Abstract
Context
Anti-Müllerian hormone (AMH) levels are increased in polycystic ovary syndrome (PCOS) patients and are associated with PCOS severity.
Objective
To evaluate the associations between serum AMH levels and in vitro fertilization (IVF)/ intracytoplasmic sperm injection (ICSI) outcomes in patients with PCOS.
Data sources
PubMed, Embase, and the Cochrane Library were searched on 11 July 2022.
Study selection
Studies reporting the association between serum AMH levels and IVF/ICSI outcomes in PCOS patients were considered for inclusion. The primary outcomes were clinical pregnancy, live birth, and ovarian hyperstimulation syndrome.
Data extraction
Data were extracted using a standardized data extraction form. Study quality was assessed independently by two groups of researchers.
Data synthesis
Nineteen studies were included in this review. Meta-analyses demonstrated that PCOS patients with a serum AMH level within the 75-100th percentile had a decreased odds of clinical pregnancy (OR: 0.77, 95% CI: 0.63–0.93) and livebirth (OR: 0.71; 95% CI: 0.58–0.87) compared to those within the 0-25th percentile. An increased AMH level was also correlated with an increased number of oocytes retrieved (SMD: 0.90, 95% CI: 0.30–1.51) and a lower odds of fertilization (OR: 0.92, 95% CI: 0.87–0.98). There was no significant difference in the number of MII oocytes (SMD: 1.85, 95% CI: -1.07–4.78), E2 on the day of hCG (SMD: 0.12; 95% CI: -0.98–1.23), or implantation (OR: 0.82, 95% CI: 0.28–2.39) between the two groups. In addition, we found significant dose–response associations between serum AMH level and clinical pregnancy, live birth, number of oocytes retrieved, and fertilization in PCOS patients.
Conclusion
AMH may have clinical utility in counseling regarding IVF/ICSI outcomes among women with PCOS who wish to undergo fertility treatment. More large-scale, high-quality cohort studies are needed to confirm these findings.
Similar content being viewed by others
Introduction
Polycystic ovary syndrome (PCOS) is characterized by hyperandrogenism, chronic anovulation, and polycystic ovaries, with various reproductive and metabolic sequelae [1]. It continues to be one of the most prevalent endocrine conditions among women of reproductive age, the leading cause of anovulatory infertility, and a significant risk factor for type 2 diabetes and mental health issues [2]. Studies have demonstrated how PCOS affects fertility and pregnancy [3, 4]. Women with PCOS have increased risks of adverse maternal and neonatal outcomes [5]. Assisted reproductive technology (ART), such as in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI), can provide effective treatment options for infertility in women with PCOS [6].
Anti-Müllerian hormone (AMH) is secreted solely by the granulosa cells of preantral and small antral follicles [7]. Known for its low intracycle and intercycle variability, the AMH level is a significantly more accurate and reliable measure of ovarian reserve than the antral follicle count (AFC) or FSH concentration, and this has led to its adoption by clinicians in the counseling of women regarding their reproductive lifespans and the impact of gonadotoxic chemotherapy, radiotherapy or surgery on the ovarian reserve [8]. Serum AMH levels are significantly higher in women with PCOS than in those with normal ovulatory function [9]. This observation has led to the hypothesis that AMH could be a valuable surrogate marker for the diagnosis of PCOS and prediction of ART outcomes. However, while it is well established that AMH is correlated with ovarian response and is a good predictor of oocyte yield following ART, it is still controversial whether it may also be associated with qualitative outcomes of ART [10, 11]. Moreover, uncertainty exists as to whether increased prepregnancy AMH levels affect the ART outcome of pregnancy in women with PCOS [12]. In a cohort trial on 2436 women with PCOS undergoing IVF/ICSI, researchers found that the live birth rate (LBR) and clinical pregnancy rate (CPR) of fresh embryo transfer cycles were lower with higher baseline AMH levels than with low or average AMH levels [13, 14]. In contrast, studies have reported a null association between serum AMH levels and IVF/ICSI outcomes in patients with PCOS [15, 16]. Specifically, they demonstrated that AMH may have a predictive role among non-PCOS patients but not among PCOS patients. In addition, although a lot of opposite conclusions have been reported so far, no studies have systematically analyzed and clarified the association between prepregnancy serum AMH and IVF/ICSI outcomes in PCOS patients. Therefore, we aimed to summarize currently available evidence regarding the association between serum AMH level and IVF/ICSI outcomes in PCOS patients.
Materials and methods
This meta-analysis is registered with PROSPERO (registration number: CRD42022300037) and was conducted in accordance with the Meta-analyses of Observational Studies in Epidemiology (MOOSE) checklist and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17, 18]. No formal ethical approval was acquired for this study.
Search strategy
Two groups of authors (TY, ZY and CG, GF) independently screened each record. PubMed, Embase, and the Cochrane library were comprehensively searched for relevant studies from the respective inceptions of these databases to 11 July 2022. Keywords including “polycystic ovary syndrome”, “in vitro fertilization”, “intracytoplasmic sperm injection”, “assisted reproductive technology”, and “anti-Müllerian hormone” and their entry terms were used in the database searches. There was no specified date, country, or language restriction. We did not include any IVF/ICSI or pregnancy outcomes in the initial search because the exact outcomes may not be present in the title or abstract but might be described in the full text instead. We also manually searched Google Scholar and examined the reference lists of all included studies and key journals in the related field to include all potentially eligible studies. After selecting studies by their titles and abstracts, the full text of potential studies was obtained and examined for eligibility.
Inclusion and exclusion criteria
The key questions for this study were based on the Populations, Exposures, Comparison, and Outcome (PECO) framework as follows: (1) the study population was PCOS patients undergoing IVF/ICSI; (2) the exposure was a higher serum AMH level (e.g., 75-100th percentile) than that of the general population of PCOS patients; (3) the comparator was a lower serum AMH level (e.g., 0-25th percentile) than that of the general population of PCOS patients; and (4) the primary outcomes of interest for this review were clinical pregnancy, livebirth, and ovarian hyperstimulation syndrome (OHSS). The secondary outcomes included E2 on the day of hCG, number of oocytes retrieved, number of MII oocytes, implantation, fertilization, obstetric outcomes, and neonatal outcomes. Case reports, case series, reviews, comments, letters, and conference abstracts were excluded.
Data extraction and risk of bias assessment
The following data were extracted from the included studies: study name, first author, year of publication, country, study design, participant inclusion and exclusion criteria, PCOS diagnostic criteria, measurement of AMH, number of participants in each group, and ovarian stimulation protocol. Study authors were contacted for additional information or missing data if necessary. Considering that all eligible studies had a cohort design, their quality (risk of bias) was assessed using the Newcastle‒Ottawa quality assessment scale (NOS), with a maximum score of 9 representing the highest quality. Studies rated with a score of more than 6 were rated as high quality. Both data extraction and risk of bias assessment were conducted independently by two groups of authors (TY, ZY and CG, GF), and all discrepancies were resolved by consultation and discussion with QL and HF.
Statistical analysis
The statistical analyses were performed using Stata/SE (version 5.1), and further analysis was performed with R (version 4.1.1). In R, the meta and dmetar packages were used to obtain pooled results. The dosresmeta package was used to conduct dose–response meta-analysis. Odds ratios (ORs) were calculated for dichotomous outcomes with a 95% confidence interval (Cl), while standardized mean differences (SMDs) with 95% CIs were calculated for continuous outcomes. Heterogeneity was checked using I2 statistics. Meaningful heterogeneity was determined if the I2 was greater than 50%. In this case, a random-effects model was used to pool studies. The robustness of the results was assessed using the leave-one-out method. If a study classified their participants into a low-AMH group (the 0-25th percentile), average-AMH group (the 25-75th percentile), and high-AMH group (the 75-100th percentile), then the high-AMH group was compared with the low-AMH group and a dose–response meta-analysis was conducted. If a study provided data based on the classification of the 75-90th percentile group and 90-100th percentile group, then the latter group was compared with the former group in our meta-analysis. We also calculated weighted mean AMH cutoff values for each group and displayed the results in forest plots.
Results
Study selection
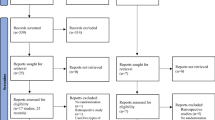
As shown in Fig. 1, 650 studies were identified by initial databases and manual searches. A total of 203 studies were removed due to duplicate records, and 365 were excluded after assessing their titles and abstracts. The remaining 82 studies underwent full-text review, and 63 studies were not eligible for inclusion. Finally, 19 studies fulfilled the eligibility criteria and were included in the qualitative analysis, with 10 included in the quantitative analysis (meta-analysis).
Study characteristics
Table 1 summarizes the characteristics of the 19 studies. All 19 studies were cohort studies, and the NOS score ranged from 5 to 9 (median 6). The classifications of the level of serum AMH were not consistent among these studies. Seven studies classified participants into the low-AMH group (the 0-25th percentile), average-AMH group (the 25-75th percentile), and high-AMH group (the 75-100th percentile) [13, 15, 16, 19,20,21,22]; three studies classified participants into the 75–90th percentile group and 90-100th percentile group [14, 23, 24]. The remaining studies did not classify participants into different groups based on AMH levels but analyzed AMH levels as a continuous variable [22, 25,26,27,28,29,30,31,32,33] and thus were not included in the quantitative analysis (meta-analysis). All 19 studies used the Rotterdam criteria for the diagnosis of PCOS.
Primary outcomes
Clinical pregnancy
Seven studies analyzed clinical pregnancy; six stratified their patients into a low-AMH group (the 0-25th percentile), average-AMH group (the 25-75th percentile), and high-AMH group (the 75-100th percentile) [13, 15, 16, 19,20,21]. The overall clinical pregnancy rate was 48.5% (414/853) and 55.0% (473/860) in the high-AMH group and the low-AMH group, respectively. Meta-analysis demonstrated that the odds of a clinical pregnancy were significantly lower if patients were classified in the high-AMH group (OR: 0.77, 95% CI: 0.64–0.93) (Fig. 2). There was a moderate degree of heterogeneity (I2 = 46%). In addition, Du et al. [25] examined clinical pregnancy rate in a cohort of 200 PCOS patients aged 25 to 36 years undergoing IVF-ET. Patients were divided into two different groups based on a cutoff AMH level of 6.99 ng/L. Their results showed that clinical pregnancy rate was significantly lower if AMH was greater than 6.99 ng/L (p = 0.001). The result of the dose–response meta-analysis is shown in Fig. 4a. We observed an inverse linear association between prepregnancy serum AMH level and clinical pregnancy in PCOS patients (p = 0.008). The heterogeneity was not significant (I2 = 44.4%). The OR (95% CI) of clinical pregnancy was 0.996 (0.994, 0.999) per 1% increase in the AMH percentile.
Live birth
Seven studies analyzed live birth; three studies stratified their patients into a low-AMH group (the 0-25th percentile), average-AMH group (the 25-75th percentile), and high-AMH group (the 75-100th percentile) [13, 15, 20]. The incidence of live birth (per treatment cycle) was 49.9% (381/763) in the low-AMH group and 48.9% (315/643) in the high-AMH group. Meta-analysis demonstrated that the odds of a live birth were significantly lower if patients were classified into the high-AMH group than if they were classified into the low-AMH group (OR: 0.71; 95% CI: 0.58–0.87) (Fig. 2). Heterogeneity was not detected (I2 = 0%). The result of the dose–response meta-analysis is shown in Fig. 4b. We observed an inverse linear association between prepregnancy serum AMH level and live birth in PCOS patients (p < 0.001). The heterogeneity was not significant (I2 = 0%). The OR (95% CI) of live birth was 0.995 (0.993, 0.998) per 1% increase in the AMH percentile.
This section contains a summary of findings that cannot be meta-analyzed. Ho et al. examined live birth rate in a cohort of 921 women with PCOS who underwent IVM priming with hCG [27]. While high AMH levels do indicate a high number of oocytes and a high oocyte maturation rate, univariate analysis did not reveal an association between AMH level and live birth after the transfer of the first embryo after IVM (OR: 1.02; 95% CI: 0.98–1.06). Notably, this result may be applicable only to the specific IVM technique used in this study, namely, the transfer of day-2 embryos, which is not a regular practice at many IVF centers. Tabibnejad et al. [26] investigated the relationship between serum AMH levels and ICSI outcomes in 50 PCOS patients with 289 embryos. In this scenario, their findings suggested that AMH was not an accurate predictor of a live birth (AUC = 0.59 [95% CI, 0.42–0.76]). However, among women with tubal factor infertility, AMH had a moderate predictive value for a live birth (AUC = 0.70 [95% CI, 0.55 to 0.85]). Guan et al. [28] analyzed the cumulative live birth rate in 160 PCOS patients of advanced age (≥ 35 years). All patients underwent their first fresh cycles and subsequent frozen cycles within one year. Their results demonstrated that patients with an AMH level above 32.12 pmol/L were likely to have a 72% (HR, 1.72; 95% CI, 1.08–2.73, p = 0.023) and 34% (HR, 1.34; 95% CI, 1.07–1.68, p = 0.010) improvement in cumulative live birth rate compared to those with AMH levels below 7.85 pmol/L and 7.85–32.12 pmol/L, respectively. Acharya et al. [32] divided their patients based on an AMH cutoff level of 12 ng/ml. Their results showed that AMH was negatively associated with live birth (OR, 0.93; 95% CI, 0.90–0.96) up to an AMH level of 12 ng/ml. Beyond 12 ng/ml, the association was attenuated (OR, 1.01; 95% CI, 0.99–1.04).
Ovarian hyperstimulation syndrome
Three studies analyzed the incidence of OHSS. Tal et al. [15] reported a retrospective cohort study in a sample of 184 women with PCOS who underwent their first fresh IVF/ICSI cycles. Women were stratified into 3 groups according to the 0-25th (< 3.32 ng/ml), 25-75th (3.32–8.27 ng/ml), or 75-100th (> 8.27 ng/ml) percentile of serum AMH concentration. The stimulation protocol included either pituitary downregulation via GnRH agonist in a long protocol or a GnRH antagonist to prevent premature ovulation. No difference regarding the OHSS incidence was found among the three groups. When Kamel et al. [33] divided patients into two groups (AMH cutoff value: 4.6 ng/ml) and used GnRH antagonist, they found that the incidence of severe OHSS was significantly higher in patients with AMH > 4.6 ng/ml (p = 0.026). In addition, Muharam et al. [30] tried to determine the cutoff value of AMH to predict hyperresponse in PCOS patients undergoing controlled ovarian stimulation. The AUC of the ROC curve was 0.626 (95% CI; sensitivity: 71.8%; specificity: 52.7%), indicating poor predictive quality.
Secondary outcomes
E2 on day of hCG
Four studies analyzed E2 on the day of hCG and stratified their patients into a low-AMH group (the 0-25th percentile), average-AMH group (the 25-75th percentile), and high-AMH group (the 75-100th percentile) [13, 15, 16, 21]. The pooled results found a null association between serum AMH levels and E2 on the day of hCG (SMD: 0.12; 95% CI: -0.98–1.23) (Fig. 3a). Sensitivity analysis demonstrated that the study by Kaya et al. [21] affected the robustness of the meta-analysis. After excluding this study, the difference in E2 on the day of hCG became significant (SMD: 0.63; 95% CI: 0.13–1.14). The result of the dose–response meta-analysis is shown in Fig. 4c. Using a linear model, we did not observe a dose–response association between prepregnancy serum AMH level and E2 on the day of hCG in PCOS patients (p = 0.901).
Number of oocytes retrieved
Eight studies analyzed the number of oocytes retrieved: four stratified their patients into a low-AMH group (the 0-25th percentile), an average-AMH group (the 25-75th percentile), and a high-AMH group (the 75-100th percentile) [13, 15, 16, 21]. The meta-analysis showed a significantly increased number of oocytes retrieved in women with PCOS who were classified into the high-AMH group compared with the low-AMH group (SMD: 0.90, 95% CI: 0.30–1.51) in a random-effects model. There was a high degree of heterogeneity (I2 = 87%) (Fig. 3b). The result of the dose–response meta-analysis is shown in Fig. 4d. Using a quadratic model, we observed a nonlinear inverted U-shaped association between prepregnancy serum AMH level and the number of oocytes retrieved in PCOS patients (p = 0.002). PCOS patients with an AMH level between the 39th percentile and the 97th percentile had a significantly increased number of oocytes retrieved. The heterogeneity was significant (I2 = 99.1%).
This section contains a summary of findings that cannot be meta-analyzed. Ho et al. [27], using the study design described earlier in this paper, reported that AMH showed a significant positive correlation with the number of oocytes by univariate analysis (coefficient = 0.28; p = 0.001). Similarly, Tabibnejad et al. [26] prospectively evaluated the number of oocytes retrieved in a cohort of 50 PCOS patients undergoing ICSI. They also found a positive correlation (Spearman’s r = 0.45, p = 0.001). However, when using multivariable analysis in a sample of 59 PCOS patients, Chen et al. [29] found a null association (r = -0.059, p = 0.685). Arslanca et al. [14] reported the same outcome in a cohort of 110 PCOS patients who underwent FET. Patients were categorized into the AMH 75-90th percentile group (n = 66) and 90-100th percentile group (n = 44), and no significant differences in terms of the number of oocytes retrieved were noted between the two groups.
Number of MII oocytes
Three studies analyzed the number of MII oocytes. Two stratified their patients into a low-AMH group (the 0-25th percentile), an average-AMH group (the 25-75th percentile), and a high-AMH group (the 75-100th percentile) [13, 21]. Although there was a trend toward a greater number of MII oocytes in the high-AMH group (SMD: 1.85, 95% CI: -1.07–4.78), the difference did not reach significance in a random-effects model. There was a high degree of heterogeneity (I2 = 97%) (Fig. 3c). In addition, Tabibnejad et al. [26] reported a significant positive correlation between AMH concentration and the number of MII oocytes (Spearman r = 0.42, p = 0.002). The result of the dose–response meta-analysis is shown in Fig. 4e. Using a linear model, we did not observe a dose–response association between prepregnancy serum AMH level and the number of MII oocytes in PCOS patients (p = 0.206).
Fertilization
Six studies reported fertilization rate, and four of those studies stratified their patients into a low-AMH group (the 0-25th percentile), average-AMH group (the 25-75th percentile), and high-AMH group (the 75-100th percentile) [13, 15, 16, 21]. The incidence of overall fertilization (per treatment cycle) was 61.9% (5656/9134) in the low-AMH group and 60.0% (6688/11140) in the high-AMH group. The meta-analysis demonstrated significantly decreased odds of fertilization in women with PCOS whose AMH was classified in the high-AMH group in a fixed-effects model (OR 0.92, 95% CI 0.87–0.98) (Fig. 3d). There was a moderate degree of heterogeneity (I2 = 36.1%). In addition, Du et al. [25] and Arabzadeh et al. [31] reported a null association between AMH and the fertilization rate (p > 0.05). The result of the dose–response meta-analysis is shown in Fig. 4f. We observed an inverse linear association between prepregnancy serum AMH level and fertilization in PCOS patients (p = 0.027). The heterogeneity was not significant (I2 = 44%). The OR (95% CI) of fertilization was 0.999 (0.998, 1.000) per 1% increase in the AMH percentile.
Implantation
Five studies analyzed implantation rate, and three of those studies stratified their patients into a low-AMH group (the 0-25th percentile), average-AMH group (the 25-75th percentile), and high-AMH group (the 75-100th percentile) [15, 16, 21]. The overall incidence of implantation was 39.7% (69/174) in the low-AMH group and 31.7% (52/164) in the high-AMH group. The meta-analysis found no association between AMH level and implantation rate (OR: 0.82, 95% CI: 0.28–2.39) (Fig. 3e). There was a high degree of heterogeneity (I2 = 79%). Du et al. [25] reported that the incidence of implantation was 41.1% (37/90) in the low-AMH group (< 6.99 ng/L) and 20.91% (23/110) in the high-AMH group (> 6.99 ng/L). Additionally, Arabzadeh et al. [31] reported that AMH was not associated with implantation rate in women with PCOS (r = -0.299, p = 0.138) but was positively associated with implantation rate in the non-PCOS control group (r = 0.305, p = 0.05). The result of the dose–response meta-analysis is shown in Fig. 4g. We did not observe a dose–response association between prepregnancy serum AMH level and implantation in PCOS patients (p = 0.735).
Cycle cancellation
Two studies analyzed cycle cancellation. Acharya et al. [32] stratified their patients with an AMH cutoff value of 12 ng/ml. In both groups, an increasing AMH level was associated with a higher probability of cycle cancellation (OR: 1.12, 95% CI: 1.10–1.15 and OR: 1.03, 95% CI: 1.01–1.05 in the AMH < 12 ng/ml group and > 12 ng/ml group, respectively). In the analysis of the reasons for cycle cancellation, the authors reported that in the AMH < 12 ng/ml group, each 1-unit increase in AMH level was associated with an 11% increase in the odds of embryo transfer cancellation because of the OHSS risk (OR, 1.11; 95% CI, 1.07–1.16). Xi et al. [16] stratified their 164 participants into 3 groups according to the < 25th (< 4.85 ng/ml), 25th to 75th (4.85–8.82 ng/ml), or > 75th (> 8.82 ng/ml) percentile of serum AMH concentration. Embryo transfers cancelled due to OHSS risk from the low-, middle-, and high-serum AMH groups were 1, 4 and 7 cases, respectively.
Obstetric outcomes
Three studies analyzed miscarriages. Liu et al. [20] examined this outcome in a cohort of 2973 infertile women, including 418 women with PCOS undergoing their first IVF treatments. The incidence of miscarriage was 8.1% (6/74) in the low-AMH group, 19.1% (29/152) in the average-AMH group, and 17.1% (12/70) in the high-AMH group. Although there were more high-quality embryos transferred in the average-AMH group than in the low-AMH group, the difference in miscarriage rate was not significant among these three groups, indicating that AMH was not associated with miscarriage rate among PCOS patients. Du et al. [25] also reported the early miscarriage rate in their two subgroups. The findings suggested that the rate of early miscarriage was significantly lower among the participants in the low-AMH group, with an incidence of early miscarriage of 6.67% (6/90) in the low-AMH group and 19.09% (21/110) in the high-AMH group (p < 0.001). Notably, this study investigated only the early miscarriage rate of patients, while the patient’s late pregnancy process was not studied.
GDM was reported in two studies [14, 25]. In the comparison of the GDM incidence between the serum AMH 75-90th percentile group and the 90-100th percentile group, there was no association between AMH and GDM. However, Du et al. [25] suggested that patients with AMH greater than 6.99 ng/ml had an increased incidence of GDM (p < 0.001). In addition, preeclampsia and PPROM were reported by only one study [14], with no association found.
Neonatal outcomes
Four studies reported preterm birth [14, 22,23,24]. Meta-analysis was performed to compare the preterm birth rate between the 75-90th percentile group and the 90-100th percentile group (AMH) (Fig. 3f), and a null association was found (OR: 1.92, 95% CI: 0.58–6.42). Meanwhile, Du et al. [22] reported that a higher AMH (75-100th percentile) was associated with an increased risk for preterm birth among women with a BMI ≥ 24 kg/m2 (OR: 2.10, 95% CI: 1.01–4.37) but not among women with a BMI < 24 kg/m2 (OR: 0.78, 95% CI: 0.35–1.73) after adjusting for multiple confounding factors, including maternal age, BMI, duration of infertility, and basal antral follicle count. This study also selected “small for gestational age”, “large for gestation age”, “low birth weight”, and “macrosomia” as outcomes of interest. In brief, no significant differences were found in the rates of these outcomes among patients in the different serum AMH groups (adjusted OR ranging from 0.91 to 1.13).
Discussion
This paper summarizes currently available evidence concerning the association between prepregnancy serum levels of AMH and IVF/ICSI outcomes among women with PCOS and substantially strengthens the theory that a higher level of AMH is associated with a subsequently lower clinical pregnancy rate and live birth rate. The findings presented here could reveal a more significant role for AMH in women with PCOS in clinical settings, and they represent a step toward more precise medicine by demonstrating the value of AMH in the analysis of the risk of adverse ART outcomes in an individual with PCOS.
Regarding the primary outcomes, our results demonstrated that serum AMH levels were negatively associated with clinical pregnancy rate and live birth rate in PCOS patients undergoing IVF/ICSI. Notably, these results were in contrast with the findings of prior studies based on the general population, which demonstrated that a higher AMH level is associated with a higher live birth rate and live birth rate. A previous meta-analysis [34] showed that the pooled diagnostic OR for AMH as a predictor of clinical pregnancy rate among 4324 women in the general population with unspecified ovarian reserve was 2.10, whereas the area under the curve (AUC) of the summary receiver operation characteristic (ROC) curve was 0.634. Thus, the role of AMH in predicting IVF/ICSI outcomes among PCOS patients is different from that in the general population. PCOS is characterized by elevated AMH levels, which are due to both the increased number of small antral follicles that express AMH the most and the overexpression of AMH and anti-Mullerian hormone receptor type 2 by their granulosa cells (GCs) [2, 35, 36]. In GCs from women with PCOS, AMH expression is upregulated by high levels of luteinizing hormone (LH), androgens, and androgen receptors. Studies have also found a positive correlation between AMH levels and PCOS severity [37, 38]. In severe PCOS, although patients do have a higher number of follicles, follicle development is suppressed, which may result in a higher number of oocytes retrieved but no increase in MII oocytes [39]. In women, AMH inhibits the recruitment of primordial follicles out of the resting oocyte pool and may suppress FSH actions, contributing to ovulatory disturbances. This is consistent with our study, as we found that patients with AMH levels in the 75-100th percentile range had an increased number of total oocytes but not MII oocytes. Next, following oocyte retrieval and during fertilization and implantation, indicators such as fertilization rate and implantation rate may also be similar regardless of AMH levels due to obesity, insulin resistance, poor luteal function, and poor endometrial receptivity [40, 41]. In the present study, when women with AMH levels within the 75-100th percentile and those with AMH levels within the 0-25th percentile were compared, the former had a decreased fertilization rate and an implantation rate comparable to that of the latter group. The ORs were 0.92 and 0.82, respectively. In the general population, researchers have found that patients with low AMH levels had a higher rate of MII oocytes [42]. This may be associated with the number of follicles that grow in the ovary. Compared with a large quantity of oocytes, a few oocytes may obtain more sufficient nutrition from the ovary to support their maturation. Additionally, increased levels of AMH cleavage have been found to be related to various metabolic parameters in both control women and women with PCOS, which has a negative impact on implantation and endometrial receptivity. Overall, these factors led to the observed lower clinical pregnancy rate and live birth rate in high-AMH PCOS patients.
PCOS patients are more likely to suffer from OHSS due to a higher sensitivity and exaggerated response to ovarian stimulation protocols, particularly COS with gonadotropins [43]. Severe OHSS can lead to serious complications, including pleural effusion, acute renal insufficiency, and venous thromboembolism, and it can even be life-threatening. Therefore, every attempt should be made to identify patients who are at the highest risk for OHSS. The present study analyzed the association between AMH and the risk of OHSS in PCOS patients and found inconsistencies in the results of prior studies. In general, AMH has a poor predictive quality in OHSS. In addition, two studies demonstrated increasing cycle cancellation events due to OHSS risk in patients with elevated AMH levels. With similar results, in a retrospective cohort study of 134 general women with elevated AMH levels (> 5 ng/ml), women with AMH > 10 ng/ml had significantly higher rates (> threefold) of OHSS [44]. In another study, AMH levels in women with OHSS were sixfold higher than those in age- and weight-matched controls [45]. Notably, available studies were very limited, and patients received different ovarian stimulation protocols, which made it difficult to generalize the results. Clinical guidelines have demonstrated that AMH values > 3.4 may be useful to predict increased OHSS risk, but this cutoff point needs further validation [46]. Overall, AMH may be useful for planning ovarian stimulation protocols and counseling patients regarding risk. However, these measures should be used with caution since clear cutoff points have not been validated in the literature.
There are some limitations to this study. First, many of the included studies did not report adjusted effect estimates, which are less biased by confounders compared to crude estimates. Second, heterogeneity was observed in part of our meta-analysis. Current evidence is hampered by the differences in basic demographic characteristics, such as age and BMI, ovulation stimulation protocols, and AMH assays. Finally, we did not discuss other pregnancy outcomes, such as the multiple pregnancy rate or cesarean delivery, in our meta-analysis due to scarce information in the current literature.
In conclusion, this study assessed currently available evidence on the association between serum AMH levels and IVF/ICSI outcomes in PCOS patients. Our results suggest that an increased serum AMH level is inversely associated with clinical pregnancy, live birth, and fertilization; a higher serum AMH level is also associated with a higher number of oocytes retrieved, though comparable number of MII oocytes, in women with PCOS undergoing ART. Thus, AMH may be a useful risk stratification tool for PCOS women undergoing IVF/ICSI and may have clinical utility in counseling regarding IVF/ICSI outcomes among women with PCOS who wish to undergo fertility treatment. More large-scale, high-quality cohort studies are needed to confirm these findings.
Availability of data and materials
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in References.
References
Bhide P, Homburg R. Anti-Müllerian hormone and polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:38–45.
Joham AE, Norman RJ, Stener-Victorin E, Legro RS, Franks S, Moran LJ, Boyle J, Teede HJ. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022;10:668–80.
Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, Lizneva D, Natterson-Horowtiz B, Teede HJ, Yildiz BO. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057.
Zheng X, Wang CC. Is polycystic ovary syndrome undervalued in China? Lancet Reg Health West Pac. 2022;25: 100513.
Sir-Petermann T. Ladrón de Guevara A, Villarroel AC, Preisler J, Echiburú B, Recabarren S: [Polycystic ovary syndrome and pregnancy]. Rev Med Chil. 2012;140:919–25.
Tso LO, Costello MF, Albuquerque LET, Andriolo RB, Macedo CR. Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2020;12:Cd006105.
Shrikhande L, Shrikhande B, Shrikhande A. AMH and Its Clinical Implications. J Obstet Gynaecol India. 2020;70:337–41.
Nelson SM, Anderson RA, Broekmans FJ, Raine-Fenning N, Fleming R, La Marca A. Anti-Müllerian hormone: clairvoyance or crystal clear? Hum Reprod. 2012;27:631–6.
Teede H, Misso M, Tassone EC, Dewailly D, Ng EH, Azziz R, Norman RJ, Andersen M, Franks S, Hoeger K, et al. Anti-Müllerian Hormone in PCOS: a review informing international guidelines. Trends Endocrinol Metab. 2019;30:467–78.
La Marca A, Giulini S, Tirelli A, Bertucci E, Marsella T, Xella S, Volpe A. Anti-Müllerian hormone measurement on any day of the menstrual cycle strongly predicts ovarian response in assisted reproductive technology. Hum Reprod. 2007;22:766–71.
Muttukrishna S, McGarrigle H, Wakim R, Khadum I, Ranieri DM, Serhal P. Antral follicle count, anti-mullerian hormone and inhibin B: predictors of ovarian response in assisted reproductive technology? BJOG. 2005;112:1384–90.
Garg D, Tal R. The role of AMH in the pathophysiology of polycystic ovarian syndrome. Reprod Biomed Online. 2016;33:15–28.
Guo Y, Liu S, Hu S, Li F, Jin L. High serum anti-mullerian hormone concentrations are associated with poor pregnancy outcome in fresh IVF/ICSI cycle but not cumulative live birth rate in PCOS patients. Front Endocrinol (Lausanne). 2021;12: 673284.
Arslanca T, Ecemis T, Kiseli M, Arslanoglu E, Kotanoglu MS, Caglar GS: Pregnancy outcome of freeze thaw cycles of polycystic ovary syndrome patients regarding the anti-Mullerian hormone percentile. J Obstet Gynaecol. 2021:1–6.
Tal R, Seifer CM, Khanimov M, Seifer DB, Tal O. High serum Antimullerian hormone levels are associated with lower live birth rates in women with polycystic ovarian syndrome undergoing assisted reproductive technology. Reprod Biol Endocrinol. 2020;18:20.
Xi W, Gong F, Lu G. Correlation of serum Anti-Müllerian hormone concentrations on day 3 of the in vitro fertilization stimulation cycle with assisted reproduction outcome in polycystic ovary syndrome patients. J Assist Reprod Genet. 2012;29:397–402.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339: b2700.
Brooke BS, Schwartz TA, Pawlik TM. MOOSE reporting guidelines for meta-analyses of observational studies. JAMA Surg. 2021;156:787–8.
Sahmay S, Guralp O, Aydogan B, Cepni I, Oral E, Irez T. Anti-Mullerian hormone and polycystic ovary syndrome: assessment of the clinical pregnancy rates in in vitro fertilization patients. Gynecol Endocrinol. 2013;29:440–3.
Liu S, Hong L, Mo M, Xiao S, Wang X, Fan X, Zhang S, Diao L, Zeng Y. Association of antimullerian hormone with polycystic ovarian syndrome phenotypes and pregnancy outcomes of in vitro fertilization cycles with fresh embryo transfer. BMC Pregnancy Childbirth. 2022;22:171.
Kaya C, Pabuccu R, Satiroglu H. Serum antimullerian hormone concentrations on day 3 of the in vitro fertilization stimulation cycle are predictive of the fertilization, implantation, and pregnancy in polycystic ovary syndrome patients undergoing assisted reproduction. Fertil Steril. 2010;94:2202–7.
Du M, Zhang J, Yu X, Guan Y. Elevated anti-mullerian hormone is an independent risk factor for preterm birth among patients with overweight polycystic ovary syndrome. Front Endocrinol (Lausanne). 2021;12: 788000.
Hu KL, Liu FT, Xu H, Li R, Qiao J. High antimullerian hormone levels are associated with preterm delivery in patients with polycystic ovary syndrome. Fertil Steril. 2020;113(444–452): e441.
Hsu JY, James KE, Bormann CL, Donahoe PK, Pépin D, Sabatini ME. Müllerian-inhibiting substance/Anti-Müllerian hormone as a predictor of preterm birth in polycystic ovary syndrome. J Clin Endocrinol Metab. 2018;103:4187–96.
Du J, Cao Y. Anti-Mullerian hormone and associated pregnancy outcomes in females with polycystic ovary syndrome undergoing In vitro fertilization-embryo transfer. BioMedica. 2021;37:240–7.
Tabibnejad N, Soleimani M, Aflatoonian A. Serum Anti-Mullerian hormone and embryo morphokinetics detecting by time-lapse imaging: a comparison between the polycystic ovarian syndrome and tubal factor infertility. Int J Reprod Biomed. 2018;16:483–90.
Ho VNA, Pham TD, Le AH, Ho TM, Vuong LN. Live birth rate after human chorionic gonadotropin priming in vitro maturation in women with polycystic ovary syndrome. J Ovarian Res. 2018;11:70.
Guan Y, Kong P, Xiao Z, Zhang J, He J, Geng W, Yan J, Sun S, Mu M, Du X, Wang X. Independent variables for determining the cumulative live birth rates of aged patients with polycystic ovary syndrome or tubal factor infertility: a retrospective cohort study. Front Endocrinol (Lausanne). 2021;12: 728051.
Chen Y, Ye B, Yang X, Zheng J, Lin J, Zhao J. Predicting the outcome of different protocols of in vitro fertilization with anti-Muüllerian hormone levels in patients with polycystic ovary syndrome. J Int Med Res. 2017;45:1138–47.
Muharam R, Prasetyo YD, Prabowo KA, Putri YI, Maidarti M, Hestiantoro A. IVF outcome with a high level of AMH: a focus on PCOS versus non-PCOS. BMC Womens Health. 2022;22:172.
Arabzadeh S, Hossein G, Rashidi BH, Hosseini MA, Zeraati H. Comparing serum basal and follicular fluid levels of anti-Müllerian hormone as a predictor of in vitro fertilization outcomes in patients with and without polycystic ovary syndrome. Ann Saudi Med. 2010;30:442–7.
Acharya KS, Harris BS, Weber JM, Truong T, Pieper C, Eaton JL: Impact of increasing antimüllerian hormone level on in vitro fertilization fresh transfer and live birth rate. F&S Reports. 2022.
Kamel A, Ramadan W, Hussein AM, Dahab S, Elsherbini MM, Lasheen YS, Abu-Hamila F. Can AMH levels predict the need for increased medication during IVF/ICSI in PCOS women? J Matern Fetal Neonatal Med. 2018;31:32–8.
Tal R, Tal O, Seifer BJ, Seifer DB. Antimüllerian hormone as predictor of implantation and clinical pregnancy after assisted conception: a systematic review and meta-analysis. Fertil Steril. 2015;103:119–130.e113.
Catteau-Jonard S, Jamin SP, Leclerc A, Gonzalès J, Dewailly D, di Clemente N. Anti-Mullerian hormone, its receptor, FSH receptor, and androgen receptor genes are overexpressed by granulosa cells from stimulated follicles in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:4456–61.
Pellatt L, Hanna L, Brincat M, Galea R, Brain H, Whitehead S, Mason H. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92:240–5.
Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P, Duhamel A, Catteau-Jonard S. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26:3123–9.
Nardo LG, Yates AP, Roberts SA, Pemberton P, Laing I. The relationships between AMH, androgens, insulin resistance and basal ovarian follicular status in non-obese subfertile women with and without polycystic ovary syndrome. Hum Reprod. 2009;24:2917–23.
Azziz R. Polycystic ovary syndrome. Obstet Gynecol. 2018;132:321–36.
Llaneza-Suarez D, Llaneza P, González C, De-La-Fuente P, García-Ochoa C, Garrido P, Castañón V, Pérez-López FR. Assessment of follicular fluid leptin levels and insulin resistance as outcome predictors in women undergoing in vitro fertilization-intracytoplasmic sperm injection. Fertil Steril. 2014;102:1619–25.
Gao L, Li M, Wang Y, Zeng Z, Xie Y, Liu G, Li J, Zhang B, Liang X, Wei L, Yang X. Overweight and high serum total cholesterol were risk factors for the outcome of IVF/ICSI cycles in PCOS patients and a PCOS-specific predictive model of live birth rate was established. J Endocrinol Invest. 2020;43:1221–8.
Dai X, Wang Y, Yang H, Gao T, Yu C, Cao F, Xia X, Wu J, Zhou X, Chen L. AMH has no role in predicting oocyte quality in women with advanced age undergoing IVF/ICSI cycles. Sci Rep. 2020;10:19750.
Kollmann M, Martins WP, Lima ML, Craciunas L, Nastri CO, Richardson A, Raine-Fenning N. Strategies for improving outcome of assisted reproduction in women with polycystic ovary syndrome: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2016;48:709–18.
Tal R, Seifer DB, Khanimov M, Malter HE, Grazi RV, Leader B. Characterization of women with elevated antimüllerian hormone levels (AMH): correlation of AMH with polycystic ovarian syndrome phenotypes and assisted reproductive technology outcomes. Am J Obstet Gynecol. 2014;211(59):e51–58.
Ocal P, Sahmay S, Cetin M, Irez T, Guralp O, Cepni I. Serum anti-Müllerian hormone and antral follicle count as predictive markers of OHSS in ART cycles. J Assist Reprod Genet. 2011;28:1197–203.
Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril 2016, 106:1634–1647.
Acknowledgements
Not applicable.
Disclosure summary
The authors declare that they have no competing interests.
Funding
This study is supported by the National Natural Science Foundation of China (Grant No. 82101711).
Author information
Authors and Affiliations
Contributions
T.Y. and Z.Y. designed the study, wrote the main manuscript, and prepared the figures and the table. G.C., G.F. and Q.L retrieved the data, and took part in discussions regarding the results. H.F. designed the study, retrieved the data, revised the manuscript critically for important intellectual content and took part in discussions regarding the results. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yuwen, T., Yang, Z., Cai, G. et al. Association between serum AMH levels and IVF/ICSI outcomes in patients with polycystic ovary syndrome: a systematic review and meta-analysis. Reprod Biol Endocrinol 21, 95 (2023). https://doi.org/10.1186/s12958-023-01153-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12958-023-01153-y