Abstract
Background
The objective of this study is to perform a meta-analysis to evaluate the associations between the BRAFV600E mutation status and aggressive clinicopathological features and poor prognostic factors in papillary thyroid cancer.
Methods
A literature search was performed within the PubMed, MEDLINE, Web of Science databases, and EMBASE databases using the Medical Subject Headings and keywords from January 2003 to July 2015. Individual study-specific odds ratios and confidence intervals were calculated, as were the Mantel-Haenszel pooled odds ratios for the combined studies.
Results
Sixty-three studies of 20,764 patients were included in the final analysis. Compared with wild-type BRAF, the BRAFV600E mutation was associated with aggressive clinicopathological factors, including extrathyroidal extension, higher TNM stage, lymph node metastasis, and recurrence, and was associated with reduced overall survival; however, there was no significant association between the presence of BRAF mutation and distant metastasis.
Conclusions
BRAF mutations are closely associated with aggressive clinicopathological characteristics and poorer prognosis in papillary thyroid cancer. Accordingly, aggressive treatment should be considered for papillary thyroid cancer patients with BRAF mutation.
Similar content being viewed by others
Background
Papillary thyroid carcinoma (PTC) is the most common malignant thyroid neoplasm, accounting for 80 % of all thyroid cancers [1]. The incidence of PTC has increased dramatically in the past decades [2], and Chen et al. demonstrated that the increasing rate of PTC resulted from an actual increase in disease incidence rather than improvements in the diagnosis such as by high-resolution ultrasound [3]. PTCs, especially papillary thyroid microcarcinomas, tend to have a good prognosis; however, even for TNM stage I patients, approximately 15 % of patients experienced recurrence during 10 years of follow-up in one previous study [4]. Therefore, the classification and targeted therapies for PTC still need more investigation.
The BRAFV600E mutation is a common mutation in PTC, with a reported frequency of 25–82.3 %. Many authors have reported that the BRAFV600E mutation is associated with aggressive clinical features and poor prognosis; however, others have suggested that there is no association between the BRAFV600E mutation and clinicopathologic features in PTCs [5–7]. Thus, it remains controversial whether the BRAFV600E mutation closely correlates with poor clinical characteristics in PTC, and it is necessary to confirm the association between BRAFV600E and the clinical prognosis of PTC using meta-analysis.
Accordingly, the present meta-analysis was designed to determine whether the BRAFV600E mutation is associated with high-risk clinicopathological factors and poor prognostic outcomes in PTC patients. Additionally, we performed subgroup analyses to assess the effects of factors that might modify these associations. The results of our meta-analysis are considered very helpful for clinical surgeons to choose the optimal surgical managements, such as whether prophylactic central neck dissection is needed, and for postoperative risk stratification of PTCs.
Methods
A comprehensive search was conducted in the PubMed, MEDLINE, and Web of Science databases from January 2003 to July 2015. The search terms and keywords used included “papillary thyroid carcinoma,” “BRAF,” “mutation,” “V600E,” “T1799A,” and “PTC.” All studies that included BRAFV600E mutation data from primary PTC tissues were further evaluated. In addition, the reference lists from single case reports and relevant reviews articles were also inspected for further eligible studies by two independent reviewers. Clinical characteristics such as extrathyroidal invasion, lymph node metastasis, TNM stage, cancer recurrence, and rates of cancer-specific death at the last follow-up were included in the present analysis. The search was restricted to English language publications only. Duplicate articles and studies with no clinicopathologic data were excluded. To avoid false-negative results, studies that determined the BRAFV600E mutation status by preoperative fine needle aspiration biopsies only were also excluded. Further, studies confined to only low-risk groups or papillary thyroid microcarcinomas were excluded, as were unpublished data that were presented at international meetings. In instances where the same study cohort was used in multiple articles, either the most recent or the most appropriately informative single article was included. For example, Fugazzola et al. [6] included only data from a single institute and contained a cohort overlapping with Fugazzola et al. [8]; therefore, we used the latter study for our analysis.
Data analyses and statistical methods
We used RevMan (version 5) to calculate the summary odds ratios (ORs) with 95 % confidence intervals (CIs), using a random-effects model when p value (chi-square test of homogeneity) <0.1 and a fixed-effects model when p value (chi-square test of homogeneity) ≥0.1. We assessed the heterogeneity of the studies using the chi-square test of heterogeneity and the I 2 measure of inconsistency. Significant heterogeneity was defined as a chi-square test p value of <0.10 or as an I 2 measure >50 % (according to a statement from Cochrane Handbook). The extent to which the combined metarisk or heterogeneity were affected by individual studies was assessed further by sequentially excluding every study from the meta-analysis. We investigated potential sources of the identified heterogeneity among the studies using a stratification process according to the country or ethnics in which the research was conducted. The potential for publication bias was assessed using a funnel plot analysis.
Results
Results of the literature search and study characteristics
Figure 1 shows the study selection process. A total of 997 abstracts and titles of full-text papers obtained through electronic searches were deemed relevant and examined in detail. Following this detailed review, 63 studies met our inclusion/exclusion criteria; these studies contributed 20,764 patients with PTC to this meta-analysis [6, 8–69]. The main features of the 63 eligible studies that investigated prognostic factors are summarized in Table 1. The funnel plots for each outcome did not suggest the presence of publication bias (data not shown).
Meta-analyses of BRAF mutation effects on clinicopathological and prognostic features
BRAF mutation and extrathyroidal extension
Fifty-two studies contained data on extrathyroidal invasion. Extrathyroidal invasion was present in 5306 (51.5 %) of 10,301 patients with BRAF mutations and in 1910 (28.7 %) of 6655 patients without BRAF mutations (Fig. 2). The pooled odds ratio (OR) from these 52 studies was 2.04 (95 % confidence interval [CI], 1.75–2.37). A random-effects model was adopted because the heterogeneity of the data was significant (p < 0.00001), and the I 2 estimate of the variance between the studies was 69 %. According to our analysis, the association between the occurrence of extrathyroidal invasion and BRAF mutation was significant (p < 0.00001).
BRAF mutation and TNM stage
TNM stage was reported for patients in 51 studies (Fig. 3). The advanced TNM stage (III/IV) was present in 2913 (37.5 %) of 7765 patients with BRAF mutations and in 1364 (23.3 %) of 5842 patients without BRAF mutations (Fig. 3). The pooled odds ratio (OR) from these 51 studies was 1.82 (95 % CI, 1.53–2.17). A random-effects model was adopted because the heterogeneity of the data was significant (p < 0.00001), and the I 2 estimate of the variance between the studies was 70 %. According to our analysis, the association between the occurrence of advanced TNM stage (III/IV) and BRAF mutations was significant (p < 0.00001).
BRAF mutation and lymph node metastasis
Lymph node metastasis was reported for patients in 51 studies (Fig. 4). Lymph node metastasis was present in 4575 (43.4 %) of 10,542 patients with BRAF mutations and in 2405 (32.5 %) of 7394 patients without BRAF mutations (Fig. 4). The pooled odds ratio (OR) from these 51 studies was 1.45 (95 % CI, 1.24–1.69). A random-effects model was adopted because the heterogeneity of the data was significant (p < 0.00001), and the I 2 estimate of the variance between the studies was 73 %. According to our analysis, the association between the occurrence of lymph node metastasis and BRAF mutations was significant (p < 0.00001).
BRAF mutation and distant metastasis
Distant metastasis was reported for patients in 19 studies (Fig. 5). Distant metastasis was present in 77 (5.4 %) of 1415 patients with BRAF mutations and in 98 (5.2 %) of 1868 patients without BRAF mutations (Fig. 5). The pooled odds ratio (OR) from these 19 studies was 1.05 (95 % CI, 0.65–1.68). A random-effects model was adopted because the heterogeneity of the data was significant (p = 0.05), and the I 2 estimate of the variance between the studies was 37 %. According to our analysis, the association between the occurrence of distant metastasis and BRAF mutations was not significant (p = 0.85).
BRAF mutation and recurrence
Recurrence was reported in 24 studies (Fig. 6). Recurrence was present in 608 (24.8 %) of 2450 patients with BRAF mutations and in 407 (14.1 %) of 2877 patients without BRAF mutations (Fig. 6). The pooled OR from these 24 studies was 2.20 (95 % CI, 1.57–3.09). A random-effects model was adopted because the heterogeneity of the data was significant (p < 0.00001), and the I 2 estimate of the variance between the studies was 74 %. According to our analysis, the association between the occurrence of recurrence and BRAF mutations was significant (p < 0.00001).
BRAF mutation and OS
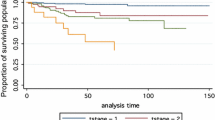
Overall survival (OS) was reported in five studies (Fig. 7). OS was present in 65 (5.1 %) of 1264 patients with BRAF mutations and in 17 (1.1 %) of 1605 patients without BRAF mutations (Fig. 7). The pooled OR from these five studies was 4.61 (95 % CI, 2.69–7.90). A fixed-effects model was adopted because the heterogeneity of the data was not significant (p = 0.77), and the I 2 estimate of the variance between the studies was 0 %. According to our analysis, the association between the occurrence of death and BRAF mutations was significant (p = <0.00001).
Subgroup analyses of the BRAF mutation effects on aggressive clinicopathological features and poor prognostic factors
Subgroup analysis was conducted according to the ethnicities of the study subjects in order to investigate the potential sources of heterogeneity and to assess whether the effects of the BRAF mutation status on aggressive clinicopathological features and poor prognosis of PTC patients were associated with geographic regions (Table 2). The heterogeneity was decreased in the subgroup analyses of extrathyroidal invasion, TNM stage, lymph node metastasis, and recurrence. Thus, the results of the subgroup analyses indicated that the BRAF mutation status may differ according to ethnicity.
Discussion
BRAFV600E was first described as a mutation related to the MEK-ERK pathway in human cancer by Davies et al. in 2002. Since then, the BRAFV600E mutation has been reported as a biological marker for the aggressiveness and prognosis of numerous cancers, including malignant melanoma and thyroid cancer among others. Among the three Raf isoforms, BRAF is the strongest downstream activator of the MEK pathway. As a downstream target of MEK, the phosphorylation of ERK can activate substrates located in both the cytoplasm and nucleus. Abnormal activation of this pathway, for example by BRAF mutation, results in the disruption of biological homeostasis and may result in tumor transformation [70, 71].
Since its initial discovery, the BRAFV600E mutation has been debatable as a diagnostic as well as prognostic indicator of PTC, as many of the studies on the topic have reported contradictory outcomes of the association between the BRAFV600E mutation and clinical characteristics and prognosis [37, 40]. Therefore, the present meta-analysis to illustrate the role of BRAF mutation in PTC was considered highly necessary.
Various clinicopathological risk factors have been reported to be related to recurrence and cancer death in PTC [72]; according to most authors’ results, we chose extrathyroidal invasion, lymph node metastasis, advanced TNM stage, and distant metastasis as reliable predictors for poor prognosis in the present analysis, as these factors not only represent aggressive cancer behavior but also poor prognosis [72, 73].
Extrathyroidal extension is associated with an increased risk of invasion into cervical structures such as the trachea, which requires more aggressive treatment. Accordingly, extrathyroidal extension is an important factor related to PTC prognosis, contributing to an increased risk of local recurrence/persistence of the disease, independent of the tumor size [74]. In our meta-analysis, the risk of extrathyroidal extension was increased by 2.04-fold in cases with positive BRAF mutation status compared to in wild-type cases. In fact, out of all included studies, only six studies showed that patients with BRAF mutation presented less frequent extrathyroidal extension than those without BRAF mutation.
The American Joint Committee on Cancer (AJCC) staging system comprises the sum of several tumor characteristics, such as the tumor size, lymph node status, and distant metastasis, which are generally considered aggressive features of cancer. AJCC stage III/IV cancers are associated with a poorer prognosis in terms of both recurrence and overall survival than stage I/II tumors [75]. According to our results, there was a significant association between high AJCC stage and BRAF mutation, with positive BRAF mutation status being associated with a 1.82-fold increased risk of stage III/IV cancers compared to wild-type cases. Out of all included studies, nine studies suggested that the risk of a high AJCC stage was lower in patients with BRAF mutation than in those without BRAF mutation; however, the other 42 studies demonstrated the opposite results, especially for patients aged >45 years, in whom positive BRAF mutation status was associated with more frequent cases of stage III/IV cancers compared to stage I/II [20, 76]. Howell et al. also reported that older age and positive mutation status were more frequently associated with TNM stage III/IV and recurrence [14].
Further, according to the present meta-analysis, the prevalence of lymph node metastasis was increased in patients with BRAF mutation, with an odds ratio of 1.45; increased risk was observed in 39 of 60 studies. Lymph node metastasis is an important risk factor for recurrence and/or persistent disease, as well as overall survival [75]. Based on these results, we suggest that the presence of BRAF mutation is a prognostic factor in PTCs. However, it is worth mentioning that the different surgical approaches and treatments used in these 60 studies probably influenced the outcome in terms of the real incidence of nodal metastasis; therefore, evaluation of the BRAF mutation status as a prognostic indicator for lymph node metastasis in PTCs should be more cautious [74].
Recurrence and persistent disease demand additional therapy and can affect the PTC patients’ quality of life. For example, recurrence increases the risk of reoperations and the exposure to a high cumulative radioiodine dose. Recently, Xing et al., in their large multicenter study, demonstrated that the BRAFV600E mutation was an independent prognostic factor for PTC recurrence in various clinicopathologic categories [77]. However, these outcomes were questioned by Bal & Ballal and Yarchoan et al. [78, 79], as the median follow-up was only 36 months and the recurrence rate was very high, which might have resulted from persistent disease rather than true recurrence. However, our meta-analysis indicated that the association between the occurrence of recurrence and BRAF mutations was significant, and these results were obtained from 24 studies including a total of 2450 patients; hence, our findings are considered more reliable.
Our present meta-analysis encompassing a total of 20,764 patients suggested that the BRAFV600E mutation is associated with several of the high-risk clinical variables used in prognostic staging systems, including extrathyroidal invasion, high TNM stage, lymph node metastasis, recurrence, and overall survival. Especially, PTCs with positive BRAFV600E mutation exhibited a 4.61-fold increased risk of death compared with cases with the wild-type form of the BRAF gene. On the other hand, one interesting finding in the present meta-analysis was that the BRAFV600E mutation was not related to distant metastasis. One potential reason for this result may be the fact that the diagnosis of distant metastasis differs between different countries and medical centers.
There were several limitations regarding our study design, especially in terms of the studies included in our analysis. Additionally, our study could not determine a causal relationship between BRAF mutations and outcomes, and BRAF mutations may correlate with some other mutations or confounders, which may turn out to be even more useful prognostic indicators. Importantly, our analysis should be interpreted with caution because of the heterogeneity in the data. Possible explanations for this heterogeneity include differences in the patient demographics and ethnicities, as well as in the thyroidectomy, approaches to lymph node dissection, pathology reporting, radioactive iodine treatment, and the time of follow-up. Therefore, our conclusions should be interpreted with caution.
Conclusions
In conclusion, our meta-analysis illustrated that the BRAFV600E mutation correlates with high-risk clinicopathological factors and poor clinical outcome. These results provide a valuable reference for physicians when assessing the prognosis of PTCs by BRAFV600E mutation analysis, and our findings may be of great value in evidence-based clinical decision-making for PTC patients.
Abbreviations
- AJCC:
-
American Joint Committee on Cancer
- PTC:
-
Papillary thyroid carcinoma
References
Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184–99.
Colonna M, Uhry Z, Guizard AV, Delafosse P, Schvartz C, Belot A, Grosclaude P. Recent trends in incidence, geographical distribution, and survival of papillary thyroid cancer in France. Cancer Epidemiol. 2015;39:511–8.
Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988-2005. Cancer. 2009;115:3801–7.
Loh KC, Greenspan FS, Gee L, Miller TR, Yeo PP. Pathological tumor-node-metastasis (pTNM) staging for papillary and follicular thyroid carcinomas: a retrospective analysis of 700 patients. J Clin Endocrinol Metab. 1997;82:3553–62.
Brzezianska E, Pastuszak-Lewandoska D, Wojciechowska K, Migdalska-Sek M, Cyniak-Magierska A, Nawrot E, Lewinski A. Investigation of V600E BRAF mutation in papillary thyroid carcinoma in the Polish population. Neuro Endocrinol Lett. 2007;28:351–9.
Fugazzola L, Mannavola D, Cirello V, Vannucchi G, Muzza M, Vicentini L, Beck-Peccoz P. BRAF mutations in an Italian cohort of thyroid cancers. Clin Endocrinol (Oxf). 2004;61:239–43.
Kim TY, Kim WB, Rhee YS, Song JY, Kim JM, Gong G, Lee S, Kim SY, Kim SC, Hong SJ, Shong YK. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2006;65:364–8.
Fugazzola L, Puxeddu E, Avenia N, Romei C, Cirello V, Cavaliere A, Faviana P, Mannavola D, Moretti S, Rossi S, et al. Correlation between B-RAFV600E mutation and clinico-pathologic parameters in papillary thyroid carcinoma: data from a multicentric Italian study and review of the literature. Endocr Relat Cancer. 2006;13:455–64.
Lee X, Gao M, Ji Y, Yu Y, Feng Y, Li Y, Zhang Y, Cheng W, Zhao W. Analysis of differential BRAF(V600E) mutational status in high aggressive papillary thyroid microcarcinoma. Ann Surg Oncol. 2009;16:240–5.
Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, Yip L, Mian C, Vianello F, Tuttle RM, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309:1493–501.
Lupi C, Giannini R, Ugolini C, Proietti A, Berti P, Minuto M, Materazzi G, Elisei R, Santoro M, Miccoli P, Basolo F. Association of BRAF V600E mutation with poor clinicopathological outcomes in 500 consecutive cases of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2007;92:4085–90.
Liu S, Zhang B, Zhao Y, Chen P, Ji M, Hou P, Shi B. Association of BRAFV600E mutation with clinicopathological features of papillary thyroid carcinoma: a study on a Chinese population. Int J Clin Exp Pathol. 2014;7:6922–8.
Kwak JY, Kim EK, Chung WY, Moon HJ, Kim MJ, Choi JR. Association of BRAFV600E mutation with poor clinical prognostic factors and US features in Korean patients with papillary thyroid microcarcinoma. Radiology. 2009;253:854–60.
Howell GM, Carty SE, Armstrong MJ, Lebeau SO, Hodak SP, Coyne C, Stang MT, McCoy KL, Nikiforova MN, Nikiforov YE, Yip L. Both BRAF V600E mutation and older age (>/= 65 years) are associated with recurrent papillary thyroid cancer. Ann Surg Oncol. 2011;18:3566–71.
Goutas N, Vlachodimitropoulos D, Bouka M, Lazaris AC, Nasioulas G, Gazouli M. BRAF and K-RAS mutation in a Greek papillary and medullary thyroid carcinoma cohort. Anticancer Res. 2008;28:305–8.
Lee JH, Lee ES, Kim YS, Won NH, Chae YS. BRAF mutation and AKAP9 expression in sporadic papillary thyroid carcinomas. Pathology. 2006;38:201–4.
Ito Y, Yoshida H, Maruo R, Morita S, Takano T, Hirokawa M, Yabuta T, Fukushima M, Inoue H, Tomoda C, et al. BRAF mutation in papillary thyroid carcinoma in a Japanese population: its lack of correlation with high-risk clinicopathological features and disease-free survival of patients. Endocr J. 2009;56:89–97.
Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, Rhoden KJ, Carson KA, Vasko V, Larin A, Tallini G, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–9.
Xing M, Clark D, Guan H, Ji M, Dackiw A, Carson KA, Kim M, Tufaro A, Ladenson P, Zeiger M, Tufano R. BRAF mutation testing of thyroid fine-needle aspiration biopsy specimens for preoperative risk stratification in papillary thyroid cancer. J Clin Oncol. 2009;27:2977–82.
Durante C, Puxeddu E, Ferretti E, Morisi R, Moretti S, Bruno R, Barbi F, Avenia N, Scipioni A, Verrienti A, et al. BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J Clin Endocrinol Metab. 2007;92:2840–3.
Nikiforova MN, Kimura ET, Gandhi M, Biddinger PW, Knauf JA, Basolo F, Zhu Z, Giannini R, Salvatore G, Fusco A, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. 2003;88:5399–404.
Puxeddu E, Moretti S, Elisei R, Romei C, Pascucci R, Martinelli M, Marino C, Avenia N, Rossi ED, Fadda G, et al. BRAF(V599E) mutation is the leading genetic event in adult sporadic papillary thyroid carcinomas. J Clin Endocrinol Metab. 2004;89:2414–20.
Ito Y, Yoshida H, Kihara M, Kobayashi K, Miya A, Miyauchi A. BRAF(V600E) mutation analysis in papillary thyroid carcinoma: is it useful for all patients? World J Surg. 2014;38:679–87.
Elisei R, Ugolini C, Viola D, Lupi C, Biagini A, Giannini R, Romei C, Miccoli P, Pinchera A, Basolo F. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93:3943–9.
Frasca F, Nucera C, Pellegriti G, Gangemi P, Attard M, Stella M, Loda M, Vella V, Giordano C, Trimarchi F, et al. BRAF(V600E) mutation and the biology of papillary thyroid cancer. Endocr Relat Cancer. 2008;15:191–205.
O'Neill CJ, Bullock M, Chou A, Sidhu SB, Delbridge LW, Robinson BG, Gill AJ, Learoyd DL, Clifton-Bligh R, Sywak MS. BRAF(V600E) mutation is associated with an increased risk of nodal recurrence requiring reoperative surgery in patients with papillary thyroid cancer. Surgery. 2010;148:1139–45. discussion 1145-1136.
Ahn D, Park JS, Sohn JH, Kim JH, Park SK, Seo AN, Park JY. BRAFV600E mutation does not serve as a prognostic factor in Korean patients with papillary thyroid carcinoma. Auris Nasus Larynx. 2012;39:198–203.
Namba H, Nakashima M, Hayashi T, Hayashida N, Maeda S, Rogounovitch TI, Ohtsuru A, Saenko VA, Kanematsu T, Yamashita S. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J Clin Endocrinol Metab. 2003;88:4393–7.
Nakayama H, Yoshida A, Nakamura Y, Hayashi H, Miyagi Y, Wada N, Rino Y, Masuda M, Imada T. Clinical significance of BRAF (V600E) mutation and Ki-67 labeling index in papillary thyroid carcinomas. Anticancer Res. 2007;27:3645–9.
Fernandez IJ, Piccin O, Sciascia S, Cavicchi O, Repaci A, Vicennati V, Fiorentino M. Clinical significance of BRAF mutation in thyroid papillary cancer. Otolaryngol Head Neck Surg. 2013;148:919–25.
Abubaker J, Jehan Z, Bavi P, Sultana M, Al-Harbi S, Ibrahim M, Al-Nuaim A, Ahmed M, Amin T, Al-Fehaily M, et al. Clinicopathological analysis of papillary thyroid cancer with PIK3CA alterations in a Middle Eastern population. J Clin Endocrinol Metab. 2008;93:611–8.
Basolo F, Torregrossa L, Giannini R, Miccoli M, Lupi C, Sensi E, Berti P, Elisei R, Vitti P, Baggiani A, Miccoli P. Correlation between the BRAF V600E mutation and tumor invasiveness in papillary thyroid carcinomas smaller than 20 millimeters: analysis of 1060 cases. J Clin Endocrinol Metab. 2010;95:4197–205.
Sapio MR, Posca D, Troncone G, Pettinato G, Palombini L, Rossi G, Fenzi G, Vitale M. Detection of BRAF mutation in thyroid papillary carcinomas by mutant allele-specific PCR amplification (MASA). Eur J Endocrinol. 2006;154:341–8.
Abrosimov A, Saenko V, Rogounovitch T, Namba H, Lushnikov E, Mitsutake N, Yamashita S. Different structural components of conventional papillary thyroid carcinoma display mostly identical BRAF status. Int J Cancer. 2007;120:196–200.
Pelizzo MR, Boschin IM, Barollo S, Pennelli G, Toniato A, Zambonin L, Vianello F, Piotto A, Ide EC, Pagetta C, et al. BRAF analysis by fine needle aspiration biopsy of thyroid nodules improves preoperative identification of papillary thyroid carcinoma and represents a prognostic factor. A mono-institutional experience. Clin Chem Lab Med. 2011;49:325–9.
Jung CK, Kang YG, Bae JS, Lim DJ, Choi YJ, Lee KY. Unique patterns of tumor growth related with the risk of lymph node metastasis in papillary thyroid carcinoma. Mod Pathol. 2010;23:1201–8.
Riesco-Eizaguirre G, Gutierrez-Martinez P, Garcia-Cabezas MA, Nistal M, Santisteban P. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I- targeting to the membrane. Endocr Relat Cancer. 2006;13:257–69.
Lin KL, Wang OC, Zhang XH, Dai XX, Hu XQ, Qu JM. The BRAF mutation is predictive of aggressive clinicopathological characteristics in papillary thyroid microcarcinoma. Ann Surg Oncol. 2010;17:3294–300.
Yip L, Nikiforova MN, Carty SE, Yim JH, Stang MT, Tublin MJ, Lebeau SO, Hodak SP, Ogilvie JB, Nikiforov YE. Optimizing surgical treatment of papillary thyroid carcinoma associated with BRAF mutation. Surgery. 2009;146:1215–23.
Liu RT, Chen YJ, Chou FF, Li CL, Wu WL, Tsai PC, Huang CC, Cheng JT. No correlation between BRAFV600E mutation and clinicopathological features of papillary thyroid carcinomas in Taiwan. Clin Endocrinol (Oxf). 2005;63:461–6.
Kim KH, Suh KS, Kang DW, Kang DY. Mutations of the BRAF gene in papillary thyroid carcinoma and in Hashimoto’s thyroiditis. Pathol Int. 2005;55:540–5.
Rivera M, Ricarte-Filho J, Tuttle RM, Ganly I, Shaha A, Knauf J, Fagin J, Ghossein R. Molecular, morphologic, and outcome analysis of thyroid carcinomas according to degree of extrathyroid extension. Thyroid. 2010;20:1085–93.
Kim J, Giuliano AE, Turner RR, Gaffney RE, Umetani N, Kitago M, Elashoff D, Hoon DS. Lymphatic mapping establishes the role of BRAF gene mutation in papillary thyroid carcinoma. Ann Surg. 2006;244:799–804.
Jo YS, Li S, Song JH, Kwon KH, Lee JC, Rha SY, Lee HJ, Sul JY, Kweon GR, Ro HK, et al. Influence of the BRAF V600E mutation on expression of vascular endothelial growth factor in papillary thyroid cancer. J Clin Endocrinol Metab. 2006;91:3667–70.
Ulisse S, Baldini E, Sorrenti S, Barollo S, Prinzi N, Catania A, Nesca A, Gnessi L, Pelizzo MR, Mian C, et al. In papillary thyroid carcinoma BRAFV600E is associated with increased expression of the urokinase plasminogen activator and its cognate receptor, but not with disease-free interval. Clin Endocrinol (Oxf). 2012;77:780–6.
Musholt TJ, Schonefeld S, Schwarz CH, Watzka FM, Musholt PB, Fottner C, Weber MM, Springer E, Schad A. Impact of pathognomonic genetic alterations on the prognosis of papillary thyroid carcinoma. ESES vienna presentation. Langenbecks Arch Surg. 2010;395:877–83.
Alzahrani AS, Xing M. Impact of lymph node metastases identified on central neck dissection (CND) on the recurrence of papillary thyroid cancer: potential role of BRAFV600E mutation in defining CND. Endocr Relat Cancer. 2013;20:13–22.
Guerra A, Fugazzola L, Marotta V, Cirillo M, Rossi S, Cirello V, Forno I, Moccia T, Budillon A, Vitale M. A high percentage of BRAFV600E alleles in papillary thyroid carcinoma predicts a poorer outcome. J Clin Endocrinol Metab. 2012;97:2333–40.
Oler G, Cerutti JM. High prevalence of BRAF mutation in a Brazilian cohort of patients with sporadic papillary thyroid carcinomas: correlation with more aggressive phenotype and decreased expression of iodide-metabolizing genes. Cancer. 2009;115:972–80.
Yim JH, Kim WG, Jeon MJ, Han JM, Kim TY, Yoon JH, Hong SJ, Song DE, Gong G, Shong YK, Kim WB. Association between expression of X-linked inhibitor of apoptosis protein and the clinical outcome in a BRAF V600E-prevalent papillary thyroid cancer population. Thyroid. 2014;24:689–94.
Han SA, Park WS, Jang JH, Min SY, Ryu JK, Song JY. BRAF mutation may predict higher necessity of postoperative radioactive iodine ablation in papillary thyroid cancer. Ann Surg Treat Res. 2014;87:174–9.
Xing M, Liu R, Liu X, Murugan AK, Zhu G, Zeiger MA, Pai S, Bishop J. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. 2014;32:2718–26.
Angell TE, Lechner MG, Jang JK, Correa AJ, LoPresti JS, Epstein AL. BRAF V600E in papillary thyroid carcinoma is associated with increased programmed death ligand 1 expression and suppressive immune cell infiltration. Thyroid. 2014;24:1385–93.
Lim JY, Hong SW, Lee YS, Kim BW, Park CS, Chang HS, Cho JY. Clinicopathologic implications of the BRAF(V600E) mutation in papillary thyroid cancer: a subgroup analysis of 3130 cases in a single center. Thyroid. 2013;23:1423–30.
Li C, Aragon Han P, Lee KC, Lee LC, Fox AC, Beninato T, Thiess M, Dy BM, Sebo TJ, Thompson GB, et al. Does BRAF V600E mutation predict aggressive features in papillary thyroid cancer? Results from four endocrine surgery centers. J Clin Endocrinol Metab. 2013;98:3702–12.
Khan MS, Pandith AA, Azad N, Hussain MU, Masoodi SR, Wani KA, Andrabi KI, Mudassar S. Impact of molecular alterations of BRAF in the pathogenesis of thyroid cancer. Mutagenesis. 2014;29:131–7.
Wang TY, Liu CL, Chen MJ, Lee JJ, Pun PC, Cheng SP. Expression of haem oxygenase-1 correlates with tumour aggressiveness and BRAF V600E expression in thyroid cancer. Histopathology. 2015;66:447–56.
Shao H, Yu X, Wang C, Wang Q, Guan H. Midkine expression is associated with clinicopathological features and BRAF mutation in papillary thyroid cancer. Endocrine. 2014;46:285–91.
He G, Zhao B, Zhang X, Gong R. Prognostic value of the BRAF V600E mutation in papillary thyroid carcinoma. Oncol Lett. 2014;7:439–43.
Russo M, Malandrino P, Nicolosi ML, Manusia M, Marturano I, Trovato MA, Pellegriti G, Frasca F, Vigneri R. The BRAF(V600E) mutation influences the short- and medium-term outcomes of classic papillary thyroid cancer, but is not an independent predictor of unfavorable outcome. Thyroid. 2014;24:1267–74.
Hong AR, Lim JA, Kim TH, Choi HS, Yoo WS, Min HS, Won JK, Lee KE, Jung KC, Park Do J, Park YJ. The frequency and clinical implications of the BRAF(V600E) mutation in papillary thyroid cancer patients in Korea over the past two decades. Endocrinol Metab (Seoul). 2014;29:505–13.
Zeng RC, Jin LP, Chen ED, Dong SY, Cai YF, Huang GL, Li Q, Jin C, Zhang XH, Wang OC. Potential relationship between Hashimoto’s thyroiditis and BRAF mutation status in papillary thyroid cancer. Head Neck.2016;38 Suppl 1:E1019-25.
Sykorova V, Dvorakova S, Ryska A, Vcelak J, Vaclavikova E, Laco J, Kodetova D, Kodet R, Cibula A, Duskova J, et al. BRAFV600E mutation in the pathogenesis of a large series of papillary thyroid carcinoma in Czech Republic. J Endocrinol Invest. 2010;33:318–24.
Czarniecka A, Rusinek D, Stobiecka E, Krajewska J, Kowal M, Kropinska A, Zebracka J, Kowalska M, Wloch J, Maciejewski A, Handkiewicz-Junak D. Occurrence of BRAF mutations in a Polish cohort of PTC patients—preliminary results. Endokrynol Pol. 2010;61:462–6.
Daliri M, Abbaszadegan MR, Bahar MM, Arabi A, Yadollahi M, Ghafari A, Taghehchian N, Zakavi SR. The role of BRAF V600E mutation as a potential marker for prognostic stratification of papillary thyroid carcinoma: a long-term follow-up study. Endocr Res. 2014;39:189–93.
Kim MH, Bae JS, Lim DJ, Lee H, Jeon SR, Park GS, Jung CK. Quantification of BRAF V600E alleles predicts papillary thyroid cancer progression. Endocr Relat Cancer. 2014;21:891–902.
Henke LE, Pfeifer JD, Ma C, Perkins SM, DeWees T, El-Mofty S, Moley JF, Nussenbaum B, Haughey BH, Baranski TJ, et al. BRAF mutation is not predictive of long-term outcome in papillary thyroid carcinoma. Cancer Med. 2015;4:791–9.
McKelvie PA, Chan F, Yu Y, Waring P, Gresshoff I, Farrell S, Williams RA. The prognostic significance of the BRAF V600E mutation in papillary thyroid carcinoma detected by mutation-specific immunohistochemistry. Pathology. 2013;45:637–44.
Lee KC, Li C, Schneider EB, Wang Y, Somervell H, Krafft M, Umbricht CB, Zeiger MA. Is BRAF mutation associated with lymph node metastasis in patients with papillary thyroid cancer? Surgery. 2012;152:977–83.
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54.
Tang KT, Lee CH. BRAF mutation in papillary thyroid carcinoma: pathogenic role and clinical implications. J Chin Med Assoc. 2010;73:113–28.
Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–62.
Sherman SI, Brierley JD, Sperling M, Ain KB, Bigos ST, Cooper DS, Haugen BR, Ho M, Klein I, Ladenson PW, National Thyroid Cancer Treatment Cooperative Study Registry Group, et al. Prospective multicenter study of thyroiscarcinoma treatment: initial analysis of staging and outcome. Cancer. 1998;83:1012–21.
Tufano RP, Teixeira GV, Bishop J, Carson KA, Xing M. BRAF mutation in papillary thyroid cancer and its value in tailoring initial treatment: a systematic review and meta-analysis. Medicine (Baltimore). 2012;91:274–86.
Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Sherman SI, Tuttle RM. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109–42.
Adeniran AJ, Zhu Z, Gandhi M, Steward DL, Fidler JP, Giordano TJ, Biddinger PW, Nikiforov YE. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol. 2006;30:216–22.
Xing M, Alzahrani AS, Carson KA, Shong YK, Kim TY, Viola D, Elisei R, Bendlova B, Yip L, Mian C, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol. 2015;33:42–50.
Bal C, Ballal S. Is there any true association between BRAF V600E mutation and recurrence, particularly in low-risk, papillary thyroid cancer? J Clin Oncol. 2015;33:2481.
Yarchoan M, LiVolsi VA, Brose MS. BRAF mutation and thyroid cancer recurrence. J Clin Oncol. 2015;33:7–8.
Publications
Liu Z, Xun X, Wang Y, Mei L, He L, Zeng W, Wang CY, Tao H. MRI and ultrasonography detection of cervical lymph node metastases in differentiated thyroid carcinoma before reoperation. Am J Transl Res. 2014;6(2):147–54.
Liu Z, Huang T. Papillary thyroid microcarcinoma: an over-treated malignancy. World J Surg. 2016;40(3):764–5.
Liu Z, Wang L, Yi P, Wang CY, Huang T. Risk factors for central lymph node metastasis of patients with papillary thyroid microcarcinoma: a meta-analysis. Int J Clin Exp Pathol. 2014;7(3):932–7.
Liu Z, Li X, Shi L, Maimaiti Y, Chen T, Li Z, Wang S, Xiong Y, Guo H, He W, Liu C, Nie X, Zeng W, Huang T. Cytokeratin 19, thyroperoxidase, HBME-1 and galectin-3 in evaluation of aggressive behavior of papillary thyroid carcinoma” Int J Clin Exp Med. 2014 Aug 15;7(8):2304-8.
Liu Z, Yu P, Xiong Y, Zeng W, Li X, Maiaiti Y, Wang S, Song H, Shi L, Liu C, Cheng B, Zhang B, Ming J, Dong F, Ge H, Nie X, Huang T. Significance of CK19, TPO, and HBME-1 expression for diagnosis of papillary thyroid carcinoma” Int J Clin Exp Med. 2015 Mar 15;8(3):4369-74.
Guo Y*, Liu Z*, Yu P, Liu C, Ming J, Zhang N, Yusufu M, Chen C, Huang T. Using foci number to predict central lymph node metastases of papillary thyroid microcarcinomas with multifocality. Int J Clin Exp Med. 2015 Jun 15;8(6):9925-30. (*contributed equally).
Liu Z, Maimaiti Y, Yu P, Xiong Y, Zeng W, Li X, Song H, Lu C, Xin Y, Zhou J, Zhang N, Ming J, Liu C, Shi W, Shi L, Li X, Nie X, Huang T. Correlation between body mass index and clinicopathological features of papillary thyroid microcarcinoma. Int J Clin Exp Med. 2015;8(9):16472–9.
Ming J*, Liu Z*, Zeng W, Maimaiti Y, Guo Y, Nie X, Chen C, Zhao X, Shi L, Liu C, Huang T. Association between BRAF and RAS mutations, and RET rearrangements and the clinical features of papillary thyroid cancer. Int J Clin Exp Pathol. 2015 Nov 1;8(11):15155-62. (*contributed equally).
9.Y• M, S Sun, Z Liu, W Zeng, C Liu, S Wang, Y Xiong, Y Guo, X Li, Y Wang, W He, T Huang. Diagnostic accuracy of ultrasonographic features for benign and malignant thyroid nodules smaller than 10 mm. Int J Clin Exp Med.
Zeng W, Sun H, Meng F, Liu Z, Xiong J, Zhou S, Li F, Hu J, Hu Z, Liu Z. Nuclear C-MYC expression level is associated with disease progression and potentially predictive of two year overall survival in prostate cancer. Int J Clin Exp Pathol. 2015;8(2):1878–88.
Zeng W, Huang L, Meng F, Liu Z, Zhou J, Sun H. Reduced-intensity and myeloablative conditioning allogeneic hematopoietic stem cell transplantation in patients with acute myeloid leukemia and myelodysplastic syndrome: a meta-analysis and systematic review. Int J Clin Exp Med. 2014;7(11):4357–68.
Zhao H, Tian Y, Liu Z, Li X, Feng M, Huang T. Correlation between iodine intake and thyroid disorders: a cross-sectional study from the South of China. Biol Trace Elem Res. 2014;162(1-3):87–94.
Zeng W, Meng F, Liu Z, Mao X, Luo L, Zheng M, Qin S, Liu W, Zhou J, Sun H, Huang L. Bortezomib-based chemotherapy regimens can improve response in newly diagnosed multiple myeloma patients with bcl-2 and survivin overexpression. Int J Clin Exp Pathol. 2014;7(7):4239–46.
Acknowledgements
No.
Funding
No.
Availability of data and materials
No.
Authors’ contributions
All authors contributed to the design of the study and writing of the manuscript. CPL, TWC, and ZML undertook the research and performed the analyses. All authors reviewed and approved the final version of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liu, C., Chen, T. & Liu, Z. Associations between BRAFV600E and prognostic factors and poor outcomes in papillary thyroid carcinoma: a meta-analysis. World J Surg Onc 14, 241 (2016). https://doi.org/10.1186/s12957-016-0979-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-016-0979-1