Abstract
Fatigue, an increasingly acknowledged symptom in various chronic diseases, has garnered heightened attention, during the medical era of bio-psycho-social model. Its persistence not only significantly compromises an individual’s quality of life but also correlates with chronic organ damage. Surprisingly, the intricate relationship between fatigue and female reproductive health, specifically infertility, remains largely unexplored. Our exploration into the existing body of evidence establishes a compelling link between fatigue with uterine and ovarian diseases, as well as conditions associated with infertility, such as rheumatism. This observation suggests a potentially pivotal role of fatigue in influencing overall female fertility. Furthermore, we propose a hypothetical mechanism elucidating the impact of fatigue on infertility from multiple perspectives, postulating that neuroendocrine, neurotransmitter, inflammatory immune, and mitochondrial dysfunction resulting from fatigue and its co-factors may further contribute to endocrine disorders, menstrual irregularities, and sexual dysfunction, ultimately leading to infertility. In addition to providing this comprehensive theoretical framework, we summarize anti-fatigue strategies and accentuate current knowledge gaps. By doing so, our aim is to offer novel insights, stimulate further research, and advance our understanding of the crucial interplay between fatigue and female reproductive health.
Similar content being viewed by others
Introduction
Infertility is a condition characterized by the inability of a woman to conceive after a year of regular, unprotected intercourse. It poses a significant global health concern, affecting approximately 15% of couples worldwide, with female factors alone accounting for at least 35% [1,2,3]. Beyond the physiological challenges, long-term infertility can lead to emotional distress, strained relationships, and societal stigma, impacting the overall well-being of couples aspiring to have children [4].
Fatigue, commonly described as extreme tiredness, diminished energy, and reduced physical and mental capacity, is a prevalent symptom in various chronic conditions [5]. While there is no consensus definition, and it overlaps with factors such as chronic pain, physical exertion, sleep disorders, and psychological stress [6, 7]. Under the bio-psycho-social medical model, the concept of “whole-person care” has been increasingly accepted and applied, and patient-reported outcomes (PROs) have received more attention in clinical research, with fatigue being an important indicator of disease [8,9,10]. This phenomenon has also appeared in the field of gynecology, thus, we began to focus on the impact of fatigue on infertility.
There is growing recognition of the potential link between fatigue and female infertility, but scientific evidence remains limited. Fatigue could impact fertility through complex mechanisms involving the neuroendocrine system [11], inflammatory-immune response [12], energy metabolism, and oxidative stress [13]. Furthermore, the development of fatigue is associated with changes in neurotransmitter metabolism and neuronal plasticity [14]. These alterations, in turn, may affect crucial reproductive processes like ovulation, implantation, and embryonic development. What’s more, managing chronic fatigue can exact a significant emotional toll, potentially leading to stress, anxiety, and depression, which may further impact fertility through hormonal imbalances [15], disrupted menstrual cycles [16], and decreased sexual desire [17].
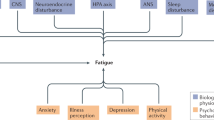
In this review, we aim to provide a comprehensive overview of the link between fatigue and female infertility, delving into the potential mechanisms through which fatigue impacts reproductive health (Fig. 1). By connecting the dots between fatigue and factors closely tied to infertility, we endeavor to unveil the complex interactions and indirect effects of fatigue on female fertility. Our objective is to offer novel insights, stimulate further research, and propel the advancement of our comprehension in this pivotal realm of female reproductive health for the benefit of graduate students in medicine and practicing physicians.
Potential mechanisms by which fatigue influences female infertility. The relationship between fatigue and female infertility is under-recognized, and it has complex crosstalk with co-factors (for example, pain and depression) through the activation of in vivo stress-responsive systems (HPA-axis, neurotransmitter system) and inflammation stimulated by different stress signals, and then contributing to hormonal dysregulation, oxidative stress, and mitochondrial metabolic dysfunction, which may be an underlying mechanism that affects female infertility. The solid lines represent reported studies, and the dotted lines represent studies that need to be developed. Double arrows represent interconnections. HPA, hypothalamic-pituitary-adrenal; 5-HT, 5-hydroxytryptamine; ROS, reactive oxygen species
Epidemiology of fatigue
Fatigue is a multifaceted symptom experienced by both healthy and unhealthy individuals, lacking a clear-cut definition. Self-reported scales are the primary measurement tools [18]. Current research suggests a key distinguishing factor between healthy and disease-related fatigue is the inability to alleviate fatigue with rest [19]. Fatigue often occurs as a comorbidity alongside various psychophysical factors, such as anxiety, depression, and pain, as demonstrated by multivariate analyses [20,21,22,23,24,25,26]. Therefore, exploring the influence of these co-factors on infertility is vital.
Though not life-threatening, fatigue severely affects the quality of life (QoL) of infertile women [27]. Despite regional variations in reported incidence [28,29,30,31,32], a recent meta-analysis estimated the global prevalence of general fatigue at 20.4% in adults and 11.7% in minors [33]. Studies from 2011 highlighted that 7–45% of the U.S. population experiences persistent fatigue [34]. A cross-sectional study involving 2063 individuals showed that over 50% of patients were troubled by fatigue in Saudi Arabia [35]. Importantly, women appear to be more susceptible to fatigue than men, with a pooled odds ratio [33]. Further, studies support that female sex may be an independent risk factor for persistent fatigue [28, 36].
Fatigue can manifest throughout a woman’s reproductive life cycle, impacting sexual maturity, pregnancy, post-partum, and perimenopause. Postpartum fatigue is well-documented [37, 38], but there’s a paucity of research on fatigue in infertile women. However, fatigue in women of reproductive age, especially in infertile women, has only begun to be mentioned in recent years. Approximately 25–60% of infertile patients are reported to be disturbed by psychological factors, including anxiety and depression [39], but the prevalence of fatigue in infertility is understudied. An observational study showed that 52 out of 140 infertile women had self-reported fatigue, and the severity of fatigue negatively affected their QoL [40]. Similarly, a study involving 149 infertile patients showed that fatigue was the most influential factor in the QoL of infertile women [41]. Taken together, more research is critically needed to address this gap.
Current state-of-the-art of fatigue in female infertility
Research on how fatigue directly impacts female infertility remains limited. However, fatigue is discussed for several chronic conditions affecting the uterus and ovaries (Table 1). Additionally, emerging studies suggest that autoimmune diseases, characterized prominently by fatigue, may adversely affect female fertility. Overall, research on fatigue associated with infertility faces multiple challenges and barriers.
Fatigue in Uterine and Ovarian diseases
Endometriosis
Endometriosis (EMT), defined as the presence of endometriotic lesions outside the uterus, is a greatly disturbing gynecologic disease that affects about 10% of women of reproductive age [42]. As early as 2018, a multicenter cross-sectional study sponsored by Wright and his colleagues found fatigue as a frequent symptom of EMT and was closely associated with insomnia, depression, pain, and occupational stress [43]. Subsequently, many observational studies have documented similar results [44]. Several evidence have indicated that the severity of EMT is associated with increased fatigue. A matched pair case-control study revealed that women with painful endometriosis reported significantly greater fatigue than those without significant pain symptoms and controls [45]. Ashrafi et al. found fatigue to be a predictor of risk for EMT through multiple logistic regression [46]. EMT-related fatigue seriously undermines women’s sense of life well-being and even their relationships with partners [47]. A scale-based observational study in Spain emphasized that moderate to severe fatigue contributed to psychological impairment, such as higher anxiety and depression, poorer sleep quality, and lower sexual function [48]. The above studies showed that fatigue is a common, secondary symptom of EMT, and is related to other somatic and psychological impairments, ultimately significantly decreasing patients’ QoL and sexual function [49].
Polycystic ovary syndrome
Polycystic ovary syndrome (PCOS) is the most common chronic endocrine disease characterized by menstrual dysfunction and ovulation disorders affecting 5–13% of women in the general population [50]. Studies have found that PCOS women often suffer from increased anxiety and depression, causing psychological fatigue and further impairing QoL [51, 52]. It is noted that patients with lower QoL parameters showed more worse marital sexual functioning [53]. A randomized clinical trial found that a psychological treatment named cognitive behavioral therapy (CBT) can significantly reduce the severity of fatigue in PCOS patients compared to the control group [51]. On the whole, fatigue almost goes unnoticed in patients with PCOS [54].
Premature Ovarian Insufficiency.
Premature ovarian insufficiency (POI) develops the breakdown of ovarian function and menopause before the age of 40, affects about 1% of women, and is one of the main causes of female infertility [55]. A cross-sectional study showed fatigue was a highly prevalent symptom closely associated with menopause in the recruited Chinese women with POI [56]. A few cross-sectional studies supported that the POI group had a higher fatigue index than controls [56, 57]. In another study, POI patients were more likely to suffer from sleep disturbance and depression, but not fatigue compared to age-matched healthy individuals [58]. Similar results were also obtained in an earlier study on premature ovarian failure [59]. Thus, the available evidence on whether patients with POI are at higher risk for fatigue is heterogeneous and needs to be studied extensively.
Fatigue in Autoimmune diseases affecting fertility
Autoimmune diseases are a large group of chronic disorders mediated by immune responses to self-antigens, such as rheumatoid arthritis (RA) [19], antiphospholipid syndrome [60], systemic lupus erythematosus [61], multiple sclerosis (MS) [62], autoimmune hypothyroidism [63]. A range of mental and somatic symptoms are present in these disorders, with fatigue being one of the most common and challenging symptoms to manage [64]. Fatigue is often indicative of disease activity in these disorders [19], while disease activity raises the risk of adverse pregnancy outcomes, particularly miscarriage [65,66,67]. MS patients with fatigue had significantly higher levels of C-reactive protein (CRP) during pregnancy than those without fatigue, suggesting that higher CRP, an indicator of systemic inflammation, may be somewhat predictive of pregnancy-related comorbidities [68]. A small single-center retrospective study revealed that more patients with MS underwent elective cesarean deliveries due to fatigue [69]. Additionally, the state of chronic fatigue directly impairs sexual function of women, which contributes significantly to the probability of fertility [17, 70], For instance, some studies showed sexual function in women with RA may be affected by pain and fatigue, potentially impacting fertility [71, 72]. Despite the shared occurrence of fatigue and female infertility in autoimmune diseases, high-quality research investigating the impact of fatigue on infertility in these conditions is needed.
Challenges of Fatigue Research in female infertility
The underrepresentation of fatigue research within female infertility remains a significant obstacle. This can be attributed to several factors:
Subjective and difficult measurement
The qualitative assessment of fatigue is relatively difficult and often subjective, and relies on the use of subjective self-report questionnaires [18, 73]. Moreover, experts in different fields may develop professional-specific questionnaires [74, 75]. The lack of standardized fatigue measurement across studies, including variations in the specific scales, recall periods, and wording, makes it challenging to compare results and draw definitive conclusions.
Focus on cross-sectional designs
Most of the studies on fatigue and infertility utilizes cross-sectional designs [76,77,78], which typically establishes causality through multiple regression analysis. The cross-sectional designs limit the ability to establish clear cause-and-effect relationships.
Potential confounding factors
Difficulties in fatigue research also arise from potential confounders of fatigue, such as emotional distress and pain [19, 79], suggesting that fatigue is not a single symptom of infertility, but rather is accompanied by a number of psychological and physical symptoms [40]. It is paramount to consider these interconnected factors to fully understand the complex relationship between fatigue and infertility.
Putative mechanisms of fatigue on female infertility
Longstanding fatigue serves as a catalyst for pathophysiologic changes in numerous systems and organs [11], posing detrimental effects on health [80], which may become the key force driving infertility. The pathogenesis of fatigue, involves the central nervous system (CNS) and autonomic nervous system (ANS), immune and inflammation, mitochondrial oxidative stress [81,82,83]. Simultaneously, the mechanisms of infertility are closely related to neuro-endocrine dysregulation, inflammatory and immune imbalances, and oxidative stress activation [62, 84]. It is clear that there are shared mechanisms for fatigue and infertility, which is worth exploring. Consequently, the forthcoming section presents evidence in support of a hypothesized mechanism illustrating overlap between fatigue and infertility.
Neuroendocrine disruption and Hormonal Imbalance
The CNS and endocrine system collaborate to maintain daily activities and keep up energy. Structural and functional dysfunctions in different regions of the brain may disrupt motor cortical excitability, hormone secretion, and the signal to the working muscles, which in turn cause fatigue [85, 86]. In particular, abnormalities in the hypothalamic-pituitary-adrenal (HPA) axis have been identified as a cause of fatigue. Notably, adrenal hormone dysregulation, such as hypocortisolemia, is frequently found in fatigued individuals, representing one facet of the imbalance of the HPA axis [87]. For instance, one study found MS patients with fatigue had significantly higher pituitary heights and widths, along with increased secretion of adrenocorticotropic hormone compared to patients without fatigue. These findings suggest CNS and endocrine systems are involved in the pathogenesis of fatigue through sophisticated regulation of brain structure and function [75].
More importantly, it is closely tied to maintaining reproductive function. First of all, gonadotrophin-releasing hormone (GnRH) from the hypothalamus, along with luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary gland, regulates follicular development and the ovarian cycle [88]. What’s more, the HPA axis has a direct inhibitory action on the hypothalamic-pituitary-ovarian axis in certain ways [15], impacting the cyclical patterns in central and ovarian hormones, including FSH, LH, E2, and P4. Besides, one early study found that uterine cortisol deficiency may mediate elevated levels of NK cells, which further impaired decidualization [89]. Dehydroepiandrosterone (DHEA), a kind of adrenal hormone, is linked to fatigue and turns out to be an important contributor to reproductive function [90]. DHEA deficiency can severely impact ovarian hormone synthesis and ovarian reserve, potentially leading to premature ovarian insufficiency (POI) [91]. Supplementation with DHEA has demonstrated positive effects on pregnancy rates, emphasizing its role in maintaining fertility [92,93,94,95]. Beyond the HPA axis, hormonal changes associated with fatigue extend to estrogen, progesterone and thyroid hormone [96,97,98,99]. Moreover, untreated maternal hypothyroidism is associated with adverse pregnancy outcomes such as miscarriage, preeclampsia, and many neonatal disorders [100].
In fact, although fatigue is a rather ill-defined physical manifestation and the relationship with female infertility has not been directly studied, evidence of neuroendocrine disruption and hormonal imbalance found in fatigue-related disorders supports their relevance to the maintenance of female fertility.
Stress and nervous system response
Perhaps, it is reasonable to suggest that fatigue and its co-factors can contribute to infertility via shared mechanistic pathways. Organisms are required to respond and adapt adequately to ubiquitous stress in an ever-changing environment, such as viral infections, trauma, and adverse life events. In the first place, the onset of fatigue and its co-factors is regulated by the in-vivo stress response system [101, 102]. When subjected to internal and external stress, the biological stress response system immediately makes corresponding alterations, including the HPA axis and the ANS system, as well as the neuroimmune system [14, 103,104,105]. Sustained stress may lead to maladaptive responses manifested as dysregulation of the above systems [106], which is specifically manifested as abnormal levels of serum catecholamines and glucocorticoids and persistent low-grade inflammation based on findings such as microglial activation in the brain and increased pro-inflammatory cytokines in the periphery [107]. Moreover, stress and stress hormones inhibit the release of GnRH, and glucocorticoids suppress LH secretion [108]. These abnormal alterations may further disrupt ovarian steroidogenesis and exacerbate inflammatory storms, ultimately being detrimental to female fertility.
Anhedonia attributed to deficits in reward processing is a shared feature in many neuropsychiatric symptoms, including fatigue, depression, and chronic pain [62]. The processing ability of brain reward circuits is disrupted by inappropriate levels of monoaminergic neurotransmission, mainly including serotonin (5-HT), norepinephrine, and dopamine with their degenerate mesocorticolimbic pathways from the midbrain to the basal ganglia, the limbic system, and the prefrontal cortex [62, 109, 110]. The lack of neurotransmitters, including 5-HT, can cause sexual dysfunction, thereby reducing fertility opportunities [15, 111].
Overall, hormonal imbalances and incorrect release of neurotransmitters caused by the nervous response system could result in infertility. Additionally, fatigue could reduce sexual desire and cause sexual dysfunction, ultimately declining the chance of pregnancy [48]. The above evidence suggests that fatigue mediate neurologic dysregulation that can aggravate infertility.
Low-grade inflammatory activation
Of great interest, fatigue is common in chronic autoimmune, inflammatory diseases [112, 113]. A recent cross-sectional study found elevating fatigue severity is closely linked to stronger signs of monocyte activation, including increased inflammatory gene expression in monocytes, higher CD8+ T-lymphocyte counts, and increased serum pro-inflammatory cytokines [114]. There is a strong correlation between fatigue severity and production of pro-inflammatory cytokines [19]. Women with fatigue often show multiple immune dysfunctions rendering them susceptible to upper respiratory tract infection, chronic lymphadenopathy, and high body temperature [115]. These dysregulations involve cell-mediated immunity, including impairment of the function of NK cells, hypo-reactivity of T cells to the antigen, the activation of monocyte macrophages, and the persistence of autoreactive cells [116]. Concurrently, they manifest as changes in many inflammation-related markers, such as increased CRP levels [68], elevation in pro-inflammatory factors like interleukin-1β, interleukin-6, interleukin-12, interleukin-2, tumor necrosis factor-alpha and interferon-gamma, increased expression of nuclear factor kappa-B, as well as decreased levels of anti-inflammatory factors like interleukin-8, interleukin-13, interleukin-15, and interleukin-23 [117]. The changes in inflammation-related profile support that patients experiencing fatigue are in a low-grade inflammatory state. In a word, inflammatory activation is undoubtedly a key step in the onset of fatigue [118].
It was reported that the increased infertility rate in RA with fatigue symptoms is due to an imbalance of inflammatory factors [72]. Indeed, the immune system serves as a crucial bridge connecting various systems within the organism, with cytokine and immune cells distributed in both in center and periphery systems. The homeostasis of immunity and inflammation is necessary to maintain a successful pregnancy. The imbalance of inflammatory factors and immune cell profiles is a notable phenomenon in patients with adverse pregnancy history [119, 120]. Such abnormalities could significantly impact pregnancy outcomes by damaging ovarian function [121], inhibiting endometrial receptivity [122], hindering trophoblast development, and interfering with immune tolerance at the maternal-fetal interface [65].
In short, inflammation is one of the most common mechanisms underlying fatigue and is also a known contributor to infertility. Fatigue may indicate active inflammation that needs to be managed in the reproductive field.
Mitochondrial dysfunction and Cellular stress
Fatigue is defined as “a lack of energy”, indicating a close relationship with energy metabolism [13], which is manifested as impacted adenosine triphosphate production [123]. Mitochondria are core organelles that have existed in most cells since biological evolution. They provide continuous energy for biological survival and function, produce adenosine triphosphate and lactic acid, and maintain the production level of reactive oxygen species (ROS) and reactive nitrogen species (RNS) and the balance of calcium and iron in cells, removing peroxides in time. Dysfunctional mitochondria can generate and release mitochondrial components, including cardiolipin, mitochondrial DNA, and mitochondrial formylated peptides. These components, once released, can act as damage-associated molecular patterns, triggering an inflammatory response through the activation of pattern recognition receptors [124]. In addition, mitochondria play a rate-limiting role in controlling steroidogenesis, and mitochondrial impairment in neurons further affects the synthesis of neuroactive steroid hormones in the brain [125]. And the collapse of mitochondria at neuronal synapse impedes the release of neurotransmitters [126, 127]. In summary, loss of neuroendocrine-immune homeostasis due to mitochondrial disruption may partially explain infertility.
Furthermore, mitochondria function as the primary origin of ROS/RNS as byproducts of nutrient metabolism. This occurrence is concomitant with various indicators of oxidative stress, including diminished activity of antioxidant enzymes such as superoxide dismutase and plasma-like glucose peroxidase, as well as reduced levels of zinc. Conversely, there is an elevation in the activity of pro-oxidative enzymes like myeloperoxidase, along with increased levels of nitro-tyrosine and heightened nitric oxide production [118]. The heightened presence of ROS/RNS assumes a pivotal role in the manifestation of fatigue. A study demonstrated that the accumulation of ROS/RNS during exercise had a detrimental impact on Na+/K+- ATPase activity, calcium conversion and sensitivity in myofibrils, and actin-myosin dynamics. These effects collectively resulted in a reduction in the generation of muscle energy, ultimately culminating in the onset of muscle fatigue [128]. Similarly, it has been proved that many experimental drugs with antioxidant properties can improve and alleviate chronic fatigue-like behaviors by changing ROS signaling pathways [129, 130]. However, excessive oxidative stress could impair the female reproductive system by destroying oocyte quality [131, 132], damaging endometrial receptivity [133], promoting trophoblast apoptosis, medicating implantation failure, and early pregnancy loss [134]. Thereby, we believe mitochondrial dysfunction and cellular stress, closely linked with fatigue, exert a profound influence on the function of the reproductive system, but need to be further studied.
Taken together, although explained from multiple perspectives, the current evidence is still insufficient to prove whether fatigue is a result or a predisposing factor for female infertility. The above hypothesized mechanisms regarding the impacts of fatigue on infertility perhaps only provide new viewpoints for researchers in the future.
The potential benefits of fatigue management on infertility
Given the potential connection between fatigue and infertility, it’s reasonable to consider whether fatigue management could benefit those experiencing infertility. An early intervention for fatigue is recommended, as a systematic review pointed out health risks associated with fatigue can occur earlier than hospitalization, illness, and death [135]. Current treatments for fatigue include dietary supplement/nutritional interventions, medications, exercise, physical therapy, and psychological interventions [136,137,138,139,140] (Fig. 2). Among them, some strategies have been reported to be beneficial for treating infertility, while others may be in favor of the health of female reproduction.
Strategies as well as mechanisms to alleviate fatigue. There are four main measures of fatigue alleviation: dietary supplements, medication, exercise and physical therapy, and psychological interventions. Relief of fatigue is achieved through anti-inflammatory, antioxidant, improved energy metabolism, and neurological modulation. The circular arrows indicate that the anti-fatigue mechanisms interact with each other and do not correspond to specific anti-fatigue measures
Anti-inflammatory and antioxidant nutrients
These nutrients, such as coenzyme Q10, L-carnitine, iron, zinc, methionine, nicotinamide adenine dinucleotide, and vitamins, help relieve fatigue [141,142,143]. Some studies have shown that supplementation with iron can significantly improve fatigue in women of reproductive age, potentially contributing to achieving successful pregnancy [144, 145]. Meanwhile, they belong to mitochondria-targeted nutrient therapy, which improves oxidative damage to ovarian and uterus. For instance, coenzyme Q10 is a key component of ATP production via oxidative phosphorylation, and improves mitochondrial membrane potential and superoxide levels, thereby rescuing ovarian reserve deficiency [146]. Zinc supplementation has been reported to significantly improve oocyte glutathione and mitochondrial activity, as well as reduce ROS levels, which facilitates oocyte maturation [147]. Vitamins have been shown to increase mitochondrial biosynthesis [148], and restore endocrine and metabolic homeostasis [149], which in turn improves follicular development and subsequent follicular quality in the PCOS model.
Medications
Some synthetic and natural drugs can combat fatigue. Glucocorticoids, relieve fatigue due to hypocortisolism and may improve ovarian function to treat infertility through regulating neuroendocrine function [150, 151]. Synbiotics, a bio-mixture of probiotics and nutrients, show potential in treating multiple symptoms of fatigue, including improving inflammation and stress responses mediated by the cytokine-HPA axis, along with mental and physical health [152], and enhance energy metabolism effectively [139].
Exercise and Physical Therapy
In addition to medication, exercise rehabilitation is used to reduce fatigue. A recent meta-analysis found that physical therapy was efficacious and safe in reducing fatigue in people with inflammatory rheumatic and musculoskeletal diseases [64]. It is believed that practicing yoga has a positive effect on reproductive organs and increases blood circulation [153]. Acupuncture treatment for infertile patients can regulate menstrual cycle and reduce fatigue, thus facilitating the process of ART cycles [154].
Psychological interventions
Furthermore, psychological interventions, particularly CBT, are applied in the management of people with fatigue [155]. The psychological vulnerability screening and additional psychological consultation for infertile women are endorsed to mitigate these mixed symptoms that are not conducive to pregnancy, such as fatigue, depression, and anxiety [156]. A quasi-experimental study found psychological interventions significantly reduced depression and fatigue of infertility women, in turn, improved their intimacy and sexual satisfaction [157]. Two randomized controlled trials from Brunei found that Nursing care reduced depression and fatigue, and improved feelings of social support and sleep quality among infertile women [158, 159]. It can be seen that psychotherapy is one of the recommended treatments for infertility patients. Herein, it is conceivable that measures to alleviate fatigue may be beneficial in treating infertility and improving reproductive outcomes.
Current gaps and future perspectives
The direct connection between fatigue and female infertility remains weak in evidence. Considering our previous discussion, it is apparent that fatigue alone is not the singular determining factor in female infertility. Unfortunately, the current concept of fatigue is vague [160], and the diagnostic criteria are not unified [161]. In different studies, fatigue can serve as an experimental concept, a symptom, a risk, a cause, and a result [6]. Moreover, the scales for assessing fatigue are varied [74, 75] and relatively subjective. There are three models for inducing fatigue, including exhaustive exercise-induced fatigue, and chemotherapy or radiotherapy-induced fatigue [162, 163]. These heterogeneities increase the difficulty of conducting research, thus making it challenging to explore fatigue in female infertility. Unlike depression, fatigue is often a concomitant symptom and is not subjectively valued, despite its significant impact on women’s QoL and reproductive health.
While anti-fatigue interventions might benefit female infertility, current studies lack thorough mechanistic exploration. The rational use of anti-fatigue approaches and their impact on female infertility and pregnancy outcomes need to be further investigated [139, 164].
In summary, the understanding of “fatigue” is weak when it comes to reproduction. Exploring the relationships between fatigue, female infertility, and adverse pregnancy outcomes is crucial, requiring more investigation in clinical trials and basic research. In clinical trials, utilizing established fatigue assessment scales and comprehensive recording of influencing factors are advised. Rigorous statistical methods, including machine learning and multivariate logistic regression, should be employed to analyze the relationship between fatigue and female infertility or pregnancy outcomes, under the premise of eliminating confounding factors. It is important to develop strict inclusion criteria for clinical populations with fatigue symptoms, implement subgroup analysis, and explore the benefits of fatigue management on fertility outcomes. Additionally, biomarker detection in blood and other humor, such as uterine fluid, may reveal insights into fatigue and pregnancy outcomes. In basic research, the use of well-established fatigue-related animal models is essential to investigate their potential impacts on reproduction.
Conclusion
Fatigue, a perplexing and disabling symptom, has gained more recognition in the medical era of bio-psycho-social model amid heightened individual psychological stress. It is thought to arise from endocrine imbalance and neurotransmitter changes in the central nervous system, immune-inflammation disruption, mitochondrial dysfunction, and excessive oxidative stress during viral infections or social-environmental stress. It is closely linked to both psychological and somatic factors, indirectly affecting female infertility. Prolonged fatigue can significantly diminish women’s quality of life and reproductive health, yet public awareness and understanding of this issue remain limited. Our study highlights the associations between fatigue and several common chronic conditions, and proposes hypotheses regarding the impact of fatigue on female infertility with the hope of calling attention to the issue of fatigue in infertile women in alignment with the whole-life care concept and providing insights for future study in this topic.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- HPA:
-
Axis: Hypothalamic-Pituitary-Adrenal Axis
- QoL:
-
Quality of Life
- MS:
-
Multiple Sclerosis
- IVF:
-
In Vitro Fertilization
- EMT:
-
Endometriosis
- PCOS:
-
Polycystic Ovary Syndrome
- CBT:
-
Cognitive Behavioral Therapy
- POI:
-
Premature Ovarian Insufficiency
- CRP:
-
C-Reactive Protein
- GnRH:
-
Gonadotrophin-Releasing Hormone
- LH:
-
Luteinizing Hormone
- FSH:
-
Follicle-Stimulating Hormone
- NK cells:
-
Natural Killer Cells
- DHEA:
-
Dehydroepiandrosterone
- CNS:
-
Central Nervous System
- ANS:
-
Autonomic Nervous System
- 5-HT:
-
Serotonin
- ECS:
-
Endocannabinoid System
- ROS/RNS:
-
Reactive Oxygen Species/ Reactive Nitrogen Species
- RA:
-
Rheumatoid Arthritis
References
Ga R, Muvvala SPR. Access to infertility care and ART treatment in India: a clinician’s perspective. Best Pract Res Clin Obstet Gynaecol. 2023;86:102302.
Sang Q, Ray PF, Wang L. Understanding the genetics of human infertility. Science. 2023;380:158–63.
Yatsenko SA, Rajkovic A. Genetics of human female infertility†. Biol Reprod. 2019;101:549–66.
Swift A, Reis P, Swanson M. Infertility-related stress and quality of life in women experiencing concurrent reproductive trauma. J Psychosom Obstet Gynaecol. 2022;43:171–6.
Zhao S, Shibata K, Hellyer PJ, Trender W, Manohar S, Hampshire A, Husain M. Rapid vigilance and episodic memory decrements in COVID-19 survivors. Brain Commun. 2022;4:fcab295.
Pattyn N, Van Cutsem J, Dessy E, Mairesse O. Bridging Exercise Science, cognitive psychology, and Medical Practice: is cognitive fatigue a remake of the Emperor’s New clothes? Front Psychol. 2018;9:1246.
Goërtz YMJ, Braamse AMJ, Spruit MA, Janssen DJA, Ebadi Z, Van Herck M, Burtin C, Peters JB, Sprangers MAG, Lamers F, et al. Fatigue in patients with chronic disease: results from the population-based lifelines Cohort Study. Sci Rep. 2021;11:20977.
Heesen C, Berger T, Riemann-Lorenz K, Krause N, Friede T, Pöttgen J, Meyer B, Lühmann D. Mobile health interventions in multiple sclerosis: a systematic review. Mult Scler. 2023;29:1709–20.
Cruz Rivera S, Aiyegbusi OL, Piani Meier D, Dunne A, Harlow DE, Henke C, Kamudoni P, Calvert MJ. The effect of disease modifying therapies on fatigue in multiple sclerosis. Mult Scler Relat Disord. 2023;79:105065.
Terwee CB, Elders PJM, Blom MT, Beulens JW, Rolandsson O, Rogge AA, Rose M, Harman N, Williamson PR, Pouwer F, et al. Patient-reported outcomes for people with diabetes: what and how to measure? A narrative review. Diabetologia. 2023;66:1357–77.
Thomas N, Gurvich C, Huang K, Gooley PR, Armstrong CW. The underlying sex differences in neuroendocrine adaptations relevant to myalgic encephalomyelitis chronic fatigue syndrome. Front Neuroendocrinol. 2022;66:100995.
Lanser L, Kink P, Egger EM, Willenbacher W, Fuchs D, Weiss G, Kurz K. Inflammation-Induced Tryptophan Breakdown is related with Anemia, fatigue, and Depression in Cancer. Front Immunol. 2020;11:249.
Zhong H, Shi J, Zhang J, Wang Q, Zhang Y, Yu P, Guan R, Feng F. Soft-Shelled Turtle Peptide Supplementation Modifies Energy Metabolism and Oxidative Stress, Enhances Exercise Endurance, and Decreases Physical Fatigue in Mice. Foods. 2022; 11.
Baker AME, Maffitt NJ, Del Vecchio A, McKeating KM, Baker MR, Baker SN, Soteropoulos DS. Neural dysregulation in post-COVID fatigue. Brain Commun. 2023;5:fcad122.
Valsamakis G, Chrousos G, Mastorakos G. Stress, female reproduction and pregnancy. Psychoneuroendocrinology. 2019;100:48–57.
Pollack B, von Saltza E, McCorkell L, Santos L, Hultman A, Cohen AK, Soares L. Female reproductive health impacts of long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: a literature review. Front Rehabil Sci. 2023;4:1122673.
Zhang L, Wu B, Ye J. Fatigue have impact on the sexual problems in Chinese females with systemic lupus erythematosus. BMC Womens Health. 2022;22:266.
Hewlett S, Dures E, Almeida C. Measures of fatigue: Bristol rheumatoid arthritis fatigue multi-dimensional questionnaire (BRAF MDQ), Bristol rheumatoid arthritis fatigue Numerical Rating scales (BRAF NRS) for severity, effect, and coping, chalder fatigue questionnaire (CFQ), Checklist Individual Strength (CIS20R and CIS8R), fatigue severity scale (FSS), Functional Assessment Chronic illness therapy (fatigue) (FACIT-F), Multi-dimensional Assessment of fatigue (MAF), multi-dimensional fatigue inventory (MFI), Pediatric Quality of Life (PedsQL) multi-dimensional fatigue Scale, Profile of fatigue (ProF), short form 36 vitality Subscale (SF-36 VT), and Visual Analog scales (VAS). Arthritis Care Res (Hoboken). 2011;63(Suppl 11):S263–286.
Davies K, Dures E, Ng WF. Fatigue in inflammatory rheumatic diseases: current knowledge and areas for future research. Nat Rev Rheumatol. 2021;17:651–64.
Möller MC, Berginström N, Ghafouri B, Holmqvist A, Löfgren M, Nordin L, Stålnacke BM. Cognitive and mental fatigue in chronic pain: cognitive functions, emotional aspects, biomarkers and neuronal correlates-protocol for a descriptive cross-sectional study. BMJ Open. 2023;13:e068011.
Valentine TR, Alschuler KN, Ehde DM, Kratz AL. Prevalence, co-occurrence, and trajectories of pain, fatigue, depression, and anxiety in the year following multiple sclerosis diagnosis. Mult Scler. 2022;28:620–31.
Clark NL, Kainth GS, Johnson M, Rangan A, Kottam L, Swainston K. Psychological interventions to improve pain, fatigue, anxiety, depression, and quality of life in children and adults with hypermobility spectrum disorders and Ehlers-Danlos syndrome: a systematic review. Rheumatol Int. 2023.
Renna ME, Shrout MR, Madison AA, Alfano CM, Povoski SP, Lipari AM, Carson WE 3rd, Malarkey WB, Kiecolt-Glaser JK. Depression and anxiety in colorectal cancer patients: ties to pain, fatigue, and inflammation. Psychooncology. 2022;31:1536–44.
Murphy HM, Fetter CM, Snow NJ, Chaves AR, Downer MB, Ploughman M. Lower corticospinal excitability and greater fatigue among people with multiple sclerosis experiencing pain. Mult Scler J Exp Transl Clin. 2023;9:20552173221143398.
Manning K, Kauffman BY, Rogers AH, Garey L, Zvolensky MJ. Fatigue severity and fatigue sensitivity: relations to anxiety, depression, pain catastrophizing, and pain severity among adults with severe fatigue and chronic low back pain. Behav Med. 2022;48:181–9.
Holten KIA, Bernklev T, Opheim R, Johansen I, Olsen BC, Lund C, Strande V, Medhus AW, Perminow G, Bengtson MB, et al. Fatigue in patients with newly diagnosed inflammatory bowel disease: results from a prospective inception cohort, the IBSEN III Study. J Crohns Colitis. 2023;17:1781–90.
Nho JH, Kim EJ. Relationships among Type-D personality, fatigue, and Quality of Life in Infertile Women. Asian Nurs Res. 2022;16:208–14.
Sunata K, Miyata J, Terai H, Matsuyama E, Watase M, Namkoong H, Asakura T, Masaki K, Chubachi S, Ohgino K et al. Asthma is a risk factor for general fatigue of long COVID in Japanese nation-wide cohort study. Allergol Int. 2023.
Amiot A, Chaibi S, Bouhnik Y, Serrero M, Filippi J, Roblin X, Bourrier A, Bouguen G, Franchimont D, Savoye G, et al. Prevalence and determinants of fatigue in patients with IBD: a cross-sectional survey from the GETAID. J Crohns Colitis. 2023;17:1418–25.
Al Maqbali M, Al Sinani M, Al Naamani Z, Al Badi K, Tanash MI. Prevalence of fatigue in patients with Cancer: a systematic review and Meta-analysis. J Pain Symptom Manage. 2021;61:167–e189114.
Zhan J, Zhang P, Wen H, Wang Y, Yan X, Zhan L, Chen H, Xu N, Lu L. Global prevalence estimates of poststroke fatigue: a systematic review and meta-analysis. Int J Stroke. 2023;18:1040–50.
Lilleholt L, Zettler I, Betsch C, Böhm R. Development and validation of the pandemic fatigue scale. Nat Commun. 2023;14:6352.
Yoon JH, Park NH, Kang YE, Ahn YC, Lee EJ, Son CG. The demographic features of fatigue in the general population worldwide: a systematic review and meta-analysis. Front Public Health. 2023;11:1192121.
Junghaenel DU, Christodoulou C, Lai JS, Stone AA. Demographic correlates of fatigue in the US general population: results from the patient-reported outcomes measurement information system (PROMIS) initiative. J Psychosom Res. 2011;71:117–23.
Al-Johani MS, Khalil R, Al-Mohaimeed YA, Al-Mundarij OM, Al-Samani AS, Al-Saqry OS, Al-Saawi AA, Al-Dhali IK, Al-Essa WA. Post-COVID-19 fatigue and health-related quality of life in Saudi Arabia: a population-based study. Front Public Health. 2023;11:1254723.
Hechenberger S, Helmlinger B, Penner IK, Pirpamer L, Fruhwirth V, Heschl B, Ropele S, Wurth S, Damulina A, Eppinger S, et al. Psychological factors and brain magnetic resonance imaging metrics associated with fatigue in persons with multiple sclerosis. J Neurol Sci. 2023;454:120833.
Corwin EJ, Arbour M. Postpartum fatigue and evidence-based interventions. MCN Am J Matern Child Nurs. 2007;32:215–20. quiz 221 – 212.
Odabas RK, Sökmen Y, Taspinar A. The effect of acupressure on postpartum fatigue in women delivering by caesarean section: a randomized controlled study. Explore (NY). 2023;19:293–9.
Boivin J, Oguz M, Duong M, Cooper O, Filipenko D, Markert M, Samuelsen C, Lenderking WR. Emotional reactions to infertility diagnosis: thematic and natural language processing analyses of the 1000 dreams survey. Reprod Biomed Online. 2023;46:399–409.
Kim YM, Nho JH. [Factors influencing infertility-related quality of life in infertile women]. Korean J Women Health Nurs. 2020;26:49–60.
Nho JH, Kim EJ. Relationships among type-D personality, fatigue, and quality of life in infertile women. Asian Nurs Res (Korean Soc Nurs Sci). 2022.
Maddern J, Grundy L, Castro J, Brierley SM. Pain in Endometriosis. Front Cell Neurosci. 2020;14:590823.
Ramin-Wright A, Schwartz ASK, Geraedts K, Rauchfuss M, Wölfler MM, Haeberlin F, von Orelli S, Eberhard M, Imthurn B, Imesch P, et al. Fatigue - a symptom in endometriosis. Hum Reprod. 2018;33:1459–65.
DiBenedetti D, Soliman AM, Gupta C, Surrey ES. Patients’ perspectives of endometriosis-related fatigue: qualitative interviews. J Patient-Reported Outcomes. 2020; 4.
Facchin F, Buggio L, Roncella E, Somigliana E, Ottolini F, Dridi D, Roberto A, Vercellini P. Sleep disturbances, fatigue and psychological health in women with endometriosis: a matched pair case-control study. Reprod Biomed Online. 2021;43:1027–34.
Ashrafi M, Sadatmahalleh SJ, Akhoond MR, Talebi M. Evaluation of risk factors Associated with endometriosis in Infertile Women. Int J Fertil Steril. 2016;10:11–21.
Alvarez-Salvago F, Lara-Ramos A, Cantarero-Villanueva I, Mazheika M, Mundo-López A, Galiano-Castillo N, Fernández-Lao C, Arroyo-Morales M, Ocón-Hernández O, Artacho-Cordón F. Chronic fatigue, physical impairments and quality of life in women with endometriosis: a case-control study. Int J Environ Res Public Health. 2020; 17.
Mundo-López A, Ocón-Hernández O, San-Sebastián AP, Galiano-Castillo N, Rodríguez-Pérez O, Arroyo-Luque MS, Arroyo-Morales M, Cantarero-Villanueva I, Fernández-Lao C, Artacho-Cordón F. Contribution of chronic fatigue to Psychosocial Status and Quality of Life in Spanish Women diagnosed with endometriosis. Int J Environ Res Public Health. 2020; 17.
Álvarez-Salvago F, Lara-Ramos A, Cantarero-Villanueva I, Mazheika M, Mundo-López A, Galiano-Castillo N, Fernández-Lao C, Arroyo-Morales M, Ocón-Hernández O, Artacho-Cordón F. Chronic fatigue, physical impairments and quality of life in women with endometriosis: a case-control study. Int J Environ Res Public Health. 2020; 17.
Guan C, Zahid S, Minhas AS, Ouyang P, Vaught A, Baker VL, Michos ED. Polycystic ovary syndrome: a risk-enhancing factor for cardiovascular disease. Fertil Steril. 2022;117:924–35.
Abdollahi L, Mirghafourvand M, Babapour JK, Mohammadi M. Effectiveness of cognitive-behavioral therapy (CBT) in improving the quality of life and psychological fatigue in women with polycystic ovarian syndrome: a randomized controlled clinical trial. J Psychosom Obstet Gynaecol. 2019;40:283–93.
Boivin MJ, Fatehi F, Phillips-Chan AE, Richardson JR, Summers AN, Foley SA. Exploratory study of a screening measure for polycystic ovarian syndrome, quality of life assessment, and neuropsychological evaluation. BMC Womens Health. 2020;20:132.
Drosdzol A, Skrzypulec V, Mazur B, Pawlińska-Chmara R. Quality of life and marital sexual satisfaction in women with polycystic ovary syndrome. Folia Histochem Cytobiol. 2007;45(Suppl 1):S93–97.
Ee C, Pirotta S, Mousa A, Moran L, Lim S. Providing lifestyle advice to women with PCOS: an overview of practical issues affecting success. BMC Endocr Disord. 2021;21:234.
Shekari S, Stankovic S, Gardner EJ, Hawkes G, Kentistou KA, Beaumont RN, Mörseburg A, Wood AR, Prague JK, Mishra GD, et al. Penetrance of pathogenic genetic variants associated with premature ovarian insufficiency. Nat Med. 2023;29:1692–9.
Huang Y, Qi T, Ma L, Li D, Li C, Lan Y, Chu K, Chen P, Xu W, Cao Y, et al. Menopausal symptoms in women with premature ovarian insufficiency: prevalence, severity, and associated factors. Menopause. 2021;28:529–37.
Benetti-Pinto CL, Menezes C, Yela DA, Cardoso TM. Sleep quality and fatigue in women with premature ovarian insufficiency receiving hormone therapy: a comparative study. Menopause. 2019;26:1141–5.
Ates S, Aydın S, Ozcan P, Bakar RZ, Cetin C. Sleep, depression, anxiety and fatigue in women with premature ovarian insufficiency. J Psychosom Obstet Gynaecol. 2022;43:482–7.
van der Stege JG, Groen H, van Zadelhoff SJ, Lambalk CB, Braat DD, van Kasteren YM, van Santbrink EJ, Apperloo MJ, Weijmar Schultz WC, Hoek A. Decreased androgen concentrations and diminished general and sexual well-being in women with premature ovarian failure. Menopause. 2008;15:23–31.
Bearne LM, Bieles J, Georgopoulou S, Andrews J, Tully A, Stolarchuk-Prowting K, Williamson T, Suarez BS, Nel L, D’Cruz D, et al. Fatigue in adults with primary antiphospholipid syndrome: findings from a mixed-methods study. Lupus. 2020;29:924–33.
Stamm B, Barbhaiya M, Siegel C, Lieber S, Lockshin M, Sammaritano L. Infertility in systemic lupus erythematosus: what rheumatologists need to know in a new age of assisted reproductive technology. Lupus Sci Med. 2022; 9.
Heitmann H, Andlauer TFM, Korn T, Mühlau M, Henningsen P, Hemmer B, Ploner M. Fatigue, depression, and pain in multiple sclerosis: how neuroinflammation translates into dysfunctional reward processing and anhedonic symptoms. Mult Scler. 2022;28:1020–7.
Louwerens M, Appelhof BC, Verloop H, Medici M, Peeters RP, Visser TJ, Boelen A, Fliers E, Smit JW, Dekkers OM. Fatigue and fatigue-related symptoms in patients treated for different causes of hypothyroidism. Eur J Endocrinol. 2012;167:809–15.
Santos EJF, Farisogullari B, Dures E, Geenen R, Machado PM. Efficacy of non-pharmacological interventions: a systematic review informing the 2023 EULAR recommendations for the management of fatigue in people with inflammatory rheumatic and musculoskeletal diseases. RMD Open 2023; 9.
Tan Y, Liu Q, Li Z, Yang S, Cui L. Pyroptosis-triggered pathogenesis: new insights on antiphospholipid syndrome. Front Immunol. 2023;14:1155222.
Tańska K, Gietka-Czernel M, Glinicki P, Kozakowski J. Thyroid autoimmunity and its negative impact on female fertility and maternal pregnancy outcomes. Front Endocrinol (Lausanne). 2022;13:1049665.
Kaplan TB, Bove R, Galetta K, Healy B, Chitnis C, Houtchens M. Effect of pregnancy loss on MS disease activity. J Neurol Sci. 2019;397:58–60.
Jalkanen A, Kauko T, Koskinen JO, Waris ME, Airas L. Elevated concentration of C-reactive protein is associated with pregnancy-related co-morbidities but not with relapse activity in multiple sclerosis. Neurol Sci. 2015;36:441–7.
Biringer K, Sivak S, Sivakova J, Ružiňák R, Martiníková M, Kantorova E, Biringerová Z, Kudela E, Kurca E. Fatigue as the limiting factor for vaginal birth in patients with multiple sclerosis. Neuro Endocrinol Lett. 2021;42:222–8.
Ghasemi V, Simbar M, Ozgoli G, Nabavi SM, Alavi Majd H. Prevalence, dimensions, and predictor factors of sexual dysfunction in women of Iran multiple sclerosis society: a cross-sectional study. Neurol Sci. 2020;41:1105–13.
Provost M, Eaton JL, Clowse MEB. Fertility and infertility in rheumatoid arthritis. Curr Opin Rheumatol. 2014;26:308–14.
Fattah A, Asadi A, Shayesteh MRH, Hesari FH, Jamalzehi S, Abbasi M, Mousavi MJ, Aslani S. Fertility and infertility implications in rheumatoid arthritis; state of the art. Inflamm Res. 2020;69:721–9.
Beckers E, Hermans K, Van Tubergen A, Boonen A. Fatigue in patients with rheumatic and musculoskeletal diseases: a scoping review on definitions, measurement instruments, determinants, consequences and interventions. RMD Open 2023; 9.
Cohen ET, Matsuda PN, Fritz NE, Allen DD, Yorke AM, Widener GL, Jewell ST, Potter K. Self-report measures of fatigue for people with multiple sclerosis: a systematic review. J Neurol Phys Ther. 2023.
Eren F, Demir A, Yilmaz SE, Ozturk S. Evaluation of the relationship between the morphometric structure of the pituitary gland and fatigue in patients with multiple sclerosis. Mult Scler Relat Disord. 2023;69:104470.
Reece JC, Neate SL, Davenport RA, Milanzi E, Nag N, Bevens W, Yu M, Jelinek GA, Simpson-Yap S. Stressful life events and depression and fatigue in people with multiple sclerosis: a cross-sectional analysis of an international cohort. Acta Neurol Belg. 2023.
Uhlir V, Stallmach A, Grunert PC. Fatigue in patients with inflammatory bowel disease-strongly influenced by depression and not identifiable through laboratory testing: a cross-sectional survey study. BMC Gastroenterol. 2023;23:288.
Walker S, Goodfellow H, Pookarnjanamorakot P, Murray E, Bindman J, Blandford A, Bradbury K, Cooper B, Hamilton FL, Hurst JR, et al. Impact of fatigue as the primary determinant of functional limitations among patients with post-COVID-19 syndrome: a cross-sectional observational study. BMJ Open. 2023;13:e069217.
Onate-Figuérez A, Avendaño-Coy J, Fernández-Canosa S, Soto-León V, López-Molina MI, Oliviero A. Factors Associated with fatigue in people with spinal cord Injury: a systematic review and Meta-analysis. Arch Phys Med Rehabil. 2023;104:132–42.
Lock AM, Bonetti DL, Campbell ADK. The psychological and physiological health effects of fatigue. Occup Med (Lond). 2018;68:502–11.
García-González D, Medino-Muñoz J, Romero-Elías M, García-Foncillas J, Ruiz-Casado A. Biological mechanisms of cancer-related fatigue in breast cancer survivors after treatment: a scoping review. J Cancer Surviv. 2023.
Penson A, Walraven I, Bronkhorst E, Grootenhuis MA, Maurice-Stam H, de Beijer I, der Loo M, Tissing WJE, van der Pal HJH, de Vries ACH, et al. Chronic fatigue in childhood cancer survivors is associated with lifestyle and psychosocial factors; a DCCSS LATER study. ESMO Open. 2023;8:102044.
Åkerstedt T, Schwarz J, Theorell-Haglöw J, Lindberg E. What do women mean by poor sleep? A large population-based sample with polysomnographical indicators, inflammation, fatigue, depression, and anxiety. Sleep Med. 2023;109:219–25.
Chitnis T, Vandercappellen J, King M, Brichetto G. Symptom Interconnectivity in multiple sclerosis: a narrative review of potential underlying Biological Disease processes. Neurol Ther. 2022;11:1043–70.
Siemionow V, Fang Y, Calabrese L, Sahgal V, Yue GH. Altered central nervous system signal during motor performance in chronic fatigue syndrome. Clin Neurophysiol. 2004;115:2372–81.
Gottschalk M, Kümpfel T, Flachenecker P, Uhr M, Trenkwalder C, Holsboer F, Weber F. Fatigue and regulation of the hypothalamo-pituitary-adrenal axis in multiple sclerosis. Arch Neurol. 2005;62:277–80.
Huang YM, Chi CW, Wu PS, Tai HC, Chien MN, Chen YJ. Adrenal gland irradiation causes fatigue accompanied by reactive changes in cortisol levels. J Clin Med. 2022; 11.
Coss D. Regulation of reproduction via tight control of gonadotropin hormone levels. Mol Cell Endocrinol. 2018;463:116–30.
Kuroda K, Venkatakrishnan R, James S, Šucurovic S, Mulac-Jericevic B, Lucas ES, Takeda S, Shmygol A, Brosens JJ, Quenby S. Elevated periimplantation uterine natural killer cell density in human endometrium is associated with impaired corticosteroid signaling in decidualizing stromal cells. J Clin Endocrinol Metab. 2013;98:4429–37.
Wronka KM, Wunsch E, Kozłowska-Petriczko K, Wójcicki M, Kruk B, Milkiewicz P. Dehydroepiandrosterone sulfate indicates decreased sulfation capacity and impaired quality of life in patients with primary sclerosing cholangitis. Pol Arch Intern Med. 2021;131:790–6.
Gleicher N, Darmon S, Molinari E, Zhang L, Hu J, Albertini DF, Barad DH. A form of secondary ovarian insufficiency (SOI) due to adrenal hypoandrogenism as new infertility diagnosis. Endocrine. 2021;72:260–7.
Ozcil MD. Dehydroepiandrosterone supplementation improves ovarian reserve and pregnancy rates in poor responders. Eur Rev Med Pharmacol Sci. 2020;24:9104–11.
Gao H, Gao L, Wang W. Advances in the cellular immunological pathogenesis and related treatment of primary ovarian insufficiency. Am J Reprod Immunol. 2022;88:e13622.
Gibson DA, Simitsidellis I, Kelepouri O, Critchley HOD, Saunders PTK. Dehydroepiandrosterone enhances decidualization in women of advanced reproductive age. Fertil Steril. 2018;109:728–e734722.
Mandal S, Mukhopadhyay P, Ghosh S. DHEA on sexual function in Sheehan Syndrome: a Randomized double-blind placebo-controlled crossover trial. J Clin Endocrinol Metab. 2022;107:e3395–402.
Cabelka CA, Baumann CW, Collins BC, Nash N, Le G, Lindsay A, Spangenburg EE, Lowe DA. Effects of ovarian hormones and estrogen receptor α on physical activity and skeletal muscle fatigue in female mice. Exp Gerontol. 2019;115:155–64.
Kanamori T, Suzuki M, Kaneko Y, Yamada K, Kubo H, Uchiyama M. Severe fatigue due to valproate-induced hypothyroidism in a case of bipolar disorder. Ann Gen Psychiatry. 2020;19:49.
Sunada N, Honda H, Nakano Y, Yamamoto K, Tokumasu K, Sakurada Y, Matsuda Y, Hasegawa T, Otsuka Y, Obika M, et al. Hormonal trends in patients suffering from long COVID symptoms. Endocr J. 2022;69:1173–81.
Sun Q, Oltra E, Dijck-Brouwer DAJ, Chillon TS, Seemann P, Asaad S, Demircan K, Espejo-Oltra JA, Sánchez-Fito T, Martín-Martínez E, et al. Autoantibodies to selenoprotein P in chronic fatigue syndrome suggest selenium transport impairment and acquired resistance to thyroid hormone. Redox Biol. 2023;65:102796.
Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP, Sullivan S, et al. Guidelines of the American thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21:1081–125.
Wu Z, Qu J, Zhang W, Liu GH. Stress, epigenetics, and aging: unraveling the intricate crosstalk. Mol Cell. 2023.
Rosenberg AGW, Dingemans VDA, Bos-Roubos AG, Luijks S, Dessens AB, Dykgraaf R, Roos-Hesselink JW, Van Rossum EFC, Van Der Lely AJ, De Graaff LCG. Associations between fatigue and endocrine and non-endocrine health problems in Turner Syndrome: Cohort Study and Review. J Clin Endocrinol Metab. 2023;108:e1649–59.
Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–71.
Strahler J, Skoluda N, Rohleder N, Nater UM. Dysregulated stress signal sensitivity and inflammatory disinhibition as a pathophysiological mechanism of stress-related chronic fatigue. Neurosci Biobehav Rev. 2016;68:298–318.
Van Booven DJ, Gamer J, Joseph A, Perez M, Zarnowski O, Pandya M, Collado F, Klimas N, Oltra E, Nathanson L. Stress-Induced Transcriptomic changes in females with myalgic Encephalomyelitis/Chronic fatigue syndrome reveal disrupted Immune signatures. Int J Mol Sci. 2023; 24.
Vignjević Petrinović S, Milošević MS, Marković D, Momčilović S. Interplay between stress and cancer-A focus on inflammation. Front Physiol. 2023;14:1119095.
Li R, Zhou Y, Zhang S, Li J, Zheng Y, Fan X. The natural (poly)phenols as modulators of microglia polarization via TLR4/NF-κB pathway exert anti-inflammatory activity in ischemic stroke. Eur J Pharmacol. 2022;914:174660.
Sakurada Y, Matsuda Y, Motohashi K, Hasegawa T, Otsuka Y, Nakano Y, Tokumasu K, Yamamoto K, Sunada N, Honda H et al. Clinical characteristics of female long COVID patients with menstrual symptoms: a retrospective study from a Japanese outpatient clinic. J Psychosom Obstet Gynecol. 2024; 45.
Park Y, Lee JJ, Koh JH, Kim MJ, Park SH, Kwok SK. Kynurenine pathway can be a potential biomarker of fatigue in primary Sjögren’s syndrome. Clin Exp Rheumatol. 2023.
Langley C, Masuda N, Godwin S, De Marco G, Smith AD, Jones R, Bruce J, Thai NJ. Dysfunction of basal ganglia functional connectivity associated with subjective and cognitive fatigue in multiple sclerosis. Front Neurosci. 2023;17:1194859.
Rasmussen AL, Larsen SV, Ozenne B, Köhler-Forsberg K, Stenbæk DS, Jørgensen MB, Giraldi A, Frokjaer VG. Sexual health and serotonin 4 receptor brain binding in unmedicated patients with depression-a NeuroPharm study. Transl Psychiatry. 2023;13:247.
Milrad SF, Hall DL, Jutagir DR, Lattie EG, Czaja SJ, Perdomo DM, Fletcher MA, Klimas N, Antoni MH. Depression, evening salivary cortisol and inflammation in chronic fatigue syndrome: a psychoneuroendocrinological structural regression model. Int J Psychophysiol. 2018;131:124–30.
Slavich GM, Sacher J. Stress, sex hormones, inflammation, and major depressive disorder: extending Social Signal Transduction Theory of Depression to account for sex differences in mood disorders. Psychopharmacology. 2019;236:3063–79.
Berentschot JC, Drexhage HA, Aynekulu Mersha DG, Wijkhuijs AJM, GeurtsvanKessel CH, Koopmans MPG, Voermans JJC, Hendriks RW, Nagtzaam NMA, de Bie M, et al. Immunological profiling in long COVID: overall low grade inflammation and T-lymphocyte senescence and increased monocyte activation correlating with increasing fatigue severity. Front Immunol. 2023;14:1254899.
Kłysiak M, Wieder-Huszla S, Branecka-Woźniak D, Karakiewicz-Krawczyk K, Napieracz-Trzosek I, Owsianowska J, Jurczak A, Cymbaluk-Płoska A. Analysis of the occurrence of Predicative Factors of Chronic Fatigue in female patients with Cancer of the Reproductive organs with respect to stage of treatment. Int J Environ Res Public Health. 2023; 20.
Brenu EW, Huth TK, Hardcastle SL, Fuller K, Kaur M, Johnston S, Ramos SB, Staines DR, Marshall-Gradisnik SM. Role of adaptive and innate immune cells in chronic fatigue syndrome/myalgic encephalomyelitis. Int Immunol. 2014;26:233–42.
Fletcher MA, Zeng XR, Barnes Z, Levis S, Klimas NG. Plasma cytokines in women with chronic fatigue syndrome. J Transl Med. 2009;7:96.
Al-Hakeim HK, Al-Rubaye HT, Al-Hadrawi DS, Almulla AF, Maes M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: a proof of concept and mechanism study. Mol Psychiatry. 2023;28:564–78.
Liang PY, Diao LH, Huang CY, Lian RC, Chen X, Li GG, Zhao J, Li YY, He XB, Zeng Y. The pro-inflammatory and anti-inflammatory cytokine profile in peripheral blood of women with recurrent implantation failure. Reprod Biomed Online. 2015;31:823–6.
Qin D, Xu H, Chen Z, Deng X, Jiang S, Zhang X, Bao S. The peripheral and decidual immune cell profiles in women with recurrent pregnancy loss. Front Immunol. 2022;13:994240.
Zhu X, Liu J, Pan H, Geng Z, Huang W, Liu T, Zhang B. Thymopentin treatment of murine premature ovarian failure via attenuation of immune cell activity and promotion of the BMP4/Smad9 signalling pathway. Int J Med Sci. 2021;18:3544–55.
Jain M, Mladova E, Shichanina A, Kirillova K, Povarova A, Scherbakova L, Samokhodskaya L, Panina O. Microbiological and cytokine profiling of menstrual blood for the Assessment of Endometrial receptivity: a pilot study. Biomedicines. 2023; 11.
Xu J, Potter M, Tomas C, Elson JL, Morten KJ, Poulton J, Wang N, Jin H, Hou Z, Huang WE. A new approach to find biomarkers in chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) by single-cell Raman Micro-spectroscopy. Analyst. 2019;144:913–20.
Barrera MJ, Aguilera S, Castro I, Carvajal P, Jara D, Molina C, González S, González MJ. Dysfunctional mitochondria as critical players in the inflammation of autoimmune diseases: potential role in Sjögren’s syndrome. Autoimmun Rev. 2021;20:102867.
Zhou YX, Wei J, Deng G, Hu A, Sun PY, Zhao X, Song BL, Luo J. Delivery of low-density lipoprotein from endocytic carriers to mitochondria supports steroidogenesis. Nat Cell Biol. 2023;25:937–49.
Verma H, Gangwar P, Yadav A, Yadav B, Rao R, Kaur S, Kumar P, Dhiman M, Taglialatela G, Mantha AK. Understanding the neuronal synapse and challenges associated with the mitochondrial dysfunction in mild cognitive impairment and Alzheimer’s disease. Mitochondrion. 2023;73:19–29.
Trigo D, Avelar C, Fernandes M, Sá J, da Cruz ESO. Mitochondria, energy, and metabolism in neuronal health and disease. FEBS Lett. 2022;596:1095–110.
Supruniuk E, Górski J, Chabowski A. Endogenous and exogenous antioxidants in skeletal muscle fatigue development during Exercise. Antioxid (Basel). 2023; 12.
Bai L, Tan C, Ren J, Liu J, Zou W, Liu G, Sheng Y. Cordyceps Militaris acidic polysaccharides improve learning and memory impairment in mice with exercise fatigue through the PI3K/NRF2/HO-1 signalling pathway. Int J Biol Macromol. 2023;227:158–72.
Ma C, Deng Y, Xiao R, Xu F, Li M, Gong Q, Gao J. Anti-fatigue effect of phlorizin on exhaustive exercise-induced oxidative injury mediated by Nrf2/ARE signaling pathway in mice. Eur J Pharmacol. 2022;918:174563.
Wang L, Tang J, Wang L, Tan F, Song H, Zhou J, Li F. Oxidative stress in oocyte aging and female reproduction. J Cell Physiol. 2021;236:7966–83.
Lai XL, Xiong WJ, Li LS, Lan MF, Zhang JX, Zhou YT, Niu D, Duan X. Zinc deficiency compromises the maturational competence of porcine oocyte by inducing mitophagy and apoptosis. Ecotoxicol Environ Saf. 2023;252:114593.
Shan H, Luo R, Guo X, Li R, Ye Z, Peng T, Liu F, Yang Z. Abnormal endometrial receptivity and oxidative stress in polycystic ovary syndrome. Front Pharmacol. 2022;13:904942.
Mauchart P, Vass RA, Nagy B, Sulyok E, Bódis J, Kovács K. Oxidative stress in assisted Reproductive techniques, with a focus on an underestimated risk factor. Curr Issues Mol Biol. 2023;45:1272–86.
Knoop V, Cloots B, Costenoble A, Debain A, Vella Azzopardi R, Vermeiren S, Jansen B, Scafoglieri A, Bautmans I. Fatigue and the prediction of negative health outcomes: a systematic review with meta-analysis. Ageing Res Rev. 2021;67:101261.
Katznelson L, Gadelha M. Glucocorticoid use in patients with adrenal insufficiency following administration of the COVID-19 vaccine: a pituitary society statement. Pituitary. 2021;24:143–5.
Vandenbulcke L, Erard M, Van Assche D, De Langhe E. The effect of physical exercise on fatigue in systemic lupus erythematosus: a systematic review. Acta Clin Belg. 2023;78:342–57.
Maunick B, Skvarc D, Olive L, Mikocka-Walus A. Effects of acceptance and commitment therapy on fatigue for patients with cancer and other chronic health conditions: a systematic review and meta-analysis. J Psychosom Res. 2023;171:111366.
Wan JJ, Qin Z, Wang PY, Sun Y, Liu X. Muscle fatigue: general understanding and treatment. Exp Mol Med. 2017;49:e384.
Spinelli FR, Berti R, Farina G, Ceccarelli F, Conti F, Crescioli C. Exercise-induced modulation of Interferon-signature: a therapeutic route toward management of systemic Lupus Erythematosus. Autoimmun Rev. 2023;22:103412.
Turk MA, Liu Y, Pope JE. Non-pharmacological interventions in the treatment of rheumatoid arthritis: a systematic review and meta-analysis. Autoimmun Rev. 2023;22:103323.
Maksoud R, Balinas C, Holden S, Cabanas H, Staines D, Marshall-Gradisnik S. A systematic review of nutraceutical interventions for mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome. J Transl Med. 2021;19:81.
Barnish M, Sheikh M, Scholey A. Nutrient therapy for the improvement of fatigue symptoms. Nutrients. 2023; 15.
Avery H, Jackson P, Haskell-Ramsay C. The effect of iron supplementation on cognition, subjective mood, well-being and fatigue in women of reproductive age: a systematic review. Proceedings of the Nutrition Society. 2020; 79:E330-E330.
Garzon S, Cacciato PM, Certelli C, Salvaggio C, Magliarditi M, Rizzo G. Iron Deficiency Anemia in pregnancy: Novel approaches for an old problem. Oman Med J. 2020;35:e166.
Shelling AN, Ahmed Nasef N. The role of lifestyle and dietary factors in the development of premature ovarian insufficiency. Antioxid (Basel). 2023; 12.
Yao Y, Tang Y, Qin H, Meng R, Zhang C, Zhang Y, Yang Y, Qiao P, Liu J, Su J. Zinc supplementation promotes oocyte maturation and subsequent embryonic development in sheep. Theriogenology. 2023;206:161–9.
Safaei Z, Bakhshalizadeh SH, Nasr Esfahani MH, Akbari Sene A, Najafzadeh V, Soleimani M, Shirazi R. Effect of vitamin D3 on mitochondrial Biogenesis in Granulosa cells derived from polycystic ovary syndrome. Int J Fertil Steril. 2020;14:143–9.
Izadi A, Ebrahimi S, Shirazi S, Taghizadeh S, Parizad M, Farzadi L, Gargari BP. Hormonal and metabolic effects of Coenzyme Q10 and/or vitamin E in patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104:319–27.
Cleare AJ. Glucocorticoids and glucocorticoid receptors: mediators of fatigue? Acta Neuropsychiatr. 2003;15:341–53.
Sasson R, Winder N, Kees S, Amsterdam A. Induction of apoptosis in granulosa cells by TNF alpha and its attenuation by glucocorticoids involve modulation of Bcl-2. Biochem Biophys Res Commun. 2002;294:51–9.
Hinchado MD, Quero-Calero CD, Otero E, Gálvez I, Ortega E. Synbiotic supplementation improves Quality of Life and Inmunoneuroendocrine Response in patients with Fibromyalgia: influence of codiagnosis with chronic fatigue syndrome. Nutrients. 2023; 15.
Demir Yıldırım A, Güngör Satılmış İ. The effects of yoga on pregnancy, stress, and anxiety in infertile individuals: a systematic review. Holist Nurs Pract. 2022;36:275–83.
Min ES, Lee MS, Lee MK, Lee M, Kim E, Song E, Hur MH. A qualitative study on the experience of acupuncture treatment in infertile women. Integr Med Res. 2021; 10.
Regev S, Schwartz D, Sarid O, Goren G, Slonim-Nevo V, Friger M, Sergienko R, Greenberg D, Monsonego A, Nemirovsky A, et al. Randomised clinical trial: psychological intervention improves work productivity and daily activity by reducing abdominal pain and fatigue in Crohn’s disease. Aliment Pharmacol Ther. 2023;57:861–71.
Szatmári A, Helembai K, Zádori J, Kovács I. Paramedical counselling in infertility treatment: its effects on anxio-depressive symptom severity, perceived stress and self-esteem. Heliyon. 2022;8:e09827.
Kim M, Moon SH, Kim JE. Effects of psychological intervention for Korean infertile women under in Vitro fertilization on infertility stress, depression, intimacy, sexual satisfaction and fatigue. Arch Psychiatr Nurs. 2020;34:211–7.
Ozcan S, Kirca N. Effects of care given in line with Levine’s conservation model on the quality of life of women receiving infertility treatment: a single blind randomized controlled trial. Health Care Women Int. 2023;44:418–39.
Kirca N, Özcan S. The effects of nursing care based on Levine’s conservation model on fatigue, depression, perceived social support, and sleep quality in infertile women: a randomized controlled trial. Int J Nurs Knowl. 2023;34:284–96.
Skau S, Sundberg K, Kuhn HG. A proposal for a Unifying Set of definitions of fatigue. Front Psychol. 2021;12:739764.
Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. 2013;80:409–16.
Yang X, Xue Y, Liu R, Zhao X, Li K, Wang J, Hou L. Dopamine release impairments Accompany Movement Vigor Deficiency in an Exercise-Induced fatigue mouse model. ACS Chem Neurosci. 2023;14:2443–9.
Dougherty JP, Wolff BS, Cullen MJ, Saligan LN, Gershengorn MC. Taltirelin alleviates fatigue-like behavior in mouse models of cancer-related fatigue. Pharmacol Res. 2017;124:1–8.
Wersocki E, Bedson J, Chen Y, LeResche L, Dunn KM. Comprehensive systematic review of long-term opioids in women with chronic noncancer pain and associated reproductive dysfunction (hypothalamic-pituitary-gonadal axis disruption). Pain. 2017;158:8–16.
Acknowledgements
The authors thank Dr. Shaolin Liang and Mrs. Yuan Sheng (STI-Zhilian Research Institute for Innovation and Digital Health, Beijing, China) for optimizing the schema graph.
Funding
The writing of this review was made possible by grants from the General Program of the National Natural Science Foundation of China (82371684, 82271672), the Interdisciplinary Innovative Talents Foundation from Renmin Hospital of Wuhan University (JCRCWL-2022-001), and the General Program of the Natural Science Foundation of Guangdong Province (2022A1515010650, 2023A1515011675).
Author information
Authors and Affiliations
Contributions
L.H.D. conceptualized the idea. T.L.Y. discussed and refined the framework. W.Z.L. drafted and revised the manuscript. X.Y.H. critically reviewed the manuscript with a focus on the autoimmune aspects. Y.Q.W. contributed to manuscript revision and language editing. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, W., Huang, X., Wei, Y. et al. Connecting the dots: the role of fatigue in female infertility. Reprod Biol Endocrinol 22, 66 (2024). https://doi.org/10.1186/s12958-024-01235-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12958-024-01235-5