Abstract
Background
Textbook outcome (TO) is a multidimensional measure used to assess the quality of surgical practice. It is a reflection of an “ideal” surgical result, based on a series of benchmarks or established reference points that may vary depending on the pathology in question. References to TO in the literature are scarce, and the few reports that are available were all published very recently. In the case of gastric surgery, there is no established consensus on the parameters that should be included in TO, a circumstance that prevents comparison between series.
Aim
To present a review of the literature on TO in gastric surgery (TOGS) and to try to establish a consensus on its definition.
Material and methods
Following the PRISMA guide, we performed an unlimited search for articles on TOGS in the MEDLINE (PubMed), EMBASE and Cochrane, Latindex, Scielo, and Koreamed databases, without language restriction, updated on December 31, 2022. The inclusion criterion was any type of study assessing TO in adult patients after oncological gastric surgery. Selected studies were assessed, and TOGS was measured. The parameters used to assess the achievement of TOGS in selected studies were also recorded.
Results
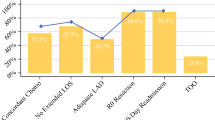
Twelve articles were included, comprising a total of 44,581 patients who had undergone an oncological gastric resection. The median rate of TOGS was 38.6%. All the publications but one included mortality as a TO variable, showing statistically significant differences in favor of the group in which TOGS was achieved. All articles included the number of nodes examined in the surgical specimen, with the assessment of fewer than 15 being associated with a low rate of TOGS achievement in five studies (41.7%). The variable postoperative complications according to the Clavien-Dindo score was the most important cause of failure to achieve TOGS in four studies (33.3%). Seven articles (58.3%) found a significant increase in long-term survival in patients who obtained TO. Advanced age, elevated ASA, and Charlson score had a negative impact on obtaining TOGS.
Conclusions
The standardization of TOGS is necessary to be able to establish comparable results between groups.
Similar content being viewed by others
Introduction
Textbook outcome (TO) is an indicator that combines a series of perioperative parameters that together define patients with an ideal postoperative course, and shows the percentage of patients in which this result is achieved [1]. The concept of TO was defined for the first time by Kolfschoten et al. in the field of colorectal cancer surgery and was based on six parameters: hospital stay, mortality, reoperation rate, readmission rate, R0, and surgery without stoma. Since then, several articles have been published on TO in various cancers requiring surgery, using definitions that have sometimes included variables other than the ones just listed [1,2,3,4,5].
The results published are quite heterogeneous because the parameters included to assess TO have varied according to the pathology; sometimes, the parameters used have varied even inside the same pathology, as in the case of gastric carcinoma, for example. Consequently, although TO is a very useful tool for assessing the quality of surgical treatment, the variation in the parameters included makes it hard to obtain valid results and conclusions [6, 7].
Esophagogastric surgery has particularly high morbidity and mortality rates. The first definition of TO in esophagogastric surgery was published by Busweiler in 2017 [1]. Without any doubt, it is one of the most demanding: it includes 10 variables, and the rates of TO obtained are much lower than those recorded in other cancers. Since Busweiler et al.’s study, the definitions of TO in gastric cancer surgery have consistently included variables such as R0 resection and the number of nodes analyzed (15 or more) [2]. However, there are variations in the definition of severe complications, hospital stay, and the period of time (i.e., 30 or 90 days) considered to assess postoperative mortality. In addition, the results of TO for all types of esophagogastric cancer surgery have frequently been presented together, in spite of the fact that they are procedures with different morbidity and mortality rates. Further, in some studies, the main objective is the comparison of high and low-volume hospitals (3,10), but in others, the focus is on the assessment of long-term survival associated with TO (1,12) [3]. Finally, after esophageal and gastric cancer surgery, a direct correlation was observed between the achievement of TO and survival [4].
Thus, TO is regarded as a high-quality indicator for measuring the results of gastric surgery (GS) and as an indicator of long-term survival. We therefore carried out a systematic review of the published literature on TO in gastric cancer surgery (TOGS) with the aim of proposing a set of common variables that make it possible to compare the results of different centers.
Methods
Adhering to PRISMA guidelines, we performed an unlimited search for articles on TOGS in the MEDLINE (PubMed), EMBASE and Cochrane database, Latindex, Scielo, and Koreamed databases, with no language restriction, updated on December 31, 2022. The search items were ((Textbook outcomes) or (Textbook outcome)) and ((Gastric) or (Stomach)) and ((Surgery)).
The sole inclusion criterion was any type of article that included adult patients in whom TO had been measured after any type of surgery for gastric cancer. Exclusion criteria were studies that combined different types of surgery (gastric and esophageal) without presenting the data for gastric surgery separately, benign gastric surgery, series of pediatric patients, duplicated series, surveys, and editorials.
The following data from the selected studies were included, if available: the author of the study, year of publication, type of study, the Ottawa-Newcastle scale score [5], number of patients included, disease, procedure (type of gastrectomy), percentage of TO, factors associated with achieving TO, the parameter least frequently achieved in obtaining TO, variation between hospitals included in multicenter studies, the relationship between TO and survival, and others (Table 1). The parameters used to define the achievement of TOGS in the selected studies were also recorded (Table 2).
The articles were included or rejected based on the pre-defined criteria and on the information obtained from the title and summary matched by three authors (SC, JMR, CV). Searches for duplicate series were performed, and in case of doubt, the article was read in full. The references of the selected articles were also checked, though no additional articles not included in the initial search were found.
The quality of the studies was assessed using the Newcastle–Ottawa scale (Table 3) [5]. Scores of 0–2 were considered poor quality, 3–5 fair, and 6–9 good or high. None of the studies were RCTs.
This systematic review has been registered in the Research Registry (reviewregistry1695).
Results
The search yielded 31 articles. Nineteen of the articles were excluded, for the following reasons: 11 were not on TO, two were invited comments on articles about TO, two evaluated esophageal TO exclusively, one included only neuroendocrine gastric tumors, one evaluated TO after bariatric surgery, and another unspecified oncological TO. Figure 1 shows the PRISMA diagram (Fig. 1).
Ten studies were considered to be good quality according to the Ottawa-Newcastle scale and two were fair (Table 3). Due to the heterogeneity in the design of the studies and the variables used, we were unable to carry out a meta-analysis of the data.
Finally, the study focused on 12 articles including 44,581 patients who had undergone resection for gastric cancer and in whom TO had been measured. Six of the studies were multicenter, and these provided the largest number of cases (N = 41,606, 93.3% of the total), five were single-center (N = 881, 8.9%), and one was conducted at two centers (N = 258, 2.6%). In four studies, the results came from population databases (N = 39,589, 88.8%) [6].
The median number of patients who attained TOGS was 38.6% IQR (34.3–49.7). The rates of TOGS achieved ranged widely (22.7–75.7%) (1,17), but were between 34 and 45% in eight of the studies. Only two studies (16.7%) surpassed the threshold of 50% TO (18,19)—one single-center study in Korea and another in Spain. The median TOGS obtained in population studies (92.5% of all patients) was 27.9% (11,13,16,20) (Table 1).
Parameters included in the assessment of TO
All the publications except one included mortality as a TO variable (16). The exception also provided the largest number of cases, although it reported mortality in the series at 30 days (1.4% TOGS group versus 4.7% non-TOGS group) and 90 days (2.3% TOGS versus 9.4% non-TOGS) and presented statistically significant differences in favor of the TOGS group [6]. Of the rest of the studies, three (25%) did not specify whether mortality was assessed at 30 or 90 days (1,20,21), six (50%) measured it at 30 postoperative days, and two (16.6%) at 90 days (3,9,12). The readmission rate was measured in ten articles (83.3%), although there was no consensus regarding the number of days required for its measurement: five (41.7%) assessed it at 30 days, one at 90 days, and in five (41.7%), the time point was not specified (2,3,10–12,14,17,18,20,21,23,24). Readmission was included as a TOGS variable in all but one study [7].
All the studies included the number of lymph nodes examined in the surgical specimen, and all but one (91.7%) applied a cutoff point ≥ 15; the exception established the cutoff point at > 16 nodes [7]. Complete resection was included in 66.7% of the studies, and R0 resection was included in all the articles reviewed (2,3,10–12,14,17,18,20,21,23,24).
Eight studies (66.7%) included the variables postoperative complications, readmission to the intensive care unit (ICU), and hospital stay in the definition of TOGS. Specifically, non-reoperation was included in nine (75%)—in three of these studies, this parameter was assessed at 30 days, and in six, the time point was not specified. Complications were measured according to the Clavien-Dindo (CD) scale in eight articles (66.7%) (3,10–12,14,17,23,24) using a CD score of ≥ II as a cutoff point to rule out TOGS. Two articles (16.6%) mentioned and included postoperative complications, but did not specify the classification used (1,20). In addition to the CD classification, one article used a Comprehensive Complication Index (CCI) score of ≥ 30 as a measure of complications [11].
Non-re-admission to the ICU was included for the evaluation of TOGS in eight (66.6%) of the studies. Length stay was included in ten studies, eight of which used the 75th percentile of the stay (≤ 21 days), one a stay of ≤ 14 days [3], and the other a stay of < 12 days [6]. Finally, the intraoperative complication variable was included in seven studies (58.3%). The rest of the variables that make up the TO are described in Table 2.
Variables associated with obtaining TOGS
The parameters most frequently associated with the failure to achieve TOGS were fewer than 15 lymph nodes examined in the surgical specimen (five studies, 41.7%) and severe complications ≥ CD II (four studies, 33.3%). In three studies (25%), the variables that most influenced the non-achievement of TO were not specified.
In seven articles (58.3%), a significant increase in long-term survival was found in patients who obtained TO. Table 1 shows the rest of the parameters included in the review. Advanced age had a negative impact on obtaining TO (2,22). ASA < 3, Charlson < 2, and CCI = 0 were other factors significantly associated with obtaining TO (3,22).
In four articles, neoadjuvant treatment was positively associated with obtaining TO (1,2,8,16). Two were European, and the other two were from the USA. One of the US articles included neoadjuvant therapy as a TOGS parameter using data from a national database [6].
Other factors positively associated with obtaining TOGS were BMI 24–29.9, weight loss < 5% pre-surgery, preoperative hemoglobin ≥ 10 g/dL, location of the tumor in the antrum, laparoscopic surgery, and non-performance of multivisceral resection.
Finally, other factors associated with lower rates of achieving TOGS were tumors located in the esophagogastric junction and diffuse-type histology. No study found statistically significant differences with regard to gender (1,3,10,11,14,17,20,23).
Discussion
TO is a tool that comprises a range of variables to assess the quality of care, and its use is currently increasing (1). This systematic review of TOGS yielded a median rate of 38.6%. Ten of the studies (83.3%) analyzed did not achieve a TOGS rate of 50%. Among the studies that surpassed this rate, the results of the only Asian series (a single-center Korean study) stand out, with a TOGS rate of 75.7% in 395 patients (18). The next highest rate was achieved in another single-center study in Spain, with a rate of 51.04% in 96 patients [8]. Multicenter studies and those that obtained data from national databases obtained even lower figures for TOGS (2,9,16). These data reopen the discussion on whether the results obtained in Asia and Western countries are comparable and highlight the drawbacks of using large population databases in which much data may be lost. The fact that other surgeries of similar complexity, such as pancreatic, liver, or colorectal surgery, obtain values close to 60% suggests that TO may be more difficult to achieve in the case of gastric cancer surgery (24,25). Probably, the inclusion of a higher number of parameters is one of the reasons for the lower rate of TO in this setting.
Although there is no internationally accepted definition of TOGS, the first article on TO in gastric surgery used ten parameters [1], a considerably higher number than the six initially described by Kolfschoten et al. for TO in colon cancer [15]. The ten variables originally described by Busweiler et al. for the definition of TOGS were complete resection, pathological R0, and > 15 lymph nodes in the surgical specimen, no intraoperative complications, no reintervention, no ICU readmission, no prolonged hospital stay (defined as the 75th percentile of stay, 21 days if applicable), no mortality or readmission at 30 days, and no severe complications, defined as CD ≥ II [1].
None of the studies published since Busweiler et al.’s initial report have included these ten parameters: the only variables included by all the studies in the review are mortality, number of nodes > 15, hospital stay, and obtaining R0. The concordance between the rest of the parameters was 91.7% for non-readmission at 30 days, 75% for CD ≥ II complications and non-reoperation, and 66.7% for complete resection and non-readmission to the ICU. Only one article included neoadjuvant therapy as an additional parameter [6].
Examination of 15 or more lymph nodes in the surgical specimen and severe complications CD ≥ II had the greatest specific weight for reducing the rate of TOGS (41.7% and 33.3%, respectively). The influence of these two variables on the TOGS rate, together with the fact that they were included in 100% and 75% of the series, respectively, support their inclusion in a consensus TOGS. Obtaining ≥ 15 lymph nodes is associated with a good surgical technique and a high level of engagement on the part of the pathologists. The type of lymphadenectomy (D2), the preparation of the specimen by the surgeon after resection and the use of indocyanine green are factors that can help to obtain this high number of nodes and thus improve TO levels [16]. As regards the inclusion of CD grade ≥ II complications, perhaps the use of only severe complications > CD IIIa would receive more widespread support, as complications of this grade are considered by many authors to be major [17]. The inclusion of parameters such as reoperation (a CD grade IIIb complication) and ICU readmission might be unnecessary if the CD classification is used: the reason for readmission to the ICU is almost always reoperation (CD IIIb) or failure of one or more organs (CD IVa-b), which are major complications in the CD classification and automatically rule out TOGS. Lastly, the R0 variable already includes the concept of complete resection, so we suggest that the latter variable may be superfluous in the TOGS assessment.
Hospital length stay was analyzed in 91.7% of the series included in our study and is another of the parameters that most influenced the achievement of TO [1]. In 72.7% of the studies, hospital stay was considered prolonged when it was greater than the 75th percentile, ranging from 12 to 21 days. However, the result of this quality indicator is highly dependent on both the implementation of ERAS protocols and the appearance of major complications and so the inclusion of this parameter in the TOGS has been questioned (29–31). The 30-day readmission rate was included in 91.7% of the studies reviewed, although there is a direct relationship between early discharge and readmission [18]; therefore, the measurement of these variables at 90 days, as reported by some authors, would improve the measurement of TOGS, since it is considered that around a third of patients are readmitted at a point later than 30 days post-surgery [19].
Gastrectomy for cancer is a major surgery with a significant morbidity rate that ranges from 4 to 45% according to series (32,34), although there is no accepted standard definition of severe postoperative complications. A growing number of studies show that the decrease in morbidity and mortality seems to be associated with the volume of gastrectomies performed annually (35–39).
Postoperative mortality after surgery for gastric cancer continues to be high, but it varies significantly between series (2–10%) (2,32,40). Although postoperative mortality was analyzed in all the series, it was only measured at 90 days in three studies (2,16,22). It is important that mortality be evaluated 90 days postoperatively, since there are notable differences between the rates at 30 or 90 days (32,41,42).
The series included in our study refer to different parameters that influenced the achievement of TOGS, such as age, ASA classification and Charlson index, neoadjuvant therapy, tumor location, histological type, BMI, preoperative hemoglobin, type of approach, and association with multivisceral resections. Some of these variables require further analysis and could be independent factors associated with the achievement of TOGS.
Although the association between advanced age and postoperative morbidity and mortality after gastric surgery is not well defined, it has been reported in a growing number of studies (43–47). In 36.3% of the studies included here, a direct correlation was found between age and the possibility of obtaining TO, although no definite cutoff point was established in the series included (2,18,24). A report by the Dutch Upper Gastrointestinal Cancer Audit (DUCA) nationwide registry showed a trend towards significance for an association between age 70 and older and postoperative 30-day or in-hospital mortality (OR 1.56; 95% CI 0.99 to 2.46) (22). Some studies have reported a statistically significant increase in postoperative mortality from age 75 onwards [20]. A Japanese study of 327,642 patients undergoing major abdominal surgery (including gastric surgery) concluded that mortality increased with age in all procedures and that respiratory complications such as pneumonia were a key factor in mortality in this subgroup of elderly patients (> 80 years) (40,46,47). In the light of the above, we consider that TOGS should be adjusted according to the age of the patient. The ASA classification and the Charlson index are risk factors for morbidity and mortality that are usually correlated with age. ASA grade < 3 and Charlson index < 2 were significantly associated with obtaining TOGS in two articles. However, the validity of these parameters has been questioned because they may be affected by interobserver variability (50,51).
The administration of neoadjuvant chemotherapy, and specifically the perioperative FLOT scheme (fluoracil, oxaliplatin, leucovorin, docetaxel), has shown its beneficial role in terms of survival in cases of locally advanced gastric cancer and with positive lymph nodes, but it is routinely administered only in European countries [12]. Therefore, it is rarely included in studies of TOGS (2,18,24). On the other hand, the administration of neoadjuvant chemotherapy was associated with greater morbidity and mortality, although this assessment has been carried out with the MAGIC scheme, which is less toxic (46,52). One might speculate that patients receiving neoadjuvant therapy are a selected group of patients who have advanced tumors but are less frail and have a good status performance (40,46). For all these reasons, neoadjuvant treatment should be included in the assessment of TOGS.
As regards the surgical approach, the meta-analyses carried out do not report differences in survival, morbidity, and oncological results between laparoscopic and open gastrectomy, although the laparoscopic approach presents advantages in terms of earlier diet food intake, less surgical site infection, and shorter hospital stay [21]. Perhaps this is why in our study the series with the highest proportion of laparoscopic surgeries are the ones that obtain the highest TO rates (2,18,21).
In our review, the location of the tumor in the esophagogastric junction and diffuse histology were associated with a lower probability of obtaining TO. Perhaps the fact that this tumor location requires a total gastrectomy increases morbidity and mortality, although this supposition is not borne out by the literature; several studies comparing total and subtotal gastrectomy have found no differences in terms of mortality, blood loss, or hospital stay (53–55). In contrast, there seems to be a correlation between the number of lymph nodes in the surgical specimen and intra-abdominal collections in the postoperative period, which is higher in total gastrectomy (53,56). Although diffuse histology is associated with a worse prognosis, it tends to occur in younger patients who usually have a higher probability of TO [22]. The weight of these two variables should be studied in greater detail in future studies of TO.
Other variables positively associated with achieving TO in some of the series studied were BMI 24–29.9, weight loss < 5% pre-surgery, preoperative hemoglobin ≥ 10 gr/dL, and no multivisceral resection [12, 13]. Gender was not associated with the achievement of TO in any of the studies reviewed (1–3,10–12, 14,17,18,20,23,24).
Achieving TO was independently associated with a statistically significant increase in survival. Six of our studies (54.5%) found a survival benefit when TO was reached, with a median survival in the TO group almost 20 months higher than in the non-TO group.
The main limitation of our study was the heterogeneity of parameters used for the evaluation of TOGS in the articles included. This meant that comparison between them is almost impossible, and we were unable to perform a meta-analysis.
In conclusion, we believe that TOGS needs to be standardized in order to be able to carry out comparisons between groups. We propose the following six parameters for creating a consensus definition of TOGS: > 15 lymph nodes in the surgical specimen, R0 resection, absence of major complications (CD > IIIa) measured at 90 days, length of stay (75th percentile), 90-day mortality, and 90-day readmission. However, the analysis of other parameters such as age or the diversity of preoperative treatment, which in some countries includes neoadjuvant therapy, suggests that this basic definition of TOGS should be adjusted to incorporate these variables.
Availability of data and materials
Non-applicable, since this is a bibliographical review.
Abbreviations
- TO:
-
Textbook outcome
- GS:
-
Gastric surgery
- TOGS:
-
Textbook outcome gastric surgery
- CD:
-
Clavien-Dindo
- ICU:
-
Intensive care unit
- ASA:
-
American Society of Anesthesiologist
- CCI:
-
Comprehensive Complication Index
- US:
-
United States
- DUCA:
-
Dutch Upper Gastrointestinal Cancer Audit
References
Busweiler LAD, Schouwenburg MG, van Berge Henegouwen MI, Kolfschoten NE, de Jong PC, Rozema T, et al. Textbook outcome as a composite measure in oesophagogastric cancer surgery. Br J Surg. 2017;104(6):742–50.
Mehta R, Tsilimigras DI, Paredes AZ, Sahara K, Moro A, Farooq A, et al. Comparing textbook outcomes among patients undergoing surgery for cancer at U. S. News & World Report ranked hospitals. J Surg Oncol. 2020;121(6):927–35.
Carbonell Morote S, Gracia Alegría E, Ruiz de la Cuesta Tapia E, Llopis Torremocha C, Ortiz Sebastián S, Estrada Caballero JL, et al. Textbook outcome en cirugía gástrica oncológica, ¿qué implicaciones tiene sobre la supervivencia? Cirugía Española. 2021. Available from: https://www.elsevier.es/es-revista-cirugia-espanola-36-avance-resumen-textbook-outcome-cirugia-gastrica-oncologica-S0009739X2100302X. Cited 2022 Mar 31.
van der Kaaij RT, de Rooij MV, van Coevorden F, Voncken FEM, Snaebjornsson P, Boot H, et al. Using textbook outcome as a measure of quality of care in oesophagogastric cancer surgery. Br J Surg. 2018;105(5):561–9.
Lo CKL, Mertz D, Loeb M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14(1):1–5.
Cibulas MA, Avila A, Mahendra AM, Samuels SK, Gannon CJ, Llaguna OH. Impact of textbook oncologic outcome attainment on survival after gastrectomy: a review of the National Cancer Database. Ann Surg Oncol. 2022;29(13):8239–48. Available from: https://pubmed.ncbi.nlm.nih.gov/35974232/. Cited 2022 Dec 8.
Spolverato G, Paro A, Capelli G, Dalmacy D, George MS, Ryan AP, et al. Surgical treatment of gastric adenocarcinoma: are we achieving textbook oncologic outcomes for our patients? J Surg Oncol. 2022;125(4):621–30. https://doi.org/10.1002/jso.26778.
Priego P, Cuadrado M, Ballestero A, Galindo J, Lobo E. Comparison of laparoscopic versus open gastrectomy for treatment of gastric cancer: analysis of a textbook outcome. J Laparoendosc Adv Surg Tech. 2019;29(4):458–64. Available from: https://www.liebertpub.com/doi/abs/10.1089/lap.2018.0489. Cited 2021 Jun 17.
van der Werf LR , Wijnhoven BPL, Fransen LFC, van Sandick JW , Nieuwenhuijzen GAP, Busweiler LAD, et al. A national cohort study evaluating the association between short-term outcomes and long-term survival after esophageal and gastric cancer surgery. Ann Surg. 2019;270(5):868–76. https://doi.org/10.1097/SLA.0000000000003520.
Dal Cero M, Román M, Grande L, Yarnoz C, Estremiana F, Gantxegi A, et al. Textbook outcome and survival after gastric cancer resection with curative intent: a population-based analysis. Eur J Surg Oncol. 2022;48(4):768–75. https://doi.org/10.1016/j.ejso.2021.10.025.
Sędłak K, Rawicz-Pruszyński K, Mlak R, Gęca K, Skórzewska M, Pelc Z, et al. Union is strength: textbook outcome with perioperative chemotherapy compliance decreases the risk of death in advanced gastric cancer patients. Eur J Surg Oncol. 2022;48(2):356–61.
Bolger JC, Al Azzawi M, Whooley J, Bolger EM, Trench L, Allen J, et al. Surgery by a minimally invasive approach is associated with improved textbook outcomes in oesophageal and gastric cancer. Eur J Surg Oncol. 2021;47(9):2332–9. Available from: https://doi.org/10.1016/j.ejso.2021.03.240.
Roh CK, Lee S, Son SY, Hur H, Han SU. Textbook outcome and survival of robotic versus laparoscopic total gastrectomy for gastric cancer : a propensity score matched cohort study. Sci Rep. 2021;0123456789:1–11. Available from: https://doi.org/10.1038/s41598-021-95017-3.
Levy J, Gupta V, Amirazodi E, Allen-Ayodabo C, Jivraj N, Jeong Y, et al. Textbook outcome and survival in patients with gastric cancer: an analysis of the population registry of esophageal and stomach tumours in Ontario (PRESTO). Ann Surg. 2022;275(1):140–8. https://doi.org/10.1097/SLA.0000000000003849.
Kolfschoten NE, Kievit J, Gooiker GA, Van Leersum NJ, Snijders HS, Eddes EH, et al. Focusing on desired outcomes of care after colon cancer resections; hospital variations in “textbook outcome.” Eur J Surg Oncol. 2013;39(2):156–63. Available from: https://doi.org/10.1016/j.ejso.2012.10.007.
Baiocchi GL, Molfino S, Molteni B, Quarti L, Arcangeli G, Manenti S, et al. Fluorescence-guided lymphadenectomy in gastric cancer: a prospective western series. Updates Surg. 2020;72(3):761–72. Available from: https://doi.org/10.1007/s13304-020-00836-0.
Clavien PA, Barkun J, De Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The clavien-dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96. Available from: https://pubmed.ncbi.nlm.nih.gov/19638912/. Cited 2021 Jun 18.
Li SS, Costantino CL, Mullen JT. Morbidity and mortality of total gastrectomy: a comprehensive analysis of 90-day outcomes. J Gastrointest Surg. 2019;23(7):1340–8. Available from: https://pubmed.ncbi.nlm.nih.gov/31062268/. Cited 2022 Dec 8.
Kim Y, Gani F, Lucas DJ, Ejaz A, Spolverato G, Canner JK, et al. Early versus late readmission after surgery among patients with employer-provided health insurance. Ann Surg. 2015;262(3):502–9. Available from: https://pubmed.ncbi.nlm.nih.gov/26258319/. Cited 2023 Jan 9.
Lacueva FJ, Escrig-Sos J, Marti-Obiol R, Zaragoza C, Mingol F, Oviedo M, et al. Short-term postoperative outcomes of gastric adenocarcinoma patients treated with curative intent in low-volume centers. World J Surg Oncol. 2022;20(1):344. Available from: https://pubmed.ncbi.nlm.nih.gov/36253780/. Cited 2022 Dec 8.
Cai J, Wei D, Gao CF, Zhang CS, Zhang H, Zhao T. A prospective randomized study comparing open versus laparoscopy-assisted D2 radical gastrectomy in advanced gastric cancer. Dig Surg. 2012;28(5–6):331–7.
Petrelli F, Berenato R, Turati L, Mennitto A, Steccanella F, Caporale M, et al. Prognostic value of diffuse versus intestinal histotype in patients with gastric cancer: a systematic review and meta-analysis. J Gastrointest Oncol. 2017;8(1):148–63. Available from: https://pubmed.ncbi.nlm.nih.gov/28280619/. Cited 2022 Dec 8.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
Silvia Carbonell-Morote: writing, review and statistic analysis. Han-Kwang Yang: review. Javier Lacueva: writing, review and statistic analysis. Juan Jesús Rubio-García: statistic analysis. Lucia Alacan-Friedrich: research date. Lea Fierley: research dat. Celia Villodre: writing, review and statistic analysis. Jose M Ramia: writing, review and statistic analysi.
Corresponding author
Ethics declarations
Ethics approval and concent to participate
Our manuscript is a bibliographical review. Our ethical committee waived the need for ethical committee approval.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Carbonell-Morote, S., Yang, HK., Lacueva, J. et al. Textbook outcome in oncological gastric surgery: a systematic review and call for an international consensus. World J Surg Onc 21, 288 (2023). https://doi.org/10.1186/s12957-023-03166-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-023-03166-8