Abstract
Background
The best method for selecting embryos ploidy is preimplantation genetic testing for aneuploidies (PGT-A). However, it takes more labour, money, and experience. As such, more approachable, non- invasive techniques were still needed. Analyses driven by artificial intelligence have been presented recently to automate and objectify picture assessments.
Methods
In present retrospective study, a total of 3448 biopsied blastocysts from 979 Time-lapse (TL)-PGT cycles were retrospectively analyzed. The “intelligent data analysis (iDA) Score” as a deep learning algorithm was used in TL incubators and assigned each blastocyst with a score between 1.0 and 9.9.
Results
Significant differences were observed in iDAScore among blastocysts with different ploidy. Additionally, multivariate logistic regression analysis showed that higher scores were significantly correlated with euploidy (p < 0.001). The Area Under the Curve (AUC) of iDAScore alone for predicting euploidy embryo is 0.612, but rose to 0.688 by adding clinical and embryonic characteristics.
Conclusions
This study provided additional information to strengthen the clinical applicability of iDAScore. This may provide a non-invasive and inexpensive alternative for patients who have no available blastocyst for biopsy or who are economically disadvantaged. However, the accuracy of embryo ploidy is still dependent on the results of next-generation sequencing technology (NGS) analysis.
Similar content being viewed by others
Background
Despite the notable advancements in assisted reproductive technology, the average live birth rate in the UK is still low, at 32% per embryo transfer (for women under 35 years old), therefore, the selection of the best embryo for transfer remains the fundamental difficulty in in vitro fertilization (IVF) field [1]. Aneuploidy is the primary cause of implantation failure and pregnancy loss [2]. Preimplantation genetic testing for aneuploidy (PGT-A), which allows the accurate analysis of all 24 chromosomes, has made it one of the best current technologies for choosing euploidy embryos [3]. However, its invasive nature due to the requirement for embryo biopsy might not be available because it is illegal or they could think it is unethical for certain people [2]. Or, they could also lack biopsy-ready embryos. Further restricting accessibility is the fact that PGT-A can cost up to $12 000 in the USA and over £3000 in the UK [4]. Additionally, improvements in PGT-A’s clinical result seem to only apply to women older than 37 years [5]. Therefore, the necessity for more non-invasively or sophisticated techniques to identify aneuploidy embryo is critical.
The introduction of Time-lapse (TL) in IVF field has provided ways to avoid some of the drawbacks of conventional morphological evaluation. It reduced the possible effects of fluctuations in temperature or gas composition, enabled continuous monitoring of embryo development, which improves our understanding of embryokinetics [6]. Therefore, several groups aimed to clarify the relationship between morphokinetic parameters got from TL and ploidy status. Some abnormal cleavage pattern was investigated, such as the degree of fragmentation, existence of direct and reverse cleavage, blastocyst contractions, and multinucleation etc [7,8,9,10,11,12]. These morphological features might help to distinguish ploidy types.
Theoretically, the artificial intelligence (AI)-powered TL evaluation represented a wealth of data that may be utilized for euploidy embryo selection [9, 13,14,15]. However, its practical applicability has to be evaluated in more well-designed research and/or sizable datasets. EmbryoScope + incubators could be connected to the software “intelligent data analysis (iDA) Score”. The deep learning algorithm used in this program, which was trained on hundreds of thousands of videos, assigned each embryo with a score between 1.0 and 9.9.
In present study, we aim to investigate the correlations between morphology and ploidy status. In particular, it is also determined whether the ability of iDAScore to discriminate embryo ploidy could be enhanced by incorporating some clinical features.
Methods
Study design
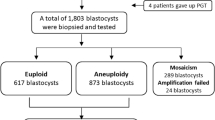
The retrospective cohort study involved 979 TL-PGT cycles conducted from 2018 to 2021 at the Reproductive Medicine Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China. A total of 3448 blastocysts were biopsied. These samples were lysed, and the DNA was fragmented and amplified. Among them, 3405 samples were amplified successful, and 42 samples were failed to amplify. In this work, the iDAScore model was used to retrospectively examine 3405 blastocysts that were cultivated in an EmbryoScope Plus (Vitrolife A/S, Denmark) incubator. In Fig. 1, the research design is displayed. On an informed consent form, each patient signed. Every patient signed an informed consent form. The study complied with the Declaration of Helsinki for Human Subjects in Medical Research and the Board of Institutional Review (No. 2019s097) approval was given by the Ethical Committee of Reproductive Medicine Center, Tongji Hospital, Tongji Medicine College, Huazhong University of Science and Technology.
Ovarian stimulation and oocyte retrieval
Controlled ovarian stimulation (COS) was performed in compliance with our previous studies [16]. Patients were regularly monitored with transvaginal ultrasonography during COS. When the leading follicle(s) measured more than 18 mm, human chorionic gonadotropin (HCG) was administered. The oocytes were retrieved 36 h after the HCG injection, guided by ultrasonography. The collection of cumulus-oocyte complexes (COCs) was performed as described in our previous studies [17].
Embryo culture and biopsy
The density gradient centrifugation method was used to optimize the semen samples [18]. The sperm concentration, motility, and morphology were evaluated using the fifth edition of the World Health Organization recommendations. During intracytoplasmic sperm injection (ICSI), the COCs were denuded two hours after retrieval, and sperm was injected four hours later. The resultant zygotes were subsequently cultured utilizing a time-lapse incubation system at G1 Plus (Vitrolife, Sweden). Each embryo was photographed every ten minutes in TL incubator. After insemination, pronuclei were inspected 16–18 h later. The culture medium was switched to G2 Plus (Vitrolife, Sweden) on the third day. The blastocysts that met the Gardner criterion (better than 3BC) were biopsied and then cryopreserved for later use on the fifth and sixth day. Rarely, the embryo was cultivated for vitrification up until the seventh day. Before performing a biopsy, a tiny hole in the zona pellucida is created using a laser. This allowed for the mechanical dissection of three to six trophectoderm cells.
Next-generation sequencing technology (NGS) analysis
The PGT cycles were conducted with NGS analysis [19]. To summarize, the samples were amplified using a single-cell whole-genome amplification (WGA) based on multiple annealing and looping-based amplification cycles (MALBACs), in accordance with commercial kit protocol (Yikon Genomics). DNA was fragmented, amplified, labelled, and purified in a sequential manner. Utilizing Life Technologies’ Ion Proton technology, the final library was sequenced at a depth of around 0.04× genomes. In order to detect variants, this sequencing speed generates repeatable copy number variations (CNVs) at ∼ 4 MB resolution. A threshold of more than 70% was established for the detection of aneuploidy. When it comes to chromosomes, the threshold for mosaic detection differs. The lower limit was 30% for chromosomes 13, 16, 18, and 21, 50% for chromosome 19, and 40% for all other chromosomes. A number that is below the lower bound denotes euploidy.
iDAScore analysis
A deep learning neural network that is trained on TL videos to predict fetal heartbeat is the iDAScore embryo scoring model. Time-lapse videos are fed into the iDAScore model, which generates an embryo score between 1.0 and 9.9. The data from the blastocysts that were included in this study were assessed retrospectively using the iDAScore model.
Statistical analysis
Continuous variables with normal distributions were expressed as mean ± SD. Categorical variables were expressed as number and percentage (%). For data with a normal distribution, one-way analysis of variance (ANOVA) was applied for multiple comparisons. The chi-square test was used to compare categorical variables between groups. Multivariable logistic regression was applied to evaluate the association between the iDAScores and ploidy, and the odds ratios (ORs) were calculated. All statistical tests were two-sided and p values less than 0.05 were considered statistically significant. All analyses were conducted in SPSS Statistics (version 23.0, IBM, Armonk, NY, USA).
Results
Clinical characteristics of PGT cycles
A total of 979 TL-PGT cycles were involved in this present study. As shown in Table 1, the average maternal age is 31.78 and the average infertility duration is 2.07 years. Measurements for basic follicle-stimulating hormone (FSH), anti-Mullerian hormone (AMH), and body mass index (BMI) of females are also present. The proportion of different sperm quality among all cycles was calculated. Additionally, gonadotropin (Gn) dosage and duration are also described in Table 1.
The iDAScore of blastocysts with different ploidy
As shown in Fig. 2A, the euploid blastocysts present 43.00%, and 44.70% were aneuploid blastocysts as well as 12.31% mosaic blastocysts. The euploid blastocysts ratio vitrified on Day 5 were higher than that vitrified on Day 6 and 7 (Fig. 2B). Besides, the blastocysts were quartered with the iDAScore. And results showed that blastocysts with a higher iDAScore contained more euploidy than that with lower iDAScore (Fig. 2C).
Subsequently, the iDAScore was analyzed with different ploidy blastocyst. Results showed that median iDAScore value is 6.3 for euploid blastocyst, 4.8 for aneuploid blastocyst and 5.3 for mosaic blastocyst (Fig. 3A). For Day 5 blastocyst, median iDAScore value is 8.0 for euploid blastocyst, 7.6 for aneuploid blastocyst and 7.8 for mosaic blastocyst (Fig. 3B). For Day 6 blastocyst, median iDAScore value is 4.2 for euploid blastocyst, 3.4 for aneuploid blastocyst and 3.8 for mosaic blastocyst (Fig. 3C).
iDAScores of blastocysts with different ploidy. (A) iDAScores of all biopsied blastocysts. (B) iDAScores of all Day5 biopsied blastocysts. (C) iDAScores of all Day6 biopsied blastocysts. (D) iDAScores of different abnormal chromosome number. (E) iDAScores of different abnormal chromosome type. *** presents p < 0.001
For the number of abnormal chromosomes, there was a significant difference of iDAScore between normal chromosome and different. However, no significant difference of iDAScore was observed among different chromosome number abnormalities (Fig. 3D).
For chromosome types, a notable dissimilarity of iDAScore was observed in the blastocytes with duplications and deletions, as well as both duplications and deletions. Conversely, no significant dissimilarity was found in the ratio of deletions compared to both duplications and deletions (Fig. 3E).
Table 2 showed the results of multi-variable logistic regression analysis for euploidy prediction. The multivariable logistic regression was adjusted for Garnder criteria, cleavage type, parental chromosome, and length of incubation. The iDAScore was significantly correlated with euploidy prediction in multivariable logistic regression.
Area under the curve (AUC) of euploidy prediction
The AUC for iDAScore prediction of euploid embryos was 0.612, and when considering the length of blastocyst incubation, the AUC increased to 0.622 (Fig. 4). Furthermore, when incorporating the embryologist’s morphological assessment, the AUC raised to 0.659. When parental chromosome results were added, the AUC further improved to 0.684. Finally, when considering the embryo’s cleavage pattern, the AUC could reach 0.688 (Fig. 4).
Discussion
In the IVF field, TL system has several benefits over simple, static morphological measurements. By obtaining frequent, microscopic, multiplanar photographs, this enclosed incubation device lessens the need to remove embryos from ideal air culture conditions. These continuous photos allow embryologists to better re-trace embryo development and enable them to make observations without having to take them out of the incubator at a specific point in time. The annotations of an embryo’s morphokinetics, obtained through retrospective study of these photos, can be correlated with outcome factors such as live birth or ploidy status. This makes it possible to choose embryos that show particular development patterns at certain points in time.
Various algorithms and morphokinetic patterns derived from TL data have shown promising results in predicting ploidy [15, 20]. A recent meta-analysis explored morphological and morphokinetic associations with embryo ploidy, revealing that morphokinetic variables such as t8, t9, and tEB were deemed more relevant to ploidy status. Although categorizing aneuploid and euploid embryos with absolute certainty is challenging due to their significant heterogeneity, prioritizing biopsy for certain embryos is conceivable. However, the labeling of particular time points is dependent on individual clinical embryologists, which can lead to some subjective biases.
Currently, AI algorithms use static optical light microscopy images to predict the ploidy state of human embryos [21]. Depending on the dataset, overall accuracy ranged from 60 to 80%, with sensitivity for predicting euploid embryos varied from 75 to 95%. There is a substantial positive association between the fraction of euploid embryos and the genetics AI score in every case, supporting the clinical utility of rating and selecting embryos within a patient cohort that are more likely to be euploid.
KATO et al. investigated the significant correlation between euploidy and the iDAScore, KIDScore Day 5, and Gardner criteria used for blastocyst evaluation [22]. Our findings mirror theirs, demonstrating a substantial correlation between euploidy rates and iDAScore (p < 0.001). In contrast, notable differences in KIDScore and Gardner criteria were evident primarily among younger patients. Another study reported AUCs of 0.60 for iDAScore in predicting euploidy, comparable to embryologists’ performance [23]. In our investigation, the combined AUC for euploidy prediction incorporating iDAScore and clinical/embryonic factors reached 0.688. However, in a retrospective simulation analysis, iDAScore v1.0 tended to assign a top-quality ranking to euploid blastocysts in 63% of instances characterized by the presence of one or more euploid and aneuploid blastocysts. Conversely, in situations featuring two or more euploid blastocysts and at least one live birth, iDAScore v1.0 raised questions regarding embryologists’ ranking decisions in 48% of the cases considered. Consequently, iDAScore may serve to objectify embryologists’ assessments.
Conclusion
This study contributes additional information to strengthen the clinical applicability of iDAScore. The AUC of iDAScore combined with clinical features reached to 0.688. This offers a potential non-invasive and cost-effective alternative for patients without available blastocyst for biopsy or those facing economic constraints. Nonetheless, the accuracy of embryo ploidy remains contingent on the results of NGS analysis. As such, more clinical trials need to be conducted in order to verify the accuracy of iDAScores in predicting embryo ploidy, as well as to provide more data in support of the iDAScore as a predictor of embryo ploidy.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- AI:
-
Artificial intelligence
- AUC:
-
Area Under the Curve
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CNVs:
-
Copy number variations
- COCs:
-
Cumulus-oocyte complexes
- COS:
-
Controlled ovarian stimulation
- FSH:
-
Follicle-stimulating hormone
- Gn:
-
Gonadotropin
- HCG:
-
Human chorionic gonadotropin
- ICSI:
-
Intracytoplasmic sperm injection
- iDAScore:
-
Intelligent data analysis Score
- IVF:
-
In in vitro fertilization
- MALBACs:
-
Multiple annealing and looping-based amplification cycles
- NGS:
-
Next-generation sequencing technology
- PGT-A:
-
Preimplantation genetic testing for aneuploidies
- TL:
-
Time-lapse
- WGA:
-
Whole-genome amplification
References
Bamford T, Barrie A, Montgomery S, Dhillon-Smith R, Campbell A, Easter C, Coomarasamy A. Morphological and morphokinetic associations with aneuploidy: a systematic review and meta-analysis. Hum Reprod Update. 2022;28(5):656–86. https://doi.org/10.1093/humupd/dmac022
Pennetta F, Lagalla C, Borini A. Embryo morphokinetic characteristics and euploidy. Curr Opin Obstet Gynecol. 2018;30(3):185–96. https://doi.org/10.1097/GCO.0000000000000453
Maxwell SM, Grifo JA. Should every embryo undergo preimplantation genetic testing for aneuploidy? A review of the modern approach to in vitro fertilization. Best Pract Res Clin Obstet Gynaecol. 2018;53:38–47. https://doi.org/10.1016/j.bpobgyn.2018.07.005
Theobald R, SenGupta S, Harper J. The status of preimplantation genetic testing in the UK and USA. Hum Reprod. 2020;35(4):986–98. https://doi.org/10.1093/humrep/deaa034
Kang HJ, Melnick AP, Stewart JD, Xu K, Rosenwaks Z. Preimplantation genetic screening: who benefits? Fertil Steril. 2016;106(3):597–602. https://doi.org/10.1016/j.fertnstert.2016.04.027
Sciorio R. Use of time-lapse monitoring in medically assisted reproduction treatments: a mini-review. Zygote. 2021;29(2):93–101. https://doi.org/10.1017/S0967199420000623
Coticchio G, Barrie A, Lagalla C, Borini A, Fishel S, Griffin D, Campbell A. Plasticity of the human preimplantation embryo: developmental dogmas, variations on themes and self-correction. Hum Reprod Update. 2021;27(5):848–65. https://doi.org/10.1093/humupd/dmab016
Desai N, Goldberg JM, Austin C, Falcone T. Are cleavage anomalies, multinucleation, or specific cell cycle kinetics observed with time-lapse imaging predictive of embryo developmental capacity or ploidy? Fertil Steril. 2018;109(4):665–74. https://doi.org/10.1016/j.fertnstert.2017.12.025
Coticchio G, Ezoe K, Lagalla C, Shimazaki K, Ohata K, Ninomiya M, Wakabayashi N, Okimura T, Uchiyama K, Kato K, Borini A. Perturbations of morphogenesis at the compaction stage affect blastocyst implantation and live birth rates. Hum Reprod. 2021;36(4):918–28. https://doi.org/10.1093/humrep/deab011
Bodri D, Sugimoto T, Yao Serna J, Kawachiya S, Kato R, Matsumoto T. Blastocyst collapse is not an independent predictor of reduced live birth: a time-lapse study. Fertil Steril. 2016;105(6):1476–e14833. https://doi.org/10.1016/j.fertnstert.2016.02.014
Van Royen E, Mangelschots K, Vercruyssen M, De Neubourg D, Valkenburg M, Ryckaert G, Gerris J. Multinucleation in cleavage stage embryos. Hum Reprod. 2003;18(5):1062–9. https://doi.org/10.1093/humrep/deg201
Kim SG, Kim YY, Park JY, Kwak SJ, Yoo CS, Park IH, Sun HG, Kim JW, Lee KH, Park HD, Chi HJ. Early fragment removal on in vitro fertilization day 2 significantly improves the subsequent development and clinical outcomes of fragmented human embryos. Clin Exp Reprod Med. 2018;45(3):122–8. https://doi.org/10.5653/cerm.2018.45.3.122
Coticchio G, Mignini Renzini M, Novara PV, Lain M, De Ponti E, Turchi D, Fadini R, Dal Canto M. Focused time-lapse analysis reveals novel aspects of human fertilization and suggests new parameters of embryo viability. Hum Reprod. 2018;33(1):23–31. https://doi.org/10.1093/humrep/dex344
Levy DM. Continuing controversy over use of epidural adrenaline in pre-eclampsia. Br J Hosp Med. 1993;49(10):745.
Reignier A, Lammers J, Barriere P, Freour T. Can time-lapse parameters predict embryo ploidy? A systematic review. Reprod Biomed Online. 2018;36(4):380–7. https://doi.org/10.1016/j.rbmo.2018.01.001
Ma BX, Zhang H, Jin L, Huang B. Neonatal outcomes of embryos cultured in a Time-Lapse incubation system: an analysis of more than 15,000 fresh transfer cycles. Reprod Sci. 2022;29(5):1524–30. https://doi.org/10.1007/s43032-021-00714-z
Ma BX, Huang B, Chen D, Jin L, Rao Q. Are early embryo cleavage kinetics affected by Energy Substrates in different culture media? Curr Med Sci. 2022;42(6):1297–304. https://doi.org/10.1007/s11596-022-2648-7
Ma BX, Yang L, Tian Y, Jin L, Huang B. Cytoplasmic strings between ICM and mTE are a positive predictor of clinical pregnancy and live birth outcomes: a time-lapse study. Front Med (Lausanne). 2022;9:934327. https://doi.org/10.3389/fmed.2022.934327
Huang B, Tan W, Li Z, Jin L. An artificial intelligence model (euploid prediction algorithm) can predict embryo ploidy status based on time-lapse data. Reprod Biol Endocrinol. 2021;19(1):185. https://doi.org/10.1186/s12958-021-00864-4
Quinn MM, Marsh P, Ribeiro S, Simbulan RK, Rosen MP. A deep dive into the morphokinetics and ploidy of low-quality blastocysts. F S Rep. 2022;3(3):231–6. https://doi.org/10.1016/j.xfre.2022.06.004
Diakiw SM, Hall JMM, VerMilyea MD, Amin J, Aizpurua J, Giardini L, Briones YG, Lim AYX, Dakka MA, Nguyen TV, Perugini D, Perugini M. Development of an artificial intelligence model for predicting the likelihood of human embryo euploidy based on blastocyst images from multiple imaging systems during IVF. Hum Reprod. 2022;37(8):1746–59. https://doi.org/10.1093/humrep/deac131
Kato K, Ueno S, Berntsen J, Kragh MF, Okimura T, Kuroda T. Does embryo categorization by existing artificial intelligence, morphokinetic or morphological embryo selection models correlate with blastocyst euploidy rates? Reprod Biomed Online. 2023;46(2):274–81. https://doi.org/10.1016/j.rbmo.2022.09.010
Cimadomo D, Chiappetta V, Innocenti F, Saturno G, Taggi M, Marconetto A, Casciani V, Albricci L, Maggiulli R, Coticchio G, Ahlstrom A, Berntsen J, Larman M, Borini A, Vaiarelli A, Ubaldi FM, Rienzi L. Towards automation in IVF: pre-clinical validation of a deep learning-based embryo grading system during PGT-A cycles. J Clin Med. 2023;12(5). https://doi.org/10.3390/jcm12051806
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
BM and GZ were responsible for experimental design, data analysis and manuscript writing. BM, YF and YY conducted data analysis. BH was responsible for coordinating the study and assembling the time-lapse data. BH and LJ contributed to the analysis of the project.All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study complied with the Declaration of Helsinki for Human Subjects in Medical Research and the Board of Institutional Review (No. 2019s097) approval was given by the Ethical Committee of Reproductive Medicine Center, Tongji Hospital, Tongji Medicine College, Huazhong University of Science and Technology.
Consent for publication
Every patient signed an informed consent form.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lei Jin and Bo Huang. Bo Huang will handle correspondence at all stages of refereeing and publication, also post-publication.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ma, BX., Zhao, GN., Yi, ZF. et al. Enhancing clinical utility: deep learning-based embryo scoring model for non-invasive aneuploidy prediction. Reprod Biol Endocrinol 22, 58 (2024). https://doi.org/10.1186/s12958-024-01230-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12958-024-01230-w