Abstract
Background
Adrenal hemorrhage (AH) is a rare condition that can result in a life-threatening medical emergency. This medical condition could be caused by several underlying factors, one of which is the use of anticoagulants. As far as we are aware, direct oral anticoagulant (DOAC) agents are a rare but possible cause of AH.
Case presentation
Herein, we described two cases of AH due to DOACs. The first case was a 35-year-old Iranian woman with a past medical history of Hashimoto thyroiditis who was being treated with apixaban due to the previous thrombosis. Her first symptoms of AH (November 2021) were strangely similar to symptoms of autoimmune Addison disease (AAD) which led to a confirmed diagnosis of autoimmune polyendocrine syndrome type 2 (APS-2). An abdominal MRI revealed an oval shape well-encapsulated cystic mass with a diameter of 20 × 14 mm with a thick and low signal intensity rim in the left adrenal gland, highly suggestive of sub-acute left-sided AH. Our second case was an 89-year-old Iranian woman who had been admitted to the hospital (August 2021) with low blood pressure and disorientation. At the beginning of her admission, the evaluation showed hyponatremia, and further evaluations confirmed adrenal insufficiency (AI). The patient reported rivaroxaban usage for deep vein thrombosis prophylaxis after femur fixation surgery. Her abdominal CT scans showed bilateral adrenal masses highly suggestive of AH. Her follow-up examination showed persistent AI after three months.
Conclusion
Given the history of our cases, physicians should be aware of AH in patients receiving DOACs, particularly in elderly patients who are at high risk of bleeding. It is also worth noting that AH can occur in any patient with any medical history and history of DOAC use, which is why patients must be closely monitored.
Similar content being viewed by others
Background
Adrenal hemorrhage(AH) is considered a rare but potentially lethal condition that can occur in both traumatic and non-traumatic conditions [1]. Formerly, AH was mostly diagnosed during post-mortem examinations, with incidence rates ranging from 0.14% to 1.1% [1,2,3,4]. Due to tremendous advancements in imaging technology, AH is now diagnosed with a higher frequency in hospitalized patients, ranging from 1.5% to 5% [1, 5, 6].
Factors that may lead to AH include focal adrenal lesions, abdominal trauma, anticoagulation therapy, congenital or acquired bleeding disorders, sepsis, and pregnancy [1, 7, 8]. AH can be presented in asymptomatic settings to fatal conditions, mostly resulting from bilateral AH [8, 9]. It has been revealed that bilateral AH through adrenal insufficiency (AI) induction can be detrimental. A key difference between unilateral and bilateral AH is that the former is mostly biochemically silent, while the latter is potentially fatal [10, 11].
Anticoagulation options have rapidly expanded over the last decade, with the United States Food and Drug Administration (FDA) approval of the first direct oral anticoagulant (DOAC) in 2001, rivaroxaban in 2011, apixaban in 2012, and edoxaban in 2015 [12, 13]. Compared to vitamin K anticoagulants (VKA), DOACs have fewer interactions with medications and diet, have a more stable pharmacokinetics profile, and do not require routine laboratory monitoring. These findings led to the replacement of DOACs with VKA [14].
Herein, we reported for the first time a case of unilateral AH attributed to apixaban treatment in a newly diagnosed patient with previously confirmed autoimmune Addison disease (AAD) and the second case of bilateral AH following administration of rivaroxaban in an elderly woman.
Case presentation
Case 1
A 35-year-old Iranian woman presented with anorexia, nausea, and non-bloody frequent vomiting 2 months ago referred to a gastroenterologist in November 2021. She was complaining of fatigue, tiredness, postural dizziness, salt craving, dry skin, progressive buccal pigmentation, memory impairment, and about 5 kg of weight loss in 2 months. The patient denied pain in her abdomen, flank, or chest. No history was reported for abdominal blunt trauma. Medical history revealed hypothyroidism in the background of Hashimoto’s thyroiditis from four years ago. Additionally, the patient had a history of right internal jugular vein thrombosis (IJVT) eight years ago that was attributed to oral contraception pill consumption and was treated with warfarin for about one year. Moreover, the patient reported left IJVT last year which occurred two weeks after the coronavirus disease of 2019 (COVID-19) infection.
She has been under treatment with levothyroxine 350 μg per week and apixaban 2.5 mg twice a day since one year ago. She was married with no children. Her menstruation was regular. Her family history was unremarkable, excluding her sister’s hypothyroidism.
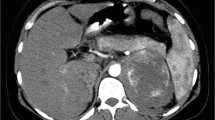
Due to her gastrointestinal symptoms and weight loss, an abdominopelvic computed tomography (CT) scan with contrast has been requested. CT showed a 32 × 22 mm left adrenal thick wall cystic mass with an enhanced peripheral rim. The right adrenal gland was normal (Fig. 1). Hence the patient was admitted to the hospital.
On physical examination, systolic blood pressure (SBP):85 mmHg, temperature(T): 37 °C, pulse rate (PR): 80 beats/min. body mass index (BMI): 16.5 kg/m2. Skin: dry, pale, no vitiligo, no ecchymosis. Pigmentation in the buccal, gums, and tongue was found. Otherwise, the physical examination was unremarkable. Table 1 shows the results of lab data at the time of admission as well as the endocrinologist’s further workup.
According to the history, physical examination, and presence of low cortisol levels (1.1 μg/dl) and high adrenocorticotropic hormone (ACTH) level, autoimmune Addison’s disease (AAD) was diagnosed. Hydrocortisone 150 mg per day was prescribed and clinical improvement occurred and BP level increased to 110/70 mmHg.
An abdominal magnetic resonance imaging (MRI) with gadolinium was ordered to determine the exact diagnosis of the adrenal cystic lesion. The MRI showed an oval shape well-encapsulated cystic mass of 20 × 14 mm with a thick and low signal intensity rim in the left adrenal gland. This mass was high signal intensity on T1 and T2 weighted images, consistent with sub-acute hemorrhage. There was an enhancement of the peripheral thick wall rim after injection of contrast. The right adrenal gland was normal (Fig. 2a).
a. Abdominal magnetic resonance imaging (MRI) with gadolinium showed an oval shape well-encapsulated cystic mass with a thick and low signal intensity rim in the left adrenal gland(A: Coronal &B: axial view of hepatobiliary phase, C: hyperintense on T1 fat-saturated). The red arrows indicate adrenal hemorrhage. b. MRI with gadolinium showed near-complete resolution of left adrenal hematoma and residual hemosiderin is noted (A: Coronal & B: axial view of hepatobiliary phase, C: hyperintense on T1 fat-saturated)
During the admission, the hemodynamics were stable. She was given prednisolone 7.5 mg daily and Fludrocortisone 0.05 mg daily. ACTH level decreased to 7.03 pg/ml after treatment. Levothyroxine was increased to 500 μg per week after AI management. Apixaban was discontinued, and she was referred to a hematologist for evaluation of thrombophilia and antiphospholipid syndrome (APLS). The hematological tests for APLS were requested but the patient refrained from performing the tests. Her follow-up laboratory tests after 3 months revealed an ACTH level of 86 pg/ml. As a follow-up, an adrenal MRI that was performed three months later revealed the disappearance of left adrenal hemorrhage and near-complete resolution of left adrenal hematoma (Fig. 2b).
Case 2
An 89-year-old Iranian woman with a history of hypertension presented with fatigue, loss of appetite, and delirium, ten days after surgery to fix her neck femur in November 2020. She was using rivaroxaban 10 mg per day for deep vein thromboembolism prophylaxis. Her SBP dropped to 90 mmHg and the oral hypotensive agent (losartan 25 mg BID) was discontinued. No history of fever, head trauma, infection symptoms, or bleeding was reported. At the time of admission, the physical examination was SBP:90 mmHg, T:37 °C, PR:96 beats/min. BMI: 26.44 kg/m2. Her Mental status was disoriented. The rest of her physical examination was otherwise unremarkable.
Due to physical examinations and her disorientation, a brain MRI was requested that revealed evidence of subdural hematoma that was not progressive in the control MRI after 3 days. Initial laboratory tests are shown in Table 2. Considering the hypotension and hyponatremia the presence of Addison’s disease (AD) was suspected so cortisol at 8 am and ACTH level was requested that confirmed the diagnosis of AD, according to the cortisol level of 0.05 μg/dl and ACTH 212 pg/ml (Table 2). Hence, hydrocortisone was initiated for the patient and her clinical symptoms improved significantly. Her sodium level increased to 137 mEq/L. During the admission hemodynamics were stable.
Adrenal CT scans with and without contrast were performed to determine the cause of AI. As shown in Fig. 3 bilateral adrenal masses were found as 38 × 25 mm on the right side and 35 × 26 mm on the left side with high density in non-contrast images (Hounsfield unit (HU):40–50), without enhancement in the portal and delayed phases, more likely to be intra-adrenal hemorrhage. Due to her AH and subdural hematoma on her MRI, the rivaroxaban has been discontinued. She has been discharged with prednisolone 7.5 mg per day and fludrocortisone 0.05 mg per day.
Follow-up adrenal CT, three months later, showed decreased size in both adrenal hemorrhages with a mean HU < 10 (Fig. 4). Her follow-up laboratory tests after 3 months revealed that her AI had persisted, with a Cortisol 8 am level of 0.4 μg/dl and an ACTH level of 204 pg/ml.
Search strategy for literature review
In a PubMed search, we searched for articles published between January 2000 and February 2021 containing the keywords "autoimmune Addison disease and adrenal hemorrhage", "DOAC".
treatment and adrenal hemorrhage", "adrenal insufficiency and adrenal hemorrhage", and "AH and long-term AI as well as "AH and autoimmune polyendocrine syndrome type 2". A comprehensive review of all relevant studies was conducted, and all references were manually checked for additional studies that may have been relevant.
Discussion
Here, we report two cases of AH post-DOAC prophylaxis. It is a novel case of unilateral AH caused by apixaban usage in a patient with newly diagnosed AAD. An APS-2 diagnosis was made that included Hashimoto hypothyroidism as well. In another case of bilateral AH after prophylactic rivaroxaban usage, the patient developed AI, which persisted during 3 months follow-up.
AH is a rare medical emergency, with an incidence rate of 0.14%-1.1% reported in some of the largest (> 25,000 cases) autopsy series [1,2,3,4, 15]. Several underlying stress conditions can cause non-traumatic AH, such as sepsis, pregnancy, post-abdominal surgery, anti-phospholipid syndrome, and, in rare cases, anticoagulation therapy [2, 16,17,18,19,20]. Bleeding is a potential risk associated with every anticoagulant medication and is the most common side effect of these medications, leading to hospitalizations and deaths [12, 21].
The most similar case to ours in the search for the etiology of AH is an article by Zachary Sanford et al. [22]. It described a case of AH in a patient with APLS who had previously been on warfarin prophylaxis, with reoccurring AH presented with right flank pain followed by retinal hemorrhage precipitated during a transition from warfarin to apixaban. It is noteworthy that this is the only case reported in which a unilateral AH occurred in a patient receiving apixaban anticoagulation [22]. We reported here the second unilateral AH due to apixaban use that was accidentally detected in CT of adrenal glands in a newly diagnosed patient with AAD and probable adrenocortical atrophy.
Clinical suspicion and prompt diagnosis of bilateral AH are clinically vital because 16–50% of patients with bilateral AH eventually develop life-threatening AI [1, 23]. CT scans with and without IV contrast or MRI with Gadolinium are commonly used to diagnose AH [24, 25].MRI is more accurate than other imaging modalities in diagnosing adrenal hematoma, and it may also differentiate between subacute and chronic hemorrhage [25, 26]. In our case with AAD, Hashimoto thyroiditis, and APS-2, the patient had left adrenal thick wall cystic mass with the enhanced peripheral rim on CT scan imaging, which suggests adrenal masses or AH. MRI findings in our case include an oval shape well-encapsulated cystic mass 20 × 14 mm with a thick and low signal intensity rim in the left adrenal gland. This mass was high signal intensity on both T1 and T2 weighted images, the findings suggestive of sub-acute AH [27].
Primary AI is the result of partial or complete bilateral adrenal cortex destruction leading to deficiency of all adrenocortical hormones (also known as AD). Tuberculosis was the most common cause of AD in the first half of the twentieth century, but AAD has recently overtaken it[23]. In patients with APLS, AH is a relatively uncommon cause of AD as a result of bilateral AH [7, 23, 28].
Diagnosis of AH is difficult due to the nonspecific clinical presentation. Therefore, having a high level of clinical suspicion plays a crucial role in facilitating an earlier diagnosis and avoiding undesirable outcomes. These signs and symptoms include pain in the back or flanks, nausea, vomiting, hypotension, and fever [29,30,31]. Our patient with AAD and unilateral AH had no symptoms related to unilateral AH excluding loss of appetite, nausea, vomiting, and buccal hyperpigmentation, which were more likely to be caused by deficiency of adrenocortical hormones. To the best of our knowledge, no other case of AAD associated with autoimmune polyendocrine syndrome type 2 (APS-2) before AH diagnosis has been reported. APS-2 is the most common autoimmune polyendocrine syndrome [32]. A hallmark of this condition is its combination of autoimmune AD with thyroid autoimmune disease and/or type 1 diabetes mellitus [32]. Similar to our first case, AD and Hashimoto thyroiditis (Schmidt syndrome) are the most common clinical combinations [32]. The presence of 21-hydroxylase autoantibodies (21OHAb), is used as a confirmation test for the diagnosis of AAD [33]. One of the limitations of our study is the country's limited access to antibody tests, which made it difficult to confirm the diagnosis based on laboratory autoimmune tests.
Due to their stable pharmacokinetic profile and fewer interactions with other medications and diets, DOACs currently do not require routine laboratory monitoring. As a result, DOACs have supplanted VKA as the first-line anticoagulant in international guidelines for managing and preventing thrombotic diseases [12,13,14, 34]. Because routine coagulation tests cannot be used to determine the degree of anticoagulation in individuals receiving a DOAC, managing bleeding can be challenging [12].To the best of our knowledge, only five cases of AH have been reported as a result of the use of the latest anticoagulant drugs (Table 3). Four with the use of rivaroxaban [10, 35,36,37], and only one through the use of apixaban [22] prophylactic treatment. However, all of the evaluated cases demonstrated AI as a result of bilateral AH. Although bilateral AH is more commonly associated with AI, the contralateral adrenal gland may become "exhausted" in unilateral AH [15]. This may result in hypocortisolemia due to decreased cortical lipids, as demonstrated in a case presented by B.Ly [37], a case of unilateral AH with confirmed AI in the laboratory test. Two of these four were under prophylactic anticoagulation following knee surgery in the patients [10, 37], and the rest were due to APLS [35, 36]. Our second case was similar to the mentioned cases because AH also occurred following rivaroxaban treatment after femur fixation surgery. In addition, she had bilateral AH, which could lead to AI.
DOACs have shown equivalent or greater efficacy and safety compared to vitamin K agonists or enoxaparin in frail or elderly subjects and after orthopedic surgeries [12]. However, because DOAC antidotes (idarucizumab for reversal of dabigatran, and andexanet alfa for reversal of the direct FXa inhibitors apixaban and rivaroxaban), are not available in our country, the elderly woman with a femoral neck fracture, was not a good candidate for DOAC agents; instead, warfarin or low molecular weight heparin (LMWH) agents with available antidotes might be a better prophylactic treatment [13, 21].
In patients with bilateral AH, the long-term follow-up of glucocorticoid and mineralocorticoid function has been assessed in only a few articles [38,39,40,41,42,43]. After a follow-up of four patients up to 19 years with acute bilateral AH and glucocorticoid insufficiency, the researchers showed the absence of need for long-term mineralocorticoid replacement. They also demonstrated the improvement in serum cortisol levels in three of the four patients and the ability of one case to function normally without cortisol replacement for 4 years. In our second case with bilateral AH, we found the need for continued adrenocortical replacement therapy after three months of follow-up.
As a limitation, we did not perform an adrenocorticotropic hormone stimulation test in our cases for confirmation of AI, however, according to the guideline released by “Endocrine Society Clinical Practice Guideline “If a corticotropin stimulation test is not feasible, using morning cortisol < 5 μg/dL in combination with high ACTH ( i.e. > twofold upper limit of the reference range) is suggestive of adrenal insufficiency [44]. Moreover, as suggested by many authors [23, 45] morning cortisol concentrations lower than 3 μg/dL are strongly predictive of adrenal insufficiency. In both of our cases with AI, the levels of cortisol were less than 1.5 μg/dL and the value of ACTH levels were more than 3 folds of the upper limits of the normal range.
In the current study, we presented two cases of AH caused by DOAC use. The first unilateral AH occurred after the therapy with apixaban in a newly diagnosed patient with AAD and the second one was a bilateral AH that occurred after the prophylactic therapy with rivaroxaban following femur fixation surgery. Any patient with any medical background might adversely develop side effects like AH when treated with DOACs, even patients that are low risk such as the young 35-year-old patient that was presented in case 1. probable adrenocortical atrophy.
Availability of data and material
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- AH:
-
Adrenal hemorrhage
- DOAC:
-
Direct oral anticoagulant
- AAD:
-
Autoimmune Addison disease
- APS-2:
-
Autoimmune Polyendocrine Syndrome type 2
- AI:
-
Adrenal Insufficiency
- VKA:
-
Vitamin K Anticoagulants
- IJVT:
-
Internal jugular vein thrombosis
- COVID-19:
-
Coronavirus Disease of 2019
- CT:
-
Computed Tomography
- SBP:
-
Systolic Blood Pressure
- T:
-
Temperature
- PR:
-
Pulse Rate
- BMI:
-
Body Mass Index
- ACTH:
-
Adrenocorticotropic Hormone
- MRI:
-
Magnetic resonance imaging
- HU:
-
Hounsfield unit
- APLS:
-
Antiphospholipid syndrome
- AD:
-
Addison disease
- LMWH:
-
Low molecular weight heparin
References
Simon DR, Palese MA. Clinical update on the management of adrenal hemorrhage. Curr Urol Rep. 2009;10:78–83.
Seow YTn, Ng ZQ, Wong SL. Anticoagulation-induced unilateral adrenal haemorrhage and pseudoaneurysm. BMJ Case Reports CP. 2019;12:e232539.
Botteri A, Orell SR. Adrenal hemorrhage and necrosis in the adult. a clinicopathological study of 23 cases. Acta Med Scand. 1964;175:409–19.
Plaut A. Adrenal necrosis and thrombosis in routine necropsies. Am J Pathol. 1955;31:93–105.
Francque SM, Schwagten VM, Ysebaert DK, Van Marck EA, Beaucourt LA. Bilateral adrenal haemorrhage and acute adrenal insufficiency in a blunt abdominal trauma: a case-report and literature review. Eur J Emerg Med. 2004;11:164–7.
You JS, Chung SP, Park YS, Chung HS, Lee HS, Yu JS. Isolated adrenal hemorrhage after minor blunt trauma. Am J Emerg Med. 2007;25(984):e985-986.
Marti JL, Millet J, Sosa JA, Roman SA, Carling T, Udelsman R. Spontaneous adrenal hemorrhage with associated masses: etiology and management in 6 cases and a review of 133 reported cases. World J Surg. 2012;36:75–82.
Karwacka IM, Obołończyk Ł, Sworczak K. Adrenal hemorrhage: A single center experience and literature review. Adv Clin Exp Med. 2018;27:681–7.
Kerkhofs TM, Roumen RM, Demeyere TB, van der Linden AN, Haak HR. Adrenal tumors with unexpected outcome: a review of the literature. Int J Endocrinol. 2015;2015:710514.
Alidoost M, Soomro R, Gubeladze A, Morabia A, Holland S, Asif A, Hossain MA. Rivaroxaban related bilateral adrenal hemorrhage: a rare complications of direct oral anticoagulants–a case reports. Am J Case Rep. 2019;20:1607.
Sacerdote MG, Johnson PT, Fishman EK. CT of the adrenal gland: the many faces of adrenal hemorrhage. Emerg Radiol. 2012;19:53–60.
Kustos SA, Fasinu PS. Direct-acting oral anticoagulants and their reversal agents—an update. Medicines. 2019;6:103.
Gunasekaran K, Rajasurya V, Devasahayam J, Singh Rahi M, Chandran A, Elango K, Talari G. A review of the incidence diagnosis and treatment of spontaneous hemorrhage in patients treated with direct oral anticoagulants. J Clin Med. 2020;9:2984.
Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:1300–5 (e1302).
Vella A, Nippoldt TB, Morris III JC. Adrenal hemorrhage: a 25-year experience at the Mayo Clinic. In Mayo Clinic Proceedings. Rochester: Elsevier; 2001. p. 161-168.
Michon A, Darnige L, Pouchot J, Arlet JB. Catastrophic antiphospholipid syndrome presenting with bilateral massive adrenal haemorrhage A case report. Joint Bone Spine. 2015;82:288–9.
Jeong JH, Lee HL, Kim JO, Tae HJ, Jung SH, Lee KN, Jun DW, Lee OY, Yoon BC, Choi HS, et al. Correlation between complicated diverticulitis and visceral fat. J Korean Med Sci. 2011;26:1339–43.
Keizer AL, Peters LW, de Vries C, Smets YFC, de Wit LT, van Pampus MG. Spontaneous adrenal haemorrhage in early pregnancy. BMJ Case Reports. 2013;2013:bcr2012008062.
Leong M, Pendyala M, Chaganti J, Al-Soufi S. A case of bilateral adrenal haemorrhage following traumatic brain injury. J Intensive Care. 2015;3:4.
McNicol RE, Bradley A, Griffin J, Duncan G, Eriksen CA, Guthrie GJ. Post-operative bilateral adrenal haemorrhage: A case report. Int J Surg Case Rep. 2014;5:1145–7.
Lancaster TR, Singer DE, Sheehan MA, Oertel LB, Maraventano SW, Hughes RA, Kistler JP. The Impact of Long-term Warfarin Therapy on Quality of Life: Evidence From a Randomized Trial. Arch Intern Med. 1991;151:1944–9.
Sanford Z, Nanjundappa A, Annie FH, Embrey S. Adrenal hemorrhage in a patient anticoagulated with apixaban with antiphospholipid syndrome. Cureus. 2019;11(7):e5108.
Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. The Lancet. 2014;383:2152–67.
Hiroi N, Yanagisawa R, Yoshida-Hiroi M, Endo T, Kawase T, Tsuchida Y, Toyama K, Shibuya K, Nakata K, Yoshino G. Retroperitoneal hemorrhage due to bilateral adrenal metastases from lung adenocarcinoma. J Endocrinol Invest. 2006;29:551–4.
Goldman HB, Howard RC, Patterson AL. Spontaneous retroperitoneal hemorrhage from a giant adrenal myelolipoma. J Urol. 1996;155:639–639.
Ishikawa H, Tachibana M, Hata M, Tazaki H, Akatsuka S, Iri H. Myelolipoma of the adrenal gland. J Urol. 1981;126:777–9.
Kawashima A, Sandler CM, Ernst RD, Takahashi N, Roubidoux MA, Goldman SM, Fishman EK, Dunnick NR. Imaging of nontraumatic hemorrhage of the adrenal gland. Radiographics. 1999;19:949–63.
Ramon I, Mathian A, Bachelot A, Hervier B, Haroche J, Boutin-Le ThiHuong D, Costedoat-Chalumeau N, Wechsler B, Karmali R, Velkeniers B, et al. Primary adrenal insufficiency due to bilateral adrenal hemorrhage-adrenal infarction in the antiphospholipid syndrome: long-term outcome of 16 patients. J Clin Endocrinol Metab. 2013;98:3179–89.
Danese CA, Viola RM. Adrenal hemorrhage during anticoagulant therapy. Ann Surg. 1974;179:70–2.
May C, Asia M, Karavitaki N, Arlt W, Guest P, O’Reilly M. Bilateral adrenal haemorrhage. QJM. 2017;110:169–71.
Park KJ, Bushmiaer M, Barnes CL. Bilateral adrenal hemorrhage in a total knee patient associated with enoxaparin usage. Arthroplasty today. 2015;1:65–8.
Husebye ES, Anderson MS, Kämpe O. Autoimmune polyendocrine syndromes. N Engl J Med. 2018;378:1132–41.
Saverino S, Falorni A. Autoimmune Addison’s disease. Best Pract Res Clin Endocrinol Metab. 2020;34:101379.
Cohen AT, Hunt BJ. Is there a role for low-dose DOACs as prophylaxis? Hematology Am Soc Hematol Educ Program. 2019;2019:187–93.
Arosemena MA, Rodriguez A, Ediriweera H. Bilateral adrenal haemorrhage secondary to rivaroxaban in a patient with antiphospholipid syndrome. BMJ Case Reports CP. 2020;13:e234947.
Comuth W, Christiansen JJ, Bloch-Münster A-M, Husted S. Bilateral adrenal gland hemorrhage in a patient treated with rivaroxaban. Blood Coag Fibrinol. 2017;28:102–4.
Ly BA, Quintero L. Adrenal insufficiency from unilateral adrenal hemorrhage in a patient on rivaroxaban thromboprophylaxis. AACE clinical case reports. 2019;5:e70–2.
Caron P. Chabannier M-Hln, Cambus J-P, Fortenfant Fo, Otal P, Suc J-M: Definitive adrenal insufficiency due to bilateral adrenal hemorrhage and primary antiphospholipid syndrome. J Clin Endocrinol Metab. 1998;83:1437–9.
Delhumeau A, Moreau X, Chapotte C, Houi N, Bigorgne J. Heparin-associated thrombocytopenia syndrome: an underestimated etiology of adrenal hemorrhage. Intensive Care Med. 1993;19:475–7.
Vengrove MA, Amoroso A. Reversible adrenal insufficiency after adrenal hemorrhage. Ann Intern Med. 1993;119:439.
Feuerstein B, Streeten DH. Recovery of adrenal function after failure resulting from traumatic bilateral adrenal hemorrhages. Ann Intern Med. 1991;115:785–6.
Taylor HC, Sachs CR, Bravo EL. Primary aldosteronism: remission and development of adrenal insufficiency after adrenal venography. Ann Intern Med. 1976;85:207–9.
Jahangir-Hekmat M, Taylor HC, Levin H, Wilbur M, Llerena LA. Adrenal insufficiency attributable to adrenal hemorrhage: long-term follow-up with reference to glucocorticoid and mineralocorticoid function and replacement. Endocr Pract. 2004;10:55–61.
Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, Husebye ES, Merke DP, Murad MH, Stratakis CA. Diagnosis and treatment of primary adrenal insufficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:364–89.
Castro MA, Freixes MC, de Miguel Novoa P, Gimeno PG, Escolá CÁ, Hanzu FA. SEEN guidelines for the management and prevention of acute adrenal insufficiency. Endocrinol Diabetes Nutr (Engl Ed). 2020;67:53–60.
Acknowledgements
We express our appreciation to the research team members and TLGS and Babak imaging center participants, especially Dr. Kaveh Samimi, for their contribution to the study.
Funding
None.
Author information
Authors and Affiliations
Contributions
ESH, YSH, and FH: wrote the manuscript; MT reviewed hematologic points and comments on expertise visions; YSH and PR reviewed and interpreted the CT and MRI; FH, YSH, SST, and ESH reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval from an ethics committee was not needed for this case report since it involved two patients.
Consent for publication
Written informed consent was obtained from the patients for publication of these two Case reports and any accompanying images. A copy of the written consent is available for review by the Series Editor of this journal.
Competing interests
There was no conflict of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sheklabadi, E., Sharifi, Y., Tabarraee, M. et al. Adrenal hemorrhage following direct oral anticoagulant (DOAC) therapy: two case reports and literature review. Thrombosis J 20, 39 (2022). https://doi.org/10.1186/s12959-022-00397-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12959-022-00397-9