Abstract
Background
Mechanical thrombectomy (MT) has become the mainstay of treatment for acute ischemic stroke (AIS) recently. This case-control study aimed to identify the pivotal role of inflammation in the prognosis of AIS patients after MT.
Methods
Altogether, 70 AIS patients who underwent MT were retrospectively recruited for this study. Receiver operating characteristic analysis was performed to demonstrate the sensitivity and specificity of the inflammatory variables for predicting prognosis. A meta-analysis was performed to pool the published results together. Stata software was used for analysis.
Results
There was no differences in pre-MT inflammatory biomarkers between patients who survived and those who died, as well as patients with modified Rankin Scale (mRS) 0–2 and mRS ≥ 3. In contrast, post-MT C-reactive protein (CRP) levels might be a potential parameter to predict death after thrombectomy [area under the curve (AUC), 95%confidence interval (CI), 0.737, 0.587–0.887; p = 0.005; optimal cutoff value = 4.565]. Moreover, post-MT monocyte count might be an appropriate parameter to predict poor long-term prognosis after thrombectomy (AUC, 95%CI, 0.704, 0.575–0.833; p = 0.017; optimal cutoff value = 0.345). A meta-analysis revealed that the pre-MT inflammatory indices, including white blood cell count (weighted mean difference, 95%CI, 1.32, 1.01—1.63), neutrophil count (1.23, 0.95—1.51), monocyte count (0.05, 0.02—0.09), neuthrophil-to-lymphocyte ratio (2.42, 1.98—2.87) and platelet-to-lymphocyte ratio (24.65, 7.99—41.32), were higher in patients with 3-month mRS ≥ 3, and the lymphocyte count (−0.31,−0.43 to −0.18) was lower in this cohort.
Conclusions
Inflammatory indices were significantly associated with the prognosis of patients undergoing MT, especially post-MT CRP and monocyte count, which can predict long-term outcomes.
Similar content being viewed by others
Background
Mechanical thrombectomy (MT) has become the mainstay of treatment for acute ischemic stroke (AIS) in recent years [1]. MT can achieve a successful recanalization rate of 41–88%, which is much higher than that yielded by traditional therapies, including intravenous thrombolysis [2]. However, the functional outcome is limited, with only 30–50% of patients who achieve successful recanalization after MT having a good prognosis [3]. In theory, the early restoration of blood flow can improve neurological disorders caused by ischemia. The mismatch between the rate of successful reperfusion and good prognosis is associated with various factors in which the inflammatory response plays an essential role [4]. It is well-known that a substantial inflammatory response occurs immediately after brain infarction, including neutrophil infiltration, lymphocyte redistribution, monocyte invasion, and proinflammatory cytokine release [5]. Several inflammatory indices, especially adopted blood sample indices such as leukocyte, neutrophil, lymphocyte, and monocyte counts, are widely used to evaluate the severity and predict the prognosis of stroke in clinical settings [6]. These inflammatory indices are also available for AIS patients undergoing MT. The inflammatory status before MT may influence brain injury after reperfusion, and the inflammatory status after MT appears to affect brain repair in the long run [7]. Therefore, both baseline and post-MT inflammatory indices are considered to be linked to the efficacy of recanalization; this has garnered the attention of researchers in recent years owing to its practical value because it appears to be a safe, low-cost, and easy-to-use strategy for predicting the prognosis of patients undergoing MT [4]. However, these studies yielded conflicting results and did not provide any convincing conclusions [7]. In this regard, we analyzed the cohort in our hospital and conducted a meta-analysis to identify the pivotal role of inflammation in the prognosis of AIS patients after MT.
Methods
Study population
Altogether, 70 AIS patients who underwent MT at Tianjin Medical University General Hospital from January 2020 to September 2022 were retrospectively recruited for this study (Figure S1). The informed consent for undergoing MT had been obtained from all subjects. There were no age or sex restrictions. Some patients underwent intravenous thrombolysis before MT. This was a retrospective case-control study which only recruits the patients screened by the inclusion/exclusion criteria. The inclusion criteria were as follows: (1) AIS patients with a National Institutes of Health Stroke Scale (NIHSS) score ≥ 6 [8]; (2) onset-to-puncture time (OPT) ≤ 6 h, or 6–24 h with mismatch volume > 15 ml as assessed by computed tomography perfusion imaging (according to Dawn Trial); (3) Alberta Stroke Program Early CT Score (ASPECTS) ≥ 6; (4) the thrombectomy sites included internal carotid artery (ICA), middle cerebral artery (MCA), basilar artery (BA), and vertebral artery (VA); and (5) the occluded vessels achieved successful recanalization (TICI 2b or 3) after thrombectomy. Exclusion criteria were as follows: (1) ischemic events caused by stent or carotid endarterectomy; (2) patients comorbid with infectious diseases (such as pneumonia, urinary infection and septicopyemia), autoimmune diseases, cancer, trauma and other complications that might influence the body immune system within 72 h after MT; (3) deaths or poor outcomes caused by cardiac diseases, digestive system diseases, and/or other medical complications; and (4) participants lacking complete data.
According to the Cochrane systematic review and the Preferred Reporting Items, a meta-analysis was performed in this study. PubMed, Embase and ClinicalTrials.gov were searched for publications written by English related to specific keywords, such as ‘thrombectomy,’ ‘inflammation,’ and ‘immunology,’ which were published prior to March 2023 (A detailed search strategy was shown in Table S1). The references of the retrieved articles were thoroughly reviewed subsequently for additional reports that might have been missed in our search. The enrolled studies fulfilled the following criteria: (1) patients treated with MT alone or MT plus thrombolysis; (2) case-control analysis for comparisons between 3-month modified Rankin Scale (mRS) ≤ 2 and mRS > 2 [9], and/or survivors and deaths in the long run; and (3) inflammatory markers presented as means ± standard deviation (SD) or median (interquartile range, IQR). Exclusion criteria were as follows: (1) cohorts comprising patients treated with thrombolysis alone; (2) inflammatory markers presented with odds ratio (OR) and relative risk (RR); and (3) end-point events including hemorrhage, edema, early neurological deterioration, and short-term mRS.
Data collection
Clinical data included demographics, OPT, risk factors, baseline stroke severity, clinical outcomes, and inflammatory biomarkers (the type of apparatus was XS800, sysmex and Backman image-800), such as white blood cells (WBC), neutrophils, lymphocytes, monocytes, C-reactive protein (CRP), and hyper-sensitive C-reaction protein (hs-CRP). The neutrophil-to-lymphocyte ratio (NLR) indicated the ratio of neutrophil count to lymphocyte count; the lymphocyte-to-monocyte ratio (LMR) indicated the ratio of lymphocyte count to monocyte count; the platelet-to-lymphocyte ratio (PLR) indicated the ratio of platelet count to lymphocyte count; and the systemic immune-inflammation index (SII) was indicated by platelet×neutrophil/lymphocyte. In addition, the severity and prognosis of stroke were evaluated using NIHSS and mRS scores at admission and follow-up.
Statistical analysis
Stata 15.1 was used for the analysis in this study. Continuous variables following a Gaussian distribution were expressed as mean ± SD and analyzed with Student’s t-test; otherwise, the data were presented as median (IQR) and analyzed using the Mann–Whitney U test. Categorical variables are presented as numbers (percentages) and were analyzed using the Pearson χ2 test or Fisher’s exact test. Furthermore, a logistic regression model was used to adjust confounding effects (including age, OPT, NIHSS at admission, thrombectomy site, pre-MT ASPECTS, all of them which were considered to be correlated with the safety and efficacy of MT). The associations between inflammatory biomarkers and baseline NIHSS were assessed using Pearson correlation. The associations between inflammatory biomarkers and clinical outcomes were assessed using Spearman’s correlation. Receiver operating characteristic (ROC) analysis was performed to demonstrate the sensitivity and specificity of the variables for predicting prognosis. The area under the ROC curve (AUC) reflected the accuracy of prediction, and the power was further evaluated with positive predictive value (+ PV), negative predictive value (-PV), positive likelihood ratio (+ LR), and negative likelihood ratio (-LR). Optimal cutoff values were considered along with maximal Youden’s index [10]. Statistical significance was set at p < 0.05.
As for meta-analysis, available data was processed using Stata software (version 15.0 SE) in this study. Only the mean (SD) was used for the pooled analysis. A funnel plot was used to evaluate publication bias (Using RevMan 5.3 software). Chi-Square test was used to assess the heterogeneity of data. Pooled analysis was conducted with the fixed-effects model using Mantel-Haenszel method when the heterogeneity was expected to be available (I2 < 50%). Otherwise, the random-effects model computed by the DerSimonian-Laird method was conducted (I2≥50%). Hypothesis testing was done using the U test expressed by Z and P-value. P-value < 0.05 were considered statistically significant.
Results
Population characteristics
A total of 70 patients were enrolled (52 males and 18 females, average age was 63.94 ± 11.59 years), of whom 87.1% had hypertension, 64.3% had diabetes, and 95.7% had hyperlipidemia. Thrombectomy sites included the ICA (31.4%), MCA (42.9%), BA (24.3%), and VA (1.4%). The average hospitalization time was 14.5 ± 4.3 days and the average follow-up time was 47.5 ± 23.9 days. The mean OPT was 6.48 ± 3.12 h and the baseline mean NIHSS score was 16.81 ± 7.25. The median (IQR) of pre-MT ASPECTS and post-MT ASPECTS were 8.0 (9.0, 10) and 6.0 (3.0, 8.0) respectively. A total of 23 patients (32.9%) died following MT, of whom 18 died during hospitalization (the average onset-to-dead time was 10.5 ± 4.3 days) and 5 during follow-up (the average onset-to-dead time was 45.4 ± 21.3 days). Patients with an mRS score 0–2 accounted for 7.1% of the patients at discharge and 21.4% at follow-up, while those with mRS 3–5 accounted for 67.1% of those at discharge and 45.7% at follow-up. The details aforementioned could be seen in Table 1. The differences of baseline characteristics between favorite and unfavorite outcomes (survivors versus deaths, and patients with follow-up mRS 0–2 versus mRS ≥ 3) were shown in Table S2 and Table S3.
Association between pre-MT inflammatory parameters and prognosis
Fifty-four patients completed serum biomarker testing before MT. Pearson correlation analysis showed no correlation between the pre-MT parameters and baseline NIHSS scores (Table S4). Spearman’s correlation analysis showed that pre-MT monocyte counts (r = 0.314, p = 0.021) were correlated with follow-up mRS scores, despite a very low correlation (Table S5).
Sixteen patients (29.6%) died after MT, and this study found no differences in pre-MT inflammatory biomarkers between patients who survived and those who died (all p-value and adjusted p-value > 0.05). Parameters (WBC counts and monocyte counts) that had between-group differences with p-value < 0.1 were included in the ROC analysis. The AUC of WBC counts was 0.660, with 95% confidence interval (CI) 0.493–0.826 (p = 0.066), and that of monocyte counts was 0.661, with 95% CI 0.512–0.811 (p = 0.063). These results indicate that the pre-MT parameters in this study were not associated with death after MT (Table 2 and Table S6).
Forty-four patients (81.5%) had mRS ≥ 3 at follow-up. Univariate analysis presented significant differences in pre-MT monocyte percentage (follow-up mRS 0–2 vs. mRS ≥ 3, 4.31 ± 2.46 vs. 5.99 ± 2.19, p = 0.037) between patients with mRS 0–2 and mRS ≥ 3 at follow-up, while the multivariate analysis did not find the differences between the two arms. Based on comparisons between patients with follow-up mRS 0–2 and mRS ≥ 3, the parameters (WBC counts and monocyte counts) that had between-group differences with p-value < 0.1 were included in the ROC analysis. The AUC of WBC counts was 0.400, with 95% CI 0.221–0.579 (p = 0.327), and that of monocyte counts was 0.689, with 95% CI 0.504–0.874 (p = 0.065). The results revealed that the pre-MT parameters in this study might be underpowered to predict the prognosis (Table 3 and Table S7).
Association between post-MT inflammatory parameters and prognosis
A total of 67 patients completed serum biomarker testing after MT. The average post-MT serum collection time was 1.8 ± 0.9 days. Spearman’s correlation analysis showed that post-MT lymphocyte percentage (r=−0.287, p = 0.019), monocyte counts (r = 0.266, p = 0.030), NLR (r = 0.272, p = 0.026) and LMR (r=−0.317, p = 0.009) were correlated with follow-up mRS scores, despite a very low correlation (Table S8).
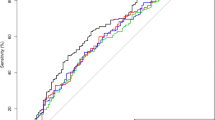
Twenty-three patients (34.3%) died after the MT. Univariate analysis showed significant differences in post-MT LMR (survivors vs. death, 2.57[1.29, 5.89] vs. 1.53[1.07, 3.55], p = 0.025) and CRP levels (2.24[0.90, 6.36] vs. 9.68[4.09, 14.90], p = 0.005) between patients who survived and those who died, while multivariate analysis indicated that post-MT WBC counts (survivors vs. death, OR, 95%CI, 0.806, 0.785–0.827, adjusted p = 0.043), CRP levels (0.824, 0.803–0.845, adjusted p = 0.008) and hs-CRP levels (0.968, 0.943–0.993, adjusted p = 0.032) were statistically different between patients who survived and those who died. ROC analysis was performed with the post-MT parameters that had between-group differences with a p-value < 0.1 (including WBC counts, monocyte counts, lymphocyte counts, NLR, LMR, SII, CRP levels, hs-CRP levels). The parameters with AUC > 0.7 included post-MT CRP levels (AUC, 95%CI, 0.737, 0.587–0.887, p = 0.005) and hs-CRP levels (0.710, 0.501–0.920, p = 0.061); however, only CRP levels reached statistical significance. Moreover, the parameter with AUC > 0.7 was further calculated with an optimal cutoff value to predict death after thrombectomy. The optimal cutoff value for CRP levels was 4.565 (+ PV = 0.609, -PV = 0.857, +LR = 2.850, -LR = 0.305). The patients were classified into two groups according to the optimal cutoff values to validate the predictive power of post-MT CRP levels for death after thrombectomy. Four out of 28 patients with post-MT CRP < 4.565 and 14 out of 23 with CRP ≥ 4.565 died after thrombectomy (p = 0.001, kappa value was 0.475). The results demonstrated that post-MT CRP levels might be a potential parameter to predict death after thrombectomy (Table 4 and Table S6).
After MT, 52 patients (77.6%) had mRS score ≥ 3 at follow-up. Univariate analysis showed that post-MT monocyte counts (follow-up mRS 0–2 vs. mRS ≥ 3, 0.20[0.12, 0.34] vs. 0.49[0.17, 0.93], p = 0.017), monocyte percentage (2.00[1.30, 3.60] vs. 5.90[1.63, 7.68], p = 0.032) and LMR (5.04[2.21, 5.90] vs. 1.60[1.07, 4.37], p = 0.008) were significantly different between patients with mRS 0–2 and mRS ≥ 3 at follow-up, while the multivariate analysis did not find the differences between the two cohorts. The post-MT monocyte counts, monocyte percentage, and LMR had between-group differences with p-value < 0.1, and were therefore further analyzed using ROC analysis. The parameter with AUC > 0.7 only included post-MT monocyte counts (AUC, 95%CI, 0.704, 0.575–0.833, p = 0.017), and it was further calculated with optimal cutoff value to predict mRS ≥ 3 at 3 months. The optimal cutoff value of monocyte counts was 0.345 (+ PV = 0.919, -PV = 0.400, +LR = 3.270, -LR = 0.433). Altogether, 18 of 30 patients with post-MT monocyte counts < 0.345 and 34 of 37 with monocyte counts ≥ 0.345 were confirmed as mRS ≥ 3 at follow-up after MT (p = 0.002, kappa value was 0.335). The results suggest that post-MT monocyte counts might be an appropriate parameter to predict poor long-term prognosis after thrombectomy (Table 5 and Table S7).
A meta-analysis of the association between inflammatory parameters and prognosis after thrombectomy
The screening strategy identified a total of 18 publications for this review (the screening flow chart is detailed in supplemental material Figure S2, and a funnel plot evaluating publication bias is shown in Figure S3) [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. The parameters with means and SD were finally included in the meta-analysis. Compared to patients with 3-month mRS < 3, the pre-MT inflammatory indices, including WBC counts (weighted mean difference, 95%CI, 1.32, 1.01—1.63), neutrophil counts (1.23, 0.95—1.51), monocyte counts (0.05, 0.02—0.09), NLR (2.42, 1.98—2.87) and PLR (24.65, 7.99—41.32), were higher in patients with 3-month mRS ≥ 3, and the lymphocyte counts (−0.31,−0.43 to −0.18) was lower in this cohort (Fig. 1). A summary of the results of the included publications is presented in Table S9 [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. The post-MT inflammatory indices were not available for meta-analysis. Even then, all the results obtained from the involved studies showed that a higher neutrophil counts and NLR, and lower lymphocyte counts are associated with poor prognosis (Table S10) [13, 15,16,17].
Discussion
The presented study showed that pre-MT monocyte counts were correlated with the follow-up mRS, however, there was no difference in the pre-MT inflammatory indices between patients who survived and those who died, as well as patients with mRS 0–2 and mRS 3–6 scores at follow-up. We also found that post-MT lymphocyte percentage, monocyte counts, NLR, and LMR were correlated with the follow-up mRS. Post-MT WBC counts, CRP levels and hs-CRP levels were significantly different between patients who survived and those who died. However, there was no difference in the post-MT inflammatory indices between patients with mRS 0–2 and mRS 3–6 scores at follow-up. In addition, ROC analysis showed that post-MT CRP levels can predict death, and post-MT monocyte counts can predict poor outcomes after MT. Hence, post-MT CRP levels and monocyte counts seemed to be more available to predict the prognosis of patients undergoing MT.
A meta-analysis of published studies showed that pre-MT inflammatory indices, including WBC counts, neutrophil counts, monocyte counts, lymphocyte counts, NLR and PLR, were capable of predicting the prognosis of patients treated with MT. In addition, the post-MT inflammatory indices including neutrophil counts, NLR, and lymphocyte counts also appeared to predict the prognosis of patients treated with MT, as demonstrated by the results of previous studies. Hence, the assessment of inflammatory indices may be imperative for predicting the long-term clinical outcomes of patients with MT.
Inflammatory markers have been widely used in the prediction of poor prognosis and complications of ischemic stroke [6, 29]. After an ischemic stroke, several proinflammatory factors, such as brain-derived antigens and damage-associated molecular patterns (DAMPs), enter the body’s circulation from the injured brain region, resulting in intense inflammatory responses that eventually disrupt the blood–brain barrier (BBB) [30]. Simultaneously, circulating neutrophils infiltrate the injured tissue within the first hour after the stroke, releasing proteolytic enzymes, such as arachidonic acid derivatives, superoxide radicals, and matrix metalloproteinase [31]. These enzymes can degrade tight junction proteins and basal lamina type-IV collagen, leading to BBB disruption and tissue damage [32]. Hence, neutrophils can promote an excessive inflammatory response and are considered to be correlated with poor prognosis in stroke patients. The stress caused by stroke during the acute phase activates the hypothalamic-pituitary-adrenal axis, leading to cortisol secretion and excessive sympathetic tone activation, both of which decrease lymphocyte levels [33]. Lymphocytes have a downregulation effect on inflammation; thus, lower lymphocyte levels might indicate milder symptoms and better prognosis [29]. Monocytes participate in inflammatory and prothrombotic pathways by interacting with platelets and endothelial cells in AIS and are also associated with the prognosis of AIS [34]. These immunological cells release various inflammatory factors, further promoting complex inflammatory responses [29]. In theory, the inflammatory response also affects the efficacy of MT after AIS. Excessive inflammatory activation disrupts the BBB and brain tissue, resulting in a high risk of intracranial hemorrhage and malignant brain edema, both of which are strongly associated with the prognosis of AIS patients [7].
Although some studies have reported an association between inflammatory indices and the prognosis of AIS patients undergoing MT, the results are diverse. We collected data from our hospital and performed a meta-analysis for this issue in this article. Most of the publications present pre-MT inflammatory indices, including pre-MT WBC counts, neutrophil counts, lymphocyte counts, monocyte counts, NLR, PLR, LMR, and SII, which were strongly associated with prognosis after MT [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. Our pooled results showed that higher pre-MT WBC counts, neutrophil counts, monocyte counts, NLR and PLR, and lower lymphocyte counts were associated with poor long-term outcomes (There were only two studies involved into the analysis of NLR, so the heterogeneity was inevitable. Even so, the involved two study reached the same conclusions that higher pre-MT NLR was associated with poor long-term outcomes). However, our findings do not yield similar results, showing that only monocyte counts was significantly different between patients with mRS 0–2 and mRS ≥ 3 at follow-up after univariate analysis but not after multivariate analysis. Moreover, our study showed no difference in the pre-MT inflammatory indices between patients who survived and those who died. We also performed ROC analysis for the pre-MT parameters that had between-group differences with a p-value < 0.1, and no parameter could be used as a predictor of prognosis or death after MT. In theory, pre-MT inflammation is capable of affecting the efficacy of MT; however, the prognosis is more likely to be influenced by a series of factors such as OPT, number of thrombectomy device passes, ASPECTS, and intracranial hemorrhage after reperfusion [35,36,37]. Many confounding factors affect the predictive power of inflammatory indices. In this respect, pre-MT assessment is ineffective, and thus we considered that pre-MT inflammatory indices might be underpowered to predict prognosis after MT.
In contrast, the post-MT assessment appears to be a promising candidate for the prediction of prognosis after MT. The influencing factors before, during, and after MT may affect the body’s inflammatory status. This multi-collinearity renders the post-MT inflammatory indices powerful for predicting prognosis after MT. As expected, our findings indicated that post-MT lymphocyte percentage, monocyte counts, NLR, and LMR were correlated with the follow-up mRS; post-MT LMR and CRP levels in those who survived were significantly different from those who died when performing univariate analysis, and post-MT WBC counts, CRP levels and hs-CRP levels were significantly different between the two arms when conducting multivariate analysis. Furthermore, univariate analysis showed that post-MT monocyte counts, monocyte percentage and LMR were significantly different between patients with mRS 0–2 and mRS ≥ 3 at follow-up, despite without statistical significance after undergoing multivariate analysis. ROC analysis showed that post-MT CRP levels, with an optimal cutoff value of 4.565, can predict death, and post-MT monocyte counts, with an optimal cutoff value of 0.345, can predict poor outcomes after MT. Previous studies have demonstrated that the post-MT inflammatory status plays an essential role in prognosis after MT [13, 15,16,17]. They regarded neutrophil counts, lymphocyte counts, and NLR as appropriate prognostic predictors. In brief, the post-MT inflammatory status may be an important factor influencing the prognosis of patients undergoing MT.
This retrospective analytical cohort had several limitations. First, the small sample size may have biased the results toward null hypothesis. Apart from post-MT CRP and monocyte counts, other inflammatory indices such as neutrophil counts, lymphocyte counts, NLR, and LMR might also predict prognosis after MT if the sample size is larger. In addition, different sample sizes were used to assess different markers, which might have influenced the results. We performed a meta-analysis with previous studies to further offset this limitation. Second, because of the retrospective nature of the study, bias in data collection and missing data might have influenced our results. This is a case-control study actually, rather than cross-section study. Whereby, the presentation percentage cannot be used as epidemic data for these populations due to high selection bias. Third, other acknowledged inflammatory markers, such as interleukin (IL)-6, IL-10, and tumor necrosis factor (TNF)-α, were not included in this study because of the limitations of data collection. These indices also appeared to be suitable for the prediction of prognosis after MT and could even be better than the indices investigated in this study; however, their predictive power is still unknown. Further investigations are warranted to address these issues. Fourth, the recruited patients had severe neurological deficits and were comorbid with several complications at admission. Therefore, the mRS scores and death rates were high in this cohort, rendering the between-group sample size unbalanced. In addition, the average follow-up time was 47.5 ± 23.9 days, rather than 30-day or 90-day. This was because we conducted the follow-up evaluation in the outpatient setting, so we could not guarantee the follow-up evaluation performed on time. This limitation might also affect our results. Fifth, other well-known predictive factors for outcome such as collateral status, clot features and penumbra were not investigated in this study. However, we considered inflammatory markers, especially the post-MT indices might had high multi-collinearity with other other predictive factors because they might also affect the body’s immune system.
Conclusions
The post-MT peripheral inflammatory markers in AIS patients undergoing MT, such as WBC counts, CRP levels and hs-CRP levels were significantly associated with the prognosis of these patients. Post-MT CRP levels can predict death, and monocyte counts can predict poor outcomes after MT, which seemed to be more available to predict the prognosis of patients undergoing MT. Previous studies also indicated that pre-MT inflammatory indices (WBC counts, neutrophil counts, monocyte counts, lymphocyte counts, NLR and PLR) as well as the post-MT inflammatory indices (neutrophil counts, NLR, and lymphocyte counts) were capable of predicting the prognosis of patients treated with MT. Considering the limitations of this study, future prospective studies with a larger sample size and a greater variety of inflammatory markers are necessary to draw more convincing conclusions.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- MT:
-
Mechanical thrombectomy
- AIS:
-
acute ischemic stroke
- OPT:
-
onset-to-puncture time
- ICA:
-
internal carotid artery
- MCA:
-
middle cerebral artery
- BA:
-
basilar artery
- VA:
-
vertebral artery
- mRS:
-
modified Rankin Scale
- SD:
-
standard deviation
- IQR:
-
interquartile range
- OR:
-
odds ratio
- RR:
-
relative risk
- WBC:
-
white blood cells
- CRP:
-
C-reactive protein
- hs-CRP:
-
hyper-sensitive C-reaction protein
- NLR:
-
neutrophil-to-lymphocyte ratio
- LMR:
-
lymphocyte-to-monocyte ratio
- PLR:
-
platelet-to-lymphocyte ratio
- SII:
-
systemic immune-inflammation index
- ROC:
-
receiver operating characteristic
- +PV:
-
positive predictive value
- -PV:
-
negative predictive value
- +LR:
-
positive likelihood ratio
- -LR:
-
negative likelihood ratio
- AUC:
-
area under the ROC curve
- DAMPs:
-
damage-associated molecular patterns
- BBB:
-
blood–brain barrier
- IL:
-
interleukin
References
Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with Acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of Acute ischemic stroke: a Guideline for Healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–418.
Campbell B, Donnan GA, Lees KR, et al. Endovascular stent thrombectomy: the new standard of care for large vessel ischaemic stroke. Lancet Neurol. 2015;14(8):846–54.
Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–31.
Mechtouff L, Debs N, Frindel C, et al. Association of blood biomarkers of inflammation with Penumbra Consumption after mechanical thrombectomy in patients with Acute ischemic stroke. Neurology. 2022;99(18):e2063–71.
Kim JY, Kawabori M, Yenari MA. Innate inflammatory responses in stroke: mechanisms and potential therapeutic targets. Curr Med Chem. 2014;21(18):2076–97.
Anrather J, Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics. 2016;13(4):661–70.
Sharma D, Spring KJ, Bhaskar S. Role of neutrophil-lymphocyte ratio in the prognosis of Acute Ischaemic Stroke after Reperfusion Therapy: a systematic review and Meta-analysis. J Cent Nerv Syst Dis. 2022;14:11795735221092518.
Kwah LK, Diong J. National Institutes of Health Stroke Scale (NIHSS). J Physiother. 2014;60(1):61.
Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38(3):1091–6.
Hajian-Tilaki K. The choice of methods in determining the optimal cut-off value for quantitative diagnostic test evaluation. Stat Methods Med Res. 2018;27(8):2374–83.
Shi Z, Guo S, Pan J, Xu C, Geng Y, Zheng S. Increased postoperative fasting glucose is Associated with unfavorable outcomes in patients treated with mechanical Thrombectomy treatment. Front Neurol. 2021;12:668363.
Kim SY, Yi HJ, Shin DS, Kim BT. Prognostic significance of platelet-to-lymphocyte and platelet-to-neutrophil ratios in patients with mechanical thrombectomy for acute ischemic stroke. J Cerebrovasc Endovasc Neurosurg. 2022;24(3):221–31.
Li SJ, Cao SS, Huang PS, Nie X, Fu Y, Liu JR. Post-operative neutrophil-to-lymphocyte ratio and outcome after thrombectomy in acute ischemic stroke. Front Neurol. 2022;13:990209.
Ma J, Guo W, Xu J, et al. Association of platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio with outcomes in stroke patients achieving successful recanalization by endovascular thrombectomy. Front Neurol. 2022;13:1039060.
Bartt R, Sercy E, Pirahanchi Y, Frei D Jr, Bar-Or D. Associations of Neutrophil-Lymphocyte ratios with reperfusion and functional outcomes in ischemic stroke after endovascular therapy. J Stroke Cerebrovasc Dis. 2022;31(12):106843.
Aly M, Abdalla RN, Batra A, et al. Follow-up neutrophil-lymphocyte ratio after stroke thrombectomy is an independent biomarker of clinical outcome. J Neurointerv Surg. 2021;13(7):609–13.
Feng Y, Bai X, Li W, et al. Postoperative neutrophil-lymphocyte ratio predicts unfavorable outcome of acute ischemic stroke patients who achieve complete reperfusion after thrombectomy. Front Immunol. 2022;13:963111.
Wang L, Cheng Q, Peng M, et al. The relationship between the platelet to leukocyte ratio and mechanical thrombectomy outcomes in acute ischemic stroke patients. Neurol Res. 2020;42(10):890–6.
Yi HJ, Sung JH, Lee DH. Systemic inflammation response index and systemic Immune-inflammation index are Associated with clinical outcomes in patients treated with mechanical thrombectomy for large artery occlusion. World Neurosurg. 2021;153:e282–9.
Chen Z, He Y, Su Y, Sun Y, Zhang Y, Chen H. Association of inflammatory and platelet volume markers with clinical outcome in patients with anterior circulation ischaemic stroke after endovascular thrombectomy. Neurol Res. 2021;43(6):503–10.
Pinčáková K, Krastev G, Haring J, Mako M, Mikulášková V, Bošák V. Low Lymphocyte-to-Monocyte Ratio as a Possible Predictor of an Unfavourable Clinical Outcome in Patients with Acute Ischemic Stroke after Mechanical Thrombectomy. Stroke Res Treat. 2022. 2022: 9243080.
Li T, Chen Z, Zhu X, et al. Neutrophil Count, Intracranial atherosclerotic stenosis, and prognosis of ischemic stroke after endovascular treatment: a mediation analysis. Front Neurol. 2020;11:605852.
Ferro D, Matias M, Neto J, et al. Neutrophil-to-lymphocyte ratio predicts cerebral edema and clinical worsening early after reperfusion therapy in stroke. Stroke. 2021;52(3):859–67.
Semerano A, Laredo C, Zhao Y, et al. Leukocytes, collateral circulation, and reperfusion in ischemic stroke patients treated with mechanical thrombectomy. Stroke. 2019;50(12):3456–64.
Boisseau W, Desilles JP, Fahed R, et al. Neutrophil count predicts poor outcome despite recanalization after endovascular therapy. Neurology. 2019;93(5):e467–75.
Li X, Wu F, Jiang C, et al. Novel peripheral blood cell ratios: effective 3-month post-mechanical thrombectomy prognostic biomarkers for acute ischemic stroke patients. J Clin Neurosci. 2021;89:56–64.
Oh SW, Yi HJ, Lee DH, Sung JH. Prognostic significance of various inflammation-based scores in patients with mechanical thrombectomy for Acute ischemic stroke. World Neurosurg. 2020;141:e710–7.
Ozgen E, Guzel M, Akpinar CK, Yucel M, Demir MT, Baydin A. The relationship between neutrophil/lymphocyte, monocyte/ /lymphocyte, platelet/lymphocyte ratios and clinical outcomes after ninety days in patients who were diagnosed as having acute ischemic stroke in the emergency room and underwent a mechanical thro. Bratisl Lek Listy. 2020;121(9):634–9.
Kim J, Song TJ, Park JH, et al. Different prognostic value of white blood cell subtypes in patients with acute cerebral infarction. Atherosclerosis. 2012;222(2):464–7.
Liesz A, Dalpke A, Mracsko E, et al. DAMP signaling is a key pathway inducing immune modulation after brain injury. J Neurosci. 2015;35(2):583–98.
Weisenburger-Lile D, Dong Y, Yger M, et al. Harmful neutrophil subsets in patients with ischemic stroke: Association with disease severity. Neurol Neuroimmunol Neuroinflamm. 2019;6(4):e571.
Gidday JM, Gasche YG, Copin JC, et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol. 2005;289(2):H558–68.
Maisel AS, Knowlton KU, Fowler P, et al. Adrenergic control of circulating lymphocyte subpopulations. Effects of congestive heart failure, dynamic exercise, and terbutaline treatment. J Clin Invest. 1990;85(2):462–7.
Carmona-Mora P, Knepp B, Jickling GC, et al. Monocyte, neutrophil, and whole blood transcriptome dynamics following ischemic stroke. BMC Med. 2023;21(1):65.
Li X, Gu F, Ding J, et al. The predictors and prognosis for unexpected reocclusion after mechanical thrombectomy: a meta-analysis. Ann Transl Med. 2020;8(23):1566.
Albers GW, Marks MP, Kemp S, et al. Thrombectomy for Stroke at 6 to 16 hours with selection by Perfusion Imaging. N Engl J Med. 2018;378(8):708–18.
Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between Deficit and Infarct. N Engl J Med. 2018;378(1):11–21.
Acknowledgements
We would like to thank all patients and doctors who participated in this study for their cooperation.
Funding
This study was sponsored by the National Natural Science Foundation of China (82201432), the Natural Science Foundation of Tianjin City (19JCYBJC27500) and Tianjin Key Medical Discipline (Specialty) Construction Project.
Author information
Authors and Affiliations
Contributions
JD and HW conceived the study, took responsibility for this paper. LS and XL collected the clinical data. XT, LZ, MZ and XY took part in the discussion of the feasibility of the experimental and clinical design. JD and HW performed the meta-analysis. JD, XT and LZ completed the article searching and screening. HW and JD drafted the manuscript. All authors critically revised it.
Corresponding author
Ethics declarations
Ethical approve
This study had been approved by the institutional ethic committee of Tianjin Medical University General Hospital in accordance with the guidelines of the 1964 Declaration of Helsinki. The informed consent for undergoing MT had been obtained from all subjects.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, H., Tian, X., Liao, Z. et al. Inflammatory biomarkers may be associated with poor outcomes after mechanical thrombectomy. Thrombosis J 22, 58 (2024). https://doi.org/10.1186/s12959-024-00630-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12959-024-00630-7