Abstract
Background
For patients with prior intra-abdominal surgery or multiple arteries, the retroperitoneal robot-assisted partial nephrectomy (rRAPN) is a better choice. The renal ventral tumor poses an additional challenge due to poor tumor exposure. This study is determined to assess the feasibility of an internal traction technique (ITT) in rRAPN for the management of renal ventral tumors.
Methods
From November 2019 to March 2021, a total of 28 patients with renal ventral tumor underwent rRAPN. All patients had prior abdominal surgery or multiple arteries. The ITT group (20 patients), which improved the tumor exposure by traction of the kidney with suture, was compared with the traditional technique group (8 patients) in terms of warm ischemia time, estimated blood loss and postoperative hospital stay, retroperitoneal drainage, R.E.N.A.L. score, and serum creatinine. Differences were considered significant when P < 0.05.
Results
All rRAPN surgeries were successful without conversion to radical nephrectomy or open partial nephrectomy. The warm ischemia time was lower in the ITT group (17.10 min vs. 24.63 min; P < 0.05). Estimated blood loss in the traditional technique group was 324.88 ± 79.42 mL, and in the ITT group, it was 117.45±35.25 mL (P < 0.05). No significant differences with regard to postoperative hospital stay, retroperitoneal drainage, R.E.N.A.L. score, and serum creatinine were observed between both groups. Surgical margins were negative and no intraoperative complications occurred in all the patients. After 10 months of follow-up, no recurrence or metastasis occurred in all cases.
Conclusion
ITT is a feasible, safe, and valid procedure in rRAPN for renal ventral tumors. Application of ITT improved the exposure and reduces warm ischemic time in comparison with the conventional procedure.
Similar content being viewed by others
Background
Partial nephrectomy has gradually become a standard surgical procedure for the treatment of renal malignancy [1,2,3]. The robot-assisted partial nephrectomy is widely used in the surgical treatment of renal tumors and most commonly performed through a transperitoneal approach [4, 5]. However, for patients with prior intra-abdominal surgery or multiple arteries, the retroperitoneal approach is a better choice [6,7,8,9]. In retroperitoneal robot-assisted partial nephrectomy (rRAPN), dealing with renal ventral tumor with poor tumor exposure would be at risk of longer warm ischemic time (WIT) and more blood loss. Patients with complex renal masses were therefore often converted to open partial nephrectomy or radical nephrectomy [10, 11]. We developed an internal traction technique (ITT) to improve exposure, which is of great value for more effective tumor resection and renal reconstruction. In this article, we present this technique and evaluate its feasibility and efficacy in a retrospective case-control comparative study.
Methods
Patients
From November 2019 to March 2021, a total of 28 consecutive patients with renal ventral tumor (≤7cm) on computed tomography or magnetic resonance imaging underwent rRAPN. Seventeen cases had prior abdominal surgery and 11 cases had multiple renal arteries. No lymph nodes or renal vessels were involved in all tumors. Tumor complexity was evaluated according to the R.E.N.A.L. score [12,13,14]. Finally, ITT was performed in 20 cases with 12 cases that had prior abdominal surgery and 8 cases that had multiple renal arteries (ITT group). Eight cases underwent conventional rRAPN (traditional technique group). The metastatic cases were excluded by computed tomography, radionuclide bone imaging, or other specific scans according to clinical indication. Blood sample analysis was performed preoperatively and postoperatively. All procedures were performed by the same surgeon. The data of patients’ demographic characteristics, tumor sizes, R.E.N.A.L scores, preoperative laboratory results, warm ischemic time, estimated blood loss, operation-related complications, pathologic results, postoperative hospital stay, and retroperitoneal drainage were collected retrospectively. All the patients were followed postoperatively according to the recommendation of the EAU guideline [15].
The study was approved by Yantai Yuhuangding Hospital Ethics Committee. Written informed consent was obtained by the participants. The patients were all informed that their clinical data might be used in future study without invasion of privacy during hospitalization.
Surgical technique
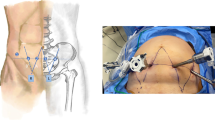
All patients underwent da Vinci-assisted partial nephrectomy. The patient was placed in the full flank position and the table fully flexed to increase the space between the 12th rib and iliac crest. The first trocar was placed at the midpoint of the midaxillary line midway between the costal margin and the iliac crest. The retroperitoneal space was first established by blunt dissection, with further extension by a handmade balloon (inflated at 800–1000 mL), and a 12-mm camera port was placed. The two 8-mm robotic instrument ports were placed in the anterior axillary line and posterior axillary line 7–8 cm from the camera port, respectively. After docking, the psoas muscle was identified as the first landmark and the camera was turned so that the muscle was in a horizontal line. Gerota’s fascia was incised after the extraperitoneal fat was removed. Both renal artery and vein were then dissected to allow for adequate closing pressure during cross clamping with bulldog clamps. The perinephric fat along the surface of the kidney was carefully separated. Most of the perirenal fat layer, especially the dorsal fat of the kidney, was removed to enhance the following traction effect. However, the perinephric fat at the lateral edge of the kidney was reserved for traction. The amount of perirenal fat retained depended on the degree of adhesion between the kidney and fat. The ultrasound probe was used to identify the borders and depth of the mass when necessary. Often, renal ventral tumor (Fig. 1) is not satisfactorily exposed (Fig. 2a). The renal artery was clamped using laparoscopic bulldog clamps and then mark the time for WIT.
The perirenal fat was pulled towards the psoas major muscle with a unidirectional barbed suture to make an appropriate surgery field and for an optimal straight tumor exposure (Fig. 2b). A Hem-o-lok clip was applied to maintain the tension of sutures.
Then, the renal tumor and kidney tissue were excised using the scissors with 0.1–0.5 cm from the tumor margin (Fig. 2c). The traction of the perirenal fat on the tumor was maintained during resection. The blood vessels and collecting system were closed with a 2-0 unidirectional barbed suture. After the final tissue bite, a Hem-o-lok clip was applied at the free end of the suture. Then, the second layer suture was continuously performed with a 2-0 unidirectional barbed suture to close the edge of the parenchyma by the same method (Fig. 2d). Additional movie files show the procedure in more detail (see Additional files 1, 2, 3, 4 and 5).
Statistical analysis
All relevant data were analyzed statistically using Student’s t-test, and P < 0.05 was considered statistically significant.
Results
The characteristics and perioperative outcomes of the study cohort are listed in Table 1. There were no significant differences between the two groups in mean age, gender, body mass index, tumor size, operation time, and R.E.N.A.L. score.
The mean WIT in the ITT group was 17.10 min which was significantly shorter (P < 0.05) than in the traditional technique group at 24.63 min. Estimated blood loss in the traditional technique group was 324.88±79.42 mL, and in the ITT group, it was 117.45±35.25 mL (P < 0.05). Suture time and separating kidney time were 1.93±0.29 min and 18.63±3.78 min, respectively. The total duration of the ITT (suture time + separating kidney time) in the ITT group was 20.55±3.72 min.
All the postoperative Scr levels were within normal limits. There were no significant differences between the ITT group and the traditional technique group in postoperative hospital stay and the retroperitoneal drainage (P > 0.05).
Pathological characteristics and postoperative complications are summarized in Table 2. On pathology, the rate of clear cell renal cell carcinoma was 90% (n = 18) in the ITT group and 87.5% (n = 7) in the traditional technique group. No positive surgical margin was found in all cases. All the patients were followed as the recommended schedule. No local recurrence was recorded. One patient in the ITT group underwent urinary tract infection and recovered after 1 week of intervention. No ileus, hemorrhage, perirenal fluid collection, or urine leak occurred.
Discussion
Laparoscopic partial nephrectomy has been shown to be an accepted, safe, and feasible treatment option for small localized renal masses [16,17,18]. With the introduction of the robotic technique, robot-assisted partial nephrectomy has become increasingly widespread for the management of small renal masses. Robot-assisted partial nephrectomy has achieved a decrease in postoperative complications and operative time compared to open partial nephrectomy [19, 20]. The robotic technique allows surgeons to overcome many of the technical challenges of pure laparoscopic surgery thereby shortening the learning curve [21,22,23]. Robot-assisted partial nephrectomy is demonstrated to be superior to conventional laparoscopic partial nephrectomy in terms of estimated blood loss and WIT, because of the 3D vision and precise dissection of the robotic system [5, 24]. A retroperitoneal approach is more suitable for patients with prior intra-abdominal surgery or multiple arteries [8, 9]. The approach avoided excessive interference of abdominal organs and reduced operation time [6, 25,26,27]. However, the renal ventral tumor with poor tumor exposure would hinder the tumor resection and extend the WIT, which limits the range of the application of rRAPN.
Feliciano et al. used an additional mechanical arm during rRAPN, which could reduce the complications and positive surgical margins caused by poor exposure [28]. However, this approach consumed additional instruments or assistants, which increased medical cost and reduced the operation space. In order to optimize tumor exposure in rRAPN, we developed a novel internal traction technique.
In this study, the psoas major exerted traction on the kidney during tumor resection and the tumor exposure was improved. With the ITT, we could stabilize the tumor in position and maintain the traction during tumor incision without adding an additional trocar. The study indicates that the WIT was significantly reduced using the new technique. WIT has been considered a significant determinant in postoperative Scr. A WIT of <25–30 min is the widely recommended standard at which any acute kidney injury is considered reversible, and multiple studies have shown worsening functional outcomes associated with WITs >25 min [29,30,31]. While ITT spent an average of 2 additional minutes for suturing, total WIT decreased. In the present study, mean WIT was 17.10 min in the ITT group, which was considered sufficiently short. Most importantly, shorter WIT may result in better renal function recovery. Separating the kidney without resecting the outer renal edge fatty tissue is the key procedure of the technique. The brittle of adherent perinephric adipose tissues determined the amount of perirenal fat retained [32]. With the approach, the renal ventral tumor was fully exposed without adding an additional trocar and the medical cost was reduced.
With the application of our technique, the precision and stability of the tumor excision were improved, which may reduce the risk of cutting into the tumor capsule. In our study, all the patients who underwent our new procedure had negative surgical margins on histology. There was no difference in complication rates between the two groups. None of the patients showed evidence of local recurrence or metastatic disease at a median follow-up of 10 months.
The limitations of our study include the small sample size and single institution nature. A larger sample size with longer follow-up periods is warranted to confirm the value of the technique.
Conclusion
Our initial experience suggests that the internal traction method is a safe and feasible procedure for the renal ventral tumors with prior intra-abdominal surgery or multiple renal arteries.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- rRAPN:
-
Retroperitoneal robot-assisted partial nephrectomy
- ITT:
-
Internal traction technique
- WIT:
-
Warm ischemic time
- BMI:
-
Body mass index
- RCC:
-
Renal cell carcinoma
- Scr:
-
Serum creatinine
References
Touijer K, Jacqmin D, Kavoussi LR, Montorsi F, Patard JJ, Rogers CG, et al. The expanding role of partial nephrectomy: a critical analysis of indications, results, and complications. Eur Urol. 2010. https://doi.org/10.1016/j.eururo.2009.10.019.
Mir MC, Derweesh I, Porpiglia F, Zargar H, Mottrie A, Autorino R. Partial nephrectomy versus radical nephrectomy for clinical T1b and T2 renal tumors: a systematic review and meta-analysis of comparative studies. Eur Urol. 2017. https://doi.org/10.1016/j.eururo.2016.08.060.
Bertolo R, Autorino R, Simone G, Derweesh I, Garisto JD, Minervini A, et al. Outcomes of robot-assisted partial nephrectomy for clinical T2 renal tumors: a multicenter analysis (ROSULA Collaborative Group). Eur Urol. 2018. https://doi.org/10.1016/j.eururo.2018.05.004.
Novara G, La Falce S, Kungulli A, Gandaglia G, Ficarra V, Mottrie A. Robot-assisted partial nephrectomy. Int J Surg. 2016. https://doi.org/10.1016/j.ijsu.2016.05.073.
Ludwig WW, Gorin MA, Pierorazio PM, Allaf ME. Frontiers in robot-assisted retroperitoneal oncological surgery. Nat Rev Urol. 2017. https://doi.org/10.1038/nrurol.2017.149.
Viterbo R, Greenberg RE, Al-Saleem T, Uzzo RG. Prior abdominal surgery and radiation do not complicate the retroperitoneoscopic approach to the kidney or adrenal gland. J Urol. 2005. https://doi.org/10.1097/01.ju.0000165654.34635.ad.
Mittakanti HR, Heulitt G, Li HF, Porter JR. Transperitoneal vs. retroperitoneal robotic partial nephrectomy: a matched-paired analysis. World J Urol. 2020. https://doi.org/10.1007/s00345-019-02903-7.
Abdullah N, Rahbar H, Barod R, Dalela D, Larson J, Johnson M, et al. Multicentre outcomes of robot-assisted partial nephrectomy after major open abdominal surgery. BJU Int. 2016. https://doi.org/10.1111/bju.13408.
Singh D, Finelli A, Rubinstein M, Desai MM, Kaouk J, Gill IS. Laparoscopic partial nephrectomy in the presence of multiple renal arteries. Urology. 2007. https://doi.org/10.1016/j.urology.2006.10.047.
Sharma V, Margreiter M. Partial nephrectomy: is there still a need for open surgery? Curr Urol Rep. 2013. https://doi.org/10.1007/s11934-012-0297-2.
Petros FG, Keskin SK, Yu KJ, Li R, Metcalfe MJ, Fellman BM, et al. Intraoperative conversion from partial to radical nephrectomy: incidence, predictive factors, and outcomes. Urology. 2018. https://doi.org/10.1016/j.urology.2018.03.017.
Borgmann H, Reiss AK, Kurosch M, Filmann N, Frees S, Mager R, et al. R.E.N.A.L. Score outperforms PADUA score, C-index and DAP score for outcome prediction of nephron sparing surgery in a selected cohort. J Urol. 2016. https://doi.org/10.1016/j.juro.2016.03.176.
Dubeux VT, Zanier JFC, Gabrich PN, Carrerette FB, Milfont JCA, Damião R. Practical evaluation of the R.E.N.A.L. score system in 150 laparoscopic nephron sparing surgeries. Int Braz J Urol. 2022. https://doi.org/10.1590/s1677-5538.Ibju.2021.0424.
Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009. https://doi.org/10.1016/j.juro.2009.05.035.
Capogrosso P, Capitanio U, La Croce G, Nini A, Salonia A, Montorsi F, et al. Follow-up after treatment for renal cell carcinoma: the evidence beyond the guidelines. Eur Urol Focus. 2016. https://doi.org/10.1016/j.euf.2015.04.001.
Bravi CA, Larcher A, Capitanio U, Mari A, Antonelli A, Artibani W, et al. Perioperative outcomes of open, laparoscopic, and robotic partial nephrectomy: a prospective multicenter observational study (The RECORd 2 Project). Eur Urol Focus. 2021. https://doi.org/10.1016/j.euf.2019.10.013.
Wang J, Lu Y, Wu G, Wang T, Wang Y, Zhao H, et al. The role of three-dimensional reconstruction in laparoscopic partial nephrectomy for complex renal tumors. World J Surg Oncol. 2019. https://doi.org/10.1186/s12957-019-1701-x.
Janssen MWW, Linxweiler J, Philipps I, Bütow Z, Siemer S, Stöckle M, et al. Kidney autotransplantation after nephrectomy and work bench surgery as an ultimate approach to nephron-sparing surgery. World J Surg Oncol. 2018. https://doi.org/10.1186/s12957-018-1338-1.
Shen Z, Xie L, Xie W, Hu H, Chen T, Xing C, et al. The comparison of perioperative outcomes of robot-assisted and open partial nephrectomy: a systematic review and meta-analysis. World J Surg Oncol. 2016. https://doi.org/10.1186/s12957-016-0971-9.
Nishimura K, Sawada Y, Sugihara N, Funaki K, Koyama K, Noda T, et al. A low RENAL Nephrometry Score can avoid the need for the intraoperative insertion of a ureteral catheter in robot-assisted partial nephrectomy. World J Surg Oncol. 2021. https://doi.org/10.1186/s12957-021-02146-0.
Larcher A, Muttin F, Peyronnet B, De Naeyer G, Khene ZE, Dell'Oglio P, et al. The learning curve for robot-assisted partial nephrectomy: impact of surgical experience on perioperative outcomes. Eur Urol. 2019. https://doi.org/10.1016/j.eururo.2018.08.042.
Omidele OO, Davoudzadeh N, Palese M. Trifecta outcomes to assess learning curve of robotic partial nephrectomy. JSLS. 2018. https://doi.org/10.4293/jsls.2017.00064.
Hanzly M, Frederick A, Creighton T, Atwood K, Mehedint D, Kauffman EC, et al. Learning curves for robot-assisted and laparoscopic partial nephrectomy. J Endourol. 2015. https://doi.org/10.1089/end.2014.0303.
Shiroki R, Fukami N, Fukaya K, Kusaka M, Natsume T, Ichihara T, et al. Robot-assisted partial nephrectomy: superiority over laparoscopic partial nephrectomy. Int J Urol. 2016. https://doi.org/10.1111/iju.13001.
Choo SH, Lee SY, Sung HH, Jeon HG, Jeong BC, Jeon SS, et al. Transperitoneal versus retroperitoneal robotic partial nephrectomy: matched-pair comparisons by nephrometry scores. World J Urol. 2014. https://doi.org/10.1007/s00345-014-1312-7.
Harke NN, Darr C, Radtke JP, von Ostau N, Schiefelbein F, Eraky A, et al. Retroperitoneal versus transperitoneal robotic partial nephrectomy: a multicenter matched-pair analysis. Eur Urol Focus. 2020. https://doi.org/10.1016/j.euf.2020.08.012.
Guo Y, Xu Q, Chen B, Liu L, Wang Y, Zhu A, et al. Clinical outcomes and effect on intraoperative blood loss and postoperative pain of patients undergoing retroperitoneal laparoscopic partial nephrectomy for complex renal tumors. World J Surg Oncol. 2021. https://doi.org/10.1186/s12957-021-02397-x.
Feliciano J, Stifelman M. Robotic retroperitoneal partial nephrectomy: a four-arm approach. JSLS. 2012. https://doi.org/10.4293/108680812x13427982376149.
Rod X, Peyronnet B, Seisen T, Pradere B, Gomez FD, Verhoest G, et al. Impact of ischaemia time on renal function after partial nephrectomy: a systematic review. BJU Int. 2016. https://doi.org/10.1111/bju.13580.
Lane BR, Gill IS, Fergany AF, Larson BT, Campbell SC. Limited warm ischemia during elective partial nephrectomy has only a marginal impact on renal functional outcomes. J Urol. 2011. https://doi.org/10.1016/j.juro.2010.12.046.
Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A, Frank I, et al. Renal function after partial nephrectomy: effect of warm ischemia relative to quantity and quality of preserved kidney. Urology. 2012. https://doi.org/10.1016/j.urology.2011.10.031.
Fang L, Li H, Zhang T, Liu R, Zhang T, Bi L, et al. Analysis of predictors of adherent perinephric fat and its impact on perioperative outcomes in laparoscopic partial nephrectomy: a retrospective case-control study. World J Surg Oncol. 2021. https://doi.org/10.1186/s12957-021-02429-6.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National Natural Science Foundation of China (Nos. 81972376).
Author information
Authors and Affiliations
Contributions
XLJ, KO, RY, JTW, and HWZ participated in its design and coordination and drafted the manuscript. XYY and DDY conceived of the study and participated in its design and coordination and helped to edit the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by Yuhuangding Hospital Ethics Committee. Written informed consent was obtained by the participants.
Consent for publication
Informed consent for publication was obtained from all authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Poor tumor exposure.
Additional file 2. Part of perirenal fat layer was removed.
Additional file 3. The renal artery was dissected and clamped.
Additional file 4. The kidney was pulled to the psoas major.
Additional file 5. The tumor was exposed clearly and excised.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jiang, XL., OuYang, K., Yang, R. et al. The application of internal traction technique in retroperitoneal robot-assisted partial nephrectomy for renal ventral tumors. World J Surg Onc 20, 213 (2022). https://doi.org/10.1186/s12957-022-02684-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-022-02684-1