Abstract
Background
With limited sample sizes and single-institution designs, how complete response (CR) after neoadjuvant chemotherapy (NAC) influences breast conserving surgery (BCS) and its value in prognosis are not clear.
Methods
A systematic research review was conducted using electronic database. The rate of clinical complete response (cCR) in BCS after NAC and these pathological CR (PCR) and non-pCR BCS patients’ local recurrence-free survival (LRFS), distance recurrence-free survival (DRFS), overall survival (OS), and disease-free survival (DFS) rates were collected. A pooled analysis was performed using a fixed or random effects model and a Q test to determine heterogeneity.
Results
Sixteen studies with a total of 4639 patients were included. The pooled data revealed that cCR patients compared with non-cCR patients had significantly higher rates of BCS, with a summary estimate odds ratios (OR) of 4.54 (95% CI 2.03–10.17). The pooled data revealed that BCS patients who achieved pCR after NAC had significantly lower rates of LRFS (RR = 0.59, 95% CI 0.38–0.92) and DRFS (RR = 0.27, 95% CI 0.13–0.55). Better DFS (RR = 0.09, 95% CI 0.04–0.25) and OS (RR = 0.36, 95% CI 0.03–3.90) were also seen, but OS was not significantly different.
Conclusions
The rate of successful BCS is higher in the cCR group than in the non-cCR group, means cCR after NAC can encourage patients to receive BCS. The achievement of pCR after NAC in BCS patients was associated with a good prognosis in terms of LRFS and DRFS, but its value in DFS and OS requires further investigation.
Similar content being viewed by others
Background
Breast cancer is known as the most common cancer with the second highest mortality in females [1]. Recently, the use of NAC has been extended to downstage tumors and enhances the BCS rate [2,3,4]. Some studies have reported equal survival benefits between preoperative and postoperative chemotherapy [5,6,7], and with the gradual improvement of postoperative radiotherapy after BCS, BCS after NAC has become more and more accepted.
The survival benefit for patients who achieve pCR after NAC is controversial. Some studies found that pCR patients have a better prognosis than non-pCR patients but Cortazar P put out in his meta-analysis that in trial-level studies, the association between pCR and both event-free survival (EFS) and OS was weak [8, 9]. Recurrence following BCS after NAC is concerned by patients and doctors. Some studies reported that BCS after NCT leads to loco-regional recurrence (LR) rates of less than 10%, but other studies reported LR rates greater than 20% [10,11,12,13]. These conclusions were drawn from studies in which the patients were not carefully separated into BCS and mastectomy treatment (MT) groups or according to CR and non-CR.
How CR influences BCS is unclear because of limited high-quality data. For these reasons, we collected data on diagnosis, treatment, and prognosis from patients who received BCS after NAC and pooled the results for analysis in this study. The goal was to explore the rate of BCS in cCR compared to non-cCR patients after NAC and if other factors influenced this outcome. We also investigated whether pCR is a prognostic factor in BCS patients relative to LRFS, DRFS, DFS, and OS compared with BCS patients who did not achieve pCR. The potential prognostic value of pCR status may be very useful in BCS patients who received NAC because it can help in the determination of follow-up treatments and monitoring.
Methods
Literature search
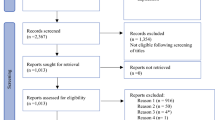
We carried out a systematic literature search using PubMed, Embase, and the Web of Science through June 20, 2017. The search headings were as follows: (breast conserving surgery OR breast conserving operation OR lumpectomy OR quadrantectomy) AND (event-free survival OR overall survival OR disease-free survival OR DFS OR OS OR treatment outcome) AND (exp breast neoplasms OR cancer OR neoplasm OR carcinoma OR exp. neoadjuvant therapy OR neoadjuvant OR exp. preoperative care) AND (cohort OR exp. cohort studies OR longitudinal OR prospective OR retrospective OR exp. clinical trial OR double-blind method OR clinical trial OR randomized controlled trial OR multicenter study OR exp. clinical trials as topic OR controlled trial OR single OR double trial OR blind trail). In all, we found 1587 articles, including 247 from PubMed, 865 from the Web of Science, and 475 from Embase (Fig. 1 ).
Study selection criteria
The published studies to be included in this analysis met the following criteria: (1) a focus on breast cancer patients who were treated with neoadjuvant chemotherapy and then received surgery, (2) the presence of evaluations of the associations between CR and outcomes in patients who received either breast conserving surgery or mastectomy surgery, (3) the presence of the necessary information to calculate outcomes (LRFS, DRFS, DFS, or OS) of interest, and (4) availability as a full-text English-language publication. When multiple publications from the same author or institution appeared that presented duplicate data, we chose the most applicable one. Systematic reviews, meta-analyses, case studies, and articles that did not meet our terms were excluded.
Data extraction
The following information was extracted from each eligible study: the first authors’ names, the country in which the data were collected, the publication year, the applied neoadjuvant chemotherapy regimens, the total number of patients, mean age, tumor size, follow-up time, the number of patients who received BCS, the number patients who achieved CR, and the survival data (percentages and numbers of events) of BCS patients who achieved CR and those who did not. Other information was also recorded if the author found it useful. We used The Newcastle-Ottawa Scale (NOS) to assess the quality of each study by two authors. The scores included three parts: the selectivity of patients (0–4), comparability of groups (0–2) and assessment of outcome (0–3). Scores > 5 were considered high-quality studies.
Statistical analysis
A meta-analysis was conducted for each endpoint of interest when there were two or more studies available. The data were pooled with the fixed-effects model by default, but if homogeneity across the tests was identified by the Q statistic and the I 2 value presented significant homogeneity as I 2 > 50% or Cochran Q < 0.1, a random effects model was used. If significant heterogeneity appeared, a subgroup analysis according to neoadjuvant chemotherapy type and a sensitivity analysis to exclude unsuitable studies were used to determine the source of heterogeneity.
Using the data on the extent to which CR influences BCS conduct, we analyzed each study and calculated pooled odds ratios (OR) and 95% CIs. LRFS, DRFS, DFS, and OS were compared between the CR group and non-CR group of BCS patients using risk ratios (RR) and 95% CIs. A 95% CI that did not include zero was considered significantly different. A power analysis was performed for each study, and the pooled results were evaluated using Power and Precision software following the manufacturer’s instructions.
We investigated whether funnel plots presented symmetry to generally assess the publication bias. All the collected data were analyzed using RevMan 5.3 analysis software (Cochrane Collaboration, Copenhagen, Denmark), and all statistical tests were two-sided with statistical significance defined as P < 0.05.
Results
We identified 1587 records, and through careful assessments, 16 studies were identified as eligible for the data abstract [10, 14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. Two studies were from the same author, but the data abstracts were different, so we included both studies [10, 14]. Among these 16 studies, five were random clinical trials, three were non-random clinical trials, and eight were retrospective cohort studies. Under the NOS quality system, all the studies’ score greater than five so the quality of the date met the analysis requirement. The details of the 16 studies included in the meta-analysis are reported in Table 1 . The specific number and power values of the studies are shown in Table 2 . Seven papers reported cCR data, and the others all reported pCR data. According to each author’s definition, four studies defined pCR as the absence of an invasive component in the primary breast tumor, regardless of pathological axillary node status, and the others defined pCR as the absence of an invasive component in both the breast and the axillary node. Among these reports with abstract data on pCR and non-pCR in BCS patients, 11 studies provided LRFS values for pCR and non-pCR individually, four studies provided DRFS values, and only two studies provided DFS and OS values. The power to detect OS was low, only 0.589. In contrast, the pooled power was greater than 0.9 for LRFS, DRFS, and DFS.
Primary endpoint: cCR and its effect on BCS conduct
Seven studies reported exact data of BCS rate in cCR and non-cCR groups. About 352 patients gained cCR, among them, 213 patients received BCS; in 1713 patients not gained cCR, about 624 patients received BCS. The pooled data revealed that cCR patients compared with non-cCR patients had significantly higher rates of BCS and the summary estimate OR was 4.54 (95% CI 2.03–10.17). Heterogeneity testing revealed I 2 = 85% with P < 0.001 (random effects model; Fig. 2 ). We then divided these studies into two groups according to neoadjuvant chemotherapy type. Neoadjuvant chemotherapy in group 1 included a taxane-anthracycline-based protocol and group 2 included an anthracycline without taxane protocol. Group 1 had the higher BCS rate in cCR patients (OR = 6.25, 95% CI 1.74–22.39) than group 2 (OR = 3.81, 95% CI 1.65–8.78), but the two groups did not show subgroup significant heterogeneity (I 2 = 0% with P = 0.53, Additional file 1 : Figure S1a).
Secondary endpoints: LR and DR rates in BCS patients who did or did not achieve pCR
Eleven studies reported LR rates in BCS patients (2197 patients). In BCS patients, the overall LR rate was 3.9% (range 0–10.8%) in pCR patients and 8.1% (range 2.3–17.2%) in non-pCR patients. The pooled data revealed that the BCS patients who achieved pCR after neoadjuvant chemotherapy had significantly lower rates of LR, with a summary estimate RR of 0.59 (95% CI 0.38–0.92). Heterogeneity testing revealed I 2 = 7% with P = 0.38 (fixed effects model, Fig. 3 a). Subgroup analysis showed that BCS patients with pCR had statistic significant differences better LRFS (RR = 0.47, 95% CI 0.26–0.83) in 5-year follow-up while not show statistic significant differences in 7-year follow-up (RR = 0.94, 95% CI 0.45–1.97, Fig. 3a).
Forest plots showing RR and the 95% CI of LRFS (a), DLFS (b), DFS (c), and OS (d) for pCR vs. non-pCR in BCS. Note: BCS breast conserving surgery, LRFS local recurrence-free survival, DRFS distant recurrence-free survival, OS overall survival, DFS disease-free survival, pCR pathology complete response, RR risk ratios, DR distant recurrence
Four studies reported DR rates in BCS patients (674 patients). In BSC patients, the overall DR rate was 3.5% (varied 0–14.3%) in pCR patients and 21.5% (varied 10.8–31.0%) in non-pCR patients. The pooled data revealed that BCS patients with pCR had significantly lower DR rates, with a summary estimate RR of 0.27 (95% CI 0.13–0.55). Heterogeneity testing revealed I 2 = 2% with P = 0.38 (fixed effects model, Fig. 3 b). In subgroup analysis, BCS patients with pCR in 5 year follow-up had significant difference in DRFS (RR = 0.12, 95% CI 0.03–0.47) while in a 7-year follow-up had no significant difference (RR = 0.46, 95% CI 0.19–1.11, Fig. 3b).
Two studies that reported LR data and another two studies that reported both LR and DR data were excluded by the sensitivity analysis because of the pCR definition they used, which was the absence of an invasive component in the primary breast tumor instead of in both the breast and the axillary node. However, the advantage of pCR in BCS remained significantly different, with an RR of 0.44 (95% CI 0.22–0.86, heterogeneity testing I 2 = 0% with P = 0.80, Additional file 1 : Figure S1b) in LRFS. The RR was 0.36 (95% CI 0.16–0.82, heterogeneity testing I 2 = 13% with P = 0.28, Additional file 1 : Figure S1c) in DRFS.
Tertiary endpoints: DFS and OS in BCS patients who did and did not achieve pCR
Only two studies provided DFS and OS rates in pCR and non-pCR patients who received BCS. The median follow-up times were 66 and 46 months. We evaluated these two studies in the meta-analysis and found that DFS rates were higher in BCS patients with pCR, with a summary estimate RR of 0.09 (95% CI 0.04–0.25, heterogeneity testing I 2 = 0% with P = 0.84, Fig. 3 c). OS was higher in pCR patients, but without a significant difference, as the RR was 0.36 (95% CI 0.03–3.90, heterogeneity testing I 2 = 63% P = 0.10, Fig. 3d).
Publication bias
The publication bias was evaluated with funnel plots. The funnel plots showed no obvious evidence for a publication bias as the plot was basically inverted and funnel-shaped with bilateral symmetry (Fig. 4 ).
Discussion
Recently, NAC has been widely used in breast cancer patients to downstage tumor size, increase the rate of resection, or increase eligibility for breast conserving surgery [29, 30]. BCS success and its prognosis were influenced by many traditional factors such as pathological features, primary tumor size, lymph node metastasis, and margin status. Patients who receive NAC exhibit one of four responses to cancer, including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Therefore, in-patients who received NAC and then BCS, whether CR can affect the operation rate of BCS and whether CR can be used as an accurate prediction marker, were discussed in this meta-analysis.
BCS rates reportedly increased overall after NAC [31]. In our study, we analyzed patients with and without cCR who received BCS and found that BCS was more likely to be successful in cCR patients. Of the seven studies included in this meta-analysis, we found significant statistical heterogeneity. NAC regimens in our study were classified as paclitaxel-epirubicin-based regimen and epirubicin without paclitaxel-based regimen. Paclitaxel-epirubicin-based regimen had higher BCS rate, and this may attribute to its superior downstaging effects [32].
Higher response and safe of BCS after tumor downstage can inspire more patients to receive BCS [33].
LRFS and DRFS were not significantly different between the BCS and MT groups [34, 35] as well as like some studies said, following neoadjuvant chemotherapy [36, 37]. However, some researchers found that BCS patients had higher LR after NAC, perhaps because the surgical margins became difficult to accurately assess [38], some studies reported contrary results that MT group had higher LR rate compared with BCS group [18, 39]. In an NSABP-B18 trial, a higher rate of LR was found in the NCT-BCS group compared to the control group, but after adjusting for tumor size and patient age, the difference was no longer significant [40]. The achievement of pCR after NAC seems to indicate a good prognosis for operable patients [41, 42], but in trial-level studies, this parameter was not recommended in the determination of EFS or OS [43]. The studies included in our analysis should be carefully compared because they used different definitions of pCR. Some studies focused on responses in the breast only, regardless of nodal involvement (ypT0/ypNx), and some used more stringent definitions that involved no residual tumor in either the breast or the node (ypT0/ypN0). We excluded studies in which only breast responses were evaluated and found that pCR patients who received BCS still had lower LR and DR rates compared with non-pCR patients who received BCS. In patients with longer follow-up time, as the included studies with limited numbers, the benefit of pCR in BCS patients’ local and distance control still in dispute. Therefore, the achievement of pCR may increase the safety of local and distant recurrence control measures in BCS patients, but how to select pCR patients before surgery needs more research such as tumor markers and accurate imaging tools.
In our study, the DFS rates were significantly higher in BCS patients with pCR. OS was higher in BCS patients with pCR, but no significant differences were observed. In another meta-analysis that included BCS and MT patients, pCR achievement following NAC indicated a good prognosis in terms of DFS and OS [42, 44]. Because of our limited data and follow-up time, the results of our study relative to DFS and OS require more data for credibility.
There are some limitations in our study. First, this analysis relied on a slightly different definition of pCR. Although we separately analyzed pCR only in the breast, pCR definition was still somewhat different compared to some studies that included in situ contents and some that did not. Second, adjuvant therapy and BCS methods including lumpectomy or quadrantectomy with sentinel lymph node biopsy or axillary dissection were mixed and matched according to the judgment of the doctor, without a united protocol. Almost all patients who received BCS also received radiotherapy, but different doses, regions, and methods were used. Adjuvant chemotherapy was administered to some patients, especially non-pCR patients. More effective treatments were used in non-pCR patients, and this may offset the advantage of pCR values in BCS eligibility. Third, the data in this study were limited. We calculated the power of each study and the pooled power, and all values were higher than 0.8, except for the data on OS. Less data was available on OS, and the follow-up times were short, so the acceptance of these results warrants caution and critical thinking.
Conclusions
Successful conduct rate of BCS was higher in the cCR group than in the non-cCR group; however, the relationship of cCR and pCR needs more research. BCS patients who achieved pCR after NAC demonstrated a good prognosis in terms of LRFS and DRFS; though also seem favors in DFS and OS, the value of pCR in long-time survival of BCS needs further investigation.
Abbreviations
- BCS:
-
Breast conserving surgery
- cCR:
-
Clinical complete response
- CR:
-
Complete response
- DFS:
-
Disease-free survival
- DR:
-
Distant recurrence
- DRFS:
-
Distant recurrence-free survival
- EFS:
-
Event-free survival
- LR:
-
Local recurrence
- LRFS:
-
Local recurrence-free survival
- MT:
-
Mastectomy
- NAC:
-
Neoadjuvant chemotherapy
- OR:
-
Odds ratios
- OS:
-
Overall survival
- pCR:
-
Pathology complete response
- RR:
-
Risk ratios
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA. 2017;67(1):7–30. https://doi.org/10.3322/caac.21387.
Bonadonna G, Veronesi U, Brambilla C, Ferrari L, Luini A, Greco M, et al. Primary chemotherapy to avoid mastectomy in tumors with diameters of three centimeters or more. J Natl Cancer Inst. 1990;82(19):1539–45.
Anderson ED, Forrest AP, Hawkins RA, Anderson TJ, Leonard RC, Chetty U. Primary systemic therapy for operable breast cancer. Br J Cancer. 1991;63(4):561–6.
Wolff AC, Davidson NE. Primary systemic therapy in operable breast cancer. J. Clin. Oncol. 2000;18(7):1558–69. https://doi.org/10.1200/jco.2000.18.7.1558.
Mauri D, Pavlidis N, Ioannidis JPA. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(3):188–94.
Mieog JSD, van der Hage JA, van de Velde CJH. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev. 2007;2 https://doi.org/10.1002/14651858.CD005002.pub2.
van der Hage JA, van de Velde CJH, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L, et al. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19(22):4224–37.
Kaufmann M, von Minckwitz G, Bear HD, Buzdar A, McGale P, Bonnefoi H, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol. 2007;18(12):1927–34. https://doi.org/10.1093/annonc/mdm201.
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72. https://doi.org/10.1016/s0140-6736(13)62422-8.
Rouzier R, Extra JM, Carton M, Falcou MC, Vincent-Salomon A, Fourquet A, et al. Primary chemotherapy for operable breast cancer: incidence and prognostic significance of ipsilateral breast tumor recurrence after breast-conserving surgery. J Clin Oncol. 2001;19(18):3828–35.
Cance WG, Carey LA, Calvo BF, Sartor C, Sawyer L, Moore DT, et al. Long-term outcome of neoadjuvant therapy for locally advanced breast carcinoma: effective clinical downstaging allows breast preservation and predicts outstanding local control and survival. Ann Surg. 2002;236(3):295–302; discussion -3. https://doi.org/10.1097/01.sla.0000027526.67560.64.
Chen AM, Meric-Bernstam F, Hunt KK, Thames HD, Oswald MJ, Outlaw ED, et al. Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol. 2004;22(12):2303–12. https://doi.org/10.1200/jco.2004.09.062.
Mauriac L, MacGrogan G, Avril A, Durand M, Floquet A, Debled M et al. Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: a unicentre randomized trial with a 124-month median follow-up. Institut Bergonie Bordeaux Groupe Sein (IBBGS) Ann Oncol 1999;10(1):47-52.
Rouzier R, Mathieu MC, Sideris L, Youmsi E, Rajan R, Garbay JR, et al. Breast-conserving surgery after neoadjuvant anthracycline-based chemotherapy for large breast tumors. Cancer. 2004;101(5):918–25. https://doi.org/10.1002/cncr.20491.
Massidda B, Atzori F, Scanu A, Contu A, Farris A, Catino AM, et al. Dose-dense primary chemotherapy, as part of multidisciplinary treatment, for inoperable stage IIIB breast cancer-long-term results of a phase II trial. Oncology. 2007;72(1–2):17–26. https://doi.org/10.1159/000111084.
Shen J, Valero V, Buchholz TA, Singletary SE, Ames FC, Ross MI, et al. Effective local control and long-term survival in patients with T4 locally advanced breast cancer treated with breast conservation therapy. Ann Surg Oncol. 2004;11(9):854–60. https://doi.org/10.1245/aso.2004.02.003.
Fastner G, Reitsamer R, Ziegler I, Zehentmayr F, Fussl C, Kopp P, et al. IOERT as anticipated tumor bed boost during breast-conserving surgery after neoadjuvant chemotherapy in locally advanced breast cancer-results of a case series after 5-year follow-up. Int J Cancer. 2015;136(5):1193–201. https://doi.org/10.1002/ijc.29064.
McIntosh SA, Ogston KN, Payne S, Miller ID, Sarkar TK, Hutcheon AW, et al. Local recurrence in patients with large and locally advanced breast cancer treated with primary chemotherapy. Am J Surg. 2003;185(6):525–31. https://doi.org/10.1016/s0002-9610(03)00078-3.
Caudle AS, T-K Y, Tucker SL, Bedrosian I, Litton JK, Gonzalez-Angulo AM, et al. Local-regional control according to surrogate markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast Cancer Res. 2012;14(3) https://doi.org/10.1186/bcr3198.
Bonadonna G, Zambetti M, Bumma C, Donadio M, Bolognesi A, della Cuna GR, et al. Multimodal treatment with primary single-agent epirubicin in operable breast cancer: 5-year experience of the Michelangelo Cooperative Group. Ann Oncol. 2002;13(7):1049–58. https://doi.org/10.1093/annonc/mdf097.
Jimbo K, Kinoshita T, Asaga S, Hojo T. Oncological safety of breast-conserving surgery after primary systemic chemotherapy in cT3-4 breast cancer patients. Surg Today. 2015;45(10):1255–62. https://doi.org/10.1007/s00595-014-1052-8.
Cho JH, Park JM, Park HS, Park S, Kim SI, Park B-W. Oncologic safety of breast-conserving surgery compared to mastectomy in patients receiving neoadjuvant chemotherapy for locally advanced breast cancer. J Surg Oncol. 2013;108(8):531–6. https://doi.org/10.1002/jso.23439.
Walker LG, Eremin JM, Aloysius MM, Vassanasiri W, Walker MB, El-Sheemy M, et al. Effects on quality of life, anti-cancer responses, breast conserving surgery and survival with neoadjuvant docetaxel: a randomised study of sequential weekly versus three-weekly docetaxel following neoadjuvant doxorubicin and cyclophosphamide in women with primary breast cancer. BMC Cancer. 2011;11:179. https://doi.org/10.1186/1471-2407-11-179.
Beriwal S, Schwartz GF, Komarnicky L, Garcia-Young JA. Breast-conserving therapy after neoadjuvant chemotherapy: long-term results. Breast J. 2006;12(2):159–64. https://doi.org/10.1111/j.1075-122X.2006.00225.x.
Noh JM, Park W, Suh CO, Keum KC, Kim YB, Shin KH, et al. Is elective nodal irradiation beneficial in patients with pathologically negative lymph nodes after neoadjuvant chemotherapy and breast-conserving surgery for clinical stage II-III breast cancer? A multicentre retrospective study (KROG 12-05). Br J Cancer. 2014;110(6):1420–6. https://doi.org/10.1038/bjc.2014.26.
Yamazaki N, Wada N, Yamauchi C, Yoneyama K. High expression of post-treatment Ki-67 status is a risk factor for locoregional recurrence following breast-conserving surgery after neoadjuvant chemotherapy. Eur J Surg Oncol. 2015;41(5):617–24. https://doi.org/10.1016/j.ejso.2015.01.036.
von Minckwitz G, Kummel S, Vogel P, Hanusch C, Eidtmann H, Hilfrich J, et al. Neoadjuvant vinorelbine-capecitabine versus docetaxel-doxorubicin-cyclophosphamide in early nonresponsive breast cancer: phase III randomized GeparTrio trial. J Natl Cancer Inst. 2008;100(8):542–51. https://doi.org/10.1093/jnci/djn085.
Criscitiello C, Azim HA, Jr., Agbor-tarh D, de Azambuja E, Piccart M, Baselga J et al. Factors associated with surgical management following neoadjuvant therapy in patients with primary HER2-positive breast cancer: results from the NeoALTTO phase III trial Ann Oncol 2013;24(8):1980-1985. doi:https://doi.org/10.1093/annonc/mdt129.
Vlastos G, Mirza NQ, Lenert JT, Hunt KK, Ames FC, Feig BW, et al. The feasibility of minimally invasive surgery for stage IIA, IIB, and IIIA breast carcinoma patients after tumor downstaging with induction chemotherapy. Cancer. 2000;88(6):1417–24.
Kuerer HM, Singletary SE, Buzdar AU, Ames FC, Valero V, Buchholz TA, et al. Surgical conservation planning after neoadjuvant chemotherapy for stage II and operable stage III breast carcinoma. Am J Surg. 2001;182(6):601–8.
Lee S, Kim SW, Kim SK, Lee KS, Kim EA, Kwon Y, et al. Locoregional recurrence of breast conserving surgery after preoperative chemotherapy in Korean women with locally advanced breast cancer. J Breast Cancer. 2011;14(4):289–95. https://doi.org/10.4048/jbc.2011.14.4.289.
Tan QW, Luo T, Zheng H, Tian TL, He P, Chen J, et al. Weekly taxane-anthracycline combination regimen versus tri-weekly anthracycline-based regimen for the treatment of locally advanced breast cancer: a randomized controlled trial. Chinese J Cancer. 2017;36(1):27. https://doi.org/10.1186/s40880-017-0196-5.
Fitzal F, Riedl O, Mittlbock M, Dubsky P, Bartsch R, Steger G, et al. Oncologic safety of breast conserving surgery after tumour downsizing by neoadjuvant therapy: a retrospective single centre cohort study. Breast Cancer Res Treat. 2011;127(1):121–8. https://doi.org/10.1007/s10549-010-1164-9.
Cai X, Liu X, Yu H, Li J, Zheng X. Breast-conserving therapy for early-stage breast cancer in Chinese women: a meta-analysis of case-control studies. Onkologie. 2012;35(3):133–9. https://doi.org/10.1159/000336969.
Zumsteg ZS, Morrow M, Arnold B, Zheng J, Zhang Z, Robson M, et al. Breast-conserving therapy achieves locoregional outcomes comparable to mastectomy in women with T1-2N0 triple-negative breast cancer. Ann Surg Oncol. 2013;20(11):3469–76. https://doi.org/10.1245/s10434-013-3011-9.
Touboul E, Lefranc JP, Blondon J, Buffat L, Deniaud E, Belkacemi Y, et al. Primary chemotherapy and preoperative irradiation for patients with stage II larger than 3 cm or locally advanced non-inflammatory breast cancer. Radiother Oncol. 1997;42(3):219–29.
Zhou X, Li Y. Local recurrence after breast-conserving surgery and mastectomy following neoadjuvant chemotherapy for locally advanced breast cancer––a meta-analysis. Breast Care (Basel, Switzerland). 2016;11(5):345–51. https://doi.org/10.1159/000450626.
Chawla A, Hunt KK, Mittendorf EA. Surgical considerations in patients receiving neoadjuvant systemic therapy. Future Oncol. (London, England). 2012;8(3):239–50. https://doi.org/10.2217/fon.12.12.
Veronesi U, Bonadonna G, Zurrida S, Galimberti V, Greco M, Brambilla C, et al. Conservation surgery after primary chemotherapy in large carcinomas of the breast. Ann Surg. 1995;222(5):612–8.
Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–85. https://doi.org/10.1200/jco.2007.15.0235.
Broglio KR, Quintana M, Foster M, Olinger M, McGlothlin A, Berry SM, et al. Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: a meta-analysis. JAMA Oncol. 2016;2(6):751–60. https://doi.org/10.1001/jamaoncol.2015.6113.
Kong X, Moran MS, Zhang N, Haffty B, Yang Q. Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favourable prognosis for breast cancer patients. Eur. J. Cancer (Oxford, England : 1990). 2011;47(14):2084–90. https://doi.org/10.1016/j.ejca.2011.06.014.
Korn EL, Sachs MC, McShane LM. Statistical controversies in clinical research: assessing pathologic complete response as a trial-level surrogate end point for early-stage breast cancer. Ann Oncol. 2016;27(1):10–5. https://doi.org/10.1093/annonc/mdv507.
Lim LY, Miao H, Lim JS, Lee SC, Bhoo-Pathy N, Yip CH, et al. Outcome after neoadjuvant chemotherapy in Asian breast cancer patients. Cancer Med. 2017;6(1):173–85. https://doi.org/10.1002/cam4.985.
Acknowledgements
Not applicable
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
Not applicable
Author information
Authors and Affiliations
Contributions
LX collected, analyzed, and interpreted the data and was a major contributor in writing the manuscript. DD and BC were the second reviewer for data collection, analysis, and risk of bias assessment. HT and WW also participated in data analysis and interpretation, as well as manuscript preparation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1: Figure S1.
Forest plots showing OR and the 95% CI for cCR compared with non-cCR in BCS groups. (a) Group 1 included a Paclitaxel-epirubicin-based taxane-anthracycline-based protocol, group 2 included an epirubicin without paclitaxel-basedanthracycline without taxane protocol; Forest plots showing RR and the 95% CI of LRFS (b), DLFS (c) removed four articles used definition of pCR as the absence of invasive component in the primary breast tumor instead of in both the breast and the axillary node for pCR vs. non-pCR in BCS. Note: BCS breast conserving surgery, LRFS local recurrence-free survival, DRFS distant recurrence-free survival, pCR pathology complete response, OR odds ratios, RR risk ratios. (TIFF 11982 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, X., Dai, D., Chen, B. et al. Oncological outcome of complete response after neoadjuvant chemotherapy for breast conserving surgery: a systematic review and meta-analysis. World J Surg Onc 15, 210 (2017). https://doi.org/10.1186/s12957-017-1273-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-017-1273-6