Abstract
Background
Sarcopenia is associated with poor outcomes in many malignancies. However, the relationship between sarcopenia and the prognosis of pancreatic cancer has not been well understood. The aim of this meta-analysis was to identify the prognostic value of preoperative sarcopenia in patients with pancreatic cancer after curative-intent surgery.
Methods
Database from PubMed, Embase, and Web of Science were searched from its inception to July 2023. The primary outcomes were overall survival (OS), progression-free survival (PFS), and the incidence of major complications. The hazard ratio (HR), odds ratio (OR), and 95% confidence intervals (CIs) were used to assess the relationship between preoperative sarcopenia and the prognosis of patients with pancreatic cancer. All statistical analyses were conducted by Review Manager 5.3 and STATA 17.0 software.
Results
A total of 23 retrospective studies involving 5888 patients were included in this meta-analysis. The pooled results demonstrated that sarcopenia was significantly associated with worse OS (HR = 1.53, P < 0.00001) and PFS (HR = 1.55, P < 0.00001). However, this association was not obvious in regard to the incidence of major complications (OR = 1.33, P = 0.11).
Conclusion
Preoperative sarcopenia was preliminarily proved to be associated with the terrible prognosis of pancreatic cancer after surgery. However, this relationship needs to be further validated in more prospective studies.
Similar content being viewed by others
Introduction
Pancreatic cancer is a highly malignant solid tumor with 5-year survival rate less than 10% [1, 2]. In recent years, its incidence and mortality are still gradually increasing, and it is predicted to be the second leading cause of cancer-related death in the USA by 2030 [3]. Although the application of systemic chemotherapy and targeted therapy has greatly benefited patients with pancreatic cancer in recent years, surgery remains the only curative-intent treatment strategy. However, postoperative survival rate is still unsatisfactory due to its large probability of recurrence and metastasis [4, 5]. Previous studies on prognosis following pancreatectomy have mainly focused on tumor-specific factors such as tumor’s differentiation, perivascular invasion, and lymph node invasion [6,7,8]. However, their predictive abilities were skeptical due to the instability of these indicators.
In recent years, there has been increasing interest in the association between body composition and prognosis due to its simplicity and practicality. Sarcopenia, referring to age-dependent reduction in skeletal muscle volume, was first described in 1989 [9]. Sarcopenia was a kind of progressive and widespread skeletal muscle disease associated with an increased likelihood of adverse outcomes, including falls, fractures, physical disability, and death [10]. It has been found to be a potential risk factor for morbidity and mortality in patients with gastrointestinal malignancies [11].
Most patients with pancreatic cancer were prone to skeletal muscle depletion, leading to reduced tolerance for postoperative adjuvant therapy [12, 13]. Several recent studies have attempted to investigate the effect of sarcopenia on the prognosis of pancreatic cancer, but the outcomes of these studies have been more or less controversial [14,15,16,17]. Evidence needs to be updated, so the aim of this systematic review and meta-analysis is to clarify the relationship between preoperative sarcopenia and the prognosis of pancreatic cancer.
Materials and methods
The systematic review and meta-analysis followed Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [18]. The registration number was INPLASY202390060. The protocol could be found in Inplasy Protocol 5298 – INPLASY.
Literature search strategy
Two independent reviewers searched PubMed, Embase, and Web of science from its inception to July 2023. The language of search results was limited to English. Subsequently, the two persons checked each other and tried to reach a consensus. The detailed search strategies are presented in the Additional file 1.
Inclusion and exclusion criteria
Inclusion criteria were as follows: patients were pathologically diagnosed with pancreatic cancer; sarcopenia was evaluated by cross-sectional computed tomography (CT) scan of the third lumbar (L3) vertebra with respective cut-off values defined by sex before surgery; the measurement method of sarcopenia included skeletal muscle index (SMI) and psoas muscle index (PMI), as described in previous studies [19, 20], which represented two most common measurement methods; the definition of cut-off values included various standards, such as receiver operating characteristic (ROC) curves, Martin’s definition [21], Prado’s definition [22], and lowest quantile; outcomes were evaluated by prognostic indicators such as overall survival (OS) and/or progression-free survival (PFS) and the incidence of postoperative complications.
Exclusion criteria were as follows: patients were pathologically diagnosed as benign or borderline pancreatic tumors; sarcopenia was assessed by methods other than CT, such as bioelectrical analysis (BIA) and dual-energy X-ray absorptiometry (DXA); the cut-off values for sarcopenia were not clearly defined; the types of studies were conference abstracts, case reports, letters, and reviews; the time to evaluate sarcopenia took place postoperatively or the treatment strategy was palliative.
Outcomes
The primary outcomes were OS, PFS, and the incidence of major complications (grade III–IV) according to the Clavien-Dindo classification [23]. Secondary outcomes were the incidence of overall complications (grade I–IV) according to the Clavien-Dindo classification, as well as surgery-specific complications including clinically relevant postoperative pancreatic fistula (CR-POPF), post-pancreatectomy hemorrhage (PPH), delayed gastric empty (DGE), and surgical site infection (SSI) [24,25,26].
Data extraction
Two investigators independently extracted the following information from each study: publishing year, the name of first author, country, sample size, perioperative treatment (including neoadjuvant and adjuvant therapy), the measurement approach of sarcopenia, the cut-off values for sarcopenia, and clinical outcomes.
Assessment of methodological quality
Two independent investigators assessed the quality of the included studies on the Newcastle–Ottawa Scale (NOS) [27]. The contents of the scale included case selection, cohort comparison, and exposure risk assessment. Only studies with NOS score of six or higher were included in the final meta-analysis.
Statistical analysis
Survival data were evaluated by hazard ratio (HR) and their 95% corresponding intervals (CIs) in multivariate regression analysis, and categorical variables by odds ratio (OR). The Cochrane’s Q-test and I2 statistics were used to assess statistical heterogeneity. The cut-off value of low, moderate, and high heterogeneity was 25%, 50% and 75%, respectively. When the value of total heterogeneity exceeded 50%, we used the random-effect model. Otherwise, the fixed-effect model was applied. Subgroup analyses stratified by measurement approach of sarcopenia (SMI or PMI), region of studies (Asia or non-Asia), and definition of cut-off values (ROC curve, Martin’s definition, Prado’s definition, and lowest quantile) were performed further to find out the source of heterogeneity. P < 0.05 was regarded as statistically significant. In order to explore the possibility of publication bias, we applied funnel plots and Egger’s test. All analyses were conducted by Review Manager 5.3 software (Copenhagen: The Nordic Cochrane Centre, The Collaboration, 2011) and STATA 17.0 software (College Station, TX).
Results
Study selection
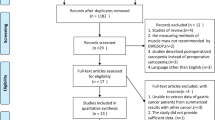
We searched 1538 articles from the electronic databases (PubMed, Embase, and Web of Science). After removing duplicates and unrelated studies, 119 full-text studies were assessed for eligibility. Eventually, 23 studies were eligible for qualitative synthesis after careful examination [14,15,16,17, 28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46]. The detailed flow diagram is shown in Fig. 1.
Basic characteristics of included studies
A total of 5888 patients with pancreatic cancer were incorporated into our meta-analysis. Publication year of studies ranged from 2012 to 2023. Seventeen (73.9%) studies were from Asian countries and only 6 (26.1%) from non-Asian countries. The majority of studies applied SMI to measure sarcopenia. And the definition of sex-related cut-off values for sarcopenia included 5 approaches, ROC curves (30.4%), Martin’s definition (13.0%), Prado’s definition (21.7%), Contal-O’Quigley method (4.3%), and lowest quantile (30.6%). The detailed information is listed in Table 1.
Primary outcomes
The relationship between preoperative sarcopenia and OS
The impact of preoperative sarcopenia on OS was explored in fifteen studies. The pooled HR demonstrated that preoperative sarcopenia was significantly associated with worse OS (HR = 1.53, 95% CI 1.41–1.67, P < 0.00001; I2 = 15%, P = 0.28) (Fig. 2). Subgroup analyses based on the measurement approach, region of studies, and different definitions of cut-off values confirmed the similar results (all P < 0.05). And all heterogeneity was moderate or low (Table 2).
The relationship between preoperative sarcopenia and PFS
Six studies evaluated the association between preoperative sarcopenia and PFS. The pooled HR showed that preoperative sarcopenia was strongly related to worse PFS (HR = 1.55, 95% CI 1.31–1.84, P < 0.00001; I2 = 0%, P = 0.67) (Fig. 3). However, we were not able to further perform subgroup analysis due to the limited available information.
The relationship between preoperative sarcopenia and the incidence of major complications
Eighteen studies including 4877 participants explored the predictive role of preoperative sarcopenia for major complications. Contrary to OS and PFS, preoperative sarcopenia was not obviously associated with high incidence of major complications (OR = 1.33, 95% CI 0.93–1.89, P = 0.11; I2 = 76%, P < 0.00001) (Fig. 4). However, interestingly, subgroup analysis stratified by the different definitions of cut-off values showed the inconsistent results. The pooled OR of those studies whose cut-off values were defined by ROC curves demonstrated preoperative sarcopenia’s strong relevance to the increased incidence of major complications (OR = 2.73, 95% CI 1.35–5.53, P = 0.005; I2 = 0%, P = 0.01), but this relevance was not shown in studies defined by the other three definitions (Fig. 5, Table 3).
Secondary outcomes
Overall and surgery-related complications
Impact of preoperative sarcopenia on overall complications was reported in six studies. Preoperative sarcopenia was not obviously related to the increased incidence of postoperative overall complications (OR = 1.33, 95% CI 0.84–2.12, P = 0.23; I2 = 75%, P = 0.001). And the increased probability of surgery-related complications, including CR-POPF, PPH, DGE, and SSI, was not observed to have a strong association with preoperative sarcopenia, either (all P > 0.05) (Additional file 2). And the results of subgroup analyses were consistent (Tables 4 and 5).
Publication bias
The symmetrical distribution of funnel plots showed no significant risk of publication bias (Additional file 3). Moreover, Egger’s regression test suggested that publication bias was insignificant for OS (P = 0.757), PFS (P = 0.684), and the incidence of major complications (P = 0.448).
Discussion
We conducted a systematic review and meta-analysis of 23 studies to investigate the relationship between preoperative sarcopenia and the prognosis of pancreatic cancer after radical surgery, including OS, PFS, and the incidence of complications (overall complications and major complications, as well as four surgical-related complications including CR-POPF, PPH, DGE, and SSI). Our results were encouraging, suggesting that preoperative sarcopenia significantly reduced survival time (OS and PFS). However, our analysis did not confirm that sarcopenia was strongly associated with high incidence of postoperative complications.
Basically consistent with our results, the first meta-analysis conducted by Mintziras et al. in patients with pancreatic ductal adenocarcinoma confirmed that sarcopenia was strongly associated with worse OS (HR = 1.49, 95% CI 1.27–1.74, P < 0.001) [47]. However, they did not exclude those patients with palliative treatment. Moreover, analyses of the incidence of major complications and CR-POPF in sarcopenia were not performed due to limited data. Bundred et al. showed that sarcopenia was not significantly associated with the incidence of postoperative complications or CR-POPF [48]. However, of the studies they included, only five and two, respectively, reported the incidence of major complications and CR-POPF. In addition, the generalization of their results was limited by the high heterogeneity caused by non-standardized measurement methods, such as BIA and DXA. CT could make up for the unavoidable disadvantage of BIA and DXA to patients caused by repeated doses of radiation, and studies have confirmed that CT scan has been shown to be more sensitive to small changes in muscle area than DXA [49, 50]. So, based on the recent consensus from the European Working Group on Sarcopenia in Older People and the Asian Working Group for Sarcopenia, CT imaging at the level of the L3 vertebra represents a standardized method to quantify the skeletal musculature [51, 52]. Thormann et al. concluded that sarcopenia was strongly relevant to dismal prognosis in both radical and palliative settings. Unfortunately, they did not conduct further subgroup analyses to explore the sources of heterogeneity [53].
The mechanism of the association between sarcopenia and poor prognosis has not been well understood. Sarcopenia is not merely a loss of muscle mass or quantity, but a disorder that reflects a disorder of immune nutritional status, and its relationship with the tumor micro-environment is still being studied [54]. Several nutritional and immune factors were found to have an important role in people with sarcopenia. Previous studies have reported that high neutrophil–lymphocyte ratio (NLR) was an independent indicator of muscle mass loss [45]. A recent meta-analysis showed that in patients with pancreatic cancer, lower NLR had better OS and PFS in patients with pancreatic cancer [55]. In addition, several studies have demonstrated that sarcopenia was associated with insulin resistance, vitamin D deficiency, elevated levels of inflammatory cytokines (such as tumor necrosis factor-alpha and interleukin-6), and decreased concentrations of muscle factors (such as interleukin-15) [56,57,58]. Under the action of the above factors, the body’s immune system is weakened, and the postoperative wound healing is poor, thus affecting the risk of postoperative complications.
Since sarcopenia is associated with unsatisfactory postoperative survival rate and high incidence of complications, perioperative intervention is important to reduce these risks. Nutritional counseling and oral nutritional supplements may also be available as intervention options for the treatment of cachexia [59, 60]. Studies have shown that in patients with gastric cancer, preoperative exercise and nutritional support programs can reduce the incidence of sarcopenia and improve postoperative outcomes [61].
To analyze the sources of heterogeneity, we performed subgroup analyses by regions of studies (Asian or non-Asian), measurement methods of sarcopenia (SMI or PMI), and definition criteria for sex-specific cut-off values, respectively. Our subgroup analyses of different study regions and measurement methods did not change the overall results. But interestingly, our research showed that under the criteria of cut-off values defined by the ROC curve, preoperative sarcopenia was strongly associated with worse OS (HR = 1.69, 95% CI 1.48–1.94, P < 0.00001) and higher incidence of complications (OR = 2.73, 95% CI 1.35–5.53, P = 0.005). In contrast, the relationship was less significant or non-significant based on the criteria of other definitions, such as the lowest quantile, Prado’s, and Martin’s definition. We speculate that this phenomenon may be related to the objectivity and accuracy of ROC curve based on the data itself, free from external interference. Therefore, this finding may provide a novel direction for more accurate definition of cut-off values for sarcopenia in the future. However, at present, no unanimously accepted cut-off values have been established for CT-based sarcopenia in Asian populations. Therefore, more large-scale studies are needed in the future to establish standardized cut-off values for sarcopenia in different populations and confirm these observations.
We have to admit that our study has several limitations. First, all the studies we included were retrospective cohort studies. In the future, large-scale randomized controlled trials are needed to further clarify the relationship between sarcopenia and the prognosis of pancreatic cancer. Second, due to the limited information available from the included studies, we did not conduct more subgroup analyses of other important indicators that may influence prognosis, such as tumor’s stage, gender, perioperative treatment (including neoadjuvant and adjuvant therapy), and surgical procedure. Finally, we did not analyze biomarkers that might affect muscle quality, such as fat infiltration and accumulation, because relevant studies were still insufficient. Sarcopenia reflects a combination of muscle quantity and mass. However, to the best of our knowledge, this study is the first meta-analysis to analyze the relationship between sarcopenia and the prognosis after radical resection of pancreatic cancer according to different definition criteria of sarcopenia cut-off values, which may provide novel direction for accurate exploration in the future.
Conclusion
Preoperative sarcopenia was preliminarily proved to be significantly associated with the poor prognosis of pancreatic cancer patients after radical surgery. However, this relationship needs to be further validated in more prospective studies.
Availability of data and materials
The current study was based on the results of relevant published studies.
Abbreviations
- PRISMA:
-
Preferred Reporting Items for Systematic Review and Meta-Analysis
- CT:
-
Computed tomography
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- BIA:
-
Bioelectrical analysis
- DXA:
-
Dual-energy X-ray absorptiometry
- CR-POPF:
-
Clinical related-postoperative pancreatic fistula
- PPH:
-
Post-pancreatectomy hemorrhage
- DGE:
-
Delayed gastric empty
- SSI:
-
Surgical site infection
- SMI:
-
Skeletal muscle index
- PMI:
-
Psoas muscle index
- NOS:
-
Newcastle-Ottawa Scale
- HR:
-
Hazard ratio
- CIs:
-
Corresponding intervals
- OR:
-
Odds ratio
- ROC:
-
Receiver operating characteristic
- NLR:
-
Neutrophil-lymphocyte ratio
References
Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008–20.
Kleeff J, Korc M, Apte M, et al. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022.
Rahib L, Smith B, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21.
Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet. 2016;388:73–85.
Groot VP, Gemenetzis G, Blair AB, et al. Defining and predicting early recurrence in 957 patients with resected pancreatic ductal adenocarcinoma. Ann Surg. 2019;269:1154–62.
Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol. 2008;15:165–74.
Zhang XP, Xu S, Gao YX, et al. Early and late recurrence patterns of pancreatic ductal adenocarcinoma after pancreaticoduodenectomy: a multicenter study. Int J Surg. 2023;109:785–93.
Liu L, Xu HX, He M, et al. A novel scoring system predicts postsurgical survival and adjuvant chemotherapeutic benefits in patients with pancreatic adenocarcinoma: implications for AJCC-TNM staging. Surgery. 2018;163:1280–94.
Rosenberg IR. Summary comments. Am J Clin Nutr. 1989;50:1231–3.
Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31.
Levolger S, van Vugt JL, de Bruin RW, IJzermans JN. Systematic review of sarcopenia in patients operated on for gastrointestinal and hepatopancreatobiliary malignancies. Br J Surg. 2015;102:1448–58.
Takeda T, Sasaki T, Suzumori C, et al. The impact of cachexia and sarcopenia in elderly pancreatic cancer patients receiving palliative chemotherapy. Int J Clin Oncol. 2021;26:1293–303.
Kim IH, Choi MH, Lee IS, Hong TH, Lee MA. Clinical significance of skeletal muscle density and sarcopenia in patients with pancreatic cancer undergoing first-line chemotherapy: a retrospective observational study. BMC Cancer. 2021;21:77.
Kim DW, Ahn H, Kim KW, et al. Prognostic value of sarcopenia and myosteatosis in patients with resectable pancreatic ductal adenocarcinoma. Korean J Radiol. 2022;23:1055–66.
Rom H, Tamir S, Van Vugt JLA, et al. Sarcopenia as a predictor of survival in patients with pancreatic adenocarcinoma after pancreatectomy. Ann Surg Oncol. 2022;29:1553–63.
Choi MH, Yoon SB, Lee K, et al. Preoperative sarcopenia and post-operative accelerated muscle loss negatively impact survival after resection of pancreatic cancer. J Cachexia Sarcopenia Muscle. 2018;9:326–34.
Ninomiya G, Fujii T, Yamada S, et al. Clinical impact of sarcopenia on prognosis in pancreatic ductal adenocarcinoma: a retrospective cohort study. Int J Surg. 2017;39:45–51.
Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006.
Hanaoka M, Yasuno M, Ishiguro M, et al. Morphologic change of the psoas muscle as a surrogate marker of sarcopenia and predictor of complications after colorectal cancer surgery. Int J Colorectal Dis. 2017;32:847–56.
Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–47.
Prado C, Lieffers J, McCargar L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–35.
Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96.
Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Stud-y Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161:584–91.
Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an Internat-ional Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20–5.
Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancrea-tic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142:761–8.
Luchini C, Stubbs B, Solmi M, et al. Assessing the quality of studies in meta-analyses: advantages and limitations of the Newcastle Ottawa Scale. World J Meta-Anal. 2017;5:80–4.
Peng P, Hyder O, Firoozmand A, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16:1478–86.
Amini N, Spolverato G, Gupta R, et al. Impact total psoas volume on short- and long-term outcomes in patients undergoing curative resection for pancreatic adenocarcinoma: a new tool to assess sarcopenia. J Gastrointest Surg. 2015;19:1593–602.
Okumura S, Kaido T, Hamaguchi Y, et al. Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery. 2015;157:1088–98.
Nishida Y, Kato Y, Kudo M, et al. Preoperative sarcopenia strongly influences the risk of postoperative pancreatic fistula formation after pancreaticoduodenectomy. J Gastrointest Surg. 2016;20:1586–94.
Okumura S, Kaido T, Hamaguchi Y, et al. Visceral adiposity and sarcopenic visceral obesity are associated with poor prognosis after resection of pancreatic cancer. Ann Surg Oncol. 2017;24:3732–40.
Takagi K, Yoshida R, Yagi T, et al. Radiographic sarcopenia predicts postoperative infectious complications in patients undergoing pancreaticoduodenectomy. BMC Surg. 2017;17:64.
El Amrani M, Vermersch M, Fulbert M, et al. Impact of sarcopenia on outcomes of patients undergoing pancreatectomy: a retrospective analysis of 107 patients. Medicine (Baltimore). 2018;97:e12076.
Tankel J, Dagan A, Vainberg E, et al. Sarcopenia is associated with a greater incidence of delayed gastric emptying following pancreaticoduodenectomy. Clin Nutr ESPEN. 2018;27:105–9.
Wagner D, Marsoner K, Tomberger A, et al. Low skeletal muscle mass outperforms the Charlson Comorbidity Index in risk prediction in patients undergoing pancreatic resections. Eur J Surg Oncol. 2018;44:658–63.
Yamane H, Abe T, Amano H, et al. Visceral adipose tissue and skeletal muscle index distribution predicts severe pancreatic fistula development after pancreaticoduodenectomy. Anticancer Res. 2018;38:1061–6.
Gruber ES, Jomrich G, Tamandl D, Gnant M, Schindl M, Sahora K. Sarcopenia and sarcopenic obesity are independent adverse prognostic factors in resectable pancreatic ductal adenocarcinoma. PLoS ONE. 2019;14:e0215915.
Ratnayake CBB, Wells C, Olsson M, Windsor JA, Pandanaboyana S. Sarcopenic obesity and post-operative morbidity after pancreatic surgery: a cohort study. ANZ J Surg. 2019;89:1587–92.
Peng YC, Wu CH, Tien YW, Lu TP, Wang YH, Chen BB. Preoperative sarcopenia is associated with poor overall survival in pancreatic cancer patients following pancreaticoduodenectomy. Eur Radiol. 2021;31:2472–81.
Ryu Y, Shin SH, Kim JH, et al. The effects of sarcopenia and sarcopenic obesity after pancreaticoduodenectomy in patients with pancreatic head cancer. HPB (Oxford). 2020;22:1782–92.
Xu JY, Li C, Zhang H, Liu Y, Wei JM. Total psoas area index is valuable to assess sarcopenia, sarcopenic overweight/obesity and predict outcomes in patients undergoing open pancreatoduodenectomy. Risk Manag Healthc Policy. 2020;13:761–70.
d’Engremont C, Grillot J, Raillat J, et al. Additive value of preoperative sarcopenia and lymphopenia for prognosis prediction in localized pancreatic ductal adenocarcinoma. Front Oncol. 2021;11:683289.
Cai ZW, Li JL, Liu M, Wang HW, Jiang CY. Low preoperative skeletal muscle index increases the risk of mortality among resectable pancreatic cancer patients: a retrospective study. World J Gastrointest Surg. 2022;14:1350–62.
Özkul B, Özkul Ö, Bilir C. Predicting the overall survival in patients with advanced pancreatic cancer by calculating L3 skeletal muscle index derived from CT. Curr Med Imaging. 2022;18:1079–85.
Shen XD, Wang X, Zheng ZJ, et al. The differential effects of sarcopenia and cachexia on overall survival for pancreatic ductal adenocarcinoma patients following pancreatectomy: a retrospective study based on a large population. Cancer Med. 2023;12:10438–48.
Mintziras I, Miligkos M, Wächter S, Manoharan J, Maurer E, Bartsch DK. Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer: systematic review and meta-analysis. Int J Surg. 2018;59:19–26.
Bundred J, Kamarajah SK, Roberts KJ. Body composition assessment and sarcopenia in patients with pancreatic cancer: a systematic review and meta-analysis. HPB (Oxford). 2019;21:1603–12.
Chen Z, Wang Z, Lohman T, et al. Dual-energy X-ray absorptiometry is a valid tool for assessing skeletal muscle mass in older women. J Nutr. 2007;137:2775–80.
Delmonico MJ, Kostek MC, Johns J, Hurley BF, Conway JM. Can dual energy X-ray absorptiometry provide a valid assessment of changes in thigh muscle mass with strength training in older adults? Eur J Clin Nutr. 2008;62:1372–8.
Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:601.
Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101.
Thormann M, Hinnerichs M, Barajas Ordonez F, et al. Sarcopenia is an independent prognostic factor in patients with pancreatic cancer-a meta-analysis. Acad Radiol. 2023;30:1552–61.
Hilmi M, Jouinot A, Burns R, et al. Body composition and sarcopenia: the next-generation of personalized oncology and pharmacology? Pharmacol Ther. 2019;196:135–59.
Zhou Y, Wei Q, Fan J, Cheng S, Ding W, Hua Z. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: a meta-analysis containing 8252 patients. Clin Chim Acta. 2018;479:181–9.
Lutz CT, Quinn LS. Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging (Albany NY). 2012;4:535–46.
Gilsanz V, Kremer A, Mo AO, Wren TA, Kremer R. Vitamin D status and its relation to muscle mass and muscle fat in young women. J Clin Endocrinol Metab. 2010;95:1595–601.
Zoico E, Rossi A, Di Francesco V, et al. Adipose tissue infiltration in skeletal muscle of healthy elderly men: relationships with body composition, insulin resistance, and inflammation at the systemic and tissue level. J Gerontol A Biol Sci Med Sci. 2010;65:295–9.
Solheim TS, Fearon KC, Blum D, Kaasa S. Non-steroidal anti-inflammatory treatment in cancer cachexia: a systematic literature review. Acta Oncol. 2013;52:6–17.
Balstad TR, Solheim TS, Strasser F, Kaasa S, Bye A. Dietary treatment of weight loss in patients with advanced cancer and cachexia: a systematic literature review. Crit Rev Oncol Hematol. 2014;91:210–21.
Kobayashi D, Ishigure K, Mochizuki Y, et al. Multi-institutional prospective feasibility study to explore tolerability and efficacy of oral nutritional supplements for patients with gastric cancer undergoing gastrectomy (CCOG1301). Gastric Cancer. 2017;20:718–27.
Acknowledgements
None.
Funding
The study was supported by Shaoxing Basic Public Welfare Project (No. 2022A14012), and Shaoxing Health Science and Technology Project (Laboratory opening plan) (No. 2022SY013).
Author information
Authors and Affiliations
Contributions
Conceptualization, Chenming Liu and Liang An; Literature research, Liang An; Software, Siyuan Zhang and Neng Wang; Writing–Original Draft Preparation, Chenming Liu and Liang An; Writing–Review & Editing, Haijun Tang; Project Administration, Haijun Tang; Funding Acquisition, Liang An and Haijun Tang.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 2: Supplementary Figure 1.
Forest plots of comparison between sarcopenia and non-sarcopenia. (A) overall complications, (B) CR-POPF, (C) PPH, (D) DGE, (E) SSI.
Additional file 3: Supplementary Figure 2.
Funnel plots for examination of publication bias. (A) overall survival, (B) major complications, (C) profession-free survival.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, C., An, L., Zhang, S. et al. Association between preoperative sarcopenia and prognosis of pancreatic cancer after curative-intent surgery: a updated systematic review and meta-analysis. World J Surg Onc 22, 38 (2024). https://doi.org/10.1186/s12957-024-03310-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-024-03310-y