Abstract
Background
Whether video-assisted thoracoscopic surgery (VATS) segmentectomy and VATS lobectomy provide similar perioperative and oncological outcomes in stage I non–small cell lung cancer (NSCLC) is still controversial.
Methods
Meta-analysis of 12 studies comparing outcomes after VATS lobectomy and VATS segmentectomy for stage I NSCLC. Data were analyzed by the RevMan 5.3 software.
Results
Disease-free survival (HR 1.19, 95% CI 0.89 to 1.33, P = 0.39), overall survival (HR 1.11, 95% CI 0.89 to 1.38, P = 0.36), postoperative complications (OR = 1.10, 95% CI 0.69 to 1.75, P = 0.7), intraoperative blood loss (MD = 3.87, 95% CI − 10.21 to 17.94, P = 0.59), operative time (MD = 10.89, 95% CI − 13.04 to 34.82, P = 0.37), air leak > 5 days (OR = 1.20, 95% CI 0.66 to 2.17, P = 0.55), and in-hospital mortality (OR = 1.67, 95% CI 0.39 to 7.16, P = 0.49) were comparable between the groups. Postoperative hospital stay (MD = − 0.69, 95% CI − 1.19 to − 0.19, P = 0.007) and number of dissected lymph nodes (MD = − 6.44, 95%CI − 9.49 to − 3.40, P < 0.0001) were significantly lower in VATS segmentectomy patients.
Conclusions
VATS segmentectomy and VATS lobectomy provide similar oncological and perioperative outcomes for stage I NSCLC patients.
This systematic review was registered on PROSPERO and can be accessed at http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID = CRD42019133398.
Similar content being viewed by others
Background
Advances in screening techniques have led to a marked increase in the number of small peripheral lung lesions being detected [1]. About 10% of these lesions turn out to be non–small cell lung cancers (NSCLC). Lobectomy with radical lymph node dissection has been the preferred management for stage I NSCLC since 1995, when the North American Lung Cancer Study Group [2] reported better survival with lobectomy than with sublobectomy. The authors of the study recommended sublobectomy only for patients with limited cardiopulmonary reserve. However, the study included patients with various clinical stages and did not discriminate sublobectomy from wedge resection and segmentectomy, and so the conclusions have been questioned by some experts. Recently, there has been a revival of interest in sublobectomy, and segmentectomy in particular, for management of stage I NSCLC. Segmentectomy preserves lung tissue and so obviously protects lung function [3, 4]; in addition, video-assisted thoracoscopic surgery (VATS) segmentectomy [5,6,7,8], which is the preferred procedure, causes less postoperative pain and requires shorter hospitalization than thoracotomy. However, it remains unclear whether perioperative safety and long-term survival are comparable between stage I NSCLC patients treated with VATS segmentectomy and VATS lobectomy. Therefore, we performed this meta-analysis to determine whether perioperative outcomes (such as postoperative complications, intraoperative blood loss, air leak) and survival (disease-free survival [DFS] and overall survival [OS]) were similar in stage I NSCLC patients treated with VATS segmentectomy and VATS lobectomy.
Materials and methods
Search strategy
Two investigators independently searched PubMed, Web of Science, ScienceDirect, The Cochrane Library, Scopus, and Google Scholar to identify relevant papers published between January 1990 and April 2019. The following keywords were used: “lobectomy AND segmentectomy” “lung cancer OR lung neoplasm OR non–small cell lung cancer OR NSCLC”, and “video-assisted thoracic surgery OR VATS”. There were no limits placed on study design or publication status (published or unpublished). The search strategy is comprehensively described in Additional file 1.
Selection criteria
Studies were eligible for inclusion in this meta-analysis if they (1) were in English, (2) only included patients with clinical stage I NSCLC, and (3) compared the perioperative and/or survival outcomes (follow-up time ≥ 5 years) of patients treated with VATS segmentectomy and VATS lobectomy. When the same data or data subsets were reported in more than one study, the one with the most details or the one most recently published was chosen. Case-only designs, case reports, systematic reviews, meta-analyses, and animal studies were excluded.
Data extraction
Two investigators independently went through each eligible study and recorded data on the following: name of first author, year of publication, geographic area, study design, DFS, OS, postoperative complications, intraoperative blood loss, operation time, postoperative hospital stay, air leak (> 5 days), in-hospital mortality, and number of lymph nodes dissected.
Quality assessment for included studies
The quality of each study was graded independently by two investigators using the Newcastle–Ottawa Scale (NOS, for nonrandomized studies). The NOS analyzes three items—selection, comparability, and exposure—to evaluate study quality. The maximum possible score is 4 for selection, 2 for comparability, and 3 for exposure. A total score of 8 or 9 indicates high quality, and a score of 6 or 7 indicates medium quality.
Details of the protocol for this systematic review were registered on PROSPERO and can be accessed at http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID = CRD42019133398. This study is presented in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement.
Statistical analysis
Statistical analysis was performed using Review Manager 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) and SPSS 18.0 (SPSS Inc., Chicago, IL, USA). Survival data (OS and DFS) were analyzed by using the hazard ratio (HR) and its standard error (SE). If the HR data were unable to be extracted directly from the included studies, we extracted data from Kaplan–Meier curves and calculated the data according to method provided by Tierney et al. [9]. The Kaplan–Meier curves were read by Engauge Digitizer version 4.1 (software downloaded from http://sourceforge.net/projects/digitizer/files/Engauge%20Digitizer/digitizer-4.1/). All calculations were performed independently by two of the authors; disagreements were settled by discussion. Higgins I2 statistic was used to evaluate heterogeneity among the included studies. If no significant heterogeneity was detected (I2 < 50%, P > 0.1), the fixed-effects model was used to pool studies; otherwise, the random-effect model was used. For some of the studies, the original data were recalculated. Funnel plots were used to assess publication bias.
Result
Included studies
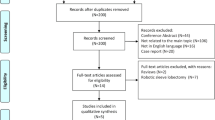
A total of 3299 publications were identified with the electronic search of the databases and the manual search of reference lists. Of these, 12 articles met our eligibility criteria (Fig. 1). These 12 articles involved a total of 2313 patients, with 750 who underwent VATS segmentectomy and 1563 who underwent VATS lobectomy. All 12 articles were retrospective studies. By the Newcastle–Ottawa Scale, six articles were graded as good quality and six as medium quality (details are presented in Additional file 2). Table 1 presents the characteristics of the 12 articles.
Primary outcome measures
Disease-free survival
Seven studies reported data on DFS. These 7 studies involved a total of 1184 patients, among whom 517 received VATS segmentectomy and 667 patients received VATS lobectomy. There was no heterogeneity among the studies (I2 = 0%, P = 0.86). The combined HR for DFS was 1.09 (95% CI 0.89 to 1.33). DFS was not significantly different between the two groups (P = 0.39, Fig. 2).
Overall survival
Nine studies reported data on OS. These 9 studies involved a total of 2160 patients, among whom 935 received VATS segmentectomy and 1225 received VATS lobectomy. There was no heterogeneity among the studies (I2 = 0%, P = 0.85). The combined HR for OS was 1.11 (95% CI 0.89 to 1.38). OS was not significantly different between the two groups (P = 0.36, Fig. 3).
Postoperative complications
Eight articles reported data on postoperative complications. These 8 studies included a total of 1515 patients, among whom 463 received VATS segmentectomy and 1052 received VATS lobectomy. There was significant heterogeneity among the studies (I2 = 62%, P = 0.01). The incidence of postoperative complications was not significantly different between the two groups (OR = 1.10, 95% CI 0.69 to 1.75, P = 0.70, Fig. 4).
Postoperative hospital stay
Six articles reported data on postoperative hospital stay. These 6 studies involved a total of 898 patients, among whom 304 received VATS segmentectomy and 594 received VATS lobectomy. There was no significant heterogeneity among the studies (I2 = 24%, P = 0.25). Postoperative hospital stay was shorter in VATS segmentectomy patients than in VATS lobectomy patients. The difference in postoperative hospital stay between the two groups was statistically significant (MD = − 6.44, 95% CI − 9.49 to − 3.40, P = 0.007, Fig. 5).
Intraoperative blood loss
Five articles reported data on intraoperative blood loss. These 5 studies involved a total of 686 patients, among whom 218 received VATS segmentectomy and 468 received VATS lobectomy. There was no significant heterogeneity between the studies (I2 = 47%, P = 0.11). The mean difference in intraoperative blood loss between the two groups was not statistically significant (MD = 3.87, 95% CI − 10.21 to 17.94, P = 0.59, Fig. 6).
Operation time
Seven articles reported data on operation time. These 7 studies involved a total of 970 patients, among whom 364 received VATS segmentectomy and 606 received VATS lobectomy. There was significant heterogeneity among the studies (I2 = 95%, P < 0.001). The mean difference in operation time between the two groups was not statistically significant (MD = 10.89, 95% CI − 13.04 to 34.82, P = 0.37, Fig. 7)
Air leak (> 5 days)
Seven articles reported data on air leak. These 7 studies involved a total of 1419 patients, among whom 411 received VATS segmentectomy and 1008 received VATS lobectomy. There was no significant heterogeneity among the studies (I2 = 48%, P = 0.07). The difference in air leak between the two groups was not statistically significant (OR = 1.20, 95% CI 0.66 to 2.17, P = 0.55, Fig. 8).
In-hospital mortality
Four articles were reported data on in-hospital mortality. These 4 studies involved a total of 665 patients, among whom 209 received VATS segmentectomy and 456 received VATS lobectomy. There was no heterogeneity among the studies (I2 = 0%, P = 0.97). The difference in incidence of air leak between the two groups was not statistically significant (OR = 1.67, 95% CI 0.39 to 7.16, P = 0.49, Fig. 9).
Number of lymph nodes dissected
Four articles reported data on dissected lymph nodes. These 4 studies involved a total of 604 patients, among whom 264 received VATS segmentectomy and 340 received VATS lobectomy. There was significant heterogeneity among the studies (I2 = 75%, P = 0.008). The number of dissected lymph nodes was more in VATS lobectomy patients. The mean difference in the number of dissected lymph nodes was statistically significant (MD = − 6.44, 95% CI − 9.49 to − 3.40, P < 0.01, Fig. 10).
Publication bias
Funnel plots (standard error of OS) demonstrated marked symmetry, indicating absence of publication bias (Fig. 11).
Discussion
A number of recent systematic reviews have shown that segmentectomy can achieve the same survival outcomes as lobectomy in stage I NSCLC patients [22, 23]. The study of International Early Lung Cancer Action Program [24] even suggests that the prognosis for clinical stage I tumors up to 2 cm in diameter is superior with segmentectomy. However, there is a paucity of studies on VATS segmentectomy [7, 25]. The differences between VATS segmentectomy and lobectomy in survival outcomes, postoperative complications, number of lymph nodes dissected, and so on have not been adequately studied. A recent meta-analysis [26] that compared VATS segmentectomy with VATS lobectomy included 8 articles, with a total of 463 patients who underwent VATS segmentectomy and 1150 patients who underwent VATS lobectomy. The authors found no significant difference in OS (HR = 1.03, 95% CI 0.76 to 1.39, P = 0.85) or DFS (HR 1.19, 95% CI 0.67 to 2.10, P = 0.56) between the two groups. However, the article did not analyze other outcomes.
Our meta-analysis included 12 studies that compared the perioperative and oncological outcomes of VATS segmentectomy versus VATS lobectomy in stage I NSCLC patients. Although those studies were retrospective in nature, they were all of moderate to high quality. We found that the outcomes were mostly comparable between patients undergoing VATS segmentectomy and VATS lobectomy. There were no significant differences between the two groups in survival (OS and DFS) or in perioperative outcomes such as operative time, intraoperative bleeding, air leak (> 5 days), postoperative complications, and in-hospital mortality. However, postoperative hospital stay and the number of lymph nodes dissected were both significantly lower in patients undergoing VATS segmentectomy.
The effect of segmentectomy on prognosis is debated. While segmentectomy preserves normal lung parenchyma and is therefore claimed to be beneficial for pulmonary function recovery, it is not clear whether the retained lung parenchyma serves to improve prognosis [27] or whether insufficient resection actually worsens prognosis [28]. Some studies show comparable prognosis with VATS segmentectomy and VATS lobectomy [29, 30], but others show poorer prognosis with the former [31, 32]. Most of the studies that demonstrated the superiority of lobectomy were not completely randomized and also did not consider other factors that could potentially affect survival, e.g., tumor size, type of procedure (wedge resection vs. segmentectomy), and the type of lymph node dissection. Our meta-analysis showed that retention of part of the lung parenchyma does not improve prognosis, and reduction in the extent of resection does not increase the risk of recurrence. These results are consistent with previous reports [4, 33, 34].
We found postoperative hospital stay to be significantly shorter after VATS segmentectomy. This was probably because patients who accepted VATS segmentectomy had more rapid lung recruitment and quicker return of lung function to optimal levels [35, 36]. The number of lymph nodes resected was significantly lower in VATS segmentectomy than in VATS lobectomy. This may have been because of the differences in the number of inter- and intra-segmental nodes dissected and also because lymph node sampling, rather than lymph node dissection, is generally adopted during VATS segmentectomy [20].
Most studies show that segmentectomy preserves more lung tissue and therefore promotes lung function recovery [33, 37, 38], but some reports suggest that the retained lung tissue provides little functional advantage [10, 39]. There could be several explanations. First, compensatory adaptation of the remaining lung may be better after lobectomy than after segmentectomy [40, 41]. Second, the intersegmental plane [42, 43] is created with an electrocautery device or an auto-suture device, but both methods have drawbacks, and perfect anatomical resection is not always possible; this may result in limited function of the retained lung. Unfortunately, data on lung function were not collected in this meta-analysis because there was no uniformity between the studies in the methods used for evaluation. However, this meta-analysis indicates that although retention of the lung parenchyma does not improve prognosis, it does accelerate postoperative recovery.
Some limitations of this study must be pointed out. First, all included studies were retrospective nonrandomized comparisons, with high probability of selection and reporting bias. Second, there was high heterogeneity among the studies with regard to postoperative complications, operation time, and number of dissected lymph nodes. Factors that may have been responsible for the heterogeneity include the level of experience of the surgeon and the shorter learning curve for VATS segmentectomy. The high heterogeneity can reduce the credibility of conclusions. Third, some studies did not report the precise clinical stage or explain the methods used for determining disease stage. PET-CT was used for staging of all patients in only three studies; in the other studies, some patients were staged with PET-CT and some by CT. The lack of uniformity in the methods and the uncertainty of the clinical stages might affect the reliability of our results. Fourth, some of the patients who underwent VATS segmentectomy were those who were considered unfit for lobectomy because of presence of comorbidities; this may have resulted in a selection bias and affected our results.
Conclusion
This meta-analysis shows that VATS segmentectomy and VATS lobectomy provide similar oncological and perioperative outcomes in stage I NSCLC patients. Well-designed large randomized clinical trials, with uniform and reliable pulmonary function indicators and staging measures (e.g., PET-CT), complete complications data, and long-term postoperative follow-up, are needed to confirm the findings of this study.
Availability of data and materials
All the data used in this study can be obtained from the original articles.
Abbreviations
- DFS:
-
Disease-free survival
- HR:
-
Hazard ratio
- MD:
-
Mean difference
- NOS:
-
Newcastle–Ottawa Scale
- NSCLC:
-
Non–small cell lung cancer
- OS:
-
Overall survival
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- SE:
-
Standard error
- VATS:
-
Video-assisted thoracoscopic surgery
References
Kim SK, Allen-Auerbach M, Goldin J, et al. Accuracy of PET/CT in characterization of solitary pulmonary lesions. J Nucl Med. 2007;48(2):214–20.
Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60(3):615–22; discussion 622-3. https://doi.org/10.1016/0003-4975(95)00537-u.
Campione A, Ligabue T, Luzzi L, et al. Comparison between segmentectomy and larger resection of stage IA non-small cell lung carcinoma. J Cardiovasc Surg (Torino). 2004;45(1):67–70.
Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg. 2006;132(4):769–75. https://doi.org/10.1016/j.jtcvs.2006.02.063.
Shiraishi T, Shirakusa T, Iwasaki A, et al. Video-assisted thoracoscopic surgery (VATS) segmentectomy for small peripheral lung cancer tumors: intermediate results. Surg Endosc. 2004;18(11):1657–62. https://doi.org/10.1007/s00464-003-9269-4.
Atkins BZ, Harpole DH, Mangum JH, et al. Pulmonary segmentectomy by thoracotomy or thoracoscopy: reduced hospital length of stay with a minimally-invasive approach. Ann Thorac Surg. 2007;84(4):1107–12; discussion 1112-3. https://doi.org/10.1016/j.athoracsur.2007.05.013.
Schuchert MJ, Pettiford BF, Pennathur AF, et al. Anatomic segmentectomy for stage I non-small-cell lung cancer: comparison of video-assisted thoracic surgery versus open approach. J Thorac Cardiovasc Surg. 2009;138(6):1318-25.e1. doi: 10.1016/j.jtcvs.2009.08.028.
Leshnower BG, Miller DF, Fernandez F, et al. Video-assisted thoracoscopic surgery segmentectomy: a safe and effective procedure. Ann Thorac Surg. 2010;89(5):1571–6. https://doi.org/10.1016/j.athoracsur.2010.01.061.
Tierney JF, Stewart LF, Ghersi DF, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. https://doi.org/10.1186/1745-6215-8-16.
Hwang Y, Kang CH, Kim HS, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy on the patients with non-small cell lung cancer: a propensity score matching study. European journal of cardio-thoracic surgery : Eur J Cardiothorac Surg. 2015;48(2):273–8. https://doi.org/10.1093/ejcts/ezu422.
Echavarria MF, Cheng AM, Velez-Cubian FO, et al. Comparison of pulmonary function tests and perioperative outcomes after robotic-assisted pulmonary lobectomy vs segmentectomy. Am J Surg. 2016;212(6):1175–82. https://doi.org/10.1016/j.amjsurg.
Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. Journal of clinical oncology. J Clin Oncol. 2014;32(23):2449–55. https://doi.org/10.1200/JCO.2013.50.8762.
Nakamura H, Taniguchi Y, Miwa K, et al. Comparison of the surgical outcomes of thoracoscopic lobectomy, segmentectomy, and wedge resection for clinical stage I non-small cell lung cancer. Thorac Cardiovasc Surg. 2011;59(3):137–41. https://doi.org/10.1055/s-0030-1250377.
Roman M, Labbouz S, Valtzoglou V, et al. Lobectomy vs. segmentectomy. A propensity score matched comparison of outcomes. Eur J Surg Oncol. 2019;45(5):845–50. https://doi.org/10.1016/j.ejso.2018.10.534.
Shapiro M, Weiser TS, Wisnivesky JP, et al. Thoracoscopic segmentectomy compares favorably with thoracoscopic lobectomy for patients with small stage I lung cancer. J Thorac Cardiovasc Surg. 2009;137(6):1388–93. https://doi.org/10.1016/j.jtcvs.2009.02.009.
Song CY, Sakai T, Kimura D, et al. Comparison of perioperative and oncological outcomes between video-assisted segmentectomy and lobectomy for patients with clinical stage IA non-small cell lung cancer: a propensity score matching study. J Thorac Dis. 2018;10(8):4891-4901. doi: 10.21037/jtd.2018.07.133.
Soukiasian HJ, Hong E, McKenna RJ Jr. Video-assisted thoracoscopic trisegmentectomy and left upper lobectomy provide equivalent survivals for stage IA and IB lung cancer. J Thorac Cardiovasc Surg. 2012;144(3):S23–6. https://doi.org/10.1016/j.jtcvs.2012.05.071.
Tsubokawa N, Tsutani Y, Miyata Y, et al. Segmentectomy versus lobectomy for radiologically pure solid clinical T1a-bN0M0 lung cancer. World J Surg. 2018;42(8):2493–501. https://doi.org/10.1007/s00268-018-4514-0.
Wang BY, Tu CC, Liu CY, et al. Single-incision thoracoscopic lobectomy and segmentectomy with radical lymph node dissection. Ann Thorac Surg. 2013;96(3):977–82. https://doi.org/10.1016/j.athoracsur.2013.05.002.
Yamashita S, Tokuishi K, Anami K, et al. Thoracoscopic segmentectomy for T1 classification of non-small cell lung cancer: a single center experience. Eur J Cardiothorac Surg. 2012;42(1):83–8. https://doi.org/10.1093/ejcts/ezr254.
Zhong C, Fang W, Mao T, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy for small-sized stage IA lung cancer. Ann Thorac Surg. 2012;94(2):362–7. https://doi.org/10.1016/j.athoracsur.2012.04.047.
Bedetti B, Bertolaccini L, Rocco R, et al. Segmentectomy versus lobectomy for stage I non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis. 2017;9(6):1615-1623. doi: 10.21037/jtd.2017.05.79.
Tan Q, Huang J, Ding Z, et al. Meta-analysis for curative effect of lobectomy and segmentectomy on non-small cell lung cancer. Int J Clin Exp Med. 2014;7(9):2599–604.
Altorki NK, Yip R, Hanaoka T, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg. 2014;147(2):754–62; Discussion 762-4. https://doi.org/10.1016/j.jtcvs.2013.09.065.
Ohtsuka T, Nomori HF, Horio HF, et al. Is major pulmonary resection by video-assisted thoracic surgery an adequate procedure in clinical stage I lung cancer? Chest. 2004;125(5):1742–6. https://doi.org/10.1378/chest.125.5.1742.
Liu Q, Wang H, Zhou D, et al. Comparison of clinical outcomes after thoracoscopic sublobectomy versus lobectomy for stage I nonsmall cell lung cancer: a meta-analysis. J Cancer Res Ther. 2016;12(2):926-31. doi: 10.4103/0973-1482.174181.
Gu Z, Wang H, Mao T, et al. Pulmonary function changes after different extent of pulmonary resection under video-assisted thoracic surgery. J Thorac Dis. 2018;10(4):2331-2337. doi: 10.21037/jtd.2018.03.163.
Sienel W, Stremmel CF, Kirschbaum AF, et al. Frequency of local recurrence following segmentectomy of stage IA non-small cell lung cancer is influenced by segment localisation and width of resection margins--implications for patient selection for segmentectomy. Eur J Cardiothorac Surg. 2007;31(3):522–7; discussion 527-8. https://doi.org/10.1016/j.ejcts.2006.12.018.
Cao C, Chandrakumar D, Gupta S, et al. Could less be more? A systematic review and meta-analysis of sublobar resections versus lobectomy for non-small cell lung cancer according to patient selection. Lung Cancer. 2015;89(2):121–32. https://doi.org/10.1016/j.lungcan.2015.05.010.
Bedetti B, Bertolaccini L, Rocco R, et al. Segmentectomy versus lobectomy for stage I non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis. 2017;9(6):1615-1623. doi: 10.21037/jtd.2017.05.79.
Bao F, Ye P, Yang Y, et al. Segmentectomy or lobectomy for early stage lung cancer: a meta-analysis. Eur J Cardiothorac Surg. 2014;46(1):1–7. https://doi.org/10.1093/ejcts/ezt554.
Zhang L, Li M, Yin R, et al. Comparison of the oncologic outcomes of anatomic segmentectomy and lobectomy for early-stage non-small cell lung cancer. Ann Thorac Surg. 2015;99(2):728–37. https://doi.org/10.1016/j.athoracsur.2014.08.080.
Keenan RJ, Landreneau RF, Maley R, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg. 2004;78(1):228–33; discussion 228-33. https://doi.org/10.1016/j.athoracsur.2004.01.024.
Martin-Ucar AE, Nakas AF, Pilling JF, et al. A case-matched study of anatomical segmentectomy versus lobectomy for stage I lung cancer in high-risk patients. Eur J Cardiothorac Surg. 2005;27(4):675–9. https://doi.org/10.1016/j.ejcts.2005.01.006.
Yoshimoto K, Nomori HF. Mori TF, et al. Quantification of the impact of segmentectomy on pulmonary function by perfusion single-photon-emission computed tomography and multidetector computed tomography. doi: J Thorac Cardiovasc Surg. 2009;137(5):1200–5. https://doi.org/10.1016/j.jtcvs.2008.10.028.
Takizawa T, Haga MF, Yagi NF, et al. Pulmonary function after segmentectomy for small peripheral carcinoma of the lung. J Thorac Cardiovasc Surg. 1999;118(3):536–41. https://doi.org/10.1016/S0022-5223(99)70193-5.
Charloux A, Quoix E. Lung segmentectomy: does it offer a real functional benefit over lobectomy? Eur Respir Rev. 2017;26(146). pii: 170079. doi: 10.1183/16000617.0079-2017.
Ueda K, Tanaka TF, Hayashi MF, et al. Computed tomography-defined functional lung volume after segmentectomy versus lobectomy. Eur J Cardiothorac Surg. 2010 Jun;37(6):1433–7. https://doi.org/10.1016/j.ejcts.2010.01.002.
Harada H, Okada MF. akamoto TF, et al. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg. 2005;80(6):2041–5. https://doi.org/10.1016/j.athoracsur.2005.06.010.
Butler JP, Loring SF, Patz SF, et al. Evidence for adult lung growth in humans. N Engl J Med. 2012;367(3):244–7. https://doi.org/10.1056/NEJMoa1203983.
Mizobuchi T, Wada HF, Sakairi YF, et al. Spirometric and radiological evaluation of the remnant lung long after major pulmonary resection: can compensatory phenomena be recognized in clinical cases? Surg Today. 2014;44(9):1735–43. https://doi.org/10.1007/s00595-013-0702-6.
Suzuki H, Morimoto J, Mizobuchi T, et al. Does segmentectomy really preserve the pulmonary function better than lobectomy for patients with early-stage lung cancer? Surg Today. 2017;47(4):463–9. https://doi.org/10.1007/s00595-016-1387-4.
Miyasaka Y, Oh SF, Takahashi NF, et al. Postoperative complications and respiratory function following segmentectomy of the lung - comparison of the methods of making an inter-segmental plane. Interact Cardiovasc Thorac Surg. 2011;12(3):426–9. https://doi.org/10.1510/icvts.2010.253989.
Acknowledgements
We would like to thank all the investigators, surgeons, and patients who participated in this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
YPW and WXZ conceived and designed this study. GMY and YM performed the literature search. JJX, DLY, and JHP performed the data analysis. WBZ and JYZ wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All analyses were based on previous published studies; thus, ethical approval and patient consent are not required.
Consent for publication
All analyses were based on previous published studies; thus, no consent for publication is required.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Search strategy.
Additional file 2.
Quality assessment of all included studies.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zeng, W., Zhang, W., Zhang, J. et al. Systematic review and meta-analysis of video-assisted thoracoscopic surgery segmentectomy versus lobectomy for stage I non–small cell lung cancer. World J Surg Onc 18, 44 (2020). https://doi.org/10.1186/s12957-020-01814-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-020-01814-x