S, Song X, Wang Y, Ru H, Shaw N, Jiang Y, Niu F, Zhu Y, Qiu W, Parvatiyar K. Structural analysis of the STING adaptor protein reveals a hydrophobic dimer interface and mode of cyclic di-GMP binding. Immunity. 2012;36:1073–86.
Abdul-Sater AA, Grajkowski A, Erdjument-Bromage H, Plumlee C, Levi A, Schreiber MT, Lee C, Shuman H, Beaucage SL, Schindler C. The overlapping host responses to bacterial cyclic dinucleotides. Microbes Infect. 2012;14:188–97.
Parvatiyar K, Zhang Z, Teles RM, Ouyang S, Jiang Y, Iyer SS, Zaver SA, Schenk M, Zeng S, Zhong W. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat Immunol. 2012;13:1155–61.
Martin M, Hiroyasu A, Guzman RM, Roberts SA, Goodman AG. Analysis of Drosophila STING reveals an evolutionarily conserved antimicrobial function. Cell Rep. 2018;23:3537–50.
Boyd CD, O’Toole GA. Second messenger regulation of biofilm formation: Breakthroughs in understanding c-di-GMP effector systems. Annu Rev Cell Dev. 2012;28:439–62.
Cotter PA, Stibitz S. c-di-GMP-mediated regulation of virulence and biofilm formation. Curr Opin Microbiol. 2007;10:17–23.
Whitney JC, Colvin KM, Marmont LS, Robinson H, Parsek MR, Howell PL. Structure of the cytoplasmic region of PelD, a degenerate diguanylate cyclase receptor that regulates exopolysaccharide production in Pseudomonas aeruginosa. J Biol Chem. 2012;287:23582–93.
Kumar M, Chatterji D. Cyclic di-GMP: a second messenger required for long-term survival, but not for biofilm formation, in Mycobacterium smegmatis. Microbiology. 2008;154:2942–55.
Groshong AM, Grassmann AA, Luthra A, McLain MA, Provatas AA, Radolf JD, et al. PlzA is a bifunctional c-di-GMP biosensor that promotes tick and mammalian host-adaptation of Borrelia burgdorferi. PLoS Pathog. 2021;17:e1009725.
Tamayo R, Pratt JT, Camilli A. Roles of cyclic diguanylate in the regulation of bacterial pathogenesis. Annu Rev Microbiol. 2007;61:131–48.
Ryan RP. Cyclic di-GMP signalling and the regulation of bacterial virulence. Microbiology. 2013;159:1286.
Serra DO, Richter AM, Hengge R. Cellulose as an architectural element in spatially structured Escherichia coli biofilms. J Bacteriol. 2013;195:5540–54.
Paul K, Nieto V, Carlquist WC, Blair DF, Harshey RM. The c-di-GMP binding protein YcgR controls flagellar motor direction and speed to affect chemotaxis by a “backstop brake” mechanism. Mol Cell. 2010;38:128–39.
Hou YJ, Yang WS, Hong Y, Zhang Y, Wang DC, Li DF. Structural insights into the mechanism of c-di-GMP-bound YcgR regulating flagellar motility in Escherichia coli. J Biol Chem. 2020;295:808–21.
Weiss CA, Winkler WC. Cyclic di-GMP Signaling in Bacillus subtilis. In microbial cyclic di-nucleotide signaling. Biofouling. 2020;33:241–60.
Tamura K, Stecher G, Kumar S. MEGA11: Molecular evolutionary genetics analysis Version 11. Mol Biol Evol. 2021;38:3022–7.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) grant funded by the Ministry of Education (2021R1A6A1A03039211 and 2022R1A2B5B01001998). This research was also supported by Basic Science Research Program through the National Research Foundation (NRF) of Korea, funded by the Ministry of Education (RS-2023–00241461 to F.Khan).
Author information
Authors and Affiliations
Contributions
F.K.: Conceptualization, Supervision, Funding, Literature Search, Writing-original Draft & Editing, G.-J. J.: Literature Search, Writing-original Draft & Editing, N.T.: Literature Search, Writing-original Draft, & Editing, and Y.-M. K.: Supervision, Funding, Writing-review & Editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Khan, F., Jeong, GJ., Tabassum, N. et al. Functional diversity of c-di-GMP receptors in prokaryotic and eukaryotic systems. Cell Commun Signal 21, 259 (2023). https://doi.org/10.1186/s12964-023-01263-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12964-023-01263-5