Abstract
Background
It remains unknown whether anticoagulation for persistent left ventricular (LV) thrombus should be continued indefinitely. Identifying patients with a high risk of thrombus unresolved may be helpful to determine the optimum anticoagulation duration. This study aimed to develop a prediction model to forecast thrombus persistence or recurrence in patients with LV thrombus.
Methods
We enrolled patients prospectively from 2020 to 2022 and retrospectively from 2013 to 2019 at the National Center of Cardiovascular Diseases of China. The two cohorts were then combined to derive predictive models of thrombus persistence/recurrence. The primary study comprised patients who received systemic oral anticoagulants and had imaging records available at the end of a 3-month follow-up period. The Lasso regression algorithm and the logistic regression were performed to select independent predictors. The calibration curve was generated and a nomogram risk prediction model was applied as a risk stratification tool.
Results
A total of 172 (64 in the prospective cohort and 108 in the retrospective cohort) patients were included, with 124 patients in a training set and 48 patients in a validation set. Six predictors were incorporated into the multivariate logistic regression prediction model. The area under the receiving operating characteristic was 0.852 in the training set and 0.631 in the validation set. Patients with protuberant thrombus and higher baseline D-dimer levels had a reduced risk of persistence/recurrence (OR 0.17, 95% CI 0.03–0.69, P = 0.025; OR 0.67, 95% CI 0.43–0.91, P = 0.030, separately), whereas thicker thrombus was linked to an increased rate of persistent thrombus (OR 1.11, 95% CI 1.05–1.20, P = 0.002). Additionally, patients with diverse diagnoses or receiving different antiplatelet treatments had different rates of LV thrombus persistence/recurrence at 3 months.
Conclusions
This prediction model provides tools to forecast the occurrence of persistent/recurrent thrombus and allows the identification of characteristics associated with unresolved thrombus. To validate the model and determine the duration of anticoagulation in patients with persistent thrombus, prospective randomized trials are necessary.
Similar content being viewed by others
Introduction
Left ventricular (LV) thrombus has been associated with up to 22% risk of embolization in the past [1]. Guidelines recommend that patients with ischemic cardiomyopathy (ICM) and LV thrombus should receive oral anticoagulation for 3 months [2], while patients with nonischemic cardiomyopathy (NICM) should be treated with oral anticoagulation for at least 3–6 months, to reduce the risk of stroke or systemic embolism events [3]. Based on the 2022 statement for LV thrombus, anticoagulation should be discontinued if patients had a resolution of LV thrombus with left ventricular ejection fraction (LVEF) improving to > 35% or major bleeding occurring [3]. However, considering patients with persistent thrombus despite anticoagulation, there are no sufficient study data to determine whether anticoagulation should be continued indefinitely. Therefore, identifying patients with a high risk of thrombus unresolved may be helpful to provide evidence on the management of anticoagulation.
Depending on a prospective trial and a retrospective study, we aimed to investigate potential factors associated with thrombus unresolved in the population of patients who received oral anticoagulation for 3 months and then provide a prediction model to determine the risk of thrombus persistence or recurrence in patients with LV thrombus.
Methods
Study design and patient population
This study was derived from two studies, including a prospective study named R-DISSOLVE (ClinicalTrials.gov: NCT04970381) and a retrospective registry study (ClinicalTrials.gov: NCT 05,006,677), carried out at Fuwai Hospital, National Center of Cardiovascular Diseases in China. R-DISSOLVE was an interventional, single-arm, open-label, investigator-initiated study between October 2020 and April 2022. The retrospective study collected data from June 2013 to December 2019 by using electronic medical records. The two study protocols were developed separately and approved by the ethics committee at the participating center. The combined study was reported in accordance with the TRIPOD checklist [4].
In the prospective study, patients with LV thrombus for less than 3 months and with systemic anticoagulation of less than 1 month were enrolled. Patients with inherited or acquired thrombophilia (e.g., antiphospholipid syndrome) were excluded since the risk of thrombus persistence/recurrence in these patients was established on a unique pathophysiological mechanism. The inclusion and exclusion criteria in the retrospective study were similar to those used for the prospective one. To increase the sample size [5,6,7], we pooled the data from the two studies to create a combined study, which was then used to develop a statistical model of thrombus persistence/recurrence prediction. Only patients having imaging records at follow-up visits and having continued systemic oral anticoagulation for at least 3 months were eligible for the primary analysis, as evidenced by objective data such as prescriptions from cardiologists.
Definitions
LV thrombus was defined as an abnormal echo mass in the left ventricular cavity, whose edge was different from the left ventricular endocardium [8]. In the prospective trial, thrombus was quantified using contrast-enhanced echocardiography (CE) at baseline and follow-up visits, whereas in the retrospective study, thrombus confirmed by transthoracic echocardiography (TTE), computer tomography (CT), or cardiac magnetic resonance imaging (CMR) was obtained. When a thrombus was detected, its morphology was categorized as either mural (if its borders are generally continuous with the adjacent endocardium) or protuberant (if its borders are distinct from the adjacent endocardium and protrude into the ventricular cavity) [3].
The primary endpoint of this combined study was the LV thrombus persistence/recurrence rate at 3 months confirmed by image techniques. The thrombus persistence was defined as the presence of a thrombus at 3 months that was comparable to the one at baseline. The thrombus recurrence was defined as the presence of thrombus at 3 months following negative images from baseline to 3 months. Safety outcomes included major bleeding according to the International Society on Thrombosis and Haemostasis [ISTH] [9] criteria and clinically related non-major hemorrhage events [10]. Additionally, stroke or embolic events were collected at 3 months.
Model development
A total of 44 variables were collected in the initial database. The data from each study were randomly split into a training set (70% of the sample) and a validation set (30% of the sample). The final sets were created by merging the training set and validation set from each study. The following five stages summarize the process of developing and validating prediction models. First, create the prediction models. The significant variables from the univariate logistic analysis and variables of interest were combined to generate Model 1. Model 2 was created using the Lasso regression algorithm to identify additional potential variables associated with prognosis. Second, assess model discrimination ability by the mean area under the receiver operating characteristic curve (AUC). Third, assess model calibration to compare the predicted with the actual rates of thrombus persistence/recurrence [11]. Fourth, assess the clinical effectiveness of models by the decision curve analysis (DCA) to quantitatively depict the net benefit of clinical decisions. Finally, draw a nomogram to visualize prediction models, which can relatively calculate the risk of thrombus persistence/recurrence at 3 months in each patient by calculating the total score of each independent factor.
Statistics analysis
Descriptive statistics were computed using the CBCgrps-Package in R [12]. Mean (standard deviation, SD) or median (interquartile range, IQR) for continuous variables and frequency (percentage) for categorical variables were reported. The Pearson chi-squared test or the Fisher exact test was performed for categorical data, and the Student unpaired t-test or the Mann–Whitney U test was applied to compare continuous variables. Odds ratios (OR) and 95% confidence intervals (CI) were estimated using regression models. To address missing data for predictor variables, multiple imputations by chained equations with predictive mean matching (MICE-Package in R) were used to create five sets of imputed data. The car package in R was used to detect collinearity between variables, and a variance inflation factor < 10 was tolerated. In addition, a restricted cubic spline curve was used between the continuous variables and the primary outcome. All analyses were scheduled for completion with R Studio and R, Version 3.5.1 (The R Project for Statistical Computing, Vienna, Austria).
Results
Study population
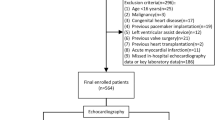
A total of 487 patients and 213 patients were screened in the retrospective and prospective study respectively (Fig. 1). At a 3 months follow-up, 108 patients and 64 patients separately were included in the combined study. Patients from the two groups were divided into a training set and a validation set with a 7:3 ratio. The final training group and validation group, respectively, comprised 124 patients and 48 patients.
Study flow diagram. A total of 172 patients were included in our analysis, which was split into a training set (n = 124, 70% of the sample) and a validation set (n = 48, 30% of the sample). †Others include 5 patients suspected of antiphospholipid antibody syndrome (n = 2) or thrombophilia (n = 3) at discharge. N, numbers of patients
The average age of the entire population was 49.8 years, and 143 out of 172 (83%) patients were male. Ischemic cardiomyopathy (ICM) was the leading underlying cause (46.5%). The median LVEF level was 30% and 126 (73.3%) patients had a mural thrombus. Regarding anticoagulation therapy, 123 (71.5%) patients received non-vitamin K antagonist oral anticoagulants (NOACs) therapy, while the remaining were with warfarin. No patients switched the type of oral anticoagulation within 3 months. Of the 123 patients, 99.2% were given rivaroxaban (49.2% with a reduced dose). In addition, 105 (61%) patients received no antiplatelet, while 44 patients received mono antiplatelet agents (36 on clopidogrel and 8 on aspirin) and 23 patients received dual antiplatelet therapy (22 on aspirin plus clopidogrel and 1 on aspirin plus ticagrelor) in the combination with anticoagulation. Baseline characteristics were presented in Table 1 and no significant differences were observed between the training group and the validation group.
Development of two models via univariate and Lasso regression
We collected 44 characteristics in the primary database. A total of 11 variables with a P value < 0.10 were selected from the univariable analysis (Table 2). By including an additional four variables of interest (diagnosis, LVEF, anticoagulation therapy, and D-dimer levels) [13,14,15,16], six predictors were finally incorporated into Model 1- diagnosis, antiplatelet therapy, the thickness of thrombi, thrombus morphology, ventricular aneurysm, and D-dimer levels (Table 3). On the other hand, ten-fold cross-validation of the Lasso coefficient profiles of 44 characteristics led to the selection of Lambda = 0.000011 as the minimum criterion for the Lasso regression (Figure S1). A total of ten variables remained after variable selection using the LASSO penalty (Fig. 2), six of which were components of Model 2 in the multivariable logistic analysis (Table 3). They were listed as follows: diagnosis, antiplatelet therapy, the thickness of thrombi, thrombus morphology, spontaneous echo contrast, and D-dimer levels.
In Model 1, the AUC was 0.852 (95% CI 0.771–0.933) in the training set and 0.631 (95% CI 0.421–0.842) in the validation set (Fig. 3). The AUC in Model 2 was similar to that in Model 1 in the training set (0.856, 95% CI 0.781–0.931) but lower in the validation set (0.617, 95% CI 0.406–0.827), while no differences were found in the comparison of two models’ AUC in the training set (P = 0.838) and the validation set (P = 0.734) (Figure S 2). To be brief, Model 1 demonstrated a stronger capacity to discriminate. Moreover, both models' DCA curves displayed a comparable range of cutoff probabilities, indicating equal clinical efficacy (Figure S3-S4). Model 1 showed a considerably better calibration of the model with more plots surrounding the ideal curves (Fig. 4, Figure S5). Above all, Model 1 was selected because it had a higher AUC-estimated predictive value and a comparable capacity to illustrate the net benefit of clinical decisions. Table 3 shows the outcome of Model 1's prediction after taking the six variables into account. Additionally, by using cross-validation, the accuracy of Model 1 was 0.838 while the Kappa value was 0.416.
ROC curves of Model 1 for predicting the risk of LV thrombus persistence/recurrence at 3 months. A Training set. B Validation set. The blue curve represents the model discrimination ability of Model 1 in the training set (AUC 0.852, 95% CI 0.771–0.933). The red curve represents the model discrimination ability of Model 1 in the validation set (AUC 0.631, 95% CI 0.421–0.842). The point in the curve represents the optimal threshold along with the corresponding specificity and sensitivity respectively. LV, left ventricular; ROC, receiver operating characteristic; AUC, area under the ROC curve
Calibration plots for predicting the risk of LV thrombus persistence/recurrence at 3 months in Model 1. A Training set. B Validation set. X-axis: predicted thrombus persistence/recurrence risk; Y-axis: actual thrombus persistence/recurrence rate. Estimates above the grey solid line represent underestimates; those below the grey solid line represent overestimates. The vertical bars represent 95%CIs. LV, left ventricular
Clinical utility
According to Model 1 (variables included diagnosis, antiplatelet therapy, thickness of thrombi, thrombus morphology, ventricular aneurysm, and D-dimer levels), the rate of persistent/recurrent LV thrombus within 3 months increased as thrombus thickness increased (OR 1.11, 95% CI 1.05–1.20, P = 0.002). It was interesting to note that patients with protuberant thrombus had a lower rate of thrombus persistence/recurrence compared to those with mural thrombus (OR 0.17, 95% CI 0.03–0.69, P = 0.025). Patients with higher baseline D-dimer levels had a lower likelihood of developing persistent or recurrent thrombus at 3 months (OR 0.67, 95% CI 0.43–0.91, P = 0.030), and there was a linear relationship between the primary end-point and the thrombus thickness or D-dimer levels (all p for non-linear > 0.05; Figure S6). In addition, the incidence of LV thrombus persistence/recurrence at 3 months differed among patients with different diagnoses or different antiplatelet therapy, and it was uncertain which diagnosis or antiplatelet therapy had more effect on the primary end-point because of their wide CIs.
Furthermore, we developed a nomogram risk prediction model that comprised independent risk factors (R2 0.38, C index 0.85, 95% CI 0.77–0.93) (Fig. 5). The scores of the items displayed in the nomogram should be added up (Table 4). For example, an echocardiogram revealed a 10 mm thick LV mural thrombus in the ventricular aneurysm in a patient with ICM who had a D-dimer level of 2 ng/mL at admission. During the hospitalization, rivaroxaban plus aspirin treatment was then administered to the patient. According to our prediction model, the overall score of the patient was 176, and the likelihood of LV thrombus persistence/recurrence within 3 months was roughly 40%.
Nomogram for the prediction of LV thrombus persistence/recurrence at 3 months in Model 1. To use the nomogram, an individual patient’s value is located on each variable axis, and a line is drawn upward to determine the number of points received for each variable value. The sum of these numbers is located on the Total Points axis, and a line is drawn downward to the last axis to determine the risk of thrombus persistence/recurrence. LV, left ventricular; ICM, ischemic cardiomyopathy; DCM, dilated cardiomyopathy
Outcome of thrombus resolution, bleeding, and major cardiovascular events
At 3 months, 38 patients (22.1%) had persistent or recurrent LV thrombus, compared to a total of 134 patients (77.9%), who had their thrombus resolved. Figure 6 depicted an illustration of a patient with persistent thrombus by CE despite receiving anticoagulant medication for 3 months. Patients in the thrombus resolution group had a higher prevalence of previous heart failure, lower LVEF and higher levels of N-Terminal pro-Brain Natriuretic Peptide (NT-proBNP), a smaller baseline thrombus, a higher proportion of spontaneous echo contrast, and a lower incidence of regional wall motion abnormality and ventricular aneurysm (Table 2).
Three patients experienced bleeding during a 3-month follow-up; one of them reported eye hemorrhage, while the other two experienced nose bleeding. During hospitalization, nine patients suffered major cardiovascular events—six patients reported having a stroke and three patients encountered a pulmonary embolism (Table S1).
Discussion
It was the first time in a prospective study and a retrospective study to predict the risk of LV thrombus persistence/recurrence among patients with oral anticoagulation for 3 months. The key findings were as follows. Patients who had thicker thrombus, mural thrombus morphology, ventricular aneurysm, or lower D-dimer levels were more likely to have persistent/recurrent LV thrombus. Therefore, irrespective of insufficient evidence, we advocated that anticoagulant therapy could be prolonged and individualized for patients with these high-risk characteristics.
The rate of thrombus persistence/recurrence in the combined study was considerably low thanks to the strict inclusion criteria in the retrospective study and the intensive monitoring in the prospective trial, which demonstrated that patients had relatively fresh thrombi prior to the enrollment and adhered to therapy well over the study period. Another significant aspect was the prospective trial's use of CE at baseline and follow-up visits, which improved the study's power to precisely identify LV thrombus. The incidence of thrombus unresolved was 31.0% in an updated meta-analysis that included 21 studies with 3057 patients over a median follow-up of 12 months [3]. According to a retrospective cohort study, 34.2% of patients with heart failure found persistent LV thrombus with a median duration of 17 months [17]. Based on these studies, it is unclear whether the rate of persistent thrombus will decrease over time given that some persistent thrombi are more likely to be calcified or organized despite anticoagulation in long-term follow-up.
In decades of research, the optimal anticoagulation treatment for LV thrombus has remained controversial. Prior recommendations suggest that anticoagulant therapy with vitamin K antagonists (VKAs) was appropriate for patients with myocardial infarction and asymptomatic LV thrombi for up to 3 months [2, 18]. The 2017 ESC guidelines for myocardial infarction recommended the duration of anticoagulation (either NOACs or VKAs) might be for 6 months guided by repeated imaging [19], in addition, the scientific statement from the American Heart Association (2022) indicated that NOACs were considered to be a reasonable alternative to VKAs in patients with LV thrombus, according to currently available data [3]. The duration of anticoagulation for persistent thrombus, meanwhile, is yet undetermined. According to consensus opinion [3], patients with persistent or recurrent thrombus should continue anticoagulation until resolution on the basis of their high compliance and frequent imaging assessments. A trial of alternative anticoagulation, on the other hand, should be addressed on a case-by-case basis. For those thrombi that are organized or calcified, discontinuing oral anticoagulation is an option because the risk of embolization is probably minimal. Consequently, long-term anticoagulation management options for patients with persistent thrombus should weigh the concerns of indefinite anticoagulation (e.g., increased pill burden and bleeding) against the potential reduction in stroke risk.
We came to the conclusion that patients with persistent thrombus shared six characteristics in addition to presumably poor adherence. It is well acknowledged that patients with different etiologies had different mechanisms in the development of LV thrombus. Based on Virchow's triad of thrombogenesis, three factors include stasis attributable to reduced ventricular function, endocardial injury, and inflammation/hypercoagulability. The interaction between these mechanisms and thrombus outcome, however, is not always easy to clarify [20, 21]. According to our finding, the rate of thrombus persistence/recurrence at 3 months differed among patients with ICM, DCM, and other cardiovascular disorders while it was unreliable to conclude whether DCM or other diseases patients had a higher likelihood of thrombus persistence than those with ICM. Indeed, researchers reported that specific causes of DCM (eg, amyloidosis, eosinophilic myocarditis) could increase the risk of LV thrombus persistent/recurrent [21, 22]. In terms of oral anticoagulation, patients taking NOACs experienced less likelihood of LV thrombus persistence at 3 months than those with VKAs, though the statistical difference was insignificant. A comparable conclusion was reached by several meta-analyses [23,24,25,26] and two small randomized clinical trials (apixaban or rivaroxaban versus warfarin) [27, 28]. Interestingly, although the CI was somewhat wide, it allowed us to conclude that patients with mono or dual antiplatelet therapy had a different LV thrombus persistence/recurrence rate at 3 months compared to those without. A prior study [13] reported that patients taking anticoagulation in combination with mono antiplatelet therapy had a higher risk of persistent thrombus than those who did not. In the study of Niku et al. [29], the rate of receiving antiplatelet therapy paired with anticoagulation was higher in patients with persistent left atrial thrombus than those with thrombus resolution (65% vs 38%, P = 0.03). Overall, for patients undergoing percutaneous coronary intervention with an indication for antiplatelet therapy and who also have an indication for oral anticoagulation, a general strategy (preferably a NOAC plus clopidogrel) may be considered on the basis of current practice and guideline recommendations [30,31,32].
In addition, patients with lower D-dimer levels in our analysis had a higher likelihood of LV thrombus persistence/recurrence at 3 months; however, the result would be adjusted by the mean D-dimer level in future work. The reason might be concluded that the thrombus of patients with a low D-dimer was neither fresh nor mobile, resulting in a persistent thrombus. The grade of thrombus mobility was found to be strongly associated with the thrombus' outcome. Limited data suggest that a large or mural thrombus has a less likelihood of thrombus resolution than a small or protuberant thrombus [33]. When evaluating the shape and the morphology of LV thrombus, the findings from Salah et al. [15], which were in agreement with our results, showed that patients with persistent thrombus had bigger baseline thrombus areas. From the consensus of the statement [3], it is unreasonable to give anticoagulation for mural thrombi even though the risk of embolization may be less than for protuberant thrombi. To conclude, a shared decision-making approach is appropriate following a risk/benefit discussion between patients and clinicians.
The major limitations were listed. First, since the sample of this study is small even when combining both the prospective and retrospective cohorts, which limits the power and utility of the model, external validations of our model are expected. Second, due to the wide CIs, it is still unclear whether patients with DCM and antiplatelet therapy are associated with a high risk of LV thrombus persistence/recurrence or not, so additional prospective research is needed to confirm the results. Third, the anticipated medication after 3 months remains unknown in the retrospective study owing to the short-term follow-up, while patients in the prospective study were informed of the appropriate anticoagulant therapy for at least 6 months. Large-scale trials are essential to determine whether indefinite anticoagulation is merited in patients with persistent/recurrent LV thrombus.
Conclusions
A prediction model comprising six variables was derived from a combination of prospective and retrospective studies. Patients were more likely to develop persistent or recurrent LV thrombus at 3 months if they had thicker thrombus, mural thrombus, ventricular aneurysm, or low baseline D-dimer levels. Considerations on the duration of anticoagulation should be based on the best clinical judgment and shared decision-making, and prospective randomized trials are necessary to validate the model.
Availability of data and materials
The data will be shared on reasonable request with the corresponding author.
References
Cruz Rodriguez JB, Okajima K, Greenberg BH. Management of left ventricular thrombus: a narrative review[J]. Ann Transl Med. 2021;9(6):520.
O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction[J]. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013;61(4):e78–140.
Levine GN, McEvoy JW, Fang JC, Ibeh C, McCarthy CP, Misra A, Shah ZI, Shenoy C, Spinler SA, Vallurupalli S, Lip GYH. Management of Patients at Risk for and With Left Ventricular Thrombus: A Scientific Statement From the American Heart Association[J]. Circulation. 2022;146(15):e205–23.
Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement[J]. BMJ. 2015;350: g7594.
Valgimigli M, Frigoli E, Heg D, Tijssen J, Jüni P, Vranckx P, Ozaki Y, Morice MC, Chevalier B, Onuma Y, Windecker S, Tonino PAL, Roffi M, Lesiak M, Mahfoud F, Bartunek J, Hildick-Smith D, Colombo A, Stanković G, Iñiguez A, Schultz C, Kornowski R, Ong PJL, Alasnag M, Rodriguez AE, Moschovitis A, Laanmets P, Donahue M, Leonardi S, Smits PC. Dual Antiplatelet Therapy after PCI in Patients at High Bleeding Risk[J]. N Engl J Med. 2021;385(18):1643–55.
Skovlund CW, Mørch LS, Kessing LV, Lidegaard Ø. Association of Hormonal Contraception With Depression[J]. JAMA Psychiat. 2016;73(11):1154–62.
Allen AM, Shah VH, Therneau TM, Venkatesh SK, Mounajjed T, Larson JJ, Mara KC, Schulte PJ, Kellogg TA, Kendrick ML, McKenzie TJ, Greiner SM, Li J, Glaser KJ, Wells ML, Chen J, Ehman RL, Yin M. The Role of Three-Dimensional Magnetic Resonance Elastography in the Diagnosis of Nonalcoholic Steatohepatitis in Obese Patients Undergoing Bariatric Surgery[J]. Hepatology. 2020;71(2):510–21.
Chaosuwannakit N, Makarawate P. Left Ventricular Thrombi: Insights from Cardiac Magnetic Resonance Imaging[J]. Tomography. 2021;7(2):180–8.
Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients[J]. J Thromb Haemost. 2005;3(4):692–4.
Kaatz S, Ahmad D, Spyropoulos AC, Schulman S. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH[J]. J Thromb Haemost. 2015;13(11):2119–26.
Ho KM. Forest and funnel plots illustrated the calibration of a prognostic model: a descriptive study[J]. J Clin Epidemiol. 2007;60(7):746–51.
Zhang Z, Gayle AA, Wang J, Zhang H, Cardinal-Fernández P. Comparing baseline characteristics between groups: an introduction to the CBCgrps package[J]. Ann Transl Med. 2017;5(24):484.
Hofer F, Kazem N, Schweitzer R, Horvat P, Winter MP, Koller L, Hengstenberg C, Sulzgruber P, Niessner A. The prognostic impact of left ventricular thrombus resolution after acute coronary syndrome and risk modulation via antithrombotic treatment strategies[J]. Clin Cardiol. 2021;44(12):1692–9.
Varwani MH, Shah J, Ngunga M, Jeilan M. Treatment and outcomes in patients with left ventricular thrombus - experiences from the Aga Khan University Hospital, Nairobi - Kenya[J]. Pan Afr Med J. 2021;39:212.
Salah Shabib Ahmed H, Ede H, Sobhy Hassan Ghonim Mahfouz A, Abdullah Ali Rahhal A, Abdulhakim Ali M, Fares Alhaj Issa A, Baki Jasem Senjar A, Mehdar Al-Saadi Al Yafei S, Rahman Mohammad Said Arabi A, Razaq Al-Qahtani A. Surrogates of the Left Ventricular Thrombus Resolution: A Retrospective Data Review[J]. Turk Kardiyol Dern Ars,2022,50(3):168–174.
Yeung W, Sia CH, Pollard T, Leow AS, Tan BY, Kaur R, Yeo TC, Tay EL, Yeo LL, Chan MY, Loh JP. Predicting mortality, thrombus recurrence and persistence in patients with post-acute myocardial infarction left ventricular thrombus[J]. J Thromb Thrombolysis. 2021;52(2):654–61.
Zhang Q, Zhang Z, Zheng H, Qu M, Li S, Yang P, Si D, Zhang W. Rivaroxaban in heart failure patients with left ventricular thrombus: A retrospective study[J]. Front Pharmacol. 2022;13:1008031.
Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, Kamel H, Kernan WN, Kittner SJ, Leira EC, Lennon O, Meschia JF, Nguyen TN, Pollak PM, Santangeli P, Sharrief AZ, Smith SC Jr, Turan TN, Williams LS. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association[J]. Stroke. 2021;52(7):e364–467.
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC)[J]. Eur Heart J. 2018;39(2):119–77.
Nunes MC, Kreuser LJ, Ribeiro AL, Sousa GR, Costa HS, Botoni FA, de Souza AC, Gomes Marques VE, Fernandez AB, Teixeira AL, da Costa Rocha MO. Prevalence and risk factors of embolic cerebrovascular events associated with Chagas heart disease[J]. Glob Heart. 2015;10(3):151–7.
Ono R, Iwahana T, Kato H, Okada S, Kobayashi Y. Literature reviews of stroke with hypereosinophilic syndrome[J]. Int J Cardiol Heart Vasc. 2021;37: 100915.
Feng D, Syed IS, Martinez M, Oh JK, Jaffe AS, Grogan M, Edwards WD, Gertz MA, Klarich KW. Intracardiac thrombosis and anticoagulation therapy in cardiac amyloidosis[J]. Circulation. 2009;119(18):2490–7.
Huang L, Tan Y, Pan Y. Systematic review of efficacy of direct oral anticoagulants and vitamin K antagonists in left ventricular thrombus[J]. ESC Heart Fail. 2022;9(5):3519–32.
Salah HM, Goel A, Saluja P, Voruganti D, Al’Aref SJ, Paydak H, et al. Direct oral anticoagulants versus warfarin in left ventricular thrombus: a systematic review and meta-analysis. Am J Ther. 2021. https://doi.org/10.1097/MJT.0000000000001432.
Michael F, Natt N, Shurrab M. Direct Oral Anticoagulants vs Vitamin K Antagonists in Left Ventricular Thrombi: A Systematic Review and Meta-analysis[J]. CJC Open. 2021;3(9):1169–81.
Cochran JM, Jia X, Kaczmarek J, Staggers KA, Rifai MA, Hamzeh IR, Birnbaum Y. Direct Oral Anticoagulants in the Treatment of Left Ventricular Thrombus: A Retrospective, Multicenter Study and Meta-Analysis of Existing Data[J]. J Cardiovasc Pharmacol Ther. 2021;26(2):173–8.
Alcalai R, Butnaru A, Moravsky G, Yagel O, Rashad R, Ibrahimli M, Planer D, Amir O, Elbaz-Greener G, Leibowitz D. Apixaban vs. warfarin in patients with left ventricular thrombus: a prospective multicentre randomized clinical trial‡[J]. Eur Heart J Cardiovasc Pharmacother. 2022;8(7):660–7.
Abdelnabi M, Saleh Y, Fareed A, Nossikof A, Wang L, Morsi M, Eshak N, Abdelkarim O, Badran H, Almaghraby A. Comparative Study of Oral Anticoagulation in Left Ventricular Thrombi (No-LVT Trial)[J]. J Am Coll Cardiol. 2021;77(12):1590–2.
Niku AD, Shiota T, Siegel RJ, Rader F. Prevalence and Resolution of Left Atrial Thrombus in Patients With Nonvalvular Atrial Fibrillation and Flutter With Oral Anticoagulation[J]. Am J Cardiol. 2019;123(1):63–8.
Angiolillo DJ, Goodman SG, Bhatt DL, Eikelboom JW, Price MJ, Moliterno DJ, Cannon CP, Tanguay JF, Granger CB, Mauri L, Holmes DR, Gibson CM, Faxon DP. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a north american perspective-2018 update[J]. Circulation. 2018;138(5):527–36.
Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, DiMaio JM, Don CW, Fremes SE, Gaudino MF, Goldberger ZD, Grant MC, Jaswal JB, Kurlansky PA, Mehran R, Metkus TS Jr, Nnacheta LC, Rao SV, Sellke FW, Sharma G, Yong CM, Zwischenberger BA. 2021 ACC/AHA/SCAI Guideline for coronary artery revascularization: a report of the american college of cardiology/american heart association joint committee on clinical practice guidelines[J]. Circulation. 2022;145(3):e18–114.
Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation[J]. Eur Heart J. 2021;42(14):1289–367.
Oh JK, Park JH, Lee JH, Kim J, Seong IW. Shape and mobility of a left ventricular thrombus are predictors of thrombus resolution[J]. Korean Circ J. 2019;49(9):829–37.
Acknowledgements
We would like to thank Fuwai hospital for providing invaluable data. We thank the help given by clinicians in enrolling patients.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Y.L., YM.Y., LT.Y., X.Q., and J.Z. put forward the concept of research. Q.Y. drafted the manuscript. Q.Y., CS.W., and X.Q. completed the data analysis. Y.L. and J.Z. contributed to the revision of the manuscript and provided suggestions for the research. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocols were approved by the Ethics Committees of Fuwai Hospital (prospective study: approval No. 2020–1380; retrospective study: approval No. 2022–1757) with written informed consent in the prospective study and oral informed consent in the retrospective study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’ s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Fig. S1. LASSO coefficient profiles of the 44 variables. A coefficient profile plot was produced against the log (Lambda) sequence. Fig. S2. ROC curves of Model 1 and Model 2 for predicting the risk of LV thrombus persistence/recurrence at 3 months. (A) Training set. (B) Validation set. The black curve represents the model discrimination ability of Model 1 either in the training set or the validation set. The red curve represents the model discrimination ability of Model 2 either in the training set or the validation set. The grey curve represents that a model has no model discrimination ability. No differences were found in the comparison of the two models’ AUC in the training set (P = 0.838) and the validation set (P = 0.734). LV, left ventricular; ROC, receiver operating characteristic; AUC, area under the ROC. Fig. S3. DCA of the nomogram for predicting the risk of LV thrombus persistence/recurrence at 3 months in Model 1. (A) Training set. (B) Validation set. X-axis: cut-off probability; Y-axis: net benefit, which is calculated across a range of threshold probabilities. The blue line represents Model 1. The grey line represents the assumption that all patients have thrombus persistence/recurrence. The black line represents the assumption that no patients have thrombus persistence/recurrence. LV, left ventricular; DCA, decision curve analysis. Fig. S4. DCA of the nomogram for predicting the risk of LV thrombus persistence/recurrence at 3 months in Model 1 and Model 2. (A) Training set. (B) Validation set. X-axis: cut-off probability; Y-axis: net benefit, which is calculated across a range of threshold probabilities. The blue line represents Model 1. The red line represents Model 2. The grey line represents the assumption that all patients have thrombus persistence/recurrence. The black line represents the assumption that no patients have thrombus persistence/recurrence. LV, left ventricular; DCA, decision curve analysis. Fig. S5. Calibration plots for predicting the risk of LV thrombus persistence/recurrence at 3 months in Model 2. (A) Training set. (B) Validation set. X-axis: predicted thrombus persistence/recurrence risk; Y-axis: actual thrombus persistence/recurrence rate. Estimates above the grey solid line represent underestimates; those below the grey solid line represent overestimates. The vertical bars represent 95%CIs. LV, left ventricular; CI, confidence interval. Fig. S6. Restricted cubic spline curve. We observed a linear relationship between LV thrombus persistence/recurrence at 3 months and continuous variables including (A) D-dimer levels and (B) thickness of thrombi (all p for nonlinear 0.05). The p values for overall association were less than 0.05 for thrombus persistence/recurrence. Both models were adjusted for cofounders in Model 1 including diagnosis, antiplatelet therapy, thrombus morphology, and ventricular aneurysm. ORs are indicated by solid lines and 95%CIs by shaded areas. LV, left ventricular; OR, odds ratio; CI, confidence interval. Table S1. Secondary outcomes at 3-month follow-up [N = 172].
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, Q., Quan, X., Wang, C. et al. A prediction model for left ventricular thrombus persistence/recurrence: based on a prospective study and a retrospective study. Thrombosis J 21, 50 (2023). https://doi.org/10.1186/s12959-023-00488-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12959-023-00488-1