Abstract
Objective
Provide an updated and comprehensive evaluation of the prognostic value of the albumin-fibrinogen ratio (AFR) and the fibrinogen-prealbumin ratio (FPR) for patients with cancer.
Materials and methods
Four databases (PubMed, Web of Science, Cochrane Library, and WanFang) were searched. The primary endpoints were overall survival (OS), disease-free survival (DFS), and progression-free survival (PFS). Pooled data were synthesized using StataMP 14 and expressed as hazard ratios (HRs) and 95% confidence intervals (CIs).
Results
This update examined 19 studies (7282 cases) that assessed the correlation of AFR with cancer prognosis. Pooled univariate and multivariate analyses indicated significant correlations of low AFR with poor OS (HR 2.18, 95%CI 1.87–2.55 and HR 1.75, 95%CI 1.54–2.00, respectively), poor DFS (HR 1.89, 95%CI 1.54–2.32 and HR 1.51, 95%CI 1.29–1.76, respectively), and poor PFS (HR 1.68, 95%CI 1.42–1.99 and HR 1.48, 95%CI 1.16–1.88, respectively). Pooled univariate and multivariate analyses of 6 studies (2232 cases) indicated high FPR significantly correlated with poor OS (HR 2.37, 95%CI 2.03–2.77 and HR 1.97, 95%CI 1.41–2.77, respectively). One study reported that high FPR correlated with poor DFS (univariate analysis: HR 2.20, 95%CI 1.35–3.57; multivariate analysis: HR 1.77, 95%CI 1.04–2.99) and one study reported a correlation of high FPR with poor PFS in univariate analysis alone (HR 1.79, 95%CI 1.11–2.88).
Conclusion
A low AFR and a high FPR correlated with increased risk of cancer mortality and recurrence. AFR and FPR may be promising prognostic markers for cancers.
Similar content being viewed by others
Introduction
Cancer incidence and mortality are rapidly increasing worldwide. There were an estimated 18.1 million newly diagnosed cancer cases and 9.6 million cancer deaths throughout the world during 2018 [1]. Research indicates that during the twenty-first century cancer will be the second-leading cause of death in the USA [2] and the single most important barrier to increasing life expectancy worldwide [1]. Moreover, the 5-year relative survival rate for all cancers is only 67%, thus indicating that prognoses of patients with cancer remain poor [3]. Therefore, evidenced-based resources are needed to improve survival from cancer and to identify patient characteristics that affect prognosis.
Malnutrition is common in cancer patients and is associated with increased morbidity and mortality [4]. Serum levels of albumin and prealbumin are common indicators of nutritional status. Albumin, which normally accounts for more than 50% of blood protein, is synthesized and secreted from the liver, and its concentration reflects the protein status of the blood and internal organs [5]. It functions as the major modulator of plasma oncotic pressure, and it also transports a variety of substances, including endogenous physiological metabolites and exogenous ligands [6]. The pretreatment serum albumin level in a cancer patient is generally used to assess nutritional status and predict prognosis [7].
Prealbumin, also known as transthyretin, has a much shorter half-life and smaller serum pool than albumin. Its main functions are to bind and transport endogenous proteins and small molecules. Prealbumin is more sensitive to changes in protein-energy status than albumin, and its concentration closely reflects recent dietary intake rather than overall nutritional status [8]. Prealbumin concentration can therefore be regarded primarily as a marker of at-risk patients who require nutritional monitoring [9]. In particular, the prealbumin level provides a quantitative measure of the efficacy of a nutritional care plan and an indication of the need to modify interventions [5].
Clinicians have long recognized specific associations of hemostatic system disorders with diverse cancers. The polypeptide fibrinogen is the central protein in the hemostasis pathway and occurs as a deposit in most tumors that occur in humans and experimental animals [10]. Fibrinogen is a 340 kDa hexameric plasma glycoprotein synthesized by the liver and consists of three pairs of non-identical polypeptide chains, the α-, β-, and γ-chains [11]. Fibrinogen deposit within the tumor stroma, especially the extracellular matrix, serves as a scaffold that supports the binding of growth factors which promote cellular responses associated with tumor cell adhesion, proliferation, and migration during cell growth and angiogenesis [12]. An elevated serum fibrinogen level is commonly associated with poor overall survival (OS) in human cancers [13].
Previous meta-analyses of cancer patients indicated that several ratios of pretreatment systemic inflammatory markers or/and nutritional markers, such as the neutrophil-lymphocyte ratio (NLR) [14], the albumin-globulin ratio (AGR) [15], and the C-reactive protein-albumin ratio (CAR) [16], can predict prognosis. Recent studies have examined the albumin-fibrinogen ratio (AFR) and/or the fibrinogen-prealbumin ratio (FPR) as prognostic indicators in cancer. For instance, a previous meta-analysis of 12 studies found that a low AFR was associated with poor prognosis in cancer [17]. However, this meta-analysis only assessed the impact of AFR on OS via multivariate analytic results and only evaluated the impact of AFR on disease-free survival (DFS) based on two studies. The impact of AFR on progression-free survival (PFS) and the impact of FPR on prognosis of cancer patients remain unknown. We conducted the present updated meta-analysis to elucidate the role of AFR in cancer prognosis and included all newly available studies to identify the prognostic value of FPR in cancers.
Materials and methods
Search strategy
Potential studies were identified by searching four online databases (PubMed, Web of Science, Cochrane Library, and WanFang) using PRISMA guidelines [18]. All studies that were published up to Oct 22, 2019, were identified using the search terms: “albumin fibrinogen ratio” or “fibrinogen albumin ratio” or “fibrinogen prealbumin ratio” or “prealbumin fibrinogen ratio.” The reference lists of initially identified studies were also scrutinized to identify additional relevant studies.
Criteria for eligibility
Eligibility criteria were defined according to the Population, Intervention, Comparison, Outcome and Study Design (PICOS) strategy. Thus, “Population” refers to cases diagnosed with cancers; “Intervention” refers to low AFR or high FPR; “Comparison” refers to the control group (without low AFR or high FPR); “Outcome” refers to OS, DFS, or PFS; and “Study design” refers to a prospective or retrospective study. A study was deemed ineligible if it was a duplicate, a review article, a comment or letter, or a case series. When there were duplicate records, the most recently published record was used.
Data collection
Data were collected independently by two authors using a predefined form. These forms were further checked by the third author, and disagreement was reconciled by consensus among all authors. The data extracted from each article included author names, publication year, country, cancer category, cancer stage, study design (prospective or retrospective), primary treatment option, optimal cut-off value and method used to select the optimal cut-off value, number of cases (total and numbers above and below the cut-off value), prognostic outcome, hazard ratios (HRs) with 95% confidence intervals (CIs), method of data analysis, data source (crude data or fitted curve), and follow-up interval. HR data were extracted from univariate and multivariate analyses if available.
Statistical analysis
When prognostic outcomes were provided as Kaplan-Meier curves, Engauge Digitizer 4.1 software was used to read the curves and identify the times of deaths. These data, defined as time-event outcomes, were used to calculate HRs and 95% CIs using the method of Tierney et al. [19]. These data were then synthesized and expressed as HRs with 95% CIs using StataMP 14. Cochran’s Q and I2 statistics were utilized to assess the heterogeneity among included studies [20]. When there was significant heterogeneity (P < 0.1 and/or I2 > 50%), pooled data were analyzed using a random-effects model; otherwise, a fixed-effects model was used. All statistical tests were two-sided, and a P value less than 0.05 was deemed statistically significant. The statistical analyses, stratification analyses, and sensitivity analyses were the same as those used in our previous publications [15, 21].
Results
Study selection
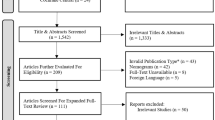
We initially identified 1805 records, and 995 of these records remained after removal of duplicates (Fig. 1). After review of the titles and abstracts, we excluded an additional 971 records. We reviewed the full text of 24 records and ultimately included 21 studies in the quantitative analysis [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42].
Characteristics of included studies
Nineteen of the included studies evaluated the role of AFR in cancer prognosis, 7 more studies than examined in the previous meta-analysis of the prognostic value of AFR in cancer [17]. In addition, 7 of the included studies examined the prognostic value of FPR in cancer prognosis. We thoroughly evaluated the prognostic value of the AFR and FPR in cancers by analysis of OS, DFS, and PFS (Table 1).
Effect of AFR on OS, DFS, and PFS
The association of AFR with OS was reported in 18 studies (7211 cases) using univariate analysis and in 17 studies (6704 cases) using multivariate analysis. Based on the univariate analyses, the pooled results of a random-effects model (I2 = 73.6%, P = 0.000) showed a significant association between low AFR and poor OS (HR 2.18, 95% CI 1.87–2.55, P = 0.00) (Fig. 2a). Based on the multivariate analyses, the pooled results of a random-effects model (I2 = 34.0%, P = 0.084) also showed a significant association between low AFR and poor OS (HR 1.75, 95% CI 1.54–2.00, P = 0.00) (Fig. 2b).
The association of AFR with DFS was reported in 5 studies (1815 cases) using univariate analysis and in 4 studies (1505 cases) using multivariate analysis. Based on the univariate analyses, the pooled results of a random-effects model (I2 = 58.7%, P = 0.046) demonstrated a significant association between low AFR and poor DFS (HR 1.89, 95%CI 1.54–2.32, P = 0.00) (Fig. 3a). Based on the multivariate analyses, the pooled results of a fixed-effects model (I2 = 0.0%, P = 0.724) also showed a correlation of low AFR with poor DFS (HR 1.51, 95%CI 1.29–1.76, P = 0.00) (Fig. 3b).
The association of AFR with PFS was reported in 6 studies (1352 cases) using univariate analysis and in 2 studies (583 cases) using multivariate analysis. According to a fixed-effects model, meta-analysis showed that low AFR was associated with poor PFS in the univariate analyses (HR 1.68, 95%CI 1.42–1.99, P = 0.00; I2 = 0.0%, P = 0.689) (Fig. 4a) and in the multivariate analyses (HR 1.48, 95%CI 1.16–1.88, P = 0.00; I2 = 0.0%, P = 0.340) (Fig. 4b).
Effect of FPR on OS, DFS, and PFS
The correlation of FPR with OS was evaluated in 6 studies (2232 cases) using both univariate and multivariate analyses. The pooled data of a fixed-effects model (I2 = 40.8%, P = 0.119) indicated a significant association between high FPR and poor OS in the univariate analysis (HR 2.37, 95%CI 2.03–2.77, P = 0.00) (Fig. 5a). The pooled data of a random-effects model (I2 = 72.2%, P = 0.001) also showed a significant relationship between high FPR and poor OS in the multivariate analysis (HR 1.97, 95%CI 1.41–2.77, P = 0.00) (Fig. 5b). Only two studies evaluated the correlation of FPR with DFS [41] and PFS [24], so we did not perform a pooled meta-analysis of these results. One of these studies examined 230 cases of hepatocellular carcinoma (HCC) and found a significant association between high FPR and poor DFS based on univariate analysis (HR 2.20, 95%CI 1.35–3.57, P = 0.001) and multivariate analysis (HR 1.77, 95%CI 1.04–2.99, P = 0.034). The other study examined 71 cases of metastatic colorectal cancer and found a significant association between high FPR and poor PFS (HR 1.79, 95%CI 1.11–2.88, P = 0.017) based on univariate analysis alone.
Subgroup meta-analysis for AFR and OS
In this update, there was heterogeneity among the studies that examined the relationship of AFR with OS. Thus, we performed subgroup analyses based on the AFR cut-off value, methods of choosing the cut-off value, study design, number of cases, cancer classification, publication time, treatment option, and data source. Our results indicated that the relationship between AFR and OS remained despite variation of these factors. At the same time, the heterogeneity was eliminated in some of the subgroup meta-analyses when classified by these factors (Tables 2 and 3).
Sensitivity analysis
In the initial meta-analysis of the relationship of AFR and OS from the multivariate analyses (Additional file 1), an apparently paradoxical plot (using crude HR with 95% CI in the original study) was present in one subgroup of the study by Li et al. [25]. Therefore, we deleted this subgroup during the meta-analysis. The sensitivity analysis (Additional files 2 and 3) indicated that all the included studies were nearly close to the central line, except the study by Li et al. [42]. In addition, the results of the sensitivity analysis indicated that omitting any single study did not change the overall effects of each pooled meta-analysis.
Discussion
Cancer is a devastating disease, and patients typically have poor prognoses. Therefore, research is needed to identify novel prognostic factors, because these factors may help to improve risk stratification and lifestyle decisions of these patients [43].
We assessed the value of ratio indexes derived from serum albumin, prealbumin, and fibrinogen—AFR and FPR—as prognostic markers for human cancers in this updated meta-analysis. Relative to the previous meta-analysis [17], this update has two strengths. First, we included 7 more studies that examined the relationship between AFR and cancer prognosis, and we also evaluated the impact of AFR on OS, DFS, and PFS using the pooled results from univariate and multivariate analyses. Second, we identified 7 additional studies that evaluated FPR as a prognostic marker in human cancers. The pooled results indicated that a high FPR correlated with poor OS, poor DFS, and poor PFS. These results thus indicated that a low AFR and a high FPR correlated with an increased risk of cancer mortality and recurrence.
We must note that the values of the AFR and FPR indexes themselves do not affect the survival outcomes of cancer patients. Instead, the underlying proteins (albumin, prealbumin, and fibrinogen) and biological processes that determine the AFR and FPR are responsible for this relationship. Serum albumin and prealbumin are two of the most commonly used indicators for assessing malnutrition, and malnutrition adversely affects the outcomes of cancer patients, in that it increases the incidence of infections, the length of hospital stay, and the risk of death [44]. However, serum albumin level is also reduced in patients with locally advanced or metastatic malignancies irrespective of the presence of malnutrition [45]. In these patients, a low albumin level has an adverse influence on the outcome of anticancer therapy [46]. Inflammation also affects the visceral synthesis of albumin and prealbumin. As a key regulator of inflammation [47], fibrinogen can induce tumor angiogenesis and metastasis by directly interacting with endothelial cells, by indirectly interacting with other regulators of angiogenesis [10], and by enhancing tumor cell invasion and metastasis through epithelial-to-mesenchymal transition (EMT) signaling [48].
Although albumin/prealbumin, and fibrinogen abnormalities are well-documented prognostic markers in cancer patients, not all cancer patients suffer from deficiencies of albumin/prealbumin and an overabundance of fibrinogen; some patients only have an albumin/prealbumin deficiency or only a fibrinogen overabundance. The ratio indexes that we used—AFR and FPR—better reflect the levels of both albumin/prealbumin and fibrinogen. That is to say, the AFR and FPR are better markers of alterations of albumin/prealbumin (representing nutrition) and fibrinogen (representing hemostasis or inflammation).
Additionally, serum albumin, prealbumin, and fibrinogen are available in the medical records of most cancer patients, and measurements are inexpensive and reproducible. Thus, use of the AFR and FPR as prognostic markers in cancers has great potential. In summary, both AFR and FPR could be promising markers of cancer prognosis. These results may help to guide future cancer treatments by identifying sub-populations with different prognoses.
There were some weaknesses in this updated meta-analysis. The main weakness is that the relationship of FPR on DFS and PFS was based on only one included study, rather than a meta-analysis. Second, there was heterogeneity among the studies included, and our pooled results were nearly all based on random-effects models. Differences in the baseline values and characteristics of patients, treatment options, and cut-off values, and other factors among studies may account for this heterogeneity. Third, there was publication bias regarding the relationship between AFR with OS (more than 10 studies), though we did not present these results or funnel plots.
Conclusions
A low AFR and a high FPR correlated with an increased risk of cancer mortality and recurrence. Thus, AFR and FPR may be promising prognostic markers for cancers.
Availability of data and materials
The data used and analyzed in the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AFR:
-
Albumin to fibrinogen
- CRLM:
-
Colorectal liver metastases
- ESCC:
-
Esophageal squamous cell carcinoma
- FPR:
-
Fibrinogen to prealbumin ratio
- HCC:
-
Hepatocellular carcinoma
- M:
-
Multivariate
- NR:
-
Not reported
- NSCLC:
-
Non-small cell lung cancer
- PFS:
-
Progression-free survival
- ROC:
-
Receiver operating characteristic
- U:
-
Univariate
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.
Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–89.
Álvaro Sanz E, Garrido Siles M, Rey Fernández L, Villatoro Roldán R, Rueda Domínguez A, Abilés J. Nutritional risk and malnutrition rates at diagnosis of cancer in patients treated in outpatient settings: early intervention protocol. Nutrition. 2019;57:148–53.
Loftus TJ, Brown MP, Slish JH, Rosenthal MD. Serum levels of prealbumin and albumin for preoperative risk stratification. Nutr Clin Pract. 2019;34(3):340–8.
Sonderby P, Bukrinski JT, Hebditch M, Peters GHJ, Curtis RA, Harris P. Self-interaction of human serum albumin: a formulation perspective. Acs Omega. 2018;3(11):16105–17.
Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. https://doi.org/10.1186/1475-2891-9-69.
Ingenbleek Y, Young VR. Significance of transthyretin in protein metabolism. Clin Chem Lab Med. 2002;40(12):1281–91.
Shenkin A. Serum prealbumin. Is it a marker of nutritional status or of risk of malnutrition? Clin Chem. 2006;52(12):2177–9.
Staton CA, Brown NJ, Lewis CE. The role of fibrinogen and related fragments in tumour angiogenesis and metastasis. Expert Opin Biol Ther. 2003;3(7):1105–20.
Shafer JA, Higgins DL. Human-fibrinogen. Crit Rev Clin Lab Sci. 1988;26(1):1–41.
Simpson-Haidaris PJ, Rybarczyk B. Tumors and fibrinogen. The role of fibrinogen as an extracellular matrix protein. Ann N Y Acad Sci. 2001;936:406–25.
Perisanidis C, Psyrri A, Cohen EE, Engelmann J, Heinze G, Perisanidis B, et al. Prognostic role of pretreatment plasma fibrinogen in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat Rev. 2015;41(10):960–70.
Mei ZB, Shi L, Wang B, Yang J, Xiao Z, Du P, et al. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: a systematic review and meta-analysis of 66 cohort studies. Cancer Treat Rev. 2017;58:1–13.
Lv GY, An L, Sun XD, Hu YL, Sun DW. Pretreatment albumin to globulin ratio can serve as a prognostic marker in human cancers: a meta-analysis. Clin Chim Acta. 2018;476:81–91.
Xu HJ, Ma Y, Deng F, Ju WB, Sun XY, Wang H. The prognostic value of C-reactive protein/albumin ratio in human malignancies: an updated meta-analysis. Onco targets Ther. 2017;10:3059–70.
Zhang Y, Xiao GL. Prognostic significance of the ratio of fibrinogen and albumin in human malignancies: a meta-analysis. Cancer Manag Res. 2019;11:3381–93.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:1–16.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Sun DW, Zhang YY, Sun XD, Chen YG, Qiu W, Ji M, et al. Prognostic value of cytokeratin 19 in hepatocellular carcinoma: a meta-analysis. Clin Chim Acta. 2015;448:161–9.
Chen QG, Zhang L, Sun F, Li SQ, You XH, Jiang YH, et al. Elevated FPR confers to radiochemoresistance and predicts clinical efficacy and outcome of metastatic colorectal cancer patients. Aging (Albany NY). 2019;11:1716–32.
Yu W, Ye Z, Fang X, Jiang X, Jiang Y. Preoperative albumin-to-fibrinogen ratio predicts chemotherapy resistance and prognosis in patients with advanced epithelial ovarian cancer. J Ovarian Res. 2019;12:88.
Zhang L, Zhang J, Wang Y, Dong Q, Piao H, Wang Q, et al. Potential prognostic factors for predicting the chemotherapeutic outcomes and prognosis of patients with metastatic colorectal cancer. J Clin Lab Anal. 2019:e22958. https://doi.org/10.1002/jcla.22958.
Li Y, Yang JN, Cheng SS, Wang Y. Prognostic significance of FA score based on plasma fibrinogen and serum albumin in patients with epithelial ovarian cancer. Cancer Manag Res. 2019;11:7697–705.
Ying J, Zhou DF, Gu TJ, Huang J, Liu H. Pretreatment albumin/fibrinogen ratio as a promising predictor for the survival of advanced non small-cell lung cancer patients undergoing first-line platinum-based chemotherapy. BMC Cancer. 2019;19(1):288. https://doi.org/10.1186/s12885-019-5490-y.
Du JH, Lu J. Circulating CEA-dNLR score predicts clinical outcome of metastatic gallbladder cancer patient. J Clin Lab Anal. 2019;33(2):e22684. https://doi.org/10.1002/jcla.22684.
Wang YY, Liu ZZ, Xu D, Liu M, Wang K, Xing BC. Fibrinogen-albumin ratio index (FARI): a more promising inflammation-based prognostic marker for patients undergoing hepatectomy for colorectal liver metastases. Ann Surg Oncol. 2019. https://doi.org/10.1245/s10434-019-07586-3.
Chen S, Yan H, Du J, Li J, Shen B, Ying H, et al. Prognostic significance of pre-resection albumin/fibrinogen ratio in patients with non-small cell lung cancer: a propensity score matching analysis. Clin Chim Acta. 2018;482:203–8.
Gao QF, Qiu JC, Huang XH, Xu YM, Li SQ, Sun F, et al. The predictive and prognostic role of a novel ADS score in esophageal squamous cell carcinoma patients undergoing esophagectomy. Cancer Cell Int. 2018;18:153. https://doi.org/10.1186/s12935-018-0648-2.
Li SQ, Jiang YH, Lin J, Zhang J, Sun F, Gao QF, et al. Albumin-to-fibrinogen ratio as a promising biomarker to predict clinical outcome of non-small cell lung cancer individuals. Cancer Med. 2018;7(4):1221–31.
Sun F, Peng HX, Gao QF, Li SQ, Zhang J, Chen QG, et al. Preoperative circulating FPR and CCF score are promising biomarkers for predicting clinical outcome of stage II-III colorectal cancer patients. Cancer Manag Res. 2018;10:2151–61.
Liang Y, Wang W, Que Y, Guan Y, Xiao W, Fang C, et al. Prognostic value of the fibrinogen/albumin ratio (FAR) in patients with operable soft tissue sarcoma. BMC Cancer. 2018;18(1):942. https://doi.org/10.1186/s12885-018-4856-x.
Xu Q, Yan Y, Gu S, Mao K, Zhang J, Huang P, et al. A novel inflammation-based prognostic score: the fibrinogen/albumin ratio predicts prognoses of patients after curative resection for hepatocellular carcinoma. J Immunol Res. 2018. https://doi.org/10.1155/2018/4925498.
Sun SY, Chen PP, Meng LX, Li L, Mo ZX, Sun CH, et al. High preoperative plasma fibrinogen and serum albumin score is associated with poor survival in operable esophageal squamous cell carcinoma. Dis Esophagus. 2019;32(1). https://doi.org/10.1093/dote/doy057.
Xu WY, Zhang HH, Xiong JP, Yang XB, Bai Y, Lin JZ, et al. Prognostic significance of the fibrinogen-to-albumin ratio in gallbladder cancer patients. World J Gastroenterol. 2018;24(29):3281–92.
Zou YX, Qiao J, Zhu HY, Lu RN, Xia Y, Cao L, et al. Albumin-to-fibrinogen ratio as an independent prognostic parameter in untreated chronic lymphocytic leukemia: a retrospective study of 191 cases. Cancer Res Treat. 2019;51(2):664–71.
Zhang J, Li SQ, Liao ZH, Jiang YH, Chen QG, Huang B, et al. Prognostic value of a novel FPR biomarker in patients with surgical stage II and III gastric cancer. Oncotarget. 2017;8(43):75195–205.
Hwang KT, Chung JK, Roh EY, Kim J, Oh S, Kim YA, et al. Prognostic influence of preoperative fibrinogen to albumin ratio for breast cancer. J Breast Cancer. 2017;20(3):254–63.
Tan ZH, Zhang M, Han Q, Wen J, Luo K, Lin P, et al. A novel blood tool of cancer prognosis in esophageal squamous cell carcinoma: the fibrinogen/albumin ratio. J Cancer. 2017;8(6):1025–9.
Zhang L, Chen QG, Li SQ, Zhang J, Min QH, Gao QF, et al. Preoperative fibrinogen to prealbumin ratio as a novel predictor for clinical outcome of hepatocellular carcinoma. Future Oncol. 2019;15(1):3227–36.
Li RT, Sun ZQ, Wang HB. Prognostic value of fibrinogen-to-prealbumin ratio in patients with advanced non-small cell lung cancer. Chin Clin Oncol. 2019;24(3):237–41 Article in Chinese.
Riley RD, Moons KGM, Snell KIE, Ensor J, Hooft L, Altman DG, et al. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ. 2019;364:k4597. https://doi.org/10.1136/bmj.k4597.
Virizuela JA, Camblor-Álvarez M, Luengo-Pérez LM, Grande E, Álvarez-Hernández J, Sendrós-Madroño MJ, et al. Nutritional support and parenteral nutrition in cancer patients: an expert consensus report. Clin Transl Oncol. 2018;20:619–29.
Deme D, Telekes A. Prognostic importance of albumin in oncology. Orv Hetil. 2018;159:96–106.
Hoogenboezem EN, Duvall CL. Harnessing albumin as a carrier for cancer therapies. Adv Drug Deliv Rev. 2018;130:73–89.
Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. 2012;34(1):43–62.
Shu YJ, Weng H, Bao RF, Wu XS, Ding Q, Cao Y, et al. Clinical and prognostic significance of preoperative plasma hyperfibrinogenemia in gallbladder cancer patients following surgical resection: a retrospective and in vitro study. BMC Cancer. 2014;14:566. https://doi.org/10.1186/1471-2407-14-566.
Acknowledgements
Not applicable.
Funding
This research was funded by the Science and Technology of Jilin Province (No. 20190101002JH).
Author information
Authors and Affiliations
Contributions
SDW and LGY conceived this research, performed the design, and analyzed the data. SDW and AL performed the data extraction, and drafted and revised the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Forest plots of the relationship between AFR and OS via multivariate analyses, before deleting the subgroup with paradoxical results (Li et al. 2019, subgroup 1).
Additional file 2.
Sensitivity analysis of the correlation of AFR with OS via univariate analyses (A) and multivariate analyses (B); with DFS from univariate analyses (C) and multivariate analyses (D); and with PFS from univariate analyses results (E) and multivariate analyses (F).
Additional file 3.
Sensitivity analysis of the correlation of FPR with OS via univariate analyses (A) and multivariate analyses (B).
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Sun, Dw., An, L. & Lv, Gy. Albumin-fibrinogen ratio and fibrinogen-prealbumin ratio as promising prognostic markers for cancers: an updated meta-analysis. World J Surg Onc 18, 9 (2020). https://doi.org/10.1186/s12957-020-1786-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-020-1786-2