Abstract
Background
Immune checkpoint inhibitors (ICIs) have dramatically prolonged survival in non-small cell lung cancer (NSCLC) patients, but little research had focused on its impact on quality of life (QoL). The purpose of our study was to compare the QoL in patients with NSCLC treated with programmed cell death protein-1/programmed cell death-ligand 1 (PD-1/PD-L1) inhibitors versus chemotherapy.
Methods
We searched for randomized controlled trials utilizing the Quality of Life Questionnaire Core 30 items (QLQ-C30) and the EuroQol Five Dimensions Questionnaire-3L (EQ-5D-3L) to assess the QoL of NSCLC treated with PD-1/PD-L1 inhibitors versus chemotherapy. We collected hazard ratios (HRs) for the time from baseline to the first clinically significant deterioration (TTD) and effect size as the difference in mean change between and within treatment groups in patients’ reported outcomes (PROs). (PROSPERO registration number: CRD42022296680).
Results
In the five trials reported by QLQ-C30, TTD was longer in PD-1/PD-L1 inhibitors compared with control groups (HR = 0.86; 95% CI = 0.76, 0.97; P = 0.013), with similar results in terms of physical function, role function, and pain. The difference in mean change between the PD-1/PD-L1 inhibitors group and the chemotherapy group in QLQ-C30 and EQ-5D VAS was 3.64 (95% CI = 1.62, 5.66; P = 0.001) and 4.74 (95% CI = 2.65, 6.83; P = 0.001), which supported PD-1/PD-L1 inhibitors. However, for the EQ-5D utility index, there was no statistically significant difference between the two groups, with a mean change difference of 0.03 (95% CI = −0.01, 0.07; P = 0.094). The mean change from baseline to follow-up in PD-1/PD-L1 inhibitors group was 2.57 (95% CI = 0.43, 4.71; P = 0.019), and chemotherapy group was −1.31 (95% CI = −3.71, 1.09; P = 0.284), correspondingly. The subgroup analysis showed that no difference was observed between open-label and double-blind trials in patients treated with chemotherapy or PD-1/PD-L1 inhibitors.
Conclusion
In conclusion, PD-1/PD-L1 inhibitors could improve the QoL of patients with NSCLC compared to chemotherapy and reduce unfavorable symptoms during treatment.
Similar content being viewed by others
Background
ICIs work by regulating T-cell cytotoxicity to tumors as well as activating cells of the innate and adaptive arms, mainly including cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), PD-1, and PD-L1 [1]. NSCLC accounts for approximately 85% of lung cancers, and PD-1/PD-L1 inhibitors alone or combined with chemotherapy are the first-line palliative treatment for unresectable NSCLC and have been incorporated into neoadjuvant therapy for resectable patients with NSCLC [2, 3]. The two phase 3 trials (CheckMate 017 and CheckMate 057) reported that 5-year PFS rates and OS rates of nivolumab-treated patients and docetaxel-treated patients in previously treated advanced NSCLC were 8.0% versus 0% and 13.4% versus 2.6%, respectively, and nivolumab showed the superiority survival benefit than docetaxel [4]. However, ICIs can also lead to immune-related adverse events by releasing autoreactive T cells, such as immune-related pneumonia, vomiting, rash, and diarrhea [5].
QoL is a subjective scale to collect the severity of patients’ symptoms at a specific time point. QoL not only is an independent prognostic factor for lung cancer patients, but also evaluates the benefit-risk ratio of immune checkpoint inhibitors [6, 7]. One of the most common questionnaires used to evaluate patients with lung cancer is the QLQ-C30, which consists of five functional scales (physical, role, cognitive, emotional, and social) and nine symptom scales (fatigue, pain, nausea and vomiting, dyspnea, appetite loss, constipation, diarrhea, sleep disturbance), as well as a global health and quality-of-life scale [8]. The EQ-5D-3L primarily consists of the EQ-5D utility index and the EQ visual analog scale (VAS). EQ-5D-3L describes five dimensions of health (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), and three levels in each dimension represent no problems, moderate problems, and extreme problems, respectively [9, 10]. EQ-5D-3L is a preference-based measure that generates an index-based summary score. The EQ-5D utility index score is typically interpreted along a continuum where 1 represents the best possible health and 0 represents dead [11, 12]. Few studies have systematically assessed the QoL of ICIs in patients with NSCLC due to the heterogeneity in the quality assessment of phases 2 to 3 clinical trials in patients with various types of cancer. A series of 10 eligible trials were included in this meta-analysis, and the QLQ-C30 and EQ-5D-3L questionnaires were applied to assess the QoL of patients with NSCLC in terms of TTD and the differences in mean change between and within groups. The study was designed to systematically investigate whether PD-1/PD-L1 inhibitors (atezolizumab, avelumab, durvalumab, nivolumab, pembrolizumab) improved the QoL of patients with NSCLC compared to chemotherapy based on published randomized controlled trials.
Materials and methods
Search strategy and study selection
Study selection corresponded with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [13]. This study was registered on PROSPERO (ID no. CRD42022296680). We searched PubMed, Embase, and the Cochrane Library databases. The search deadline was on 15 January 2022. Search terms were as follows: ((atezolizumab) OR (avelumab) OR (durvalumab) OR (nivolumab) OR (pembrolizumab)) AND ((non-small cell lung cancer) OR (carcinoma, non-small cell lung) OR (carcinomas, non-small cell lung) OR (lung carcinoma, non-small cell) OR ((non-small cell lung carcinomas) OR (non-small cell lung carcinoma) OR (lung carcinomas, non-small cell) OR (carcinoma, non-small cell lung) OR (non-small cell lung carcinoma) OR (non-small cell lung cancer)) AND ((life quality) OR (health-related quality of life) OR (health-related quality of life) OR (hrqol)).
Inclusion criteria were as below:
-
(a)
Patients with NSCLC were studied.
-
(b)
The trial group received PD-1/PD-L1 inhibitors excluding single CTLA-4, while the control group received standard platinum-based chemotherapy.
-
(c)
PROs were reported.
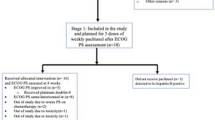
We excluded reviews, case reports, and retrospective studies (Fig. 1).
Data extraction
The extracted data includes trial name, first author, phase, masking, study type, NCT number, QoL measure, ECOG PS, follow-up, intervention arm, comparison arm, TTD, and difference in mean change between and within groups from baseline to follow-up (Table 1).
Statistical analysis
The primary outcomes of our study are as follows: (a) TTD was defined as the time from baseline to first clinically significant deterioration in PROs. A ≥ 10 points score changes for the QLQ-C30, 0.08 for the EQ-5D utility index, and 7 points for the EQ-5D VAS were deemed clinically relevant. (b) The pooled difference mean change is between and within groups from baseline to follow-up, which was all assessed by QLQ-C30 and EQ-5D-3L. We collected hazard ratios (HRs) for TTD and performed effect size as the difference in mean change in PROs between and within treatment groups. We performed a methodological quality assessment of the enrolled trials using the Cochrane risk-of-bias tool, which consists of six items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and incomplete outcome data, selective reporting, and other bias. Each risk-of-bias item was classified as high risk, low risk, and unclear risk [24]. We use the Consolidated Standards of Reporting Trials (CONSORT) PRO checklist to assess the quality of PRO [25]. Two authors (WL and QZ) independently extracted data and performed quality assessment in this process, and discrepancies were resolved by consensus (CHX). Each pooled effect size was estimated by means of the random effects model according to the DerSimonian and Laird method [26]. Heterogeneity was assessed using Cochran’s Q statistic and I2 statistic. I2 values of 25%, 50%, and 75% represented low, moderate, and high heterogeneity, respectively. Beggʼs and Eggerʼs tests were used to assess publication bias for meta-analyses for QoL. Sensitivity analyses were also conducted. P < 0.05 was considered statistically significant difference. All analyses were performed by using the Stata 16.0 software except for quality assessment which was performed using Review Manager 5.3.
Results
Literature search and characteristics of the studies
We searched the PubMed, Embase, and Cochrane Library databases for a total of 1973 publications according to the PICOS principles, of which 577 duplicates were removed, and a total of 10 publications met the inclusion criteria after screening (Fig. 1). There were eight phase 3 trials in this study; one was phase II/III, and the other was not explained in the original study; among those, clinical trials were performed with pembrolizumab (n = 4), nivolumab (n = 3), durvalumab (n = 2), and atezolizumab (n = 1). Eastern Cooperative Oncology Group Performance Status (ECOG PS) was 0-1. The follow-up period ranged from 12 to 66 weeks with a median of 22.5 weeks. PRO tools involved in these trials included EQ-5D-3L, EORTC QLQ-C30, EORTC QLQ-LC13, and LCSS, the most common of which are EORTC QLQ-C30 and EQ-5D-3L (n = 7, 70%) (Table 1). Quality assessment was reported in Fig. 2.
Time from baseline to first deterioration
A total of 5 articles report TTD results in PROs using QLQ-C30 [17, 19, 20, 22, 23]. The TTD was significantly longer with PD-1/PD-L1 inhibitors than with platinum-based chemotherapy in QoL, with an HR of 0.86 (95% CI = 0.76, 0.97; P = 0.013; Fig. 3A). There was no heterogeneity (Q = 3.28; I2 = 0.0%; P = 0.512). The p-value of Egger’s test is 0.057. Moreover, PD-1/PD-L1 inhibitors also demonstrated to delay TTD in physical function, pain, and role function on the QLQ-C30, with an HR of 0.79 (95% CI = 0.69, 0.90; P = 0.000; I2 = 67.0%; Fig. 3B), with an HR of 0.78 (95% CI = 0.69, 0.89; P = 0.000; I2 = 0.0%; Fig. 3C), with an HR of 0.84 (95% CI = 0.73, 0.96; P = 0.012; I2 = 74.3%; Fig. 3D), respectively. Sensitivity analysis of TTD was shown in Fig. 4 A–D.
Forest plot of hazard ratios for the time from baseline to first deterioration in the Quality of Life Questionnaire Core 30 items on quality of life (A), physical function (B), pain (C), and role function (D). A Random effect: P = 0.013; Egger’s test: P = 0.057; Begg’s test: P = 0.027. B Random effect: P = 0.000; Egger’s test: P = 0.020; Begg’s test: P = 0.089. C Random effect: P = 0.000; Egger’s test: P = 0.226; Begg’s test: P = 0.308. D Random effect: P = 0.012; Egger’s test: P = 0.293; Begg’s test: P = 0.308
Sensitivity analyses of time from baseline to first deterioration in the Quality of Life Questionnaire Core 30 items on quality of life (A), physical function (B), pain (C), and role function (D). Sensitivity analyses of mean change from baseline to follow-up between groups for the Quality of Life Questionnaire Core 30 items (E), EQ-5D VAS (F), and EQ-5D utility index (G). Sensitivity analyses of difference in mean change from baseline to follow-up within groups: PD-1/PD-L1 inhibitors (H) and controls (I)
The difference of mean changes between groups
The mean changes from baseline to follow-up in QoL between groups were reported with the QLQ-C30 in six trials [14, 16, 17, 19, 20, 22], the EQ-5D utility index in three trials [15, 18, 21], and the EQ-5D VAS in four trials [14, 17, 18, 21]. The difference in mean change between the PD-1/PD-L1 inhibitors group and the chemotherapy group in QLQ-C30 was 3.64 (95% CI = 1.62, 5.66; P = 0.00; Fig. 5A) favoring PD-1/PD-L1 inhibitors, with no heterogeneity (Q = 9.30; I2 = 46.24%; P = 0.10) and no publication bias (Begg’s test P = 1.000, Egger’s test P = 0.431). In addition, the same trend was also found for the EQ-5D VAS with a mean difference of 4.74 (95% CI = 2.65, 6.83; P = 0.00; Fig. 5B) and with no heterogeneity (Q = 3.06; I2 = 1.83%; P = 0.383). However, the pooled difference in mean change between PD-1/PD-L1 inhibitors and control groups in the EQ-5D utility index was 0.03 (95% CI = −0.01, 0.07; P = 0.094; I2 = 0.00%; Fig. 5C) with no statistically significant difference. Sensitivity analysis of the difference of mean changes between groups was shown in Fig. 4 E–G.
Forest plot of the difference in mean change from baseline to follow-up between groups for the Quality of Life Questionnaire Core 30 items (A), EQ-5D VAS (B), and EQ-5D utility index (C). A Random effect: P = 0.00; Egger’s test: P = 0.431; Begg’s test: P = 1.000. B Random effect: P = 0.00; Egger’s test: P = 0.128; Begg’s test: P = 0.296. C Random effect: P = 0.094; Begg’s test: P = 0.296. Forest plot of the difference in mean change from baseline to follow-up within groups: PD-1/PD-L1 inhibitors (D) and controls (E). D Random effect: P = 0.019; Egger’s test: P = 0.969; Begg’s test: P = 1.000. E Random effect: P = 0.284; Egger’s test: P = 0.141; Begg’s test: P = 0.462
The difference of mean changes within groups
There were eight trials accessible for the within-group analysis [14, 16, 19, 20, 22]. The pooled mean change from baseline to follow-up in PD-1/PD-L1 inhibitors in the QLQ-C30 was 2.57 (95% CI = 0.43, 4.71; P = 0.019, I2 = 75.79%) and in chemotherapy was −1.31 (95% CI = −3.71, 1.09; P = 0.284; I2 = 69.76%, Fig. 5 D–E), suggesting a change within the PD-1/PD-L1 inhibitors group. Statistically significant heterogeneity was observed in both studies (Q = 16.52, I2 = 75.79%, P = 0.002; Q = 13.23, I2 = 69.76%, P = 0.010). Sensitivity analysis of the difference of mean changes within groups was shown in Fig. 4 H–I.
Subgroup analysis
To further evaluate the impact of masking on QoL, we compared the effect of open-label trials versus double-blind trials in patients with NSCLC who underwent PD-1/PD-L1 inhibitors and chemotherapy in the QLQ-C30. The pooled mean change from baseline to follow-up in PD-1/PD-L1 inhibitors using open-label method was 2.74 (95% CI = −5.20, 10.67), with significant heterogeneity (I2 = 92.16%, P < 0.01). The pooled mean change of double-blind trials in PD-1/PD-L1 inhibitors group was 2.65 (95% CI = 1.29, 14.01; P = 0.25; I2 = 27.70%, Fig. 6A), suggesting low heterogeneity and beneficial change within the PD-1/PD-L1 inhibitors group [14, 16, 19, 20, 22]. The pooled mean change from baseline to follow-up in control groups using open-label method was −2.65 (95% CI = −5.43, 0.13), with low heterogeneity (I2 = 26.53%, P = 0.24). The pooled mean change of double-blind trials in control groups was −0.63 (95% CI = −3.75, 2.50, P = 0.02; I2 = 73.88%, Fig. 6B), suggesting high heterogeneity within the control group [14, 16, 19, 20, 22]. Whether open-label or double-blind trials, the mean changes from baseline to follow-up in QoL between groups both supported the PD-1/PD-L1 inhibitors group (Fig. 6C) [14, 16, 19, 20, 22].
Discussion
This study compared the TTD and the difference of mean changes in PROs in patients with NSCLC receiving PD-1/PD-L1 inhibitors and chemotherapy. Regardless of QLQ-C30 or EQ-5D-3L, the results of the meta-analysis suggested that NSCLC patients with PD-1/PD-L1 inhibitors had a more favorable difference in mean change from baseline to follow-up compared to those with chemotherapy and had a significantly delayed clinical deterioration. The changes of QoL between groups showed that PD-1/PD-L1 inhibitors were more beneficial than chemotherapy; furthermore, there was a statistically significant favorable change among NSCLC treated with PD-1/PD-L1 inhibitors in the QLQ-C30 during the follow-up duration. EQ-5D-3L is the preference-based measure that could generate health utilities from results. By contrast, QLQ-C30 is the cancer-specific quality-of-life questionnaire, which focuses on non-preference-based patient outcomes and is usually applied in clinical trials. Therefore, different measures probably generate different results [27]. The subgroup analysis showed that no difference was observed between open-label and double-blind trials in patients treated with chemotherapy or PD-1/PD-L1 inhibitors. Furthermore, a preliminary analysis of PROs of identical oncology drugs found no overestimation of improvements in open-label trials compared to blinded trials [28]. Due to the small sample size, these results should be interpreted cautiously, and more data are needed for analysis. Recently, a meta-analysis demonstrated that ICIs were positively associated with higher levels of QoL and longer time to deterioration in cancer patients, which was consistent with our results, but the study incorporated a variety of cancer types and was not specific to PD-1/PD-L1 inhibitors, thus was not as homogenous as our study [29]. The adverse reactions often occur late in the course of therapy; the QLQ-C30 and EQ-5D-3L focus on the era of chemotherapy, which fails to include specific symptoms of ICIs, so account for ICI-treated patients may benefit more than chemotherapy-treated patients. Zaim R. et al. reported that nivolumab alleviated symptom burden and improved health-related quality of life of advanced NSCLC [30]. Nishijima T. F. et al. similarly found that patients treated with PD-1/PD-L1 inhibitors had higher QoL and fewer adverse effects than chemotherapy, yet there was no significant change in the mean difference in patients with PD-1/PD-L1 inhibitors, which contradicted our findings [31]. The reason may be associated with the different subjects, small sample size, and the failure to include specific symptom toxicities associated with ICIs in lung cancer [32].
To the best of our knowledge, our study is the first systematic review and meta-analysis examining the impact of PD-1/PD-L1 inhibitors and standard chemotherapy on QoL in patients with NSCLC. However, there are some limitations to our study. Firstly, these data were extracted from published articles and were not original. Secondly, fewer articles were eligible for inclusion, and subgroup analysis of heterogeneous studies was difficult to conduct with some publication bias. Thirdly, as QoL questionnaires require patients to complete at a point time, the patients are vulnerable to losing follow-up due to the disease progression. Fourthly, it is difficult to directly compare different trials at different time points, and other functions and symptoms of PROs cannot be studied due to a lack of data and heterogeneity. Lastly, despite questionnaires reporting quality of life are currently considered to be of high quality, there are currently no international guidelines for statistical analysis of QOL in cancer patients using ICIs, and there is still a lack of confirmation of research hypotheses and questionnaires in cancer patients treated with ICIs in methodological aspects [33]. ECOG PS ≥ 2 was almost excluded from clinical trials, and future studies should be focused on older and weak patients [34, 35].
Conclusion
Our meta-analysis reported that patients with NSCLC treated with PD-1/PD-L1 inhibitors had higher QOL and fewer adverse symptoms than those with standard chemotherapy, indicating that early social, psychological, and spiritual support can improve the quality of life. The shift from traditional therapy to immunotherapy in cancer treatment will take the consideration of the patient-centered quality-of-life assessment into account, which can have a positive impact on the development of oncological care in the future. It has reference value for clinicians in the real world, and more studies will be needed to validate it.
Availability of data and materials
All data in this manuscript are available for readers.
References
Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–49.
Kang J, Zhang C, Zhong W. Neoadjuvant immunotherapy for non-small cell lung cancer: state of the art. Cancer Commun (Lond). 2021;41(4):287–302.
Mielgo-Rubio X, Uribelarrea EA, Cortés LQ, et al. Immunotherapy in non-small cell lung cancer: update and new insights. J Clin Transl Res. 2021;7:1–21.
Borghaei H, Gettinger S, Vokes EE, et al. Five-year outcomes from the randomized, phase III trials CheckMate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol. 2021;39(7):723–33.
Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–68.
Bouazza YB, Chiairi I, Kharbouchi OE, et al. Patient-reported outcome measures (PROMs) in the management of lung cancer: a systematic review. Lung Cancer. 2017;113:140–51.
Polanski J, Jankowska-Polanska B, Rosinczuk J, et al. Quality of life of patients with lung cancer. Onco Targets Ther. 2016;9:1023–8.
Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76.
Zhu J, Yan XX, Liu CC, et al. Comparing EQ-5D-3L and EQ-5D-5L performance in common cancers: suggestions for instrument choosing. Qual Life Res. 2021;30:841–54.
Khan I, Morris S, Pashayan N, et al. Comparing the mapping between EQ-5D-5L, EQ-5D-3L and the EORTC-QLQ-C30 in non-small cell lung cancer patients. Health Qual Life Outcomes. 2016;14:60.
Pickard AS, Wilke CT, Lin HW, et al. Health utilities using the EQ-5D in studies of cancer. Pharmacoeconomics. 2007;25(5):365–84.
Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70.
Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Barlesi F, Garon EB, Kim DW, et al. Health-related quality of life in KEYNOTE-010: a phase II/III study of pembrolizumab versus docetaxel in patients with previously treated advanced, programmed death ligand 1-expressing NSCLC. J Thorac Oncol. 2019;14:793–801.
Reck M, Brahmer J, Bennett B, et al. Evaluation of health-related quality of life and symptoms in patients with advanced non-squamous non-small cell lung cancer treated with nivolumab or docetaxel in CheckMate 057. Eur J Cancer. 2018;102:23–30.
Brahmer JR, Abreu DR, Robinson AG, et al. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): a multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol. 2017;18:1600–9.
Bordoni R, Ciardiello F, von Pawel J, et al. Patient-reported outcomes in OAK: a phase III study of atezolizumab versus docetaxel in advanced non-small cell lung cancer. Clin Lung Cancer. 2018;19:441–9.
Reck M, Taylor F, Penrod JR, et al. Impact of nivolumab versus docetaxel on health-related quality of life and symptoms in patients with advanced squamous non-small cell lung cancer: results from the CheckMate 017 study. J Thorac Oncol. 2018;13:194–204.
Mazieres J, Kowalski D, Luft A, et al. Health-related quality of life with carboplatin-paclitaxel or nab-paclitaxel with or without pembrolizumab in patients with metastatic squamous none small-cell lung cancer. J Clin Oncol. 2019;38(3):271e80.
Hui R, Özgüroğlu M, Villegas A, et al. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study. Lancet Oncol. 2019;20:1670–80.
Perol M, Dixmier A, Barlesi F, et al. Health-related quality of life (HRQoL) of non-small cell lung cancer (NSCLC) patients treated with nivolumab in real-life: the EVIDENS study. Ann Oncol. 2019;30:ii48.
Garassino MC, Gadgeel S, Esteban E, et al. Patient-reported outcomes following pembrolizumab or placebo plus pemetrexed and platinum in patients with previously untreated, metastatic, non-squamous non-small-cell lung cancer (KEYNOTE-189): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:387–97.
Garon EB, Cho BC, Reinmuth N, et al. Patient-reported outcomes with durvalumab with or without tremelimumab versus standard chemotherapy as first-line treatment of metastatic non-small-cell lung cancer (MYSTIC). Clin Lung Cancer. 2021;22:301–12.
Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Cochrane; 2021. https://training.cochrane.org/handbook/current. Accessed 22 Oct 2021.
Calvert M, Blazeby J, Altman DG, et al. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA. 2013;309:814–22.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Gray LA, Alava MH, Wailoo AJ. Mapping the EORTC QLQ-C30 to EQ-5D-3L in patients with breast cancer. BMC Cancer. 2021;21(1):1237.
Chakravarti PB, Basch EM, Hirshfield KM, et al. Exploring open-label bias in patient-reported outcome (PRO) emotional domain scores in cancer trials. J Clin Oncol. 2018;36:e18702.
Boutros A, Bruzzone M, Tanda ET, et al. Health-related quality of life in cancer patients treated with immune checkpoint inhibitors in randomised controlled trials: a systematic review and meta-analysis. Eur J Cancer. 2021;159:154–66.
Zaim R, Redekop K, Uyl-de GC. Analysis of patient reported outcomes included in the registrational clinical trials of nivolumab for advanced non-small cell lung cancer. Transl Oncol. 2022;20:101418.
Nishijima TF, Shachar SS, Muss HB, et al. Patient-reported outcomes with PD-1/PD-L1 inhibitors for advanced cancer: a meta-analysis. Oncologist. 2019;24:e565–73.
Hall ET, Singhal S, Dickerson J, et al. Patient-reported outcomes for cancer patients receiving checkpoint inhibitors: opportunities for palliative care-a systematic review. J Pain Symptom Manag. 2019;58:137–56.
Faury S, Foucaud J. Health-related quality of life in cancer patients treated with immune checkpoint inhibitors: a systematic review on reporting of methods in randomized controlled trials. PLoS One. 2020;15:e0227344.
Gridelli C, Peters S, Mok T, et al. First-line immunotherapy in advanced non-small-cell lung cancer patients with ECOG performance status 2: results of an International Expert Panel Meeting by the Italian Association of Thoracic Oncology. ESMO Open. 2021;7:100355.
Passaro A, Attili I, Morganti S, et al. Clinical features affecting survival in metastatic NSCLC treated with immunotherapy: a critical review of published data. Cancer Treat Rev. 2020;89:102085.
Acknowledgements
Not applicable.
Funding
The study was funded by the Major Program of Nanjing Medical Science and Technique Development Foundation (ZKX17044). The funders had no role in the study design, data collection, analysis, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
WL and QZ searched the database, judged study eligibility, and extracted data. WL, TZ, and LL analyzed data and wrote the paper. CX designed the study and revised this paper. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, W., Zhang, Q., Zhang, T. et al. Quality of life in patients with non-small cell lung cancer treated with PD-1/PD-L1 inhibitors: a systematic review and meta-analysis. World J Surg Onc 20, 333 (2022). https://doi.org/10.1186/s12957-022-02800-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-022-02800-1