Abstract
Background
Ovarian clear cell carcinoma (OCCC) is one of the most lethal types of ovarian cancer. Early-stage OCCC can be cured by surgery; however, advanced-stage disease shows poor prognosis due to chemoresistance unlike the more common high-grade serous carcinoma.
Methods
We explored the differential roles of the Wip1–p38–p53 DNA damage response pathway in respective early- or advanced-stage OCCC by immunohistochemistry of Wip1, phospho-p38, p53, and phospho-p53 from consecutive 143 patients.
Results
High Wip1 expression correlated with positive p53 (p=0.011), which in turn correlated with low nuclear phospho-p38 expression (p=0.0094). In the early stages, positive p53 showed trends toward worse overall survival (OS) (p=0.062), whereas in the advanced stages, high Wip1 correlated with worse OS (p=0.0012). The univariate and multivariate analyses of prognostic factors indicated that high Wip1 was significant and independent for worse OS (p=0.011) in the advanced stages, but not in the early stages. Additionally, high Wip1 showed trends toward shorter treatment-free interval (TFI) in the advanced stages, but not in the early stages (p=0.083 vs. 0.93). Furthermore, high Wip1 was significantly associated with positive p53 only in the patients with shorter TFI (<6 months), but not in those with longer TFI (≥6 months) (p=0.036 vs. 0.34).
Conclusions
Wip1 appears to play a crucial role for the prognosis of OCCC through chemoresistance specifically in the advanced stages, implicating that Wip1 possibly serves as a reasonable therapeutic target for improving chemoresistance and poor prognosis of advanced-stage OCCC.
Similar content being viewed by others
Background
Ovarian clear cell carcinoma (OCCC), one of the most lethal types of ovarian cancer, tends to be diagnosed at an early stage which can be cured by surgery. However, advanced-stage OCCC shows poor prognosis due to resistance to platinum-based chemotherapy unlike the more common high-grade serous carcinoma [1,2,3]. To date, various molecular markers have been suggested to predict prognosis and to serve as possible therapeutic targets in ovarian cancer [4,5,6,7,8]. However, no consensus has been reached yet, especially on specific markers for predicting refractory biological properties of advanced-stage OCCC. DNA damage response is one of the important pathways to ensure genomic integrity. When chronic DNA damage is not repairable, the cells either undergo apoptosis or extend proliferative block. Cells with dysfunctional cell cycle checkpoints and/or apoptotic responses potentially lead to the immortalization of genomic aberrations and tumorigenesis. Prominent events in the early responses induced by DNA damage include the activation of the stress-responsive p38 cascades [9] and the activation of the tumor suppressor p53 [10]. The main function of p38 was confirmed to induce apoptosis [11], and p53 plays a central role in maintaining genomic integrity by preventing the progression of cell cycle or inducing apoptosis after cellular stresses including DNA damage [12]. Wip1 is an oncogene, which is a negative regulator of the p38–p53 signaling pathway through direct and indirect mechanisms. Wip1 directly dephosphorylates and inactivates p53. Wip1 also inactivates p53 through dephosphorylating and inactivating modulators such as p38, Chk1/2, and ATM [13, 14]. Wip1 amplification has been suggested to be associated with a poor prognosis of OCCC [15, 16]. However, the prognostic significance of the Wip1–p38–p53 DNA damage response pathway in OCCC remains to be elucidated. Thus, the aim of our study was to explore the differential prognostic roles of this pathway in early/advanced-stage OCCC. Our findings provide useful information for formulating novel therapeutic strategies for improving the chemoresistance and poor prognosis in advanced-stage OCCC.
Methods
Patients and specimens
All patients diagnosed with OCCC who received primary surgery between 1987 and 2016 at the University of Tsukuba Hospital were identified through our database. A total of 143 patients with tumors of pure clear cell histology or mixed histology with clear cell component >50% were included, and the medical records were reviewed. All samples were obtained by the opt-out approach according to the study protocol approved by the Ethics Committee University of Tsukuba Hospital (H26-118). A median follow-up period excluding patients who died was 103 months (range, 7–250 months). Follow-up data were retrieved until 2020-12-1. Overall survival (OS) was defined as the interval between the primary treatment and the last follow-up. Treatment-free interval (TFI) was defined as the interval between the end of the primary adjuvant chemotherapy and recurrence. Staging was conducted based on the criteria of the International Federation of Gynecology and Obstetrics (FIGO, 2014). Treatment of patients was described previously [17]. Table 1 summarizes the patient characteristics.
Immunohistochemistry (IHC)
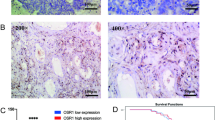
IHC procedures were described previously [17]. Antibodies used were Wip1 (F-10) (mouse monoclonal, 1:100, Santa Cruz, Dallas, TX, USA), p53 (DO-7) (mouse monoclonal, 1:200, Dako, Tokyo, Japan), phospho-p53 (S15) (rabbit polyclonal, 1:1000, Abcam, Cambridge, UK), and phospho-p38 MAPK (Thr180/Tyr182) (D3F9) (rabbit monoclonal, 1:1000, Cell Signaling, Danvers, MA, USA). The representative images of staining are displayed in Fig. 1 [17].
IHC scoring
Semiquantitative immunoreactions were assigned by two investigators (CX and TM), and any discrepancies were resolved by conferring over a microscope. For Wip1 and phospho-p53, the nuclear staining was scored by multiplying the percentages of positive tumor cells (0, no positive cell; 1, <10%; 2, 10–50%; and 3, 50%< positive tumor cells) by the most prevalent degree of staining (0, no staining; 1, weak; 2, moderate; and 3, strong). For phosho-p38, nuclear and cytoplasmic staining was separately scored in the same way. P53 staining was evaluated as previously described [17].
Statistical analyses
Differences in proportions were compared by Fisher’s exact test. Differences in continuous variables were compared by the Mann-Whitney U test. The optimal cut-off values of IHC scores for the relationships with OS were determined by the K-Adaptive partitioning method (Table 2) [18]. Survival curves were generated by the Kaplan-Meier method and statistically compared by the log-rank test. The univariate and multivariate analyses were conducted using the Cox proportional hazard model. P-values less than 0.05 were considered statistically significant. All statistical analyses were performed using R version 3.5.3.
Results
We first examined the relationships among the expressions of Wip1, p53, phospho-p53, and nuclear/cytoplasmic phospho-p38. High Wip1 expression was found to be significantly associated with positive p53 expression, which was significantly associated with low nuclear phospho-p38 expression (p=0.011 and 0.0094; Fig. 2). We further examined those relationships separately in the patients with or without lymph node metastasis. High Wip1 was significantly associated with positive p53, which was significantly associated with low nuclear phospho-p38 only in the patients without lymph node metastasis (p=0.029 and 0.028; Fig. 3A), but no significant association was found in those with lymph node metastasis (Fig. 3B).
Secondly, we examined the associations between the protein expressions and clinicopathological factors. In the early-stage diseases, positive peritoneal cytology was significantly associated both with low Wip1 expression and with high phospho-p53 expression (p=0.011 and 0.017; Table 3). In the advanced-stage diseases, low cytoplasmic phospho-p38 was significantly associated with present residual tumor and showed a trend toward stage IV disease (p=0.036 and 0.073, respectively; Table 4). Low phospho-p53 also showed a trend toward present residual tumor (p=0.077; Table 4).
Next, we compared the patient OS according to the protein expressions. High Wip1 expression showed a significant association with worse OS in the advanced stages, but no significant difference in the early stages (p=0.0012 and 0.46, respectively; Fig. 4F, A). Positive p53 showed a trend toward worse OS in the early stages, but no difference in the advanced stages (p=0.062 and 0.96, respectively; Fig. 4B, G). As regards phospho-p53, high expression showed a trend toward better OS in the advanced stages (p=0.083; Fig. 4H). As for cytoplasmic phospho-p38, high cytoplasmic phospho-p38 showed a trend toward better OS, but no difference in the early stages (p=0.089 and 0.55, respectively; Fig. 4J, E).
Kaplan-Meier curves for overall survival based on the protein expressions. A Patients with early-stage tumors expressing high vs. low Wip1 (n=18 vs. 84). B Patients with early-stage tumors expressing positive vs. negative p53 (n=21 vs. 81). C Patients with early-stage tumors expressing high vs. low phospho-p53 (n=57 vs. 45). D Patients with early-stage tumors expressing high vs. low nuclear phospho-p38 (n=50 vs. 52). E Patients with early-stage tumors expressing high vs. low cytoplasmic phospho-p38 (n=9 vs. 93). F Patients with advanced-stage tumors expressing high vs. low Wip1 (n=5 vs. 36). G Patients with advanced-stage tumors expressing positive vs. negative p53 (n=14 vs. 27). H Patients with advanced-stage tumors expressing high vs. low phospho-p53 (n=38 vs. 3). I Patients with advanced-stage tumors expressing high vs. low nuclear phospho-p38 (n=38 vs. 3). J Patients with advanced-stage tumors expressing high vs. low cytoplasmic phospho-p38 (n=30 vs. 11). OS, overall survival
Subsequently, we performed the univariate and multivariate analyses of various prognostic factors for OS. In the early-stage diseases, positive peritoneal cytology and present residual tumor were found to be significant and independent for poor OS (p=0.00043 and 0.0074, respectively; Table 5), whereas in the advanced stages, high Wip1 expression was found to be significant and independent for poor OS (p=0.011; Table 6).
Lastly, we compared TFI according to the protein expressions. Interestingly, positive Wip1 showed a trend toward shorter TFI in advanced stages, but no difference in early stages (p=0.083 and 0.93, respectively; Fig. 5). The other proteins showed no association with TFI in either the early or advanced stages (Fig. 5). Furthermore, we examined the relationships between the protein expressions and the p53 status, separately in the patients with longer TFI (≥ 6 months: chemosensitive) and those with shorter TFI (< 6 months: chemoresistant). High Wip1 was significantly associated with positive p53 only in the chemoresistant group, but not in the chemosensitive group (p=0.036 and 0.34, respectively; Fig. 6).
Comparison of treatment-free interval (days) between patients. A Early-stage tumors expressing low vs. high Wip1 (n=19 vs. 3), negative vs. high p53 (n=15 vs. 7), low vs. high phospho-p53 (n=2 vs. 20), low vs. high nuclear p38 (n=11 vs. 11), and low vs. high cytoplasmic p38 (n=20 vs. 2). B Advanced-stage tumors expressing low vs. high Wip1 (n=24 vs. 5), negative vs. high p53 (n=18 vs. 11), low vs. high phospho-p53 (n=3 vs. 26), low vs. high nuclear p38 (n=1 vs. 28), and low vs. high cytoplasmic p38 (n=8 vs. 21)
Discussion
Previously, high mRNA expression of PPM1D, which encodes Wip1, was reported to significantly correlate with poor survival in OCCC [15, 16]. Recently, abnormal p53 status has been reported to be significantly associated with poor survival in OCCC [19, 20]. Accordingly, the DNA damage response pathway seems to be playing critical roles for the survival of OCCC. Thus, we explored the prognostic significance of the Wip1–p38–p53 axis in the disease. Our IHC analyses indicated that p53 positivity was significantly associated both with high Wip1 expression and with low nuclear phospho-p38 expression (Fig. 2). Wip1 downregulates p53 and nuclear p38 upregulates p53 in the DNA damage response pathway, and positive p53 should correspond to the aberrant inactive protein. Although the detailed mechanism underlying the protein expression profile requires further elucidation, this pathway is suggested to function significantly in the pathogenesis of OCCC.
In our analyses of the associations between the protein expressions and the clinicopathological factors, low cytoplasmic phospho-p38 was found to be significantly associated with present residual tumor and showed a trend toward stage IV disease in the advanced-stage diseases (Table 4). Moreover, patients with low cytoplasmic phospho-p38 showed a trend toward worse OS compared with those with high cytoplasmic phospho-p38 (Fig. 4J). P38 has been reported to activate MMP-2/9 and increase invasive capacity in various types of tumor cells [11, 21]. Therefore, our result may keep in line with this published finding, as upregulation of nuclear p38 may exert tumor progression through transcription, and low cytoplasmic phospho-p38 may correspond to the activated nuclear p38 through nucleocytoplasmic shuttling.
Our survival analyses indicated that patients with positive p53 showed a trend toward worse OS compared with those with negative p53 in the early-stage diseases, while no difference in OS was observed according to p53 status in the advanced-stage diseases (Fig. 4B, G). In our univariate analysis for prognostic factors as well, positive p53 showed a trend toward worse OS in the early-stage diseases in addition to the well-known significant prognostic factors, peritoneal cytology, and residual tumor (Table 5) [22, 23], but not in the advanced-stage diseases (Table 6). Although abnormal p53 status is reported to be associated with poor survival [19, 20], our findings suggest that the prognostic role of p53 in OCCC may be confined to the early-stage diseases.
Patients with high Wip1 showed significantly worse OS compared with those with low Wip1 in the advanced-stage diseases, but not in the early-stage diseases (Fig. 4F, A). Furthermore, the univariate analysis indicated that high Wip1 was a significant factor for poor OS in addition to the well-known prognostic factor, residual tumor in the advanced-stage diseases (Table 6). The subsequent multivariate analysis revealed that high Wip1 was significant and independent for poor OS in the advanced-stage diseases (Table 6). However, no clinicopathologic factor was found to be associated with Wip1 expression in the advanced stages (Table 4). We further compared TFI based on the protein expressions, attempting to identify the underlying mechanism of the prognostic role of Wip1. High Wip1 showed a trend toward shorter TFI in the advanced-stage diseases but not in the early-stage diseases (Fig. 5). Moreover, high Wip1 was significantly associated with positive p53 only in the patients with shorter TFI, but not in those with longer TFI (Fig. 6). These findings suggest that Wip1 may be involved in chemoresistance in the advanced disease of OCCC, as TFI is well known to be an important surrogate marker for the chemosensitivity in ovarian cancer [24,25,26]. This hypothesis may be supported by the published findings that Wip1 is involved in chemoresistance in other types of cancer cells [27, 28]. Chemoresistance is known to be related with low activity of cellular proliferation. Therefore, our finding that both low Wip1 and high phospho-p53 were significantly associated with positive peritoneal cytology (Table 3) seems consistent with the hypothesis, as Wip1 and its downstream target p53 may be related also with indolent tumor progression. Additionally, our finding that positive p53 was found to be significantly associated both with high Wip1 and with low nuclear phospho-p38 only in the patients without lymph node metastasis, but not in those with lymph node metastasis, also suggests the possible involvement of the Wip1–p38–p53 pathway in indolent tumor progression (Fig. 3).
The present study contains a couple of limitations. First, the retrospective design causes possible selection biases. Second, the evaluation for the protein expressions is based only on the semiquantitative immunohistochemical analysis, and bioinformatics analysis is also lacking. Third, the sample number is relatively small. Nevertheless, our above findings keeping in line with multiple publications may support the validity of our study.
Conclusions
Early-stage OCCC can be cured by complete surgical resection, while advanced-stage OCCC shows poor prognosis due to the chemoresistance of residual tumor [1,2,3]. Therefore, our above findings suggest that Wip1 may be a reasonable therapeutic target for improving the poor prognosis of advanced-stage OCCC through enhancing chemosensitivity. A combination of Wip1 inhibitor with chemotherapeutic agents may be useful for advanced-stage OCCC tumors expressing high Wip1. As regards p53-mutated high-Wip1 OCCC, a combination of Wip1 inhibitor with APR-246 [29,30,31] may be useful by both reactivating mutant p53 and inhibiting the Wip1-mediated downregulation of p53. Further basic and clinical studies are warranted to verify our proposal in order to develop novel strategies for overcoming chemoresistance of OCCC.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- FIGO:
-
International Federation of Gynecology and Obstetrics
- IHC:
-
Immunohistochemistry
- OCCC:
-
Ovarian clear cell carcinoma
- OS:
-
Overall survival
- TFI:
-
Treatment-free interval
References
Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, et al. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer. 2000;88:2584–9.
Takano M, Kikuchi Y, Yaegashi N, Kuzuya K, Ueki M, Tsuda H, et al. Clear cell carcinoma of the ovary: a retrospective multicentre experience of 254 patients with complete surgical staging. Br J Cancer. 2006;94:1369–74.
Itamochi H, Kigawa J, Sugiyama T, Kikuchi Y, Suzuki M, Terakawa N. Low proliferation activity may be associated with chemoresistance in clear cell carcinoma of the ovary. Obstet Gynecol. 2002;100:281–7.
Amano T, Chano T, Isono T, Kimura F, Kushima R, Murakami T. Abundance of mitochondrial superoxide dismutase is a negative predictive biomarker for endometriosis-associated ovarian cancers. World J Surg Oncol. 2019;17:24.
Li W, Ma JA, Sheng X, Xiao C. Screening of CXC chemokines in the microenvironment of ovarian cancer and the biological function of CXCL10. World J Surg Oncol. 2021;19:329.
Zhan Y, Wu X, Zheng G, Jin J, Li C, Yu G, et al. Proline-rich protein 11 overexpression is associated with a more aggressive phenotype and poor overall survival in ovarian cancer patients. World J Surg Oncol. 2020;18:318.
Feng S, Luo S, Ji C, Shi J. miR-29c-3p regulates proliferation and migration in ovarian cancer by targeting KIF4A. World J Surg Oncol. 2020;18:315.
Liu X, Liu C, Zhang A, Wang Q, Ge J, Li Q, et al. Long non-coding RNA SDCBP2-AS1 delays the progression of ovarian cancer via microRNA-100-5p-targeted EPDR1. World J Surg Oncol. 2021;19:199.
Kyriakis JM, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271:24313–6.
Ko LJ, Prives C. p53: puzzle and paradigm. Genes Dev. 1996;10:1054–72.
Koul HK, Pal M, Koul S. Role of p38 MAP kinase signal transduction in solid tumors. Genes Cancer. 2013;4:342–59.
Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–31.
Wang ZP, Tian Y, Lin J. Role of wild-type p53-induced phosphatase 1 in cancer. Oncol Lett. 2017;14:3893–8.
Goloudina AR, Kochetkova EY, Pospelova TV, Demidov ON. Wip1 phosphatase: between p53 and MAPK kinases pathways. Oncotarget. 2016;7:31563–71.
Hirasawa A, Saito-Ohara F, Inoue J, Aoki D, Susumu N, Yokoyama T, et al. Association of 17q21-q24 gain in ovarian clear cell adenocarcinomas with poor prognosis and identification of PPM1D and APPBP2 as likely amplification targets. Clin Cancer Res. 2003;9:1995–2004.
Tan DS, Lambros MB, Rayter S, Natrajan R, Vatcheva R, Gao Q, et al. PPM1D is a potential therapeutic target in ovarian clear cell carcinomas. Clin Cancer Res. 2009;15:2269–80.
Abe A, Minaguchi T, Ochi H, Onuki M, Okada S, Matsumoto K, et al. PIK3CA overexpression is a possible prognostic factor for favorable survival in ovarian clear cell carcinoma. Hum Pathol. 2013;44:199–207.
Eo SH, Kang HJ, Hong SM, Cho H. K-adaptive partitioning for survival data, with an application to cancer staging. arXiv preprint arXiv. 2013;13064615.
Leskela S, Romero I, Cristobal E, Perez-Mies B, Rosa-Rosa JM, Gutierrez-Pecharroman A, et al. The frequency and prognostic significance of the histologic type in early-stage ovarian carcinoma: a reclassification study by the Spanish Group for Ovarian Cancer Research (GEICO). Am J Surg Pathol. 2020;44:149–61.
Parra-Herran C, Bassiouny D, Lerner-Ellis J, Olkhov-Mitsel E, Ismiil N, Hogen L, et al. p53, mismatch repair protein, and POLE abnormalities in ovarian clear cell carcinoma: an outcome-based clinicopathologic analysis. Am J Surg Pathol. 2019;43:1591–9.
Kumar B, Koul S, Petersen J, Khandrika L, Hwa JS, Meacham RB, et al. p38 mitogen-activated protein kinase-driven MAPKAPK2 regulates invasion of bladder cancer by modulation of MMP-2 and MMP-9 activity. Cancer Res. 2010;70:832–41.
Takano M, Sugiyama T, Yaegashi N, Suzuki M, Tsuda H, Sagae S, et al. The impact of complete surgical staging upon survival in early-stage ovarian clear cell carcinoma: a multi-institutional retrospective study. Int J Gynecol Cancer. 2009;19:1353–7.
Yamazaki H, Todo Y, Shimada C, Takeshita S, Minobe S, Okamoto K, et al. Therapeutic significance of full lymphadenectomy in early-stage ovarian clear cell carcinoma. J Gynecol Oncol. 2018;29:e19.
Colombo N, Gore M. Treatment of recurrent ovarian cancer relapsing 6-12 months post platinum-based chemotherapy. Crit Rev Oncol Hematol. 2007;64:129–38.
Harries M, Gore M. Part II: chemotherapy for epithelial ovarian cancer-treatment of recurrent disease. Lancet Oncol. 2002;3:537–45.
Markman M, Rothman R, Hakes T, Reichman B, Hoskins W, Rubin S, et al. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol. 1991;9:389–93.
Wang L, Mosel AJ, Oakley GG, Peng A. Deficient DNA damage signaling leads to chemoresistance to cisplatin in oral cancer. Mol Cancer Ther. 2012;11:2401–9.
Xia ZS, Wu D, Zhong W, Lu XJ, Yu T, Chen QK. Wip1 gene silencing enhances the chemosensitivity of human colon cancer cells. Oncol Lett. 2017;14:1875–83.
Aryee DN, Niedan S, Ban J, Schwentner R, Muehlbacher K, Kauer M, et al. Variability in functional p53 reactivation by PRIMA-1(Met)/APR-246 in Ewing sarcoma. Br J Cancer. 2013;109:2696–704.
Lambert JM, Gorzov P, Veprintsev DB, Soderqvist M, Segerback D, Bergman J, et al. PRIMA-1 reactivates mutant p53 by covalent binding to the core domain. Cancer Cell. 2009;15:376–88.
Lehmann S, Bykov VJ, Ali D, Andren O, Cherif H, Tidefelt U, et al. Targeting p53 in vivo: a first-in-human study with p53-targeting compound APR-246 in refractory hematologic malignancies and prostate cancer. J Clin Oncol. 2012;30:3633–9.
Acknowledgements
Not applicable.
Funding
This study did not receive any specific grant from funding agencies.
Author information
Authors and Affiliations
Contributions
CX performed the experiments and drafted the manuscript; TM analyzed the data and revised the manuscript; TM, NQ, KF, AS, HI, AS, NT, AA, SN, HO, and TS critically reviewed the manuscript; TM, KF, AS, HI, AS, NT, AA, SN, HO, and TS treated patients; TS supervised the study. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee University of Tsukuba Hospital (H26-118). The committee waived the requirement for informed consent due to the opt-out approach in accordance with national regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xu, C., Minaguchi, T., Qi, N. et al. Differential roles of the Wip1–p38–p53 DNA damage response pathway in early/advanced-stage ovarian clear cell carcinomas. World J Surg Onc 20, 139 (2022). https://doi.org/10.1186/s12957-022-02600-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-022-02600-7