Abstract
Background
Pulmonary sarcomatoid carcinoma (PSC) is a rare and unconventional non-small-cell lung cancer (NSCLC) that appears to be aggressive, with a poor prognosis and response to conventional treatment. Approximately 30% of PSCs have potentially targetable genomic alterations, but few studies have involved RET gene fusions, and corresponding targeted therapies are lacking.
Case presentation
In this report, we describe a patient with PSC harboring a KIF5B-RET gene fusion who was initially diagnosed with stage IVb lung cancer. Due to the poor performance status, the patient was unable to tolerate any radiotherapy or chemotherapy. Based on the next-generation sequencing (NGS) result of RET gene fusion, the patient was treated with pralsetinib. Two months after the treatment, the patient achieved a partial response.
Conclusions
Our case indicates that RET is one of the main driver oncogenes of PSC and provides useful information for precise RET inhibitor administration in the future. Thus, the use of comprehensive genomic profiling may provide important treatment options for PSC.
Similar content being viewed by others
Introduction
Pulmonary sarcomatoid carcinoma (PSC) is a rare and highly invasive tumor with an extremely low incidence of less than 1% in all lung cancers [1,2,3]. In accordance with the 2021 World Health Organization (WHO) classification of lung tumors, PSC, a poorly differentiated non-small-cell lung cancer (NSCLC), can be divided into five subtypes: pleomorphic carcinoma, spindle cell carcinoma, giant cell carcinoma, carcinosarcoma, and pulmonary blastoma [4]. Compared with other NSCLC subtypes, patients with PSC have a more aggressive clinical course and a poorer prognosis with the 5-year overall survival (OS) ranging between 10 and 21% [5,6,7].
Currently available treatment options for PSC are limited due to resistance to chemotherapy, low responsiveness to radiotherapy and extremely quick recurrence after surgical resection [8,9,10,11]. Schorock et al. demonstrated that approximately 30% of PSCs are accompanied by potentially targetable genomic alterations, providing a comprehensive genomic understanding for developing targeted therapeutic strategies [12]. The most frequently mutated genes across different studies include TP53, KRAS, CDKN2A, PTEN, MET, EGFR, BRAF, and HER2 [12,13,14]. In a cohort of PSC cases, 79% (44/56) of the patients harbored mutations in TP53, and 57% of the patients harbored mutations in genes of the receptor tyrosine kinase (RTK)/RAS pathway: EGFR (16%), KRAS (14%), MET (13%), BRAF (7%), NF1 (5%), and NRAS (4%) [14]. Schorock et al. revealed a 0.8% (1/12) RET proto-oncogene amplification in PSC [12], and Liang et al. found two PSC patients (2/32) with RET fusions, KIF5B-RET and TUBD1-RET [13]. Nevertheless, detailed treatments of PSC patients with RET alterations were not provided in either of these studies. Here, we report a case of PSC with a KIF5B-RET fusion that exhibited a remarkable response to the selective RET inhibitor pralsetinib.
Case presentation
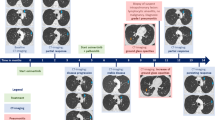
A 52-year-old nonsmoking female was admitted to our hospital due to cough and bilateral low back pain for one month. A chest computed tomography (CT) scan showed a mass in the right upper lobe (4.6 × 3.2 cm) (Fig. 1a) and multiple enlarged lymph nodes in the mediastinum 4R (Fig. 1b), mediastinum and bilateral axillary; a small nodule (1.0 × 0.6 cm) was seen in the left upper lobe; and there was a pathological fracture of the 12th thoracic (T12) vertebral body. Cranial magnetic resonance imaging (MRI) showed multiple intracranial space-occupying lesions (Fig. 1c), considering lung cancer with multiple metastases to the brain, bone and lymph nodes (clinical disease stage: IVb, cT2N2M1c). After the statement of informed consent, CT-guided percutaneous needle biopsy of the lung mass was performed. Microscopically, the tumor cells were mostly poorly differentiated with an almost spindle cell-like morphology (Fig. 2a). On immunohistochemistry, tumor cells were positive for TTF-1 (Fig. 2b) and vimentin, weakly positive for PCK (Fig. 2c) and EMA (Fig. 2d), and negative for P40 (Fig. 2e), SMA, S100 and desmin. Eventually, sarcomatoid carcinoma (spindle cell carcinoma) was diagnosed. The tumor proportion score (TPS) of programmed cell death ligand 1 (PD-L1) expression was 60% (Fig. 2f). DNA-based next-generation sequencing (NGS) revealed the presence of the KIF5B (15)-RET (12) fusion (3.05% abundance in tissue) (Fig. 3a), which was verified by ARMS RT–PCR assay (Amoy Diagnostics, Xiamen, China) (Fig. 3b). Due to the poor performance status and severe intestinal obstructive symptoms, the patient was unable to tolerate any radiotherapy or chemotherapy. On the basis of her RET fusion status, RET tyrosine kinase inhibitor treatment with pralsetinib, 125 mg three times daily, commenced on January 24th, 2022. Two months after initiation of the treatment, the examinations showed an excellent partial response, including the significant reduction of the mass (2.8 × 1.2 cm) in the right upper lobe (Fig. 1d), the marked decrease in the size of lymph nodes in the mediastinum 4R (Fig. 1e) and the shrink of the metastatic lesion in the right parieto-occipital region (Fig. 1f). And the clinical symptoms were relieved. The patient remains under follow-up.
The image changes before and after treatment. The CT scan before treatment showed the mass in the right upper lobe sized 4.6 × 3.2 cm (a), mediastinal 4R enlarged lymph nodes (b). Contrast enhanced MRI of brain before treatment revealed multiple intracranial space-occupying lesions (c). The CT examination after treatment with pralsetinib displayed the mass sized 2.8 × 1.2 cm in the right upper lobe (d), marked reduction in the size of lymph node in mediastinum 4R (e). Brain MRI after treatment demonstrated the shrink of the right parieto-occipital region (f). Red arrows indicate the tumor or lymph node lesions
Microscopic images of the PSC. a The tumor demonstrated a spindle cell-like morphology with poor differentiation (× 200). b The tumor cells showed a positive nuclear signal for TTF-1 (× 200). c The tumor cells showed a weakly positive cytoplasmic signal for PCK (× 200). d The tumor cells showed a weakly positive cytoplasmic signal for EMA (× 200). e The tumor cells showed a negative cytoplasmic signal for P40 (× 200). f PD-L1 TPS immunohistochemistry analysis showed at least 60% (× 200)
Discussion
PSC is a unique subtype of NSCLC with an exceptionally poor prognosis and resistance to traditional chemotherapy. However, oncogenic mutations, fusions, and copy number alterations of driver oncogenes specified in the NCCN NSCLC guidelines have been identified in 30% of cases. In recent years, a higher frequency of MET exon 14 splicing site mutations has been reported in PSC, with a prevalence ranging from 4.9% to 31.8%, compared to 2.62% in all NSCLC. With limited reports, the frequency of MET amplification in PSC ranges from 4.8 to 13.6%, while the MET protein overexpression rate in PSC ranges from 17 to 40.9% [15,16,17,18,19]. Lu et al. demonstrated that the objective response rate (ORR) of savolitinib in PSCs harboring MET exon 14 splicing site mutations was 40.0% (10/25) [20]. In addition, Ann Valter et al. reported a case of PSC harboring the ALK-EML4 fusion gene that displayed a good response to crizotinib [21]. Moreover, in the report by Zou et al., a PSC patient with an EGFR exon 21 L858R gene mutation was successfully treated with erlotinib after failing chemoradiotherapy and remained progression-free for 6 months [22].
To date, RET rearrangement has been identified in approximately 1–2% of NSCLC patients, involving the most common RET fusions: KIF5B-RET (70–90%) and CCDC6-RET (10–25%), followed by NCOA4-RET, TRIM33-RET, ZNF477P-RET, ERCC1-RET, HTR4-RET, and CLIP1-RET (18%) [23,24,25,26]. Specifically in PSC, RET amplification was reported by Schorock et al. [12], and KIF5B-RET along with TUBD1-RET fusion was identified by Liang et al. [13]. Even so, neither study provided treatment details for PSC patients with RET alterations. Currently, drugs such as selpercatinib and pralsetinib are FDA-approved RET kinase inhibitors for the treatment of NSCLC. The clinically important effects on the overall response rate (ORR) of selpercatinib were observed in a multicenter, open-label, multicohort clinical trial (LIBRETTO-001, NCT03157128) in patients whose tumors had RET alterations. ORRs within RET fusion–positive NSCLC patients were 64% in prior platinum-treated patients and 85% in treatment-naive patients [27]. In addition, pralsetinib has recently been reported to be a new, well-tolerated, promising treatment option for RET fusion-positive NSCLC patients, with an ORR ranging from 61% (prior platinum-treated patients) to 70% (treatment-naive patients) (ARROW, NCT03037385) [28]. Nevertheless, neither of these studies explicitly stated that PSC was involved.
To our knowledge, this is the first case report describing a clinical response to pralsetinib in a patient with PSC harboring a KIF5B-RET fusion, which demonstrates that RET is one of the main driver oncogenes of PSC and is sensitive to matched targeted therapy. Furthermore, comprehensive genomic profiling may provide important treatment options for a historically poorly characterized and difficult-to-treat disease.
Availability of data and materials
Data sharing does not apply to this article as no datasets were generated to analyzed during the current study.
Abbreviations
- PSC:
-
Pulmonary sarcomatoid carcinoma
- NSCLC:
-
Non-small-cell lung cancer
- NGS:
-
Next-generation sequencing
- WHO:
-
World Health Organization
- OS:
-
Overall survival
- CT:
-
Computed tomography
- ORR:
-
Objective response rate
References
Zheng Y, Fu Y, Zhong Q, Deng R, Zhang Y. The treatment of advanced pulmonary sarcomatoid carcinoma. Future Oncol. 2022;18(6):727–38.
Nicholson AG, Tsao MS, Beasley MB, Borczuk AC, Brambilla E, Cooper WA, et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J Thorac Oncol. 2022;17(3):362–87.
Smadhi H, Boudaya MS, Abdannadher M, BenAbdelghaffar H, Kamoun H, Ayadi A, et al. Pulmonary Sarcomatoid carcinoma: a surgical diagnosis and prognostic factors. Tunis Med. 2019;97(1):128–32.
Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10(9):1243–60.
Chen M, Yang Q, Xu Z, Luo B, Li F, Yu Y, et al. Survival Analysis and Prediction Model for Pulmonary Sarcomatoid Carcinoma Based on SEER Database. Front Oncol. 2021;11:630885.
Steuer CE, Behera M, Liu Y, Fu C, Gillespie TW, Saba NF, et al. Pulmonary sarcomatoid carcinoma: an analysis of the national cancer data base. Clin Lung Cancer. 2017;18(3):286–92.
Chen J, He Q, Liu J, Xiao Y, Xiao C, Chen K, et al. CD8+ tumor-infiltrating lymphocytes as a novel prognostic biomarker in lung sarcomatoid carcinoma, a rare subtype of lung cancer. Cancer Manag Res. 2018;10:3505–11.
Yendamuri S, Caty L, Pine M, Adem S, Bogner P, Miller A, et al. Outcomes of sarcomatoid carcinoma of the lung: a Surveillance, Epidemiology, and End Results Database analysis. Surgery. 2012;152(3):397–402.
Ung M, Rouquette I, Filleron T, Taillandy K, Brouchet L, Bennouna J, et al. Characteristics and clinical outcomes of sarcomatoid carcinoma of the lung. Clin Lung Cancer. 2016;17(5):391–7.
Vieira T, Girard N, Ung M, Monnet I, Cazes A, Bonnette P, et al. Efficacy of first-line chemotherapy in patients with advanced lung sarcomatoid carcinoma. J Thorac Oncol. 2013;8(12):1574–7.
Yuki T, Sakuma T, Ohbayashi C, Yoshimura M, Tsubota N, Okita Y, et al. Pleomorphic carcinoma of the lung: a surgical outcome. J Thorac Cardiovasc Surg. 2007;134(2):399–404.
Schrock AB, Li SD, Frampton GM, Suh J, Braun E, Mehra R, et al. Pulmonary sarcomatoid carcinomas commonly harbor either potentially targetable genomic alterations or high tumor mutational burden as observed by comprehensive genomic profiling. J Thorac Oncol. 2017;12(6):932–42.
Liang X, Li Q, Xu B, Hu S, Wang Q, Li Y, et al. Mutation landscape and tumor mutation burden analysis of Chinese patients with pulmonary sarcomatoid carcinomas. Int J Clin Oncol. 2019;24(9):1061–8.
Yang Z, Xu J, Li L, Li R, Wang Y, Tian Y, et al. Integrated molecular characterization reveals potential therapeutic strategies for pulmonary sarcomatoid carcinoma. Nat Commun. 2020;11(1):4878.
Liu X, Jia Y, Stoopler MB, Shen Y, Cheng H, Chen J, et al. Next-generation sequencing of pulmonary sarcomatoid carcinoma reveals high frequency of actionable MET gene mutations. J Clin Oncol. 2016;34(8):794–802.
Saffroy R, Fallet V, Girard N, Mazieres J, Sibilot DM, Lantuejoul S, et al. MET exon 14 mutations as targets in routine molecular analysis of primary sarcomatoid carcinoma of the lung. Oncotarget. 2017;8(26):42428–37.
Tong JH, Yeung SF, Chan AW, Chung LY, Chau SL, Lung RW, et al. MET amplification and Exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res. 2016;22(12):3048–56.
Liu XW, Chen XR, Rong YM, Lyu N, Xu CW, Wang F, et al. MET exon 14 skipping mutation, amplification and overexpression in pulmonary sarcomatoid carcinoma: A multi-center study. Transl Oncol. 2020;13(12):100868.
Mignard X, Ruppert AM, Antoine M, Vasseur J, Girard N, Mazieres J, et al. c-MET Overexpression as a poor predictor of MET amplifications or Exon 14 mutations in lung sarcomatoid carcinomas. J Thorac Oncol. 2018;13(12):1962–7.
Lu S, Fang J, Li X, Cao L, Zhou J, Guo Q, et al. Once-daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non-small-cell lung cancers harbouring MET exon 14 skipping alterations: a multicentre, single-arm, open-label, phase 2 study. Lancet Respir Med. 2021;9(10):1154–64.
Valter A, Roosipuu R, Tamm H, Padrik P. An anaplastic lymphoma kinase (ALK) fusion oncogene positive metastatic sarcomatoid carcinoma of the lung with good response to crizotinib. AME Case Rep. 2018;2:2.
Zou F, Xie G, Ma JA, Zhou DA, Jiang YI, Zheng JY. Epidermal growth factor receptor mutation heterogeneity analysis of pulmonary sarcomatoid carcinoma successfully treated with erlotinib: A case report. Oncol Lett. 2015;9(5):2239–43.
Subbiah V, Yang D, Velcheti V, Drilon A, Meric-Bernstam F. State-of-the-art strategies for targeting RET-dependent cancers. J Clin Oncol. 2020;38(11):1209–21.
Ju YS, Lee WC, Shin JY, Lee S, Bleazard T, Won JK, et al. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res. 2012;22(3):436–45.
Sarfaty M, Moore A, Neiman V, Dudnik E, Ilouze M, Gottfried M, et al. RET fusion lung carcinoma: response to therapy and clinical features in a case series of 14 patients. Clin Lung Cancer. 2017;18(4):e223–e32.
Gautschi O, Milia J, Filleron T, Wolf J, Carbone DP, Owen D, et al. Targeting RET in patients with RET-rearranged lung cancers: results from the global, multicenter RET registry. J Clin Oncol. 2017;35(13):1403–10.
Bradford D, Larkins E, Mushti SL, Rodriguez L, Skinner AM, Helms WS, et al. FDA approval summary: selpercatinib for the treatment of lung and thyroid cancers with RET gene mutations or fusions. Clin Cancer Res. 2021;27(8):2130–5.
Gainor JF, Curigliano G, Kim DW, Lee DH, Besse B, Baik CS, et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021;22(7):959–69.
Acknowledgements
The authors would like to thank all people involved in this work.
Code availability
Not applicable.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81773022 and 82072333); Natural Science Foundation of Hubei Province (No. 2020CFB808).
Author information
Authors and Affiliations
Contributions
Ying Wu: methodology, writing—original draft. Zhecheng Yan: investigation, data curation, formal analysis. Juan Pan: methodology, investigation. Xiaona Chang: resources, project administration. Bo Huang: resources, project administration. Danju Luo: resources, project administration. Rui Meng: resources, project administration. Heshui Shi: resources. Jun Fan: conceptualization, supervision, project administration. Xiu Nie: conceptualization, supervision, funding acquisition. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by appropriate institutional of Wuhan Union Hospital and Huazhong University of Science and Technology. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Consent for publication
We have obtained consent to publish from the patient.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, Y., Yan, Z., Pan, J. et al. Partial response to pralsetinib in an advanced pulmonary sarcomatoid carcinoma patient harboring a KIF5B-RET rearrangement: a case report. World J Surg Onc 20, 386 (2022). https://doi.org/10.1186/s12957-022-02848-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-022-02848-z