Abstract
Background
The ten-eleven translocation 1 (TET1), which is essential for active DNA demethylation, plays a multifaceted role in the pathogenesis of colorectal cancer. The study has demonstrated the association of TET1 mutations with a high response to immune checkpoint inhibitors (ICIs) in diverse cancers. However, the relationship between TET1 mutations and the response to ICIs in colon cancer is still lacking.
Methods
The prognosis, predictive markers, immune characteristics, mutation number of DNA damage repair (DDR) pathways, pathway enrichment, and drug sensitivity conditions were all compared between TET1-mutated and wild-type patients with colon adenocarcinoma (COAD).
Results
The overall survival of patients with TET1 mutations in the ICI-treated cohort was significantly longer than those without (p = 0.0059). Compared with the wild-type patients, TET1-mutated patients had higher tumor mutational burden and neoantigen load, enhanced abundance of tumor-infiltrating immune cells, increased expression of immune-related genes, and mutation number of DDR pathways. Additionally, the patients with TET1 mutations were found to be more sensitive to lapatinib and 5-fluorouracil.
Conclusion
These findings suggest that TET1 mutations may serve as a potential biomarker for the response to ICIs in COAD patients.
Similar content being viewed by others
Background
Colorectal cancer (CRC) is the third most commonly diagnosed malignant tumor and the fourth most deadly cancer worldwide, with nearly 0.9 million deaths each year [1, 2]. Its burden is projected to increase by 60% to over 2.2 million new cases and 1.1 million deaths by 2030 [3]. Compared with rectal cancer, colon cancer is more frequent, particularly in industrialized countries [4]. Each year, approximately 0.25 million new cases of colon cancer are diagnosed in Europe, representing around 9% of all the malignancies [4]. Despite significant advances in early detection and surgery, the 5-year survival rate of patients with metastatic CRC remains low, maintaining at around 14% [5].
In recent years, immunotherapy, a therapeutic schedule that makes use of the body’s own immune system to attack cancer, has shown promise for certain cancers, such as lung cancer and melanoma [6,7,8]. In a specific subset of CRC with mismatch-repair deficiency (dMMR) and microsatellite instability-high (MSI-H), immune checkpoint inhibitors (ICIs) are also found effective [9]. Unfortunately, ICIs do not respond well in CRC patients with mismatch-repair proficiency (pMMR) and microsatellite instability-low (MSI-L) [9]. Increasing evidence suggests the association of DNA methylation with immunotherapy/anti-immunity [10, 11]. The aberration of DNA methylation is considered a determinant of the tumor response to host immune activity, and its loss can facilitate immune evasion of tumors with high mutation and copy number load [12]. In a multicenter, retrospective study, DNA methylation signature was identified to be correlated with improved progression-free survival and overall survival (OS) in patients with stage IV non-small cell lung cancer (NSCLC) treated with anti-programmed death-1 (PD-1) agents [13]. Nevertheless, there are few studies reporting the correlation between genomic aberrations of DNA methylation-associated genes and response to ICIs.
As a member of the ten-eleven translocation (TET) family of oxygenases, TET1 can oxidize 5-methylcytosine (5-mC) to 5-hydroxymethylcytosine (5-hmC), which is essential for active DNA demethylation [14]. It plays a multifaceted role in the pathogenesis of CRC and can inhibit the proliferation of colon cancer by damaging β-catenin signal pathway [15, 16]. A previous study has demonstrated the association of TET1 mutations with high responses to ICIs in NSCLC, bladder cancer, head and neck squamous cell carcinoma (HNSCC), melanoma, and esophagogastric cancer [17]. However, the relationship between TET1 mutations and the response to ICIs in colon cancer is lacking.
In this study, we comprehensively characterized the landscape of TET1 mutations in ICI-treated and non-ICI-treated colon adenocarcinoma (COAD) cohorts and then compared the copy number variants (CNVs) and prognosis between TET1-mutated and wild-type patients. Additionally, the tumor immunogenicity and antitumor immunity were also analyzed between TET1-mutated and wild-type patients.
Methods
Data source
In this study, 85 COAD patients treated by anti-PD-(L)1 monotherapy or anti-cytotoxic T-cell lymphocyte-4 (CTLA-4) combined with anti-PD-(L)1 therapy were selected from Memorial Sloan Kettering Cancer Center (MSKCC) and were considered as the ICI-treated cohort. The detailed information of these patients, such as clinical annotation, mutation data, and response data, was accessed from the cBioPortal for Cancer Genomics (http://www.cbioportal.org) [18]. As described by Hira Rizvi, the information on somatic mutations from MSKCC database was analyzed using targeted next-generation sequencing (NGS) [19].
Another 315 COAD patients not treated with any ICIs were from The Cancer Genome Atlas (TCGA), serving as the non-ICI-treated cohort. The data of gene expression (Illumina HiSeq, RNA-Seq), somatic mutations, OS, and disease-free survival (DFS) from the cBioPortal were analyzed. In addition, the Genomics of Drug Sensitivity in Cancer (GDSC) dataset used in this study was obtained from GDSC database (https://www.cancerrxgene.org) comprising the data of somatic mutations and drug sensitivity on COAD cell lines [20].
CNV analysis
The Affymetrix single-nucleotide polymorphism (SNP) 6.0 microarray data of the non-ICI-treated cohort were accessed from the cBioPortal. The CNV segments with GISTIC 2.0 were analyzed using GenePattern (https://cloud.genepattern.org/gp/pages/index.jsf) [21]. Default values were endowed for all the CISTIC 2.0 variables.
Prognostic assessment
According to the status of TET1 mutations, COAD patients in both cohorts were divided into TET1-mutated and wild-type groups. In the ICI-treated cohort, the OS between two groups was evaluated using Kaplan-Meier method and compared by the logrank test. In the non-ICI-treated cohort, both OS and DFS were assessed. The tumor mutational burden (TMB) in the non-ICI-treated cohort was calculated by utilizing nonsynonymous mutations as the raw mutation count and dividing by 38 Mb [22], but in the ICI-treated cohort, the TMB score was used.
Analysis of tumor immunogenicity and antitumor immunity
In the non-ICI-treated cohort, the abundance of tumor-infiltrating immune cells was estimated using tumor immune estimation resource (TIMER) algorithm [23]. Wilcoxon test was employed to compare the expression levels of genes related to cytolytic activity, chemokines, immune checkpoints, immune cells, antibody presentation, and antitumor immunity between TET1-mutated and wild-type groups. In this study, cytolytic activity was defined as the log average (geometric mean) of granzyme A and perforin 1 expression in transcripts per million [24]. The antitumor immune response could be reflected through the cancer immunity cycle, and the activities of the primary steps in this cancer immunity cycle were evaluated using a single sample gene set enrichment analysis (GSEA) according to the gene expression of individual samples [25].
DNA damage repair (DDR) pathway mutations and pathway enrichment
The Molecular Signatures Database (MSigDB) of the Broad Institute comprised the gene sets related to DDR pathways. In both ICI-treated and non-ICI-treated cohorts, the number of nonsynonymous mutations in DDR pathway-associated genes was compared between TET1-mutated and wild-type groups.
In the non-ICI-treated cohort, the genes with differences in expression between TET1-mutated and wild-type groups were first identified through the edgeR package and then input into the clusterProfiler package in R for GSEA.
Efficacy of targeted drugs and drug sensitivity analysis
Computational analysis of resistance (CARE), a computational method focusing on targeted therapies, was applied to assess the efficacy of targeted drugs. It could deduce the genome-wide transcriptomic signatures of drug efficacy based on cell line compound screens and identify genome-scale biomarkers for the response to targeted therapies [26]. The CARE score for each gene showed the correlation between molecular mutations and drug efficacy. Positive CARE scores represented the presence of mutations related to drug responses, while negative scores showed the presence of mutations associated with drug resistance.
The cell lines corresponding to the code of COAD in the GDCS database were assigned into two groups based on the status of TET1 mutations, and then the cells in these two groups were compared for the sensitivity to molecular-targeted drugs and chemotherapy drugs.
Statistical analysis
Enumeration data including gene mutations, gender, and drug types were compared using chi-square test or Fisher’s exact test, while measurement data like age, TMB, neoantigen load, abundance of immune cells, and immune-associated gene expression were compared by Mann-Whitney U-test. Clinical outcomes were assessed using Kaplan-Meier method and compared by the logrank test. All the statistical analyses used in this study were managed by R software (version 4.02, R Foundation, Vienna, Austria). The value of p ≤ 0.05 was considered statistically significant.
Results
Landscape of TET1 mutations in COAD
In the ICI-treated COAD cohort (n = 85), there were 7 patients harboring TET1 mutations, with the mutational frequency of 8.2%. The common mutated genes in these 7 patients were listed in Table S1. Among TET1 wild-type patients (n = 78), the top 20 mutant genes included APC (75%), TP53 (56%), KRAS (53%), PIK3CA (34%), ARID1A (27%), KMT2D (26%), PTPRS (21%), and more, while in TET1-mutated patients (n = 7), the top 20 mutant genes comprised KMT2D (86%), PTPRS (86%), KRAS (71%), KMT2C (71%), ARID1A (57%), ZFHX (57%), FAT1 (57%), PTCH1 (57%), and more (Fig. 1a). By comparison to the frequencies of mutant genes and clinical features between TET1 wild-type and mutated groups, the differences were pronounced in APC, KMT2D, PTPRS, RNF43, KMT2C, ZFHX3, FAT1, and PTCH1 mutations.
Landscape of TET1 mutations in COAD. A The frequencies of mutant genes and clinical features between TET1 wild-type and mutated patients in the ICI-treated cohort (MSKCC-COAD). B The frequencies of mutant genes and clinical features between TET1 wild-type and mutated patients in the non-ICI-treated cohort (TCGA-COAD). C Comparison on the mutation sites of TET1 gene in MSKCC- and TCGA-COAD cohorts
In the non-ICI-treated COAD cohort (n = 315), TET1 mutations occurred in 22 cases, with the mutational frequency of 7.0%. The frequent mutated genes in these 22 patients were described in Table S2. The frequencies of mutant genes and clinical features between TET1-mutated and wild-type groups were compared in Fig. 1b, where significant differences were presented in the majority of mutant genes including APC, TP53, TTN, SYNE1, MUC16, PIKDCA, and more. In addition, the mutation sites of TET1 gene in the ICI-treated cohort and non-ICI-treated cohort were analyzed. It can be seen that N1611Tfs*13 missense mutations occurred in these two cohorts (Fig. 1c).
CNV comparison
By comparing the CNVs at the chromosome arm level between TET1-mutated and wild-type patients in the non-ICI-treated cohort, we found that TET1 wild-type patients had distinct amplifications at 8p11.23, 8p24.21, 11p15.5, 13q12.2, and 17q12, as well as deletions at 4q22.1, 5q12.1, and 6q26 than those with TET1 mutations (Fig. 2). The amplifications in TET1-mutated patients were indistinctive, and some deletions were more severe, especially at 16q23.1.
Comparison of the prognosis and predictive biomarkers
In the ICI-treated cohort, the TET1-mutated patients had longer OS than the wild-type ones (p = 0.0059, Fig. 3a), while in the non-ICI-treated cohort, no significant differences were shown in both OS and DFS between TET1-mutated and wild-type patients (p = 0.290, Fig. 3b; p = 0.720, Fig. 3c). These findings suggested that COAD patients with TET1 mutations might benefit from ICI therapy.
Comparison on the prognosis and predictive biomarkers between TET1-mutated and wild-type patients. A Analysis of the overall survival (OS) in the ICI-treated cohort (MSKCC-COAD), OS (B), and disease-free survival (C) in the non-ICI-treated cohort (TCGA-COAD). D Tumor mutational burden (TMB) levels in the ICI-treated cohort and TMB and neoantigen load in the non-ICI-treated cohort
Considering that TMB and neoantigen load could serve as potential predictive biomarkers for the response to ICIs, the TMB and neoantigen load between TET1-mutated and wild-type patients were compared in this study. The results showed that the TMB and neoantigen load were significantly higher in TET1-mutated patients than in wild-type patients (Fig. 3d).
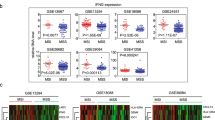
Comparison of the tumor immunogenicity and antitumor immunity
For identifying the immune features of TET1-mutated COAD, the expression levels of immune-related genes were compared between TET1-mutated and wild-type patients. By analyzing the abundance of tumor-infiltrating immune cells, we found that the CD8+ T cells, B cells, myeloid dendritic cells, and neutrophils were more abundant in TET1-mutated patients than in TET1 wild-type patients (Fig. 4a). Compared with TET1 wild-type patients, TET1-mutated patients had significantly increased expression of mRNA associated with immune cell markers (CD8+ T cells, CD8A, GZMA, GZMM, IFNG, PRF1, TBX21; natural killer cells, CRTAM, NCR1, PRR5L; Th1 cells, CTLA4, SLAMF1; dendritic cells, FGL2, IL-21R, LILRB4, SIGLEC1, SLAMF8, SLC5A3; macrophages, C1QA, CLEC5A, CYBB, LILRA2, MARCO, MS4A6A; Fig. 4b), chemokines (CCL3, CXCL10, CXCL16, CXCL9, and CCL5; Fig. 4c), immune checkpoints (CD274, CD80, CD86, TIM3, CTLA4, LAG3, PDCD1, and TIGIT; Fig. 4c), antigen presentation-related molecules (HLA-DRA, HLA-DRA1, HLA-DPB1, HLA-DQA1; Fig. 4c), and cytolytic activity (Fig. 4d) [27, 28]. In addition, regarding the antitumor immunity, TET1-mutated patients also showed higher expression levels in the release of cancer cell antigens and CD8+ T-cell recruiting than wild-type patients (Fig. 4e).
Comparison on tumor immunogenicity and antitumor immunity between TET1-mutated and wild-type patients in the non-ICI-treated cohort. A The abundance of tumor-infiltrating immune cells. B Immune cell markers. C Chemokines, immune checkpoints, and antigen presentation-related molecules. D Cytolytic activity. E The process of antitumor immune response
Comparison of the number of DDR pathway mutations
By contrast to TET1 wild-type patients, TET1-mutated patients in the ICI-treated cohort were found to have a significantly increased mutation number of DDR pathways, including nucleotide excision repair (NER), Fanconi anemia (FA), homologous recombination (HR), and nonhomologous end joining (NHEJ). In the non-ICI-treated cohort, the mutation number of six DDR pathways in TET1-mutated patients was significantly more than that in TET1 wild-type patients, such as base excision repair (BER), NER, mismatch repair (MMR), FA, HR, and NHEJ. In both cohorts, the total number of DDR pathway mutations was markedly higher in TET1-mutated patients than in wild-type ones (Fig. S1).
GSEA of TET1 mutations in COAD
The non-ICI-treated cohort was used to investigate whether TET1 mutations were involved in immune-related pathways. GSEA results revealed that the upregulation of fatty acid biosynthetic process and metabolic process was significantly enriched in TET1-mutated patients, while the downregulation of antigen processing and presentation, natural killer cell-mediated cytotoxicity, PD-L1 expression and PD-1 checkpoint pathway, interferon-gamma-mediated signaling pathway, interferon-gamma production, response to I interferon, and type I interferon signaling pathway was primarily enriched in TET1 wild-type patients (Fig. 5).
Association of TET1 mutations with drug responses and drug selection of COAD cells
In this study, the cell line data of antitumor drugs from Cancer Genome Project (CGP) were used to assess the association of TET1 mutations with drug responses. The results showed that TET1 mutations in COAD were associated with the drug resistance (Fig. 6a and b). Through analysis of GDSC dataset, TET1-mutated patients were found to be more sensitive to the molecular-targeted drug lapatinib and chemotherapy drug 5-fluorouracil, while oxaliplatin, irinotecan, cetuximab, dabrafenib, and trametinib were relatively resistant in TET1-mutated patients (Fig. 6c). These findings may provide some evidence for the drug selection in COAD patients with TET1 mutations.
Discussion
In the present study, MSKCC, TCGA, and GDSC datasets were used to investigate the effect of TET1 mutations on the response to ICIs in COAD patients. The results revealed that TET1-mutated patients had better OS, higher TMB and neoantigen load, reinforced tumor immunogenicity, activated antitumor immunity, and more mutation number of DDR pathways than those without. In addition, the patients with TET1 mutations were characterized by upregulated fatty acid biosynthetic process and metabolic process and more sensitive to lapatinib and 5-fluorouracil. These findings suggested that TET1 mutations might be a potent predictive biomarker of immune responses and contribute to the enhancement of immune responses in COAD patients.
Recently, epigenetic characteristics have emerged as a leading feature of cancers. The gene expression is under the influence of epigenetic modifications, and abnormal epigenetic modifications play important roles in tumorigenesis and progression, especially DNA cytosine hydroxymethylation [29, 30]. TET enzymes can result in DNA demethylation and gene regulation by catalyzing the oxidation of 5-mC to 5-hmC [31]. The loss of 5-hmC related to TET downregulation and/or its functional changes is considered as a hallmark of cancer [32]. In multiple cancer types like colon cancer, this dysregulation has been identified [16]. There is evidence showing that TET proteins are involved in shaping the course of immune responses [33]. Of 21 DNA methylation-associated genes detected, mutations in TET1 were observed to be significantly associated with better responses to ICIs in patients with NSCLC, bladder cancer, HNSCC, melanoma, and esophagogastric cancer [17], but its potential role in colon cancer was not analyzed. This study first assessed the relationship between TET1 mutations and responses to ICIs in COAD patients. The results exhibited that the ICI-treated patients with TET1 mutations had better OS than those without, while survival benefits were not significantly presented in the non-ICI-treated cohort, indicating that COAD patients with TET1 mutations might benefit from ICI therapies.
The tumor immunogenicity is essential for the initiation of antitumor immune responses. High-frequency somatic mutations can improve the immune killing capabilities of T cells to tumor cells by inducing tumor cells to generate more neoantigens [34]. The tumor immunogenicity is influenced by several factors like antigen processing efficiency and presentation of dendritic cells in tumor microenvironment associated with the efficacy of ICIs in CRC patients [35, 36]. For instance, enhanced CD8+ cell response was correlated with ICI therapies [37]. Moreover, T-cell inflamed gene expression profile was found to be associated with the clinical benefits of patients treated with ICIs [38]. In the study made by Jiang et al., the tumor cytotoxic T-lymphocyte levels were evaluated via the average expression levels of CD8A, CD8B, GZMA, GZMB, and PRF1, and the results showed that the survival time of highly infiltrated cytotoxic T-lymphocyte patients receiving ICIs was prolonged distinctly. CD8+ T cells were also involved in the reinforcement of immune infiltration and antitumor immunity through chemokines including CXCL9 and CXCL10 [39]. Hence, higher TMB and neoantigen load, more abundant CD8+ T cells, B cells, myeloid dendritic cells and neutrophils, and increased expression of mRNA related to cytolytic activity, chemokines, immune checkpoints, immune cell markers, and more might explain why ICIs were more effective in patients with TET1 mutations than those without.
DDR pathways play crucial roles in maintaining genomic integrity. Their aberrations can promote genomic instability and are related to high TMB. Studies have demonstrated that the mutations in DDR pathways may be a potent biomarker for predicting the response to ICIs [40, 41]. In the present study, patients with TET1 mutations in both cohorts were found to have a significantly increased mutation number of DDR pathways than those without. This might be a reason for high responses to ICIs in TET1-mutated patients. Additionally, our results also revealed the upregulation of fatty acid biosynthetic process and metabolic process in TET1-mutated patients. During tumor progression, fatty acids secreted into the microenvironment can affect the function of infiltrating immune cells and phenotypes with exacerbated cancer-stromal interactions [42]. Recent studies have shown the extraordinary importance of fatty acid cellular metabolism on T-cell differentiation [43]. In clinical settings, targeting metabolic pathways was identified to have a beneficial effect on the T-cell response [44].
One major superiority of the present study was that it first reported the correlation between TET1 mutations and the response to ICIs in COAD patients. Nevertheless, some limitations still needed to be illustrated. First, this was a retrospective study with small sample size, which might affect the statistical power. Second, the effects of TET1 mutations on the CNVs, tumor immunogenicity, antitumor immunity, GSEA, and drug sensitivity were not analyzed in the ICI-treated cohort due to use of a single-targeted NGS panel and the absence of gene expression data. Third, the patients included in the ICI-treated cohort lacked the information on the line of treatment and were treated with anti-PD-(L)1 monotherapy or CTLA-4 combined with anti-PD-(L)1 therapy; this treatment difference may influence the response to ICIs. In the future, more large-scale, prospective studies should be conducted to verify our results.
Conclusions
The present study revealed that ICI-treated COAD patients with TET1 mutations had better OS than those without, highlighting the importance of TET1 mutations in COAD patients receiving ICIs. Moreover, in the context of TET1 mutations, the tumor immunogenicity and antitumor immunity were reinforced. In short, TET1 mutations may serve as a potential biomarker for the response to ICIs in COAD patients.
Availability of data and materials
The data that support the findings of this study are available from the first author and corresponding author upon reasonable request.
Abbreviations
- CRC:
-
Colorectal cancer
- dMMR:
-
Mismatch-repair deficiency
- MSI-H:
-
Microsatellite instability-high
- ICIs:
-
Immune checkpoint inhibitors
- pMMR:
-
Mismatch-repair proficiency
- MSI-L:
-
Microsatellite instability-low
- PD-1:
-
Programmed death-1
- NSCLC:
-
Non-small cell lung cancer
- TET:
-
Ten-eleven translocation
- 5-mC:
-
5-methylcytosine
- 5-hmC:
-
5-hydroxymethylcytosine
- HNSCC:
-
Head and neck squamous cell carcinoma
- COAD:
-
Colon adenocarcinoma
- CNVs:
-
Copy number variants
- CTLA-4:
-
Cytotoxic T-cell lymphocyte-4
- MSKCC:
-
Memorial Sloan Kettering Cancer Center
- NGS:
-
Next-generation sequencing
- TCGA:
-
The Cancer Genome Atlas
- OS:
-
Overall survival
- DFS:
-
Disease-free survival
- GDSC:
-
Genomics of Drug Sensitivity in Cancer
- TMB:
-
Tumor mutational burden
- TIMER:
-
Tumor immune estimation resource
- DDR:
-
DNA damage repair
- MSigDB:
-
Molecular Signatures Database
- GSEA:
-
Gene set enrichment analysis
- CARE:
-
Computational analysis of resistance
- NER:
-
Nucleotide excision repair
- FA:
-
Fanconi anemia
- HR:
-
Homologous recombination
- NHEJ:
-
Nonhomologous end joining
- BER:
-
Base excision repair
- MMR:
-
Mismatch repair
References
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467–80.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–91.
Labianca R, Beretta GD, Kildani B, et al. Colon cancer. Crit Rev Oncol Hematol. 2010;74:106–33.
American Cancer Society. Cancer facts & figures. Atlanta: American Cancer Society; 2019. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancerfacts-and-figures/2019/cancer-facts-and-figures-2019.pdf. Accessed 10 Mar 2021.
Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378:1789–801.
Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–92.
Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;390:1853–62.
Lichtenstern CR, Ngu RK, Shalapour S, Karin M. Immunotherapy, inflammation and colorectal cancer. Cells. 2020;9:618.
Jones PA, Ohtani H, Chakravarthy A, De Carvalho DD. Epigenetic therapy in immune-oncology. Nat Rev Cancer. 2019;19:151–61.
Chiappinelli KB, Zahnow CA, Ahuja N, Baylin SB. Combining epigenetic and immunotherapy to combat cancer. Cancer Res. 2016;76:1683–9.
Jung H, Kim HS, Kim JY, et al. DNA methylation loss promotes immune evasion of tumours with high mutation and copy number load. Nat Commun. 2019;10:4278.
Duruisseaux M, Martínez-Cardús A, Calleja-Cervantes ME, et al. Epigenetic prediction of response to anti-PD-1 treatment in non-small-cell lung cancer: a multicentre, retrospective analysis. Lancet Respir Med. 2018;6:771–81.
Cheng YW, Chou CJ, Yang PM. Ten-eleven translocation 1 (TET1) gene is a potential target of miR-21-5p in human colorectal cancer. Surg Oncol. 2018;27:76–81.
Tian Y, Pan F, Sun X, et al. Association of TET1 expression with colorectal cancer progression. Scand J Gastroenterol. 2017;52:312–20.
Guo H, Zhu H, Zhang J, Wan B, Shen Z. TET1 suppresses colon cancer proliferation by impairing β-catenin signal pathway. J Cell Biochem. 2019;120:12559–65.
Wu HX, Chen YX, Wang ZX, et al. Alteration in TET1 as potential biomarker for immune checkpoint blockade in multiple cancers. J Immunother Cancer. 2019;7:264.
Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–4.
Rizvi H, Sanchez-Vega F, La K, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36:633–41.
Yang W, Soares J, Greninger P, et al. Genomics of drug sensitivity in cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 2013;41:D955–61.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40.
Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34.
Li T, Fu J, Zeng Z, et al. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48:W509–14.
Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61.
Xu L, Deng C, Pang B, et al. TIP: a web server for resolving tumor immunophenotype profiling. Cancer Res. 2018;78:6575–80.
Jiang P, Lee W, Li X, et al. Genome-scale signatures of gene interaction from compound screens predict clinical efficacy of targeted cancer therapies. Cell Syst. 2018;6(3):343–4.e5.
Thorsson V, Gibbs DL, Brown SD, et al. The immune landscape of cancer. Immunity. 2018;48(4):812–830.e14.
Huang W, Lin A, Luo P, et al. EPHA5 mutation predicts the durable clinical benefit of immune checkpoint inhibitors in patients with lung adenocarcinoma. Cancer Gene Ther. 2021;28(7-8):864–74.
Berdasco M, Esteller M. Aberrant epigenetic landscape in cancer: how cellular identity goes awry. Dev Cell. 2010;19:698–711.
Münzel M, Globisch D, Carell T. 5-Hydroxymethylcytosine, the sixth base of the genome. Angew Chem Int Ed Engl. 2011;50:6460–8.
Pastor WA, Aravind L, Rao A. TETonic shift: biological roles of TET proteins in DNA demethylation and transcription. Nat Rev Mol Cell Biol. 2013;14:341–56.
Collignon E, Canale A, Al Wardi C, et al. Immunity drives TET1 regulation in cancer through NF-κB. Sci Adv. 2018;4:eaap7309.
Lio CJ, Rao A. TET Enzymes and 5hmC in adaptive and innate immune systems. Front Immunol. 2019;10:210.
Gubin MM, Artyomov MN, Mardis ER, Schreiber RD. Tumor neoantigens: building a framework for personalized cancer immunotherapy. J Clin Invest. 2015;125:3413–21.
Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–8.
Lin A, Zhang J, Luo P. Crosstalk between the MSI status and tumor microenvironment in colorectal cancer. Front Immunol. 2020;11:2039.
Kurtulus S, Madi A, Escobar G, et al. Checkpoint blockade immunotherapy induces dynamic changes in PD-1-CD8+ tumor-infiltrating T cells. Immunity. 2019;50:181–94.e6.
Jiang P, Gu S, Pan D, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 2018;24:1550–8.
Peng D, Kryczek I, Nagarsheth N, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527:249–53.
Teo MY, Seier K, Ostrovnaya I, et al. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J Clin Oncol. 2018;36:1685–94.
Wang Z, Zhao J, Wang G, et al. Comutations in DNA damage response pathways serve as potential biomarkers for immune checkpoint blockade. Cancer Res. 2018;78:6486–96.
Corn KC, Windham MA, Rafat M. Lipids in the tumor microenvironment:from cancer progression to treatment. Prog Lipid Res. 2020;80:101055.
Lochner M, Berod L, Sparwasser T. Fatty acid metabolism in the regulation of T cell function. Trends Immunol. 2015;36:81–91.
Kidani Y, Elsaesser H, Hock MB, et al. Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nat Immunol. 2013;14:489–99.
Acknowledgements
Not applicable.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. This study was supported by The Collaborative Innovation Major Project of Zhengzhou (Grant No. 20XTZX08017).
Author information
Authors and Affiliations
Contributions
TZQ was responsible for study conceptualization and writing. XXW, FRD, XJH, and FJS participated in data collection, data analysis, and data interpretation. CS and JZ were responsible for reviewing the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Comparison on the number of DDR pathway mutations between TET1-mutated and wild-type patients in the ICI-treated (MSKCC) and non-ICI-treated (TCGA) COAD cohorts. Table S1. The common mutated genes in 7 COAD patients harboring TET1 mutations from the ICI-treated cohort (MSKCC). Table S2. The common mutated genes in 22 COAD patients with TET1 mutations from the non-ICI-treated cohort (TCGA).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Qiu, T., Wang, X., Du, F. et al. TET1 mutations as a predictive biomarker for immune checkpoint inhibitors in colon adenocarcinoma. World J Surg Onc 20, 115 (2022). https://doi.org/10.1186/s12957-022-02581-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-022-02581-7