Abstract
Background
Giant cell tumor of bone (GCTB) is an intermediate tumor known to be locally aggressive, but rarely metastasizing. To plan a prospective study of GCTB, we performed a questionnaire survey for institutions participating in the Bone and Soft Tissue Tumor Study Group (BSTTSG) in the Japan Clinical Oncology Group (JCOG) in 2015.
Methods
We reviewed 158 consecutive patients with primary GCTB treated with curettage without perioperative denosumab from 2008 to 2010 in Japan. We investigated local and distant recurrence rates after definitive curettage. We also investigated the recurrence rate after treatment with preoperative and/or postoperative denosumab with curettage in recent years. There were 40 patients treated with perioperative denosumab, and the factors affecting recurrence in them were investigated.
Results
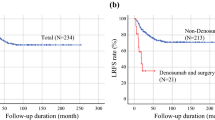
Answers were available from 24 of 30 institutions (80.0%) participating in JCOG BSTTSG. Thirty (19.0%) and 4 (2.5%) of 158 patients developed local and distant recurrence after curettage without perioperative denosumab from 2008 to 2010, respectively. Campanacci grade and embolization before surgery were significantly associated with increasing incidence of local recurrence after curettage (p = 0.034 and p = 0.022, respectively). In patients treated with perioperative desnosumab, 120 mg denosumab was administered subcutaneously for a median 6 (2–41) and 6 (1–14) times in preoperative and postoperative settings, respectively. The recurrence rates were 6 of 21 (28.6%), 2 of 9 (22.2%), and 0 of 10 (0.0%) in the preoperative, postoperative, and both pre- and postoperative denosumab treatment groups, respectively. With all of the preoperative treatments, administration exceeding five times was significantly associated with a decreased incidence of local recurrence after curettage (p < 0.001).
Conclusion
The recurrence rate of GCTB was still high after curettage, especially in Campanacci grade III, and improvements in the therapeutic strategy are needed in this cohort. There is a possibility that a sufficient dose of preoperative denosumab can reduce recurrence after curettage. Recently, we have started a clinical trial, JCOG1610, to investigate the efficacy of preoperative denosumab in patients who can be treated with curettage in GCTB.
Similar content being viewed by others
Background
Giant cell tumor of bone (GCTB) is an intermediate tumor known to be locally aggressive, but rarely metastasizing in the WHO classification [1]. GCTB possibly originates from the metaphyseal region [2], and accounts for 4–5% of all skeletal neoplasms in Japan. Local and distant recurrence rates were reported in 24.8–30.8% [3,4,5,6] and 2% [7, 8] of the patients after curettage, respectively. To reduce local recurrence and preserve the adjacent joint, adjuvant treatments such as high-speed burr [3], phenol [5, 9], ethanol, liquid nitrogen [5], and polymethyl methacrylate (PMMA) [3, 5, 9] have been reported. Even though there were some reports of GCTB in Japan [7, 10, 11], the recent clinical results of GCTB after curettage in multiple institutions in Japan have not been well documented.
Denosumab is a fully human monoclonal antibody that inhibits the receptor activator of NF-κB (RANK) ligand (RANKL) and then interrupts RANK-RANKL interactions. In GCTB, the stromal cells and osteoclast-like giant cells express RANKL and RANK, respectively, and the RANK-RANKL interaction is considered to be necessary for the differentiation and activation of osteoclasts [12]. Therefore, the RANK-RANKL interaction has a critical role for bone destruction in GCTB, and dramatic change was observed after treatment of denosumab in GCTB. Multinucleated osteoclast-like giant cells and stromal cells were decreased after denosumab treatment for GCTB [13]. A recent phase 2 study demonstrated the effects of denosumab for patients with unresectable GCTB and salvageable GCTB whose surgery was associated with severe morbidity [14]. Denosumab was accepted for health insurance coverage in Japan in 2014. However, the role of denosumab in patients with GCTB who can be treated by curettage has not been well defined.
We performed a questionnaire survey for institutions participating in the Bone and Soft Tissue Tumor Study Group (BSTTSG) in the Japan Clinical Oncology Group (JCOG) in 2015 for planning a clinical trial of JCOG1610, a randomized phase III study of preoperative denosumab with curettage for GCTB. The first aim of the present study was to identify the historical outcome after curettage for GCTB without perioperative desnosumab in Japan. The second purpose was to identify the clinical use of perioperative denosumab and the factors influencing local recurrence after perioperative denosumab with curettage.
Methods
Patients
We reviewed 158 patients with GCTB treated by curettage without perioperative desnosumab from 2008 to 2010 in institutions participating in the JCOG BSTTSG. We also reviewed 40 patients with GCTB treated with curettage and perioperative denosumab.
Methods
We performed a questionnaire survey for institutions participating in the BSTTSG in JCOG in April and June 2015. This questionnaire survey was performed for planning a clinical trial of JCOG 1610 (UMIN000029451), a randomized phase III study of preoperative denosumab with curettage for GCTB. We retrospectively reviewed clinical records and filled out the questionnaire. The questionnaire included standard treatments (e.g., local adjuvant, reconstruction) of curettage for GCTB in each institution, details (e.g., number of extremities, Campanacci grade, pathological fracture at presentation, and embolization before surgery) of GCTB treated with curettage from 2008 to 2010, and clinical results (e.g., number of local recurrences, distant recurrences, and death) after the curettage, and details (e.g., sites, Campanacci grade, pathological fracture at presentation, time to the recurrence, final joint preservation, and embolization before surgery) of the patients with local recurrence. We also asked about perioperative use of denosumab for GCTB. The questionnaire included the indications of denosumab for GCTB in each institution, the number of patients treated with preoperative, postoperative, and both pre- and postoperative denosumab, respectively, number of times of perioperative denosumab administration, and clinical results of local recurrence after the perioperative denosumab with curettage. This study was approved by the ethics committee of Nagoya University Graduate School and School of Medicine (Nagoya, Japan) and a waiver of informed consent was provided.
Statistics
A chi-square test was used to analyze the correlation of various clinical factors with recurrence. Clinical factors such as sites (extremity, trunk), Campanacci grade (I, II, III), pathological fracture at presentation (yes, no), timing of denosumab (preoperative only vs postoperative only, both preoperative and postoperative vs preoperative or postoperative, preoperative only vs both preoperative and postoperative, postoperative only vs both preoperative and postoperative), and number of times of denosumab administration (5>, 5≦) were analyzed as related to the frequency of recurrence. p values of < 0.05 were considered significant. Statistical analysis was done using IBM SPSS Statistics 24.0 software (IBM, Armonk, NY, USA).
Results
Responses to the questionnaire were available from 24 of 30 institutions (80.0%) participating to JCOG BSTTSG. Standard treatments of curettage for GCTB in the 24 institutions are summarized in Table 1. As a local adjuvant therapy, high-speed burr was used after curettage in 22 of 24 (92%) institutions followed by ethanol (8 of 24 institutions, 33%), liquid nitrogen (6 of 24 institutions, 25%), and phenol (3 of 24 institutions, 13%). Autologous bone graft and polymethyl methacrylate (PMMA) were used for reconstruction after curettage in 18 (75%) and 17 (71%) of 24 institutions, respectively. Characteristics of the patients with GCTB were summarized in Table 2. Primary tumor sites were in an extremity in 151 of 158 (96%) patients and trunk in 7 of 158 (4%). Sixteen of 158 (10%) evaluated as Campanacci grade I, 97 of 158 (61%) as Campanacci grade II, and 45 of 158 (29%) as Campanacci grade III.
Thirty of 158 (19.0%) developed local recurrence, and 4 of 158 (2.5%) developed distant recurrence after curettage without perioperative denosumab. In extremities, 29 of 151 (19.2%) developed local recurrence, and 4 of 151 (2.6%) developed distant recurrence after curettage. There were no deaths after curettage. Campanacci grade and embolization before surgery were significantly associated with an increased incidence of local recurrence after curettage (p = 0.034 and p = 0.022, respectively) (Table 3). Demographics of local recurrent patients after curettage for GCTB were summarized in Table 4. Local recurrence occurred in 1 of 16 (6.2%) in Campanacci grade I, 15 of 97 (15.5%) in Campanacci grade II, and 14 of 45 (31.1%) in Campanacci grade III. Median time to local recurrence was 15.5 months (5–69 months) after curettage, and joint preservation was achieved in 26 of 30 patients (86.7%).
The indications of denosumab for GCTB at the 24 institutions are summarized in Table 5. As a general policy, denosumab was used perioperatively at 6 of 24 (25%) institutions. Actually, 40 patients were treated with perioperative denosumab in 16 institutions. The number of GCTB patients treated with perioperative denosumab and that of the institutions where they were treated were listed in Table 6. Denosumab was administered subcutaneously at 120 mg, but the dosing interval was not included in the questionnaire. Median number of times of denosumab administration were 6 (2–41) and 6 (1–14) in the preoperative and postoperative settings, respectively. The local recurrences were observed in 6 of 21 (28.6%), 2 of 9 (22.2%), and 0 of 10 (0.0%) patients treated with the preoperative, postoperative, and both preoperative and postoperative denosumab, respectively. In 31 patients treated with any preoperative denosumab, administration exceeding 5 times was significantly associated with a decreased incidence of local recurrence after curettage (p < 0.001) (Table 7). Question for toxicity or side effects during perioperative denosumab were not included in the questionnaire survey.
Discussion
To plan a clinical trial JCOG 1610, a randomized phase III study of preoperative denosumab with curettage for GCTB, we conducted a questionnaire survey to comprehend the historical clinical results after curettage of GCTB without perioperative denosumab. Although the clinical outcomes after curettage of GCTB have been reported sporadically in Japan [7, 10, 11], the more recent clinical results of GCTB after curettage in multiple institutions in Japan are not as clear. To determine the recent perioperative use in Japan, we also reviewed patients with GCTB treated with curettage and perioperative denosumab. Even though denosumab was accepted for health insurance coverage in Japan in 2014, the risk/benefit ratio of denosumab when used for patients with GCTB who are treatable by curettage is not well defined.
There are some limitations in our study. First, because of its questionnaire format, we did not have data regarding the follow-up period after curettage. We investigated GCTB patients treated from 2008 to 2010 and performed this questionnaire survey in 2015, meaning that the follow-up period can be considered adequate given that most recurrences in GCTB occur within 5 years [3,4,5,6] and recurrent GCTB is usually treated at the same institution where the first surgery was performed. Second, there was a lack of important data such as size of tumor, detailed sites, and Campanacci grade of GCTB treated with perioperative denosumab, which could act as confounding factors. Because our study is a questionnaire survey, we could not conduct an additional survey due to unlinkable anonymizing of our data. Third, we could not perform multivariate analysis because of the small number of recurrences and lack of information regarding other important clinical factors. Finally, we could not determine whether the patients treated with preoperative denosumab were all suitable for curettage from the time of their initial consultation.
As a local adjuvant therapy, a high-speed burr was used after curettage in 92% of institutions followed by ethanol (33%), liquid nitrogen (25%), and phenol (13%) in our study. Clinical results of these local adjuvant therapies have been reported [3, 5, 9], but it is difficult to determine the advantage of each treatment. Generally, the high-speed burr is easier to use than drug therapies such as ethanol, liquid nitrogen, and phenol, accounting for its extensive use in Japan.
In our study, autologous bone graft and PMMA were used for reconstruction after curettage in 75% and 71% of institutions, respectively. Some reports demonstrated the clinical benefit of PMMA for decreasing local recurrence after curettage of GCTB [3, 4, 6, 9]. Autologous bone graft is widely used in Japan because the lack of a bone bank precludes routine use of allogenic bone.
In our study, the local recurrence rate after curettage was 19.0%. Previous reports showed local recurrence of GCTB in 24.8–30.8% after curettage [3,4,5,6], and so our local recurrence rate is slightly better than that documented in these previous reports. Past reports have shown some clinical factors affecting local recurrence such as tumor extension, surgical margin, local adjuvant therapy, Campanacci grade, use of PMMA, and soft tissue progression on multivariate analyses [4, 5, 9]. In our study, Campanacci grade was well balanced similar to previous studies [9, 15], and we ascribe the better local recurrence rate achieved in our study to the wide use of local adjuvant treatments as well as PMMA in many institutions.
In our study, Campanacci grade was significantly associated with increasing incidence of local recurrence after curettage, and this result was similar to that noted in previous studies [4]. Embolization before surgery was also significantly associated with the increasing incidence of local recurrence after curettage, but it was difficult to interpret. Since the embolization is usually used for patients with GCTB which is large and expected bleeding, there was a possibility that these factors had affected the result. However, in the present study, one of the recurrent cases after embolization had GCTB in metatarsal bone (Table 4). Our study included only a small number of cases of GCTB in the trunk and a previous report showed a high local recurrence rate of 43.3% in axial cases after curettage [4]. However, there was no difference in the local recurrence rate between location in the trunk (1 of 7 patients, 14.3%) and extremity (29 of 151 patients, 19.2%) in our study. There was no significant relation between pathological fracture at first visit and local recurrence in our study. A past report also could not demonstrate an effect of pathological fracture on local recurrence in a meta-analysis [16]. Local recurrent GCTB is known to be highly re-recurrent after curettage with rates of re-recurrence of 32 to 34% [17, 18]. Our study included no patients after recurrence, and our analysis was limited to primary tumors. Distant recurrence rate was 2.6% after curettage in our study, and this result was similar to that noted in previous studies [7, 8].
In our study, 16 of 24 (67%) institutions actually performed perioperative use of denosumab with curettage, and the recurrence rates were 6 of 21 (28.6%), 2 of 9 (22.2%), and 0 of 10 (0.0%) with preoperative, postoperative, and both pre- and postoperative treatments with curettage, respectively. One study on the perioperative use of denosumab for GCTB with curettage demonstrated local recurrence in 17 of 116 patients (15%) with a median follow-up period of 13.0 months [19]. However, the study included patients with unresectable GCTB and salvageable GCTB whose surgery was associated with severe morbidity, and the effect of denosumab for patients with GCTB who can be treated by curettage at the first visit was not clear. Our study may have also included some patients who could not be treated by curettage at first, but we could not identify those patients due to the questionnaire format used.
Median numbers of administration times of denosumab were 6 (2–41) and 6 (1–14) in the preoperative and postoperative settings, respectively in our study. Some reports demonstrated histopathological changes after 6 months treatment with denosumab [20, 21], but 6 months are thought to be too long to use it as a post- and/or preoperative treatment in patients with GCTB which can be treated by curettage. In our study, more than five administration times was significantly associated with a decreased incidence of local recurrence after curettage in 31 patients treated with preoperative or both pre- and postoperative denosumab, meaning that a sufficiently high dose of preoperative denosumab can suppress local recurrence after curettage. When used as a running dose, five times administration of denosumab takes 3 months. This relatively short administration period is associated with major benefits, both economic and social, for patients, and this dose is specified in JCOG1610.
The clinical use of perioperative denosumab is complicated by various issues such as economic problem, side effects, and pregnancy. The cost of one-shot denosumab (120 mg) for GCTB is 46,685 yen (approximately 420 dollar) in Japan as of October 2017. In the previous phase 2 trial of GCTB, denosumab caused diverse side effects such as arthralgia (20%), headache (18%), nausea (17%), fatigue (16%), back pain (15%), extremity pain (15%), hypocalcemia (5%), and osteonecrosis of jaw (1%) of any grade [14]. The use of denosumab for pregnant women should be avoided because it was reported to increase postnatal mortality, decreased body weight gain, and decreased growth/development in a study of infants exposed in utero in cynomolgus monkeys [22]. This may affect the clinical use of denosumab for premenopausal women. In addition, there is a report that denosumab treatment in postmenopausal women with osteoporosis did not interfere with fracture healing [23], but the effects of denosumab on pathological fracture healing and final joint preservation have not been well understood in GCTB patients. Malignant transformation occurs in less than 1% of GCTB [1], and recently, malignant transformation was reported after treatment with denosumab [24] and requires particular caution. In addition, recent study showed a higher rate of recurrence in the GCTB treated with denosumab and curettage compared to historical control without denosumab in retrospective study [25]. For these reasons, perioperative treatment of denosumab should not be done unless an advantage is considered or proved in GCTB which can be treated by curettage.
At present, we have started a clinical trial, JCOG1610 (UMIN000029451), to investigate the efficacy of preoperative denosumab in patients with GCTB which can be treated with curettage. The primary aim of JCOG1610 is to confirm the effects of preoperative denosumab on recurrence after curettage. A previous report demonstrated that proliferation of stromal cells cultured from clinical specimens following denosumab treatment was approximately 50% slower than that of specimens from untreated patients [20]. Even though denosumab did not completely prevent proliferation of stromal cells which have been considered as genuine tumor cells [20], there is a possibility that preoperative denosumab may decrease local and distant recurrences after the curettage of the tumor stromal cells biologically suppressed by denosumab. Secondary endpoints of JCOG1610 include overall survival, joint-preserved survival, local relapse-free survival, metastasis-free survival, adverse events, serious adverse events, surgical and postoperative complications, and discontinuance of denosumab. Systemic denosumab treatment can affect joint-preserved survival, and both local and distant recurrence.
Conclusions
In conclusion, the recurrence rate of GCTB after curettage was 19.0% in Japan and especially high in Campanacci grade III; therefore, improvements in the therapeutic strategy are needed in this cohort. There is a possibility that a sufficient dose of preoperative denosumab can reduce recurrence after curettage. Recently, we have started JCOG1610 to investigate the efficacy of preoperative denosumab in patients with GCTB which can be treated with curettage.
Abbreviations
- BSTTSG:
-
Bone and Soft Tissue Tumor Study Group
- GCTB:
-
Giant cell tumor of bone
- JCOG:
-
Japan clinical oncology group
- PMMA:
-
Polymethyl methacrylate
- RANK:
-
Receptor activator of NF-κB
- RANKL:
-
Receptor activator of NF-κB ligand
References
Fletcher CDM. WHO classification of tumours of soft tissue and bone. 4th ed. Lyon: IARC Press; 2013.
Futamura N, Urakawa H, Tsukushi S, Arai E, Kozawa E, Ishiguro N, Nishida Y. Giant cell tumor of bone arising in long bones possibly originates from the metaphyseal region. Oncol Lett. 2016;11:2629–34.
Balke M, Schremper L, Gebert C, Ahrens H, Streitbuerger A, Koehler G, Hardes J, Gosheger G. Giant cell tumor of bone: treatment and outcome of 214 cases. J Cancer Res Clin Oncol. 2008;134:969–78.
Gaston CL, Bhumbra R, Watanuki M, Abudu AT, Carter SR, Jeys LM, Tillman RM, Grimer RJ. Does the addition of cement improve the rate of local recurrence after curettage of giant cell tumours in bone? J Bone Joint Surg Br. 2011;93:1665–9.
van der Heijden L, van der Geest IC, Schreuder HW, van de Sande MA, Dijkstra PD. Liquid nitrogen or phenolization for giant cell tumor of bone?: a comparative cohort study of various standard treatments at two tertiary referral centers. J Bone Joint Surg Am. 2014;96:e35.
Gao ZH, Yin JQ, Xie XB, Zou CY, Huang G, Wang J, Shen JN. Local control of giant cell tumors of the long bone after aggressive curettage with and without bone cement. BMC Musculoskelet Disord. 2014;15:330.
Masui F, Ushigome S, Fujii K. Giant cell tumor of bone: a clinicopathologic study of prognostic factors. Pathol Int. 1998;48:723–9.
Siebenrock KA, Unni KK, Rock MG. Giant-cell tumour of bone metastasising to the lungs. A long-term follow-up. J Bone Joint Surg Br. 1998;80:43–7.
Arbeitsgemeinschaft K, Becker WT, Dohle J, Bernd L, Braun A, Cserhati M, Enderle A, Hovy L, Matejovsky Z, Szendroi M, et al. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy. J Bone Joint Surg Am. 2008;90:1060–7.
Muramatsu K, Ihara K, Taguchi T. Treatment of giant cell tumor of long bones: clinical outcome and reconstructive strategy for lower and upper limbs. Orthopedics. 2009;32:491.
Oda Y, Miura H, Tsuneyoshi M, Iwamoto Y. Giant cell tumor of bone: oncological and functional results of long-term follow-up. Jpn J Clin Oncol. 1998;28:323–8.
Atkins GJ, Haynes DR, Graves SE, Evdokiou A, Hay S, Bouralexis S, Findlay DM. Expression of osteoclast differentiation signals by stromal elements of giant cell tumors. J Bone Miner Res. 2000;15:640–9.
Branstetter DG, Nelson SD, Manivel JC, Blay JY, Chawla S, Thomas DM, Jun S, Jacobs I. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin Cancer Res. 2012;18:4415–24.
Chawla S, Henshaw R, Seeger L, Choy E, Blay JY, Ferrari S, Kroep J, Grimer R, Reichardt P, Rutkowski P, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013;14:901–8.
Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69:106–14.
Salunke AA, Chen Y, Chen X, Tan JH, Singh G, Tai BC, Khin LW, Puhaindran ME. Does pathological fracture affect the rate of local recurrence in patients with a giant cell tumour of bone?: a meta-analysis. Bone Joint J. 2015;97-B:1566–71.
Takeuchi A, Tsuchiya H, Niu X, Ueda T, Jeon DG, Wang EH, Asavamongkolkul A, Kusuzaki K, Sakayama K, Kang YK. The prognostic factors of recurrent GCT: a cooperative study by the Eastern Asian Musculoskeletal Oncology Group. J Orthop Sci. 2011;16:196–202.
Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res. 2011;469:1181–7.
Rutkowski P, Ferrari S, Grimer RJ, Stalley PD, Dijkstra SP, Pienkowski A, Vaz G, Wunder JS, Seeger LL, Feng A, et al. Surgical downstaging in an open-label phase II trial of denosumab in patients with giant cell tumor of bone. Ann Surg Oncol. 2015;22:2860–8.
Mak IW, Evaniew N, Popovic S, Tozer R, Ghert M. A translational study of the neoplastic cells of Giant cell tumor of bone following neoadjuvant denosumab. J Bone Joint Surg Am. 2014;96:e127.
Thomas D, Henshaw R, Skubitz K, Chawla S, Staddon A, Blay JY, Roudier M, Smith J, Ye Z, Sohn W, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11:275–80.
Bussiere JL, Pyrah I, Boyce R, Branstetter D, Loomis M, Andrews-Cleavenger D, Farman C, Elliott G, Chellman G. Reproductive toxicity of denosumab in cynomolgus monkeys. Reprod Toxicol. 2013;42:27–40.
Adami S, Libanati C, Boonen S, Cummings SR, Ho PR, Wang A, Siris E, Lane J, Group FF-HW, Adachi JD, et al. Denosumab treatment in postmenopausal women with osteoporosis does not interfere with fracture-healing: results from the FREEDOM trial. J Bone Joint Surg Am. 2012;94:2113–9.
Aponte-Tinao LA, Piuzzi NS, Roitman P, Farfalli GL. A high-grade sarcoma arising in a patient with recurrent benign giant cell tumor of the proximal tibia while receiving treatment with denosumab. Clin Orthop Relat Res. 2015;473:3050–5.
Errani C, Tsukamoto S, Leone G, Righi A, Akahane M, Tanaka Y, Donati DM. Denosumab may increase the risk of local recurrence in patients with giant-cell tumor of bone treated with curettage. J Bone Joint Surg Am. 2018;100:496–504.
Acknowledgements
We thank young researchers in JCOG BSTTSG, Dr. Shintaro Iwata, Dr. Makoto Endo, Dr. Tomoya Matsunobu, Dr. Tomoki Nakamura, Dr. Fumihiko Nakatani, and Dr. Kazuya Oshima for the kind advice regarding this questionnaire survey.
Funding
We do not have a funding for this questionnaire study.
Availability of data and materials
The datasets supporting the conclusions of the article are included within the article.
Author information
Authors and Affiliations
Contributions
HU was involved in the conception and design of the study. TY, SM, TT, KA, MW, AT, NN, YM, AK, TK, TK, ME, HH, HH, ST, YN, TA, TM, MT, AN, HY, KS, MK, and KH were responsible in the acquisition of data and HU in the analysis of data. HU drafted the article, and all authors edited and revised it for important intellectual content. HU, KT, HH, YI, and TO take responsibility for the integrity of the work as a whole, from inception to finished article. All authors approved the final version to be published.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of Nagoya University Graduate School and School of Medicine (Nagoya, Japan) and a waiver of informed consent was provided.
Consent for publication
Not applicable.
Competing interests
None of the authors have any financial or personal relationships with any other persons or organizations that could potentially and/or inappropriately influence their work and conclusion.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Urakawa, H., Yonemoto, T., Matsumoto, S. et al. Clinical outcome of primary giant cell tumor of bone after curettage with or without perioperative denosumab in Japan: from a questionnaire for JCOG 1610 study. World J Surg Onc 16, 160 (2018). https://doi.org/10.1186/s12957-018-1459-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-018-1459-6