Abstract
Background
Adding anti-epidermal growth factor receptor (anti-EGFR) target agents to conversion therapy may improve the resection rates and survival of patients with potentially resectable metastatic colorectal cancer (mCRC). This study aims to analyze the efficacy and safety of additional anti-EGFR target agents.
Methods
A systematic search was conducted on PubMed, Web of Science, Embase, and Cochrane Library. And all relevant studies published in English before January 2023 were collected to explore the impact of additional anti-EGFR targeted agent on the efficacy and safety of patients with potentially resectable mCRC (PROSPERO: CRD42022340523, https://www.crd.york.ac.uk/PROSPERO/).
Results
This study included a total of 8 articles, including 2618 patients. The overall response rate (ORR) and R0 resection rates of the experimental group were higher than those of the control group, while there was no significant difference in progression-free survival (PFS) and overall survival (OS) between the two groups. In RAS/KRAS wild-type patients, the ORR (RR: 1.20, 95% Cl: 1.02–1.41, p = 0.03), R0 resection rate (RR: 1.60, 95% Cl: 1.17–2.20, p = 0.003), PFS (HR: 0.80, 95% Cl: 0.68–0.93, p = 0.003), and OS (HR: 0.87, 95% Cl: 0.76–0.99, p = 0.031) of the experimental group were higher than those of the control group. While in KRAS mutant patients, there was no statistical difference between the two groups in ORR, R0 resection rate, PFS, and OS.
Conclusion
The addition of anti-EGFR targeted agents can improve the prognosis of RAS/KRAS wild-type patients with potentially resectable mCRC, while KRAS mutant patients may not benefit. In addition, the overall safety factor was controllable.
Similar content being viewed by others
Introduction
As the third most common tumor in the world, colorectal cancer (CRC) was diagnosed in approximately 20 billion cases in 2020 and kill nearly 10 billion people each year, ranking it second in cancer-related mortality (https://www.iarc.fr/faq/latest global-cancer-data-2020-qa/). About 33% of patients with CRC will have metastases, with the most common metastatic site being liver metastasis, significantly reducing the expected 5-year overall survival rate (OS) and becoming the main cause of death [1, 2]. For patients with metastatic colorectal cancer (mCRC), complete surgical resection of the metastatic site is the only possible treatment, allowing the patient to achieve an almost radical effect. However, some patients have tumors that were initially unresectable due to the location, number, size, or other influencing factors of their tumors [3]. In recent years, the advances in chemotherapy and targeted drugs have opened up the possibility of conversion therapy for mCRC. Specifically, conversion therapy is systematic treatment administered preoperatively to reduce the size of the tumor and convert the initially unresectable metastatic lesions into resectable ones [4]. Moreover, the prognosis of patients who underwent resection after transformation was almost the same as that of patients who underwent initial resection [5, 6].
Conversion therapy is performed by chemotherapy with or without targeted drugs. The specific treatment plan needs to be formulated according to the status of patients, the mutation type of the tumor, and the location of the primary tumor (left side or right side) [7, 8]. In addition, for some giant liver metastases, some studies have suggested that hepatic artery intubation chemotherapy and hepatic artery embolization chemotherapy can also achieve high response rates and R0 resection rates [9]. Recently, studies have shown improved R0 resection rates as well as survival in patients treated with targeted therapy in combination with chemotherapy [10, 11], but the randomized phase 3 COIN study did not report that the addition of targeted therapeutic agents to the treatment regimen increased R0 resection rates [12]. In addition, different molecular subtypes respond differently to drugs [13]. Anti-epidermal growth factor receptor (anti-EGFR) target agent plus cytotoxic drugs are one of the conversion therapy for potentially resectable mCRC. They are monoclonal antibodies, including cetuximab and panitumumab, which target the EGFR receptor and block intracellular signaling, thereby inhibiting cancer cell proliferation [14].
There are few meta-analyses analyzing the role of anti-EGFR targeted agents in patients with potentially resectable mCRC, and although both cetuximab and panitumumab are anti-EGFR targeted agents, there are limited data assessing the efficacy and safety of panitumumab in patients with potentially resectable mCRC. Therefore, the present study focused on the efficacy and safety of adding anti-EGFR targeted agents in patient with potentially resectable mCRC.
Methods
Search strategy
Four electronic databases, including PubMed, Embase, Cochrane, and Web of Science, have been researched. This systematic study explored the efficacy and safety of anti-EGFR targeted agents in combination with chemotherapy in patients with potentially resectable mCRC. Articles published in English from inception until January 2023 were searched. The following search terms and keywords were used in the retrieval process: “anti-EGFR targeted agents,” “epidermal growth factor receptor targeted agents,” “panitumumab,” “cetuximab,” “colorectal liver metastasis,” and “metastatic colorectal cancer” (Supplementary Table 1). In addition, data could also be derived from the reference lists of studies, and this meta-analysis complies with the Preferred Reporting Items Systematic reviews and Meta-Analyses (PRISMA) guidelines for the systematic review and meta-analysis [15]. The study protocol has registered with the PROSPERO: CRD42022340523 (https://www.crd.york.ac.uk/PROSPERO/).
Selection criteria
Inclusion criteria were based on PICOS principles (participants, intervention, comparison, outcomes, and study design): (1) participants: patients with potentially resectable mCRC; (2) intervention: use of anti-EGFR targeted agents in combination with chemotherapy in the experimental group; (3) comparison: patients in the control group received chemotherapy only; (4) outcomes: data on at least one of the progression-free survival (PFS), overall survival, and objective response rate (ORR) was reported in results; and (5) study design: randomized controlled trial (RCT).
The following exclusion criteria were used: (1) patients with resectable mCRC were included in the study; (2) R0 resection rate was not reported in study; (3) data could not be extracted, or the extracted data could not be further processed; and (4) the article was not published in English. If the studies were conducted for the same clinical trial, the present study would contain the most recent or comprehensive version.
In general, the present study requires that included in the study must be patients with mCRC who are likely to be resected after conversion therapy and excludes patients with initially resectable mCRC.
Data extraction and quality assessment
Articles that met the inclusion criteria were further analyzed by two independent authors. Extracted information was recorded in a standardized Microsoft Excel form (Microsoft Corp): author, year of publication, country, number of patients, treatment regimen, follow-up time, and prognostic outcomes of the studies. Any discrepancy between the two independent authors was judged by a third researcher to reach consensus. The meta-analysis concentrates on prognostic endpoints, including PFS, OS, ORR, R0 resection, and adverse events (AEs). In addition, the quality of RCTs was evaluated by Cochrane Collaboration’s tool in seven aspects: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. The risk of bias was classified as “high risk,” “low risk,” and “unclear risk.” In the end, only high-quality articles were included in the analysis.
Statistical analysis
To statistically analyze the prognosis and surgical resection rate of anti-EGFR targeted agents combined with chemotherapy, hazard ratio (HR) as well as 95% confidence intervals (CI) for PFS and OS was extracted from the included articles in this study to evaluate the prognostic impact of anti-EGFR targeted agents plus chemotherapy. Furthermore, ORR, R0 resection, and AEs were measured by risk ratio (RR) and 95%CI. Statistical heterogeneity was determined by using chi-square test and I2 statistic, with p < 0.1 or I2 > 50% indicating high heterogeneity. Due to the different conditions of patients and the use of different drugs, the random-effect model was used to improve the reliability of the results. Begg’s test was used to evaluate publication bias. Sensitivity analysis was used to verify the stability of the results. All the statistical tests were performed with RevMan 5.4 and STATA version 10.0 (Stata Corporation, College Station, TX, USA). The p-value is two sided, and the results of p < 0.05 were deemed to be statistically significant.
Results
Eligible research and inclusion characteristics
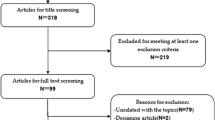
Figure 1 shows the flow chart of the screening process. A total of 16,757 citations were obtained by searching PubMed, Cochrane, Embase, and Web of Science as well as two articles obtained from the references of other articles. After the removal of duplicate citations, 6642 citations remained. Among them, 6620 citations were excluded because their titles or abstracts obviously did not meet the inclusion criteria of present study. After reading the full text of the remaining 22 articles, we found that 14 of the 22 articles were excluded due to the following reasons: 7 articles used treatment regimens that did not meet the requirements, 5 studies were excluded due to the discovery of updated publication on the same trail, 1 article did not report the relevant results required for this study, and the data of 1 article could not be extracted. Therefore, 8 articles [10,11,12,13, 16,17,18,19] with a total of 2618 patients were finally included in this meta-analysis.
The 8 studies included in present study were RCTs, including 6 studies from Europe and 2 studies from Asia. All patients in the experimental group received anti-EGFR targeted agents in combination with chemotherapy, with cetuximab in 6 studies and panitumumab in the other 2 studies. As far as the chemotherapy regime was concerned, only 1 study of the chemotherapy regime used triplet chemotherapy regimen, and the other 7 studies used doublet chemotherapy. All 8 articles reported the PFS, OS, and ORR of patients after treatment. More detailed information is provided in Table 1 and Supplementary Table 2. Detailed information on the quality assessment of the 8 articles included in present study is shown in Supplementary Fig. 1 and Supplementary Fig. 2.
Objective response rate and R0 resection rate
Eight articles reported the ORR and R0 resection rates of patients. In general, patients receiving anti-EGFR targeted agents combined with chemotherapy have higher ORR (RR: 1.24, 95% Cl: 1.13–1.37, p < 0.001) and R0 resection rate (RR: 1.63, 95% Cl: 1.27–2.09, p < 0.001) than those in the control group. Among RAS/KRAS wild-type patients, the ORR (RR: 1.20, 95% Cl: 1.02–1.41, p = 0.03) and R0 resection rate (RR: 1.60, 95% Cl: 1.17–2.20, p = 0.003) were higher in the experimental group than in the control group. In this study, 4 articles provided ORR after treatment in patients with KRAS mutant, and 2 articles provided R0 resection rate. Among patients with KRAS mutant, there was no statistically significant difference in ORR (RR: 0.99, 95% Cl: 0.78–1.25, p = 0.94) and R0 resection rate (RR: 2.90, 95% Cl: 0.46–18.54, p = 0.26) between the experimental group and the control group. Details are shown in Table 2.
In addition, according to the drug types of anti-EGFR targeted agents, data on ORR and R0 resection rates in patients treated with cetuximab combined with chemotherapy were mentioned in 6 articles, and the ORR and R0 resection rates of patients treated with panitumumab combined with chemotherapy were mentioned in 2 articles. Compared to the control group, statistical analysis showed that patients receiving cetuximab in combination with chemotherapy had higher ORR (RR: 1.28, 95% Cl: 1.13–1.45, p < 0.001) and R0 resection rate (RR: 1.69, 95% Cl: 1.26–2.28, p < 0.001). There was no significant difference in ORR (RR: 1.18, 95% Cl: 0.98–1.42, p = 0.08) and R0 resection rate (RR: 1.66, 95% Cl: 0.80–3.43, p = 0.18) between patients in the experimental group receiving panitumumab in combination with chemotherapy and those in the control group receiving chemotherapy only. Details are shown in Table 2.
Effect of anti-EGFR targeted agents on survival
The 8 articles included in present study provided detailed data on PFS and OS (Figs. 2 and 3). As for the PFS (HR: 0.89, 95% Cl: 0.79–1.01, p = 0.066) and OS (HR: 0.93, 95% Cl: 0.83–1.04, p = 0.188) of patients in the experimental group and the control group, the differences between the two were not statistically significant. In order to further explore the impact of genetic status on the prognosis of patients, this study divided them into RAS/KRAS wild type and KRAS mutant. In present study, 8 articles reported PFS of patients with RAS/KRAS wild type after treatment, and 4 articles reported PFS of patients with KRAS mutant. Statistical analysis showed that, comparing with the control group, the PFS was higher in patients with RAS/KRAS wild type in the experimental group (HR: 0.80, 95% Cl: 0.68–0.93, p = 0.003). However, for patients with KRAS mutant, there was no statistical difference in PFS between the two groups (HR: 0.88, 95% Cl: 0.59–1.31, p = 0.526). The OS for RAS/KRAS wild-type patients can be found in the 8 articles and for KRAS mutant patients in 5 articles. For RAS/KRAS wild-type patients, the OS was higher in the experimental group with anti-EGFR targeted agents than in the control group (HR: 0.87, 95% Cl: 0.76–0.99, p = 0.031). However, in patients with KRAS mutant, the difference between the two groups was not statistically significant (HR: 1.07, 95% Cl: 0.95–1.20, p = 0.250). More detailed information is in Figs. 4 and 5.
Grade 3 or higher adverse events
In this study, 8 articles reported on relevant contents of AEs. Grade 3 or higher AEs are listed in Table 3. Four articles reported the total AEs of patients. The pooled analysis results showed that the incidence of AEs was higher in the experimental group than in the control group (RR: 1.22, 95% Cl: 1.05–1.42, p = 0.01). Compared with the control group, statistical analysis showed that anti-EGFR targeted agents with chemotherapy increased the incidence of some AEs in patients, such as diarrhea (RR: 1.71), fatigue (RR: 1.54), rash (RR: 26.60), skin reactions (RR: 44.52), hemoglobin (RR: 2.43), hypermagnesemia (RR: 13.10), stomatitis (RR: 2.90), cardiac events (RR: 29.62), and infusion-related reactions (RR: 2.32).
Publication bias and sensitivity analysis
The present study evaluated the potential publication bias of articles reporting PFS and OS according to the genetic profile (RAS/KRAS wild type and RAS/KRAS mutant), and no significant publication bias was found. Details are shown in Supplementary Fig. 3 (p = 0.174), Supplementary Fig. 4 (p = 0.308), Supplementary Fig. 5 (p = 0.174), and Supplementary Fig. 6 (p = 0.806). In addition, the sensitivity analysis results of present study were generally stable. Details are shown in Supplementary Fig. 7, Supplementary Fig. 8, Supplementary Fig. 9, and Supplementary Fig. 10.
Discussion
For mCRC patients with different initial conditions (initially resectable, potentially resectable, and unresectable), the addition of anti-EGFR targeted agents has different status. In turn, the determination of resectability needs to be evaluated by a professional multidisciplinary team [20, 21]. With the continuous progress of research, the applicability of resectable metastatic lesions is becoming more extensive [22]. At present, these definitions of resectability are only at the technical level and are based on the possibility of completely removing all visible metastatic tumors, leaving an adequately functioning parenchyma [23]. It includes the following: in terms of tumor, the tumor is required to be resectable, and the margin of resection is negative, and the tumor is required to respond to preoperative chemotherapy, and extrahepatic diseases should be controlled; in terms of liver condition, patients are required to have two contiguous functional liver segments with preserved blood flow of vein, artery, portal vein, and biliary tract; in addition, the number of liver lesions and other factors that are related to poor prognosis after conversion resection also needs attention [24,25,26,27].
For patients with initially resectable mCRC, in the New EPOC study, Bridgewater et al. found that patients receiving cetuximab in combination with chemotherapy had lower survival rates than those receiving chemotherapy alone. Therefore, this study suggests that cetuximab should not be used as neoadjuvant therapy for patients with resectable mCRC [28]. For patients with initially unresectable mCRC, there is currently no clear standard to distinguish between those who are suitable for palliative care only and those who have the opportunity to undergo surgery after receiving conversion treatment. Therefore, they may both be potentially resectable mCRC patients. However, with the progress of research, some patients with tumors (that were) previously considered unresectable can now benefit from the removal of metastatic lesions through conversion treatment. For example, in the VOLFI trial included in present study, some patients who were initially assigned to cohort 1 (where tumor resection was considered impossible) still achieved resection after receiving anti-EGFR targeted agents in combination with chemotherapy [13]. Moreover, even if the patient has a wider range of metastatic lesions beyond the liver, there is still a chance of achieving R0 resection after receiving conversion treatment [29]. This meta-analysis examines the impact of additional anti-EGFR targeted agents on the rate of conversion resection in patients with potentially resectable mCRC and analyzed the efficacy and safety of this treatment regime.
The pooled analysis results of present study showed that additional anti-EGFR targeted agents could improve ORR and R0 resection rates in patients with potentially resectable mCRC, which is consistent with the results of many previous studies [10, 13, 19]. A higher ORR may imply a higher rate of conversion resection [30]. From a genetic perspective, the addition of anti-EGFR targeted agents might improve the ORR and R0 resection rates of RAS/KRAS wild-type patients, but KRAS mutant patients do not received this benefit. Here, attention needs to be paid to the correlation between targeted therapy and genes. Innocenti et al. noted that low tumor mutation burden and RAS and BRAF mutations were negative prognostic factors in the first-line treatment for patients with mCRC [31]. Mutations in KRAS resulted in sustained activation of the protein it encoded, so that even if upstream EGFR overexpression is blocked, downstream events cannot be regulated, and tumor growth and proliferation continue [32]. And studies have shown that during the treatment of anti-EGFR targeted agents, RAS mutant clones may exist in the initially RAS wild-type tumors, leading to resistance to anti-EGFR targeted agents [33]. The exception was tumors carrying KRASG13D mutation, where neurofibromin (NF1) ensures the normal expression of KRAS protein, while KRASG13D has weak interaction with NF1 and cannot competitively inhibit NF1. Therefore, even if KRASG13D is mutated, it can still rely on EGFR to exert its effect [32].
In addition, the present study also explored the effects of different anti-EGFR targeted agents (cetuximab vs panitumumab) on ORR and R0 resection rates. The results showed that the addition of cetuximab could benefit patients in terms of response rate and resection rate, and the results of many previous studies also support this conclusion [11, 34]. Unfortunately, similar conclusion was not observed in the pooled analysis results related to panitumumab. The research data related to panitumumab was limited, and the impact of genotype on the outcomes still needs to be considered here. In the two trials included in this study, the effect of treatment with additional panizumab on mCRC was investigated. Modert et al. studied patients with RAS wild-type mCRC [13], whereas in the PRIME trial included in present study, both KRAS wild-type and KRAS mutant patients were included in it [17, 35]. Therefore, this suggested that KRAS testing was essential for selecting suitable patients for anti-EGFR targeted therapy.
In terms of survival, the results of the pooled analysis showed that the addition of anti-EGFR targeted agents did not improve PFS and OS in patients with potentially resectable mCRC. Based on different gene types, the results of the subgroup analysis indicated that RAS/KRAS wild-type patients could achieve survival benefits after receiving anti-EGFR targeted agents combined with chemotherapy, while KRAS mutant patients could not improve their PFS or OS through additional anti-EGFR targeted agents. These results are supported by many previous studies [36,37,38]. When exploring the relationship between response rate, resection rate, and prognosis, present study found that in RAS/KRAS wild-type patients, additional anti-EGFR targeted agents improved not only the ORR and R0 resection rates of patients but also their survival rates, while KRAS mutant patients did not benefit in these aspects. This may further emphasize the importance of molecular screening in transformation therapy, while more profound screening such as BRAF also needs to be taken seriously [39, 40].
In addition, the combined chemotherapy regimen can also have an impact on the prognosis of patients, with some studies suggesting that triplet chemotherapy can improve responses rates and tumor resection rates and bring survival benefits compared to doublet chemotherapy [22, 41, 42]. Also, it has been suggested that this intensive therapy can increase the incidence of AEs [22, 43]. Recently, Ychou et al. published a comparison of the results of doublet chemotherapy or triplet chemotherapy combined with targeted therapy. The subjects of this study were generally in good condition and had a median age of 60 years. However, no significant benefit in terms of tumor resection rate and OS was observed in the triplet chemotherapy group in this study compared to the doublet chemotherapy group [44], which was consistent with the findings of Carrato et al. [45]. This makes us wonder whether the benefits of triple drug chemotherapy have been overestimated.
In terms of the safety of anti-EGFR targeted agents combined with chemotherapy, present study showed that compared to simple chemotherapy, additional anti-EGFR targeted agents increased the incidence of AEs above grade 3, mainly manifested in diarrhea, fatigue, rash, skin reactions, hemoglobin, hypermagnesemia, stomatitis, cardiac events, and infusion-related reactions. The overall safety factor was manageable. Studies have suggested that higher levels of skin toxicity are associated with improved ORR, and that skin toxicity can be considered as a surrogate indicator of the efficacy of cetuximab [10, 19, 46].
There are some published meta-analyses similar to this study, but we found that there are still many differences between these studies and present study. In terms of intervention, the study by Kong et al. did not explore the impact of panitumumab on patients with potentially resectable mCRC, and there are some differences in retrieval strategies and data processing between present study and those of Kong et al., so the results need further confirmation [47]. As far as the participants in the study are concerned, participants in Li et al., Wu et al., and Mastrantoni et al. were mCRC patients [48, 49] and did not limit participants to patients with potentially resectable mCRC. Present study limited the participants to patients with potentially resectable mCRC and conducted a systematic search of four major databases and rigorous data analysis, ultimately including only data from RCT for analysis so that more targeted research can be conducted on these patients.
There are some limitations to present study: firstly, due to a lack of data, other molecular features of the tumor [16, 34], chemotherapy regimen [7], and the location of the primary tumor [50] were not explored in present study, all of which may have influenced the results. Secondly, the follow-up times of the trials included in present study was not consistent, and we were unable to evaluate whether there was a correlation between various outcomes and time. Nevertheless, present study is the first to systematically explore the efficacy and safety of additional anti-EGFR targeted agents in patients with potentially resectable mCRC using a meta-analysis. The impact of genotype and drug on outcomes were further explored by subgroup analysis.
Conclusion
The meta-analysis showed that additional anti-EGFR targeted agents could improve ORR, R0 resection rate, and survival rate in RAS/KRAS wild-type patients with potentially resectable mCRC, with a manageable overall safety margin. Meanwhile, the results of present study demonstrate the importance of molecular screening. With the progress of research, more patients may be offered radical treatment under conversion therapy in the future.
Availability of data and materials
The original contributions presented in the study are included in the article/supplementary material, and further inquiries can be directed to the corresponding authors.
Abbreviations
- CRC:
-
Colorectal cancer
- mCRC:
-
Metastatic colorectal cancer
- Anti-EGFR:
-
Anti-epidermal growth factor receptor
- ORR:
-
Overall response rate
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- RCT:
-
Randomized controlled trial
- AEs:
-
Adverse events
- HR:
-
Hazard ratio
- CI:
-
Confidence intervals
- RR:
-
Risk ratio
- NF1:
-
Neurofibromin
References
Väyrynen V, Wirta EV, Seppälä T, Sihvo E, Mecklin JP, Vasala K, Kellokumpu I. Incidence and management of patients with colorectal cancer and synchronous and metachronous colorectal metastases: a population-based study. BJS Open. 2020;4(4):685–92.
Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(12):1208–15.
Haraldsdottir S, Wu C, Bloomston M, Goldberg RM. What is the optimal neo-adjuvant treatment for liver metastasis? Ther Adv Med Oncol. 2013;5(4):221–34.
Le DT, Kim TW, Van Cutsem E, Geva R, Jäger D, Hara H, Burge M, O’Neil B, Kavan P, Yoshino T, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol. 2020;38(1):11–9.
Viganò L, Russolillo N, Ferrero A, Langella S, Sperti E, Capussotti L. Evolution of long-term outcome of liver resection for colorectal metastases: analysis of actual 5-year survival rates over two decades. Ann Surg Oncol. 2012;19(6):2035–44.
Phelip JM, Tougeron D, Léonard D, Benhaim L, Desolneux G, Dupré A, Michel P, Penna C, Tournigand C, Louvet C, et al. Metastatic colorectal cancer (mCRC): French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR). Dig Liver Dis. 2019;51(10):1357–63.
Folprecht G, Gruenberger T, Bechstein WO, Raab H-R, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher J, Weitz J, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11(1):38–47.
Gruenberger T, Bridgewater J, Chau I, García Alfonso P, Rivoire M, Mudan S, Lasserre S, Hermann F, Waterkamp D, Adam R. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol. 2015;26(4):702–8.
Liu DM, Thakor AS, Baerlocher M, Alshammari MT, Lim H, Kos S, Kennedy AS, Wasan H. A review of conventional and drug-eluting chemoembolization in the treatment of colorectal liver metastases: principles and proof. Future Oncol. 2015;11(9):1421–8.
Ye L-C, Liu T-S, Ren L, Wei Y, Zhu D-X, Zai S-Y, Ye Q-H, Yu Y, Xu B, Qin X-Y, et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol. 2013;31(16):1931–8.
Qin S, Li J, Wang L, Xu J, Cheng Y, Bai Y, Li W, Xu N, Lin L-Z, Wu Q, et al. Efficacy and tolerability of first-line cetuximab plus leucovorin, fluorouracil, and oxaliplatin (FOLFOX-4) versus FOLFOX-4 in patients with RAS wild-type metastatic colorectal cancer: the open-label, randomized, phase III TAILOR trial. J Clin Oncol. 2018;36(30):3031–9.
Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377(9783):2103–14.
Modest DP, Martens UM, Riera-Knorrenschild J, Greeve J, Florschütz A, Wessendorf S, Ettrich T, Kanzler S, Nörenberg D, Ricke J, et al. FOLFOXIRI plus panitumumab as first-line treatment of RAS wild-type metastatic colorectal cancer: the randomized, open-label, phase II VOLFI study (AIO KRK0109). J Clin Oncol. 2019;37(35):3401–11.
Sihver W, Pietzsch J, Krause M, Baumann M, Steinbach J, Pietzsch H-J. Radiolabeled cetuximab conjugates for EGFR targeted cancer diagnostics and therapy. Pharmaceuticals (Basel). 2014;7(3):311–38.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A, Celik I, Schlichting M, Koralewski P. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22(7):1535–46.
Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25(7):1346–55.
Tveit KM, Guren T, Glimelius B, Pfeiffer P, Sorbye H, Pyrhonen S, Sigurdsson F, Kure E, Ikdahl T, Skovlund E, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol. 2012;30(15):1755–62.
Van Cutsem E, Köhne C-H, Hitre E, Zaluski J, Chang Chien C-R, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–17.
Charnley RM. Effect of specialist decision-making on treatment strategies for colorectal liver metastases (Br J Surg 2012; 99: 1263–1269). Br J Surg. 2012;99(9):1269–70.
Morris VK, Kennedy EB, Baxter NN, Benson AB, Cercek A, Cho M, Ciombor KK, Cremolini C, Davis A, Deming DA, et al. Treatment of metastatic colorectal cancer: ASCO guideline. J Clin Oncol. 2023;41(3):678–700.
Khan AS, Garcia-Aroz S, Ansari MA, Atiq SM, Senter-Zapata M, Fowler K, Doyle MB, Chapman WC. Assessment and optimization of liver volume before major hepatic resection: current guidelines and a narrative review. Int J Surg. 2018;52:74–81.
Jones RP, Vauthey JN, Adam R, Rees M, Berry D, Jackson R, Grimes N, Fenwick SW, Poston GJ, Malik HZ. Effect of specialist decision-making on treatment strategies for colorectal liver metastases. Br J Surg. 2012;99(9):1263–9.
Nygaard MS, Jul MS, Debrabant B, Madsen GI, Qvist N, Ellebæk MB. Remote ischemic postconditioning has a detrimental effect and remote ischemic preconditioning seems to have no effect on small intestinal anastomotic strength. Scand J Gastroenterol. 2022;57(7):768–74.
Bliss LA, Strong EA, Gamblin TC. Surgical resectability of multisite metastatic colorectal cancer: pushing the limits while appropriately selecting patients. J Surg Oncol. 2019;119(5):623–8.
Ciardiello D, Vitiello PP, Cardone C, Martini G, Troiani T, Martinelli E, Ciardiello F. Immunotherapy of colorectal cancer: challenges for therapeutic efficacy. Cancer Treat Rev. 2019;76:22–32.
Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–422.
Bridgewater JA, Pugh SA, Maishman T, Eminton Z, Mellor J, Whitehead A, Stanton L, Radford M, Corkhill A, Griffiths GO, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(3):398–411.
Köhne CH, Poston G, Folprecht G, Ciardiello F, Ronga P, Beier F, Van Cutsem E. FOLFIRI plus cetuximab in patients with liver-limited or non-liver-limited RAS wild-type metastatic colorectal cancer: a retrospective subgroup analysis of the CRYSTAL study. Eur J Surg Oncol. 2016;42(10):1540–7.
Jeyarajah DR, Doyle MBM, Espat NJ, Hansen PD, Iannitti DA, Kim J, Thambi-Pillai T, Visser BC. Role of yttrium-90 selective internal radiation therapy in the treatment of liver-dominant metastatic colorectal cancer: an evidence-based expert consensus algorithm. J Gastrointest Oncol. 2020;11(2):443–60.
Innocenti F, Ou F-S, Qu X, Zemla TJ, Niedzwiecki D, Tam R, Mahajan S, Goldberg RM, Bertagnolli MM, Blanke CD, et al. Mutational analysis of patients with colorectal cancer in CALGB/SWOG 80405 identifies new roles of microsatellite instability and tumor mutational burden for patient outcome. J Clin Oncol. 2019;37(14):1217–27.
McFall T, Diedrich JK, Mengistu M, Littlechild SL, Paskvan KV, Sisk-Hackworth L, et al. A systems mechanism for KRAS mutant allele-specific responses to targeted therapy. Sci Signal. 2019;12(600):eaaw8288.
Cheng R, Li F, Zhang M, Xia X, Wu J, Gao X, Zhou H, Zhang Z, Huang N, Yang X, et al. A novel protein RASON encoded by a lncRNA controls oncogenic RAS signaling in KRAS mutant cancers. Cell Res. 2023;33(1):30–45.
Van Cutsem E, Lenz H-J, Köhne C-H, Heinemann V, Tejpar S, Melezínek I, Beier F, Stroh C, Rougier P, van Krieken JH, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33(7):692–700.
Douillard J-Y, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28(31):4697–705.
De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V, Papamichael D, Laurent-Puig P, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11(8):753–62.
Fornaro L, Lonardi S, Masi G, Loupakis F, Bergamo F, Salvatore L, Cremolini C, Schirripa M, Vivaldi C, Aprile G, et al. FOLFOXIRI in combination with panitumumab as first-line treatment in quadruple wild-type (KRAS, NRAS, HRAS, BRAF) metastatic colorectal cancer patients: a phase II trial by the Gruppo Oncologico Nord Ovest (GONO). Ann Oncol. 2013;24(8):2062–7.
Douillard J-Y, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023–34.
Rowland A, Dias MM, Wiese MD, Kichenadasse G, McKinnon RA, Karapetis CS, Sorich MJ. Meta-analysis of BRAF mutation as a predictive biomarker of benefit from anti-EGFR monoclonal antibody therapy for RAS wild-type metastatic colorectal cancer. Br J Cancer. 2015;112(12):1888–94.
Pietrantonio F, Petrelli F, Coinu A, Di Bartolomeo M, Borgonovo K, Maggi C, Cabiddu M, Iacovelli R, Bossi I, Lonati V, et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer. 2015;51(5):587–94.
Assenat E, Desseigne F, Thezenas S, Viret F, Mineur L, Kramar A, Samalin E, Portales F, Bibeau F, Crapez-Lopez E, et al. Cetuximab plus FOLFIRINOX (ERBIRINOX) as first-line treatment for unresectable metastatic colorectal cancer: a phase II trial. Oncologist. 2011;16(11):1557–64.
Garufi C, Torsello A, Tumolo S, Ettorre GM, Zeuli M, Campanella C, Vennarecci G, Mottolese M, Sperduti I, Cognetti F. Cetuximab plus chronomodulated irinotecan, 5-fluorouracil, leucovorin and oxaliplatin as neoadjuvant chemotherapy in colorectal liver metastases: POCHER trial. Br J Cancer. 2010;103(10):1542–7.
Poston G, Adam R, Xu J, Byrne B, Esser R, Malik H, Wasan H, Xu J. The role of cetuximab in converting initially unresectable colorectal cancer liver metastases for resection. Eur J Surg Oncol. 2017;43(11):2001–11.
Ychou M, Rivoire M, Thezenas S, Guimbaud R, Ghiringhelli F, Mercier-Blas A, Mineur L, Francois E, Khemissa F, Chauvenet M, et al. Chemotherapy (doublet or triplet) plus targeted therapy by RAS status as conversion therapy in colorectal cancer patients with initially unresectable liver-only metastases. The UNICANCER PRODIGE-14 randomised clinical trial. Br J Cancer. 2022;126(9):1264–70.
Carrato A, Abad A, Massuti B, Grávalos C, Escudero P, Longo-Muñoz F, Manzano J-L, Gómez A, Safont MJ, Gallego J, et al. First-line panitumumab plus FOLFOX4 or FOLFIRI in colorectal cancer with multiple or unresectable liver metastases: a randomised, phase II trial (PLANET-TTD). Eur J Cancer. 2017;81:191–202.
Lièvre A, Bachet J-B, Boige V, Cayre A, Le Corre D, Buc E, Ychou M, Bouché O, Landi B, Louvet C, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26(3):374–9.
Kong Y, Hong L, Xu X. Potentially resectable mCRC-treated with cetuximab combined with chemotherapy. J Coll Physicians Surg Pak. 2020;30(11):1206–12.
Li R, Liang M, Liang X, Yang L, Su M, Lai KP. Chemotherapeutic effectiveness of combining cetuximab for metastatic colorectal cancer treatment: a system review and meta-analysis. Front Oncol. 2020;10:868.
Mastrantoni L, Beccia V, Caira G, Trovato G, Calegari MA, Basso M, Salvatore L, Pozzo C, Tortora G, Bria E, et al. Maintenance strategies after anti-EGFR-based induction in metastatic colorectal cancer: a systematic review and Bayesian network meta-analysis. Crit Rev Oncol Hematol. 2023;191:104106.
Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer. 2017;70:87–98.
Acknowledgements
Not applicable
Funding
The authors have no financial support to declare.
Author information
Authors and Affiliations
Contributions
JJZ designed the research process. YW and XYL searched the database for corresponding articles. DYW and YJH extracted useful information from the articles above. MFW and YJC used statistical software for analysis. MTZ and YTS drafted the meta-analysis. TMH and JJZ polished this article. All authors had read and approved the manuscript and ensured that this was the case.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Fig. 1.
A review of authors’ judgements about each risk of bias item for each included study.
Additional file 2: Supplementary Fig. 2.
A review of authors’ judgements about each risk of bias item presented as percentages across all included studies.
Additional file 3: Supplementary Fig. 3.
Begg’s test of the PFS for additional anti-EGFR target agents on RAS/KRAS wild-type patients (p=0.174).
Additional file 4: Supplementary Fig. 4.
Begg’s test of the PFS for additional anti-EGFR target agents on KRAS mutant patients (p=0.308).
Additional file 5: Supplementary Fig. 5.
Begg’s test of the OS for additional anti-EGFR target agents on RAS/KRAS wild-type patients (p=0.174).
Additional file 6: Supplementary Fig. 6.
Begg’s test of the OS for additional anti-EGFR target agents on KRAS mutant patients (p=0.806).
Additional file 7: Supplementary Fig. 7.
Sensitivity analysis of the PFS for additional anti-EGFR target agents on RAS/KRAS wild-type patients.
Additional file 8: Supplementary Fig. 8.
Sensitivity analysis of the PFS for additional anti-EGFR target agents on KRAS mutant patients.
Additional file 9: Supplementary Fig. 9.
Sensitivity analysis of the OS for additional anti-EGFR target agents on RAS/KRAS wild-type patients.
Additional file 10: Supplementary Fig. 10.
Sensitivity analysis of the OS for additional anti-EGFR target agents on KRAS mutant patients.
Additional file 11: Supplementary Table 1.
Literature search strategy.
Additional file 12: upplementary Table 2.
Characteristics of all the studies included in the meta-analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, Y., Li, X., Huang, T. et al. The efficacy and safety of anti-EGFR target agents in patients with potentially resectable metastatic colorectal cancer: a meta-analysis of randomized controlled trials. World J Surg Onc 21, 340 (2023). https://doi.org/10.1186/s12957-023-03222-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-023-03222-3