Abstract
Background
This study aimed to investigate the usefulness of computed tomography (CT) texture analysis in the diagnosis of lateral pelvic lymph node (LPLN) metastasis of rectal cancer.
Methods
This was a retrospective cohort study of 45 patients with rectal cancer who underwent surgery with LPLN dissection at Tokushima University Hospital from January 2017 to December 2021. The texture analysis of the LPLNs was performed on preoperative CT images, and 18 parameters were calculated. The correlation between each parameter and pathological LPLN metastasis was evaluated. The texture parameters were compared between pathologically metastasis-positive LPLNs and metastasis-negative LPLNs.
Results
A total of 40 LPLNs were extracted from 25 patients by preoperative CT scans. No LPLNs could be identified in the remaining 19 patients. Eight of the 25 patients had pathologically positive LPLN metastasis. Extracted LPLNs were analyzed by the texture analysis. Pathologically metastasis-positive LPLNs had significantly lower mean Hounsfield unit, gray-level co-occurrence matrix (GLCM) energy, and GLCM Entropy_log2 values, and a significantly larger volume than pathologically metastasis-negative LPLNs. Multivariate analysis revealed that the independent predictive factors for LPLN metastasis were volume (a conventional parameter) (odds ratio 7.81, 95% confidence interval 1.42–43.1, p value 0.018) and GLCM Entropy_log2 (a texture parameter) (odds ratio 12.7, 95% confidence interval 1.28–126.0, p value 0.030). The combination of both parameters improved the diagnostic specificity while maintaining the sensitivity compared with each parameter alone.
Conclusion
Combining the CT texture analysis with conventional diagnostic imaging may increase the accuracy of the diagnosis of LPLN metastasis of rectal cancer.
Similar content being viewed by others
Background
Colorectal cancer is the third most common cancer in men and the second most common cancer in women worldwide, with high morbidity and mortality rates [1]. Furthermore, rectal cancer accounts for 40% of colorectal cancer [2]. Although the outcome of rectal cancer has been improved by total mesorectal excision and neoadjuvant chemoradiotherapy, the local recurrence rates are still as high as 7.4–12.6% [3]. The local recurrence rate of rectal cancer is related to lateral pelvic lymph node (LPLN) metastasis [4], which is reportedly detected in 7.3% of patients with lower rectal cancer [5]. Japanese guidelines consider LPLN metastasis a local condition and recommend LPLN dissection [6], whereas Western guidelines consider LPLN metastasis a systemic disease and recommend neoadjuvant chemoradiotherapy [7]. Regardless of whether the Japanese or Western strategy is performed, it is important to properly assess the condition of the LPLNs prior to treatment.
Currently, the main method used to predict the LPLN metastasis is size-based diagnosis using the largest short-axis diameter of the lymph node on computed tomography (CT) or magnetic resonance imaging (MRI) [8]. However, Ogawa et al. reported that the accuracy of the short-axis diameter on MRI for predicting LPLN metastasis is 77.6% [9], which is not satisfactory.
The texture analysis is an image-processing algorithm that quantifies tissue heterogeneity by evaluating the distribution of the texture coarseness, density, and irregularity within a lesion and is expected to provide a more detailed and quantitative characterization of lesions than visual analysis. Several studies have reported the usefulness of the texture analysis in predicting lymph node metastasis of various cancers [10]. However, to our knowledge, the value of the CT texture analysis in the prediction of LPLN metastasis of rectal cancer has not been reported. The present study aimed to investigate the usefulness of the CT texture analysis in the prediction of LPLN metastasis of rectal cancer.
Methods
Patients
This was a retrospective cohort study of 45 patients with rectal cancer who underwent surgery at Tokushima University Hospital from January 2017 to December 2021. The inclusion criteria were lower rectal cancer of clinical stage ≥ T2 or LPLN metastasis in patients with recurrent rectal cancer who underwent LPLN dissection. The exclusion criterion was the absence or unavailability of contrast-enhanced CT images. All patients who underwent preoperative chemotherapy were treated with FOLFOXIRI (irinotecan: 150 mg/m2/90 min, leucovorin: 200 mg/m2 for 120 min, oxaliplatin: 85 mg/m2 for 120 min, 5-fluorouracil: 2400 mg/m2 for 44 h) regimen with bevacizumab (5 mg/kg for 90 min) or panitumumab (60 mg/kg for 60 min). All patients who underwent preoperative chemoradiotherapy (CRT) received S-1 orally (80 mg/m2/day on days 1–5, 8–12, 15–19, and 22–26) and infusions of oxaliplatin (50 mg/m2 on days 1, 8, 15, and 22) and bevacizumab (5 mg/kg on days 1 and 15). The total radiation dose was 40 Gy delivered in daily fractions of 2 Gy via the four-field technique. Surgery was performed 6–9 weeks after completing preoperative CRT.
CT scan protocol
A 320-detector row CT scanner (Aquilion ONE™/GENESIS Edition, Canon Medical Systems, Tokyo, Japan) was used. The scanning parameters were tube voltage, 120 kV; tube current, auto exposure control; tube rotation speed, 0.5 per second; slice thickness, 0.5 mm; reconstruction kernel, FC14; image reconstruction interval, 0.5 mm; helical pitch, 129; and interpolation method, 180°. Dynamic contrast-enhanced CT was performed using a bolus-tracking technique, where a region of interest (ROI) was placed on the abdominal aorta and the trigger threshold inside the ROI was set at 120 Hounsfield units (HU). The first-phase scan was started 8 s after the threshold was achieved following the administration of contrast material. The second-phase image was obtained 15 s after the first arterial phase. CT was performed within 1 month prior to surgery.
CT texture analysis
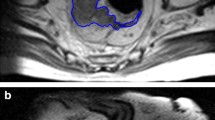
To calculate the texture parameters, DICOM image files of the LPLNs were imported into the software package LIFEx (version 7.1.0, http://www.lifexsoft.org/). All clinical results were blinded during the measurement. The ROI was manually traced on the axial slice of the first-phase CT image that yielded the maximum LPLN area, as already reported (Fig. 1) [11]. Texture parameters of the ROI were automatically extracted using LIFEx software.
Analysis of the texture parameters on the axial first-phase computed tomography (CT) image that yielded the maximum lateral pelvic lymph node area. A An enlarged lateral pelvic lymph node. B The region of interest was drawn manually along the outer edge of the lymph node and the texture parameters were extracted
Eighteen texture parameters were extracted, including conventional parameters, histogram-based parameters, shape-based parameters, and gray-level co-occurrence matrix (GLCM) parameters. The minimum (HU min), mean attenuation (HU mean), standard deviation (HU std), and maximum values of HU (HU max) were evaluated as conventional parameters. The skewness, kurtosis, entropy_log10, entropy_log2, and energy values were evaluated as histogram-based parameters. The volume and sphericity values were evaluated as shape-based parameters. The homogeneity, energy, contrast, correlation, entropy_log10, entropy_log2, and dissimilarity values were evaluated as GLCM parameters.
Histopathologic analysis
All patients underwent robot-assisted surgery with LPLN dissection, and all resected tumors and lymph nodes were evaluated by pathologists in accordance with the Seventh Edition of the American Joint Committee on Cancer TNM grading system.
Statistical analysis
All statistical analyses were performed with EZR (version 1.54) (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (version 4.03) (R Foundation for Statistical Computing, Vienna, Austria) [12]. EZR is a modified version of R commander (version 2.7–1) designed to add statistical functions frequently used in biostatistics. Categorical variables were analyzed with Fisher’s exact test. Continuous variables were analyzed with the Mann–Whitney U test. Multivariate analysis was carried out based on the logistic regression model using a forward stepwise selection. The P values of < 0.05 were considered statistically significant.
Results
Patient characteristics
A total of 45 patients were evaluated. A total of 40 LPLNs were extracted from 25 patients by preoperative CT scans. No LPLNs could be identified in the remaining 19 patients. Eight of the 25 patients had pathologically positive LPLN metastasis. The comparison of patient characteristics between the patients with pathologically positive LPLN metastasis and the patients with pathologically negative LPLN metastasis is summarized in Table 1. The patients with pathologically positive LPLN metastasis showed significantly higher pathological tumor depth of invasion (pT) and pathological lymph node metastasis (pN), and lower differentiation of the tumor than the patients with pathologically negative LPLN metastasis.
Comparison of texture parameters
Extracted LPLNs were analyzed by texture analysis. Fifteen of the 40 LPLNs showed pathologic metastasis. None of the patients whose LPLNs could not be identified by CT scans had pathologic lymph node metastasis. The texture parameters were compared between pathologically metastasis-positive LPLNs and metastasis-negative LPLNs (Table 2). Pathologically, metastasis-positive LPLNs had significantly lower mean HU, GLCM Energy, and GLCM Entropy_log2 values and a significantly larger volume than pathologically metastasis-negative LPLNs.
The cutoff values for the prediction of LPLN metastasis were 2.546 for the mean HU, 85.0 for the volume, 5.164 for the GLCM Energy, and 1.73 for the GLCM Entropy_log2; the areas under the receiver operating characteristic curves for each parameter were 0.739, 0.773, 0.773, and 0.792, respectively (Fig. 2).
Multivariate analysis
The parameters that showed significant differences in univariate analysis were divided into two groups by cutoff values, and multivariate analysis was performed. Volume and GLCM Entropy_log2 were identified as independent predictive factors for LPLN metastasis (Table 3). The sensitivity and specificity of a positive result for both factors were 66.7% and 84.0%, respectively. The sensitivity and negative predictive value of a negative result for both factors were 100%, respectively.
Discussion
The present study identified volume (a conventional parameter) and GLCM Entropy_log2 (a texture parameter) as independent predictive factors for LPLN metastasis. The cutoff value of the volume was 85.0 ml, with a sensitivity and specificity of 80.0% and 76.0%, respectively. Some studies have reported the size-based diagnosis of LPLN metastasis on CT or MRI. Sato et al. reported that the maximum short-axis LPLN diameter was significantly larger in metastasis-positive cases than in metastasis-negative cases [13]; the cutoff value was 7.0 mm, with a sensitivity and specificity of 100.0% and 84.2%, respectively. Ishibe et al. reported that the sensitivity and specificity of the diagnosis of LPLN metastasis using a cutoff value of the LPLN diameter of 10 mm on MRI were 43.8% and 98.5%, respectively [14]. However, in actual clinical practice, size-based diagnosis of LPLN metastasis has limitations because even lymph nodes smaller than the cutoff value may be positive for metastasis on histopathological examination.
The present study showed that the GLCM Entropy_log2 value may be useful in the diagnosis of LPLN metastasis using CT texture analysis. The cutoff value of the GLCM Entropy_log2 was 1.730, and the sensitivity and specificity were 93.3% and 60.0%, respectively. Texture analysis has recently been widely applied in diagnostic imaging. Liu et al. evaluated a total of 1054 lymph nodes in patients with colorectal mucinous adenocarcinoma and demonstrated that some CT texture parameters were of significance in differentiating lymph node metastasis [15]. Song and Yin evaluated 108 patients with rectal cancer and demonstrated that texture features obtained from T2-weighted MRI may be valuable for identifying lymph node invasion [16]. He et al. evaluated 199 patients with colorectal cancer and developed machine-learning models for the prediction of regional lymph node metastasis based on 18F-fluorodeoxyglucose positron emission tomography (PET)/CT and PET-based radiomic features [17]. However, to the best of our knowledge, there have been no reports of texture analysis for predicting LPLN metastasis of rectal cancer.
The combination of the volume and the GLCM Entropy_log2 value showed better predictive ability for LPLNM in this study. Following the results of this study, we consider that positive for both factors is an absolute indication o LPLND, that positive for either one of the indicators is a relative indication of LPLND, and that a negative for both factors indicates the possibility of omitting LPLND.
The present study had some limitations. First, the sample size was small. Smaller sample sizes may affect the reliability of results due to increased variability and possible bias. Second, this study included patients who underwent preoperative chemotherapy or chemoradiotherapy. Zhang et al. have reported that histogram and GLCM features were associated with the response to chemotherapy for liver metastasis of colorectal cancer [18]. Liu et al. also reported that GLCM features were associated with the response to chemoradiotherapy for locally advanced rectal cancer [19]. Preoperative treatment may have affected the parameters of the texture analysis in this study as well. Third, the study was a retrospective study. A prospective study is needed to determine the accuracy of the results.
Conclusion
In the present study, the combination of the volume and the GLCM Entropy_log2 value showed better predictive ability for LPLNM in this study. The specificity was improved by the positivity of both factors, and the sensitivity was improved by the negativity of both factors. Combining texture analysis with conventional diagnostic imaging may lead to a more accurate prediction of LPLN metastasis of rectal cancer.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- LPLN:
-
Lateral pelvic lymph node
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- ROI:
-
Region of interest
- HU:
-
Hounsfield units
- GLCM:
-
Gray-level co-occurrence matrix
- PET:
-
Positron emission tomography
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A, Global cancer statistics,. CA: A Cancer Journal for Clinicians. Wiley. 2012;2015(65):87–108.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-86 (Wiley-Liss Inc).
Fujita S, Mizusawa J, Kanemitsu Y, Ito M, Kinugasa Y, Komori K, et al. Mesorectal excision with or without lateral lymph node dissection for clinical stage ii/iii lower rectal cancer (JCOG0212). Annals Surg. 2017;266:201–7. Available from: https://journals.lww.com/annalsofsurgery/Fulltext/2017/08000/Mesorectal_Excision_With_or_Without_Lateral_Lymph.1.aspx. Lippincott Williams and Wilkins (Cited 28 Mar 2022)
Kim TH, Jeong SY, Choi DH, Kim DY, Jung KH, Moon SH, et al. Lateral lymph node metastasis is a major cause of locoregional recurrence in rectal cancer treated with preoperative chemoradiotherapy and curative resection. Ann Surg Oncol. 2008;15:729–37.
Komori K, Fujita S, Mizusawa J, Kanemitsu Y, Ito M, Shiomi A, et al. Predictive factors of pathological lateral pelvic lymph node metastasis in patients without clinical lateral pelvic lymph node metastasis (clinical stage II/III): the analysis of data from the clinical trial (JCOG0212). Eur J Surg Oncol. 2019;45:336–40.
Akiyoshi T, Watanabe T, Miyata S, Kotake K, Muto T, Sugihara K. Results of a Japanese nationwide multi-institutional study on lateral pelvic lymph node metastasis in low rectal cancer: is it regional or distant disease? Annals of Surg. 2012;255:1129–34. Available from: https://journals.lww.com/annalsofsurgery/Fulltext/2012/06000/Results_of_a_Japanese_Nationwide.19.aspx. (Cited 28 Mar 2022)
van de Velde CJH, Boelens PG, Borras JM, Coebergh JW, Cervantes A, Blomqvist L, et al. EURECCA colorectal: Multidisciplinary management: European consensus conference colon & rectum. Eur J Cancer. 2014;50:1.e1-1.e34 (Elsevier Ltd).
Akiyoshi T, Matsueda K, Hiratsuka M, Unno T, Nagata J, Nagasaki T, et al. Indications for lateral pelvic lymph node dissection based on magnetic resonance imaging before and after preoperative chemoradiotherapy in patients with advanced low-rectal cancer. Annals Surg Oncol. 2015;22:614–20 (Springer New York LLC).
Ogawa S, Hida J ichi, Ike H, Kinugasa T, Ota M, Shinto E, et al. Prediction of lateral pelvic lymph node metastasis from lower rectal cancer using magnetic resonance imaging and risk factors for metastasis: multicenter study of the Lymph Node Committee of the Japanese Society for Cancer of the Colon and Rectum. Int J Colorectal Dis. 2017;32:1479–87 (Springer Verlag).
Gu Y, She Y, Xie D, Dai C, Ren Y, Fan Z, et al. A texture analysis–based prediction model for lymph node metastasis in stage IA lung adenocarcinoma. Ann Thorac Surg. 2018;106:214–20 (Elsevier USA).
Nioche C, Orlhac F, Boughdad S, Reuz E S, Goya-Outi J, Robert C, et al. Convergence and technologies: resource report LIFEx: a freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. [cited 2022 Aug 11]; Available from: http://cancerres.aacrjournals.org/ (Cited 11 Aug 2022)
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Sato K, Morohashi H, Tsushima F, Sakamoto Y, Miura T, Fujita H, et al. Dual energy CT is useful for the prediction of mesenteric and lateral pelvic lymph node metastasis in rectal cancer. Mol Clin Oncol. 2019;10(6):625-30.
Ishibe A, Ota M, Watanabe J, Suwa Y, Suzuki S, Kanazawa A, et al. Prediction of lateral pelvic lymph-node metastasis in low rectal cancer by magnetic resonance imaging. World J Surg. 2016;40:995–1001 (Springer New York LLC).
Liu Y, Dou Y, Lu F, Liu L. A study of radiomics parameters from dual-energy computed tomography images for lymph node metastasis evaluation in colorectal mucinous adenocarcinoma. Medicine. 2020;99:e19251 (NLM (Medline)).
Song L, Yin J. Application of texture analysis based on sagittal fat-suppression and oblique axial T2-weighted magnetic resonance imaging to identify lymph node invasion status of rectal cancer. Frontiers Oncol. Frontiers Media S.A.; 2020;10:1364.
He J, Wang Q, Zhang Y, Wu H, Zhou Y, Zhao S. Preoperative prediction of regional lymph node metastasis of colorectal cancer based on 18F-FDG PET/CT and machine learning. Annals Nucl Med. 2021;35:617–27 (Springer Japan).
Zhang H, Li W, Hu F, Sun Y, Hu T, Tong T, et al. MR texture analysis: potential imaging biomarker for predicting the chemotherapeutic response of patients with colorectal liver metastases Abdominal Radiology. Abdom Radiol. 2019;44:65–71. Available from: https://doi.org/10.1007/s00261-018-1682-1.
Liu S, Wen L, Hou J, Nie S, Zhou J, Cao F, et al. Predicting the pathological response to chemoradiotherapy of non-mucinous rectal cancer using pretreatment texture features based on intravoxel incoherent motion diffusion-weighted imaging. Abdom Radiol. 2019;44:2689–98. Available from: https://link.springer.com/article/10.1007/s00261-019-02032-0. (Cited 28 Mar 2022)
Acknowledgements
We thank Kelly Zammit, BVSc, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
TN, MS, and KY were involved in the study design and data interpretation. TT, MN, HK, CT, YW, and TY were involved in the data analysis. TN wrote the main manuscript text, figures and tables. All authors revised the manuscript, approved the final manuscript to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol for this research project was approved by the Ethics Committee of Tokushima University (approval no. 3215–1) and conforms to the provisions of the Declaration of Helsinki.
Consent for publication
Informed consent was obtained from all patients.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nakao, T., Shimada, M., Yoshikawa, K. et al. Computed tomography texture analysis for the prediction of lateral pelvic lymph node metastasis of rectal cancer. World J Surg Onc 20, 281 (2022). https://doi.org/10.1186/s12957-022-02750-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-022-02750-8