Abstract
Background
Non-small cell lung cancer (NSCLC) is the most frequent cause of cancer deaths worldwide. The targeted therapy had made important progress in recent years, but few potential predictive biomarkers for prognosis of NSCLC patients were identified. Angiopoietin-2 (Ang-2), a cytokine upregulated in tumor endothelial cells and some tumor cells including NSCLC, is a partial agonist and antagonist of angiopoietin-1 (Ang-1). Ang-1 is another ligand for the tyrosine kinase receptor Tie2; it promotes recruitment of pericytes and smooth muscle cells, stabilizing vascular networks by binding to Tie2. Although many studies mainly considered that Ang-2 correlated with progression and prognosis of NSCLC significantly, there are much conflicting and controversial data. Therefore, we conducted a meta-analysis to assess the relationship between Ang-2 and prognosis, a clinical outcome of NSCLC.
Methods
The search was based on major databases from PubMed, Cochrane Library, EMBASE, and CNKI, and 20 eligible publications (range from 2002 to 2015) are included in our meta-analysis with 2011 NSCLC patients in total. These studies illuminated the correlation between the expression of Ang-2 and NSCLC, based on either prognostic factors or clinicopathological features. Pooled calculations were carried out on the odds ratio (OR) and the corresponding 95 % confidence interval (CI) to perform this meta-analysis, and all statistical analyses were carried out by STATA 12.0 and Review Manager 5.3.
Results
According to our results, the expression of Ang-2 in NSCLC tissues was significantly higher than that in normal lung tissues, indicating that Ang-2 over-expression may be a predictive marker (pooled OR = 5.09, corresponding 95 % confidence interval (95 % CI) 3.10–8.36, p = 0.000). In addition, our pooled data showed that Ang-2 expression was positively correlated with tumor stages (pooled OR = 3.58, 95 % CI 2.40–5.35, p = 0.000), differentiation (pooled OR = 0.65, 95 % CI 0.45–0.94, p = 0.02), lymphatic invasion (pooled OR = 3.15, 95 % CI 1.97–5.03, p = 0.000), and poor survival (pooled OR = 1.93, 95 % CI 1.47–2.52, p = 0.000) of NSCLC, but seems to have no significant impact on tumor size (pooled OR = 1.09, 95 % CI 0.59–2.00, p = 0.78).
Conclusions
These results demonstrate that Ang-2 expression significantly correlated with poor prognosis for patients with NSCLC.
Similar content being viewed by others
Background
Lung cancer is the leading cause of cancer deaths worldwide, whose 5-year survival rate is only 16 % [1]. In China, nearly 560,000 people die because of lung cancer; unfortunately, the incidence and mortality of lung cancer is still on the rise, especially non-small cell lung cancer (NSCLC), which makes up 80 % of lung cancer [2]. Due to the emergence of inhibitors of epidermal growth factor receptors (EGFRs), anaplastic lymphoma kinase (ALK) and other targeted drugs, the survival of patients improved greatly [3–5]. Although the targeted therapy of NSCLC makes important progress, efforts to identify new additional prognostic and predictive biomarkers for NSCLC may help to stratify cancer patients, monitor tumor progression as well as response to the therapy.
Recent evidence suggests that angiogenesis is an established hallmark of many solid tumors, while inhibiting proangiogenic factors demonstrates a potential avenue for the treatment [6]. Notably, several studies have proved that angiogenesis results from an imbalance between angiogenic and antiangiogenic factors. Vascular endothelial growth factor (VEGF), as the best known angiogenic factor, promotes proliferation and migration of endothelial cell [7, 8]. In addition, it is also known that angiopoietin-2 (Ang-2), a cytokine upregulated in tumor endothelial cells and some tumor cells including NSCLC, stimulates tumor angiogenesis in collaboration with VEGF and other proangiogenic factors [9].
Angiopoietin-1 (Ang-1) and Ang-2 are ligands for the tyrosine kinase receptor Tie2; they are not only widely expressed on many embryonic tissues, but also expressed on some cancer cells. There is a complicated and homeostatic balance between Ang-1 and Ang-2; some studies suggest that Ang-2, a partial agonist and inhibitor of Ang-1, normally restrains Ang-1-induced activation of the Tie2 receptor [10]. However, other scholars believed that Ang-2 delivers proangiogenic activity through binding the Tie2 receptor with the presence of VEGF [11, 12]. Accumulated evidence shows that the Tie2-signaling pathway is involved in NSCLC and is a potential therapeutic target [13]. For example, AMG 386, neutralizing the interaction between angiopoietins (Ang-1/2) and their Tie2 receptors, inhibits tumor angiogenesis and tumor growth successfully [14, 15].
While the role of Ang-2 in angiogenesis and tumor therapy is well-established, it is also found that Ang-2 expression is associated with prognosis of various tumors, such as chronic lymphocytic leukemia [16], hepatocellular carcinoma [17], colorectal cancer [18], and melanoma [19]. For example, Volkova et al. analyzed Ang-2 of serum samples in colorectal cancer patients (n = 344) and confirmed serum Ang-2 as a significant predictor for outcome of colorectal cancer, as well as metastatic CRC treated with bevacizumab-containing therapy [18].
According to available data, previous studies mainly considered that Ang-2 correlated with progression and prognosis of NSCLC significantly. For instance, Coelho et al. [20] detected circulating Ang-2 messenger RNA (mRNA) in NSCLC patients’ blood samples (n = 92) and found that a highly circulating Ang-2 mRNA level serving as a significantly unfavorable prognostic factor in NSCLC overall survival is a unique and practical diagnostic tool to determine prognosis in NSCLC. Furthermore, Fawzy et al. [21] suggested that serum angiopoietin-2 is a useful marker for the diagnosis of NSCLC by ELISA technique. Additionally, another study involving 335 unselected stage I-IIIA NSCLC patients described the relationship between the Ang-2 expression and survival; illuminated Ang-4 and Ang-2 were independently associated with survival, and the expression of VEGF was strongly associated with that of Ang-2 [22].
In view of the previous studies, they emphasize that Ang-2 is significantly relevant to the clinical outcome of NSCLC [23, 24]. However, Kabalak et al. [25] used the ELISA method to measure serum VEGF and Ang-2 levels collected from 100 lung cancer patients (87 NSCLC) and then found that VEGF and Ang-2 showed a weak positive correlation; in addition, a higher Ang-2 level in patients may be useful for differential diagnosis, whereas there was no relation between Ang-2 levels and survival days significantly. Reinmuth et al. [26] analyzed tissue samples of 72 patients with primary stages I and II NSCLC and found that neither the expression of Ang-2 nor VEGF was associated with survival.
Although Ang-2 is implicated in prognosis and clinical outcome of NSCLC, other studies did report conflicting results that Ang-2 had no effect on survival. Therefore, it remains unknown whether this discrepancy is caused by limited sample sizes, tumor differentiation, tumor stage, and lymphatic invasion. In this study, a meta-analysis was performed to assess the relationship between Ang-2 expression and prognosis, a clinical outcome of NSCLC.
Methods
Search strategy
This meta-analysis was carried out in accordance with the guidelines of the meta-analysis of PRISMA (preferred reporting items for systematic reviews and meta-analyses). In this study, we took a comprehensive search strategy (“angiopoietin-2 OR ANGPT2 protein” and “Carcinoma, Non Small Cell Lung OR Lung Carcinoma, Non-Small-Cell OR Non small Cell Lung Cancer”) through an electronic search on PubMed, Cochrane Library, EMBASE, and CNKI. We also performed a manual search for the articles in the references. Articles, incepted and ended on November 2015, were identified by two investigators (Xuan ZX and Zhang S) independently. Studies included in the meta-analysis had to meet the following criteria: (1) all patients were pathologically diagnosed as NSCLC in clinic; (2) the content of Ang-2 was detected in all patients; and (3) the relationship between the Ang-2 expression and the prognosis of NSCLC patients was investigated. Articles were excluded based on any of the following criteria: (1) duplicated articles or data; (2) no clinical specimens; and (3) abstracts, unpublished studies, reviews.
Data extraction
Data was extracted independently by two investigators (Xuan ZX and Zhang S), using a standard form. Any discrepancies were solved via discussion, and all the data were subject to consensus. We extracted information including first author’s name, year of publication, country of the study population, case of the study population, type of tumor, stage, analysis method, evaluation method, the follow-up time (months), outcome indexes, and hazard ratio (HR) (95 % CI). Some studies provided HR and p; we used the following mathematical formula: b = ln(HR), std = b/inverse_normal_distribution(p/2), 95 % CI = exp(b ± 1.96*std) to calculate the 95 % CI.
Qualitative assessment
Quality assessment of each available study was performed using the Newcastle–Ottawa Quality Assessment Scale (NOS) for the Cochrane Non-Randomized Studies. Included studies were scored for both bias and NOS independently by two authors (Xuan ZX and Zhang S), and referral to the panel made failing consensus agreement, and a score of 0–9 could be determined to indicate the quality of each study (Table 1). Studies labeled with five or more stars were considered to be of high quality.
Statistical analysis
The differences in the studies were shown by the odds ratio (OR) and the corresponding 95 % confidence interval (CI), and no overlap of the 95 % CI with 1 indicated a statistical significance. In order to quantify the aggregation of survival results, HR and their 95 % CI were combined to give the effective value, and HR was calculated from data, which were reported directly or presented in the form of Kaplan–Meier survival curve. In our study, the heterogeneity of included studies was tested by the I 2 statistic based on Cochran’s Q test, I 2 < 50 % or p > 0.1 was considered to be of low heterogeneity, and the fixed-effect model was used to calculated the pooled OR; otherwise, a random-effect model was used because of the significant heterogeneity. We also evaluated the stability of the results through removing each study at a time. Two-sided p < 0.05 was considered statistically significant. All statistical analyses were carried out by STATA 12.0 and Review Manager 5.3.
Results
Study characteristics
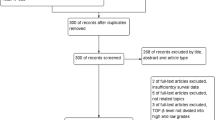
The flowchart of the study selection process is presented in Fig. 1. Eleven articles are excluded for no comparative data based on the difference of Ang-2 expression, or no assessment of relationship between the Ang-2 expression and the prognosis of NSCLC. Twenty eligible publications are included in our meta-analysis with 2011 NSCLC patients in total [20, 22, 27–44]. These eligible articles were published from 2002 to 2015. The features of the eligible studies are shown in Table 1. These studies mainly illuminated the correlation between the expression of Ang-2 and NSCLC, based on either prognostic factors or clinicopathological features. One of the 20 studies was from the USA, 11 studies were from China, three studies were from Japan, one study was from France, one study was from Norway, two studies were from South Korea, and one study was from Portugal. Of them, the NOS score is from 5 to 8, so all of the articles were regarded as high quality.
Different expression of Ang-2 between NSCLC tissues and normal tissues
For the meta-analysis of two-category variables, there were six articles, which assessed the correlation between Ang-2 expression and NSCLC [28, 31, 41–44], our results demonstrated that Ang-2 expressions in NSCLC tissues were significantly higher than normal lung tissues (pooled OR = 5.09, 95 % CI: 3.10–8.36, p = 0.000 and I 2 = 0 %; Fig. 2a). In addition, five articles detected Ang-2 through ELISA in NSCLC serum or normal serum, to analyze the relationship between Ang-2 expression and NSCLC [30, 32, 34, 35, 39]. Due to the high heterogeneity among these studies (P = 0.000, I 2 = 81 %), we chose the random-effect model. As showed in the Fig. 2b, all the content of Ang-2 in NSCLC tissues was higher than that in normal tissues, and Ang-2 over-expression was associated with NSCLC (control: pooled OR = 0.87, 95 % CI: 0.56-1.18, P = 0.000).
The difference of the Ang-2 expression on NSCLC patients and control. a For the meta-analysis of two-category variables, six articles assessed the correlation between Ang-2 expression and NSCLC. b Five articles detected Ang-2 content through ELISA in NSCLC serum or normal serum, to analyze the relationship between Ang-2 expression and NSCLC
Correlation of Ang-2 expression and clinicopathological features of NSCLC
There were seven studies investigating the relationship between Ang-2 expression and tumor stages, TNM stages I and II were considered as low stage and III and IV as high stage [28, 29, 31, 38, 40, 41, 44]. Their pooled analysis revealed that Ang-2 expression significantly associated with tumor stages (pooled OR = 3.58, 95 % CI: 2.40–5.35, p = 0.000 and I 2 = 0 %; Fig. 3a). Eight studies illuminated the association of Ang-2 expression with tumor differentiation [28, 29, 31, 38, 40–43]; the combined OR revealed that there was a significant association between Ang-2 expression and tumor differentiation (pooled OR = 0.65, 95 % CI: 0.45–0.94, P = 0.02 and I 2 = 21 %; Fig. 3b). The association between Ang-2 expression and lymphatic invasion is illustrated in Fig. 3c. Ang-2 expression had a significant relationship with lymphatic invasion in NSCLC patients [28, 31, 40, 41, 43, 44], with the pooled OR of 3.15 (95 % CI: 1.97–5.03, p = 0.000 and I 2 = 26 %). To explore the relevance between Ang-2 expression and tumor size, tumors were classified based on the tumor size (cutoff value was 3 or 5 cm), and three studies were included, and the combined OR showed that Ang-2 expression was not significantly associated with tumor size [28, 31, 40], with a pooled OR estimate of 1.09 (95 % CI: 0.59–2.00, p = 0.78 and I 2 = 0 %; Fig. 3d).
Association of Ang-2 expression with clinicopathological parameters. a Seven studies investigated the relationship between Ang-2 expression and tumor stages, and their pooled results revealed that Ang-2 expression significantly associated with tumor stages. b Eight studies illuminated the association of Ang-2 expression with tumor differentiation; the combined OR revealed that there was a significant association between Ang-2 expression and tumor differentiation. c Ang-2 expression had a significant relationship with lymphatic invasion in NSCLC patients. d The combined OR showed that Ang-2 expression was not significantly associated with tumor size
Correlation of Ang-2 expression and survival of NSCLC patients
The cytoplasmic staining of NSCLC tissues was scored with regard to density as well: 1 = low, 2 = intermediate, and 3 = high, and high expression for Ang-2 was defined as ≥2.5. In our meta-analysis, the NSCLC patients in five studies were divided into the Ang-2 H (high expression) group and the Ang-2 L (lower expression) group via the Ang-2 level, and the overall survival in both groups was calculated [22, 31, 36–38]. The collected data were significantly heterogeneous (p = 0.02, I 2 = 65 %). Thus, a random model was used. This meta-analysis showed that the overall survival in Ang-2 L group is tendentiously higher than that of the Ang-2 H group (pooled OR = 0.65, 95 % CI: 0.34–1.21, p = 0.17; Fig. 4a).
Association of Ang-2 expression with survival. a NSCLC patients in five studies were divided into Ang-2 H (high expression) group and Ang-2 L (lower expression) group via the Ang-2 level, and results showed that the overall survival in the Ang-2 L group is tendentiously higher than that of the Ang-2 H group. b The pooled data from these five studies suggested that Ang-2 expression was significantly associated with poor survival of NSCLC
Five studies provided sufficient information about the correlation of Ang-2 expression and survival of NSCLC patients, especially the hazard ratio (HR) for overall survival (OS) or disease-free survival (DFS) and 95 % confidence interval (CI) [20, 22, 27, 33, 34]. The study of Daly provided HR = 2.12 and p = 0.094, we used mathematical formula to calculate the 95 % CI, that was 0.88–5.11. Then, pooled data from these five studies suggested that Ang-2 expression was significantly associated with poor survival of NSCLC (Fig. 4b). There was no significant heterogeneity (I 2 = 0 %, p = 0.995), and the pooled HR was 1.93 (95 % CI: 1.47–2.52), indicating that Ang-2 expression predicted worse prognosis in NSCLC.
Sensitivity analysis was carried out to confirm the stability of the result. As shown in Table 2, the pooled HRs ranged from 1.90 (95 % CI: 1.40–2.57, p = 0.000) to 1.97 (95 % CI: 1.47–2.63, p = 0.000), and relative heterogeneity remained insignificant. Therefore, there was no individual study that could significantly influence the result of HR when any individual study was removed from our meta-analysis.
Discussion
NSCLC is one of common neoplastic diseases, and increasing evidence proves that there is poor prognosis and limited therapeutic options [45]. Therefore, it is urgent to identify reliable biomarkers, which may be helpful in predicting prognosis and guiding surveillance in NSCLC [46]. To our knowledge, tumor angiogenesis, characterized by the formation of new irregular blood vessels derived from preexisting vascular network, plays a central role in NSCLC tumor growth. Recently, the angiopoietins have been shown to be important regulators of neovascularization and endothelial cell survival in malignant tumors [47]. Considered as the major activating ligand to the tyrosine kinase receptor Tie2, Ang-1 usually recruits and sustains peri-endothelial supporting cells to promote endothelial cell survival and vessel stabilization. In general, Ang-2 has been recognized as a naturally occurring antagonist to Ang-1 and prevents Tie2 activation [9, 47]. However, some studies have suggested that Ang-2 can either agonize or antagonize the Tie2 receptor, thereby leading to vascular sprouting or regression [48].
More and more studies indicated that Ang-2 expression is significantly linked with prognosis of NSCLC [22]. Nevertheless, some studies suggested that Ang-2 levels did not correlate with survival days, and the association between Ang-2 and the prognosis of NSCLC remained controversial [25, 26, 49]. Therefore, we conduct this meta-analysis to systematically determine the association of Ang-2 levels with clinicopathological features and prognosis of NSCLC.
Firstly, we assessed the difference of Ang-2 expression between NSCLC tissues and normal tissues. Our result revealed that the expression of Ang-2 in NSCLC tissues was significantly higher than that in normal lung tissues either through two-category variables or continuous variable analysis, and Ang-2 over-expression may be a predictive marker because of the correlation with NSCLC. Then, we also studied the pooled association between Ang-2 expression and clinicopathological features. The pooled data of our results indicated that Ang-2 expression was positively correlated with TNM stages, tumor differentiation, and lymphatic invasion of NSCLC. These results of meta-analysis supported the hypothesis that Ang-2 might contribute to malignant progression of NSCLC, which subsequently leads to a poorer prognosis. Furthermore, the correlation of Ang-2 expression and survival of NSCLC patients was also assessed; the pooled data indicated that Ang-2 expression significantly predicted poor survival (Table 3). Moreover, removing one individual study could not significantly change the linear trend and the stability of the result.
Conclusions
In conclusion, we conducted a systematical and comprehensive meta-analysis to assess the relationship between Ang-2 expression and prognosis, a clinical outcome of NSCLC, and the prognostic significance of Ang-2 expression for NSCLC was identified by comparing the depth of tumor stages, tumor differentiation, lymphatic invasion, and other clinicopathological features. Eventually, our data showed that Ang-2 expression is significantly linked with poor prognosis for patients with NSCLC.
However, it was shown that Ang-2 expression did not significantly link with tumor size, but there were only three studies included, and tumors divided into two portions based on tumor size were 3 or 5 cm, so the research about relationship between tumor size and Ang-2 expression should be studied in the future. Additionally, current data about Ang-2 expression and NSCLC prognosis is limited, and some studies containing negative conclusions were not published. Only large studies illuminating the prognostic value of Ang-2 in NSCLC substantiated our conclusions; Ang-2 expression may act as a potential predictor for prognosis of patients with NSCLC. Ang-2 will provide useful information for a therapeutic target to treat NSCLC at the moment.
Abbreviations
- NSCLC:
-
Non-small cell lung cancer
- Ang-2:
-
Angiopoietin-2
- Ang-1:
-
Angiopoietin-1
- EGFRs:
-
Epidermal growth factor receptors
- ALK:
-
Anaplastic lymphoma kinase
- NOS:
-
Newcastle–Ottawa Quality Assessment Scale
- OR:
-
Odds ratio
- 95 % CI:
-
Corresponding 95 % confidence interval
- DFS:
-
Disease-free survival
- HR:
-
Hazard ratio
References
Minna JD, Gazdar AF, Sprang SR, et al. A bull’s eye for targeted lung cancer therapy. Science. 2004;304(5676):1458–61.
Fan H, Shao ZY, Xiao YY, et al. Incidence and survival of non-small cell lung cancer in Shanghai: a population-based cohort study. BMJ Open. 2015;5(12):e009419.
Lastwika KJ, Wilson W 3rd, Li QK, et al. Control of PD-L1 expression by oncogenic activation of the AKT/mTOR pathway in non-small cell lung cancer. Cancer Res. 2015. Epub ahead of print.
Gasparini P, Cascione L, Landi L, et al. microRNA classifiers are powerful diagnostic/prognostic tools in ALK-, EGFR-, and KRAS-driven lung cancers. Proc Natl Acad Sci U S A. 2015;112(48):14924–9.
Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26.
Kerbel RS. Therapeutic implications of intrinsic or induced angiogenic growth factor redundancy in tumors revealed. Cancer Cell. 2005;8(4):269–71.
Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307.
Xuan ZX, Li LN, Zhang Q, et al. Fully human anti-VEGFR2 monoclonal antibody reduces tumor angiogenesis and inhibits tumor growth. Int J Oncol. 2014;45(6):2411–20.
Gerald D, Chintharlapalli S, Augustin HG, et al. Angiopoietin-2: an attractive target for improved antiangiogenic tumor therapy. Cancer Res. 2013;73(6):1649–57.
Felcht M, Luck R, Schering A, et al. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest. 2012;122(6):1991–2005.
Danza K, Pilato B, Lacalamita R, et al. Angiogenetic axis angiopoietins/Tie2 and VEGF in familial breast cancer. Eur J Hum Genet. 2013;21(8):824–30.
Nasarre P, Thomas M, Kruse K, et al. Host-derived angiopoietin-2 affects early stages of tumor development and vessel maturation but is dispensable for later stages of tumor growth. Cancer Res. 2009;69(4):1324–33.
Gu Y, Körbel C, Scheuer C, et al. Tubeimoside-1 suppresses tumor angiogenesis by stimulation of proteasomal VEGFR2 and Tie2 degradation in a non-small cell lung cancer xenograft model. Oncotarget. 2015. doi:10.18632/oncotarget.6676.
Monk BJ, Poveda A, Vergote I, et al. Anti-angiopoietin therapy with trebananib for recurrent ovarian cancer (TRINOVA-1): a randomised, multicentre, double- blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15(8):799–808.
Gourley C. Trebananib: an alternative anti-angiogenic strategy. Lancet Oncol. 2014;15(8):776–7.
Maffei R, Martinelli S, Santachiara R, et al. Angiopoietin-2 plasma dosage predicts time to first treatment and overall survival in chronic lymphocytic leukemia. Blood. 2010;116(4):584–92.
Bupathi M, Kaseb A, Janku F. Angiopoietin 2 as a therapeutic target in hepatocellular carcinoma treatment: current perspectives. Onco Targets Ther. 2014;7:1927–32.
Volkova E, Willis JA, Wells JE, et al. Association of angiopoietin-2, C-reactive protein and markers of obesity and insulin resistance with survival outcome in colorectal cancer. Br J Cancer. 2011;104(1):51–9.
Helfrich I, Edler L, Sucker A, et al. Angiopoietin-2 levels are associated with disease progression in metastatic malignant melanoma. Clin Cancer Res. 2009;15(4):1384–92.
Coelho AL, Araújo A, Gomes M, et al. Circulating Ang-2 mRNA expression levels: looking ahead to a new prognostic factor for NSCLC. PLoS One. 2014;9(2):e90009.
Fawzy A, Gaafar R, Kasem F, et al. Importance of serum levels of angiopoietin-2 and survivin biomarkers in non-small cell lung cancer. J Egypt Natl Canc Inst. 2012;24(1):41–5.
Andersen S, Donnem T, Al-Shibli K, et al. Prognostic impacts of angiopoietins in NSCLC tumor cells and stroma: VEGF-A impact is strongly associated with Ang-2. PLoS One. 2011;6(5):e19773.
Shijubo N, Kojima H, Nagata M, et al. Tumor angiogenesis of non-small cell lung cancer. Microsc Res Tech. 2003;60(2):186–98.
Hall RD, Le TM, Haggstrom DE, et al. Angiogenesis inhibition as a therapeutic strategy in non-small cell lung cancer (NSCLC). Transl Lung Cancer Res. 2015;4(5):515–23.
Akın Kabalak P, Çiledağ A, Demir N, et al. Prognostic significance of serum vascular endothelial growth factor and angiopoietin-2 in patients with lung cancer. Tuberk Toraks. 2015;63(2):71–7.
Reinmuth N, Piegelbrock E, Raedel M, et al. Prognostic significance of vessel architecture and vascular stability in non-small cell lung cancer. Lung Cancer. 2007;55(1):53–60.
Daly S, Kubasiak JC, Rinewalt D, et al. Circulating angiogenesis biomarkers are associated with disease progression in lung adenocarcinoma. Ann Thorac Surg. 2014;98(6):1968–75.
Huang T, Lu C, Lei Z, et al. Expression of Ang-2, HIF-2α and VEGF in non-small cell lung carcinoma and its clinical significance. Chin J Clin. 2012;6(21):6685–9 (published in Chinese).
Jiang AY, Yu RZ, Luo HL, et al. Expression of Tie2 and Ang2 in non-small cell lung cancer and its clinical significance. Evaluation and analysis of drug-use in hospitals of China. 2015;15(5):571–3. (published in Chinese).
Li MQ, Shi XK, Long Q, et al. Clinical significance of tumor Ang-2 for lung cancer detection. Heilongjiang Med J. 2015;28(1):17–9 (published in Chinese).
Liu HY. The expression of Ang-2, CD105 and CD34 in NSCLC and their prognostic significance. Shandong University Master’s Thesis. 2010. (published in Chinese).
Liu YY, Wang SW. Expressions and significance of VEGF and Ang-2 in serum of patients with NSCLC. Mod Oncol. 2014;23(19):2774–7 (published in Chinese).
Massabeau C, Rouquette I, Lauwers-Cances V, et al. Basic fibroblast growth factor-2/beta3 integrin expression profile: signature of local progression after chemoradiotherapy for patients with locally advanced non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;75(3):696–702.
Park JH, Park KJ, Kim YS, et al. Serum angiopoietin-2 as a clinical marker for lung cancer. Chest. 2007;132(1):200–6.
Park JH, Choi H, Kim YB, et al. Serum angiopoietin-1 as a prognostic marker in resected early stage lung cancer. Lung Cancer. 2009;66(3):359–64.
Sasaki H, Suzuki A, Shitara M, et al. Angiopoietin-like protein ANGPTL2 gene expression is correlated with lymph node metastasis in lung cancer. Oncol Lett. 2012;4(6):1325–8.
Takanami I. Overexpression of Ang-2 mRNA in non-small cell lung cancer: association with angiogenesis and poor prognosis. Oncol Rep. 2004;12(4):849–53.
Tanaka F, Ishikawa S, Yanagihara K, et al. Expression of angiopoietins and its clinical significance in non-small cell lung cancer. Cancer Res. 2002;62(23):7124–9.
Wan RG, Chen DB, Zhou SH. Clinical significance of serum angiopoietin-2 levels in non-small cell lung cancer. Zhejiang J Lab Med. 2008;6(2):10–1 (published in Chinese).
Wang L, Geng T, Guo X, et al. Co-expression of immunoglobulin-like transcript 4 and angiopoietin-like proteins in human non-small cell lung cancer. Mol Med Rep. 2015;11(4):2789–96.
Xiao HM, Wu LP, Hao JL, et al. The expression and their correlations of survivin and angiogenin-2 in non-small cell lung cancer. Chin J Clin Ration Drug Use. 2010;3(1):43–4 (published in Chinese).
Xing LH, Zhang ZX, Xu YJ. The expression of angiogenin-2 gene in non-small cell lung cancer tissue and significance. Chin J Tuberc Respir Dis. 2003;26(11):730–1 (published in Chinese).
Yuan B. Effect of hypoxia-inducible factor-1α on angiopoietin-2 expression in non-small cell lung cancer. Huazhong University of Science and Technology Master’s Thesis. 2007. (published in Chinese).
Zhang XB, Xia XZ. The expression of angiogenin-2 (Ang-2) in non-small cell lung cancer (NSCLC) and the relationship between tumor angiogenesis. China Practical Medicine. 2008;3(12):106–8 (published in Chinese).
Gadgeel SM. Personalized therapy of non-small cell lung cancer (NSCLC). Adv Exp Med Biol. 2016;890:203–22.
Gure AO, Chua R, Williamson B, et al. Cancer-testis genes are coordinately expressed and are markers of poor outcome in non-small cell lung cancer. Clin Cancer Res. 2005;11(22):8055–62.
Mazzieri R, Pucci F, Moi D, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19(4):512–26.
Thurston G, Daly C. The complex role of angiopoietin-2 in the angiopoietin-tie signaling pathway. Cold Spring Harb Perspect Med. 2012;2(9):a006550.
Eroglu Z, Stein CA, Pal SK. Targeting angiopoietin-2 signaling in cancer therapy. Expert Opin Investig Drugs. 2013;22(7):813–25.
Acknowledgements
None.
Funding
This research was supported by grants from the General Research Program of Medical and Health of Zhejiang Province (2016KYA028) and Zhejiang Provincial Natural Science Foundation of China (LY16H310008 and LY16H310009).
Availability of data and materials
The authors wish to share any specific data upon request. As of now, there is no additional data, other than the abovementioned manuscript.
Authors’ contributions
XZX conceived and designed the analysis. ZS, YSJ, and WW analyzed the data. XZX and YJ wrote the paper. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable. The manuscript does not report on or involve the use of any animal or human data or tissue, and this section is not applicable to this submission.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Xuan, ZX., Zhang, S., Yuan, SJ. et al. Prognostic value of angiopoietin-2 in non-small cell lung cancer patients: a meta-analysis. World J Surg Onc 14, 237 (2016). https://doi.org/10.1186/s12957-016-0992-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-016-0992-4