Abstract
Background
Reports of synchronous multiple primary cancers in patients with oral cancer have recently been increasing because of progress in radiographic diagnostic techniques. Multiple primary cancers in patients with oral cavity cancer mainly occur in the head and neck region, lung, and esophagus. 2-[18F]-fluoro-2-deoxy-d-glucose positron emission tomography is usually used to identify synchronous multiple primary cancers.
Case presentation
We herein describe a 69-year-old woman diagnosed with synchronous quadruple multiple primary cancers, namely a squamous cell carcinoma of the mobile tongue, invasive ductal carcinoma of the right breast, intraductal carcinoma of the left breast, and chromophobe renal cell carcinoma of the right kidney. We removed the four tumors over three surgical procedures to reduce the surgical risk because the patient had diabetes mellitus. To the best of our knowledge, this combination of multiple primary cancers has not been reported to date. Importantly, we followed this case for 5 years after surgery. The patient was alive and well with no clinical or radiologic signs of recurrent or metastatic disease at the time of this writing.
Conclusions
In the present case, the kidney cancer could not be detected by 2-[18F]-fluoro-2-deoxy-d-glucose positron emission tomography but could be detected by contrast-enhanced computed tomography. To avoid overlooking multiple primary cancers of the kidney, we suggest that contrast-enhanced computed tomography should cover a region extending to the inferior margin of the kidney, rather than only to the liver, in patients with oral cavity cancer.
Similar content being viewed by others
Background
Multiple primary cancers (MPCs) are defined as more than two cancers detected in an individual patient. MPCs can be classified as either synchronous or metachronous according to their timing of diagnosis [1–4]. Reports of synchronous MPCs in patients with oral cancer have been increasing because of recent progress in radiographic diagnostic techniques. MPCs in patients with oral cavity cancer mainly occur in the head and neck region, lung, and esophagus (HNLE) [5, 6]. 2-[18F]-fluoro-2-deoxy-d-glucose positron emission tomography (FDG-PET) is usually used to identify synchronous MPCs [7, 8].
We herein report a case involving a patient with synchronous quadruple MPCs, namely a squamous cell carcinoma (SCC) of the tongue, ductal carcinoma of the bilateral breasts, and chromophobe renal cell carcinoma (RCC) of the right kidney. The kidney cancer could not be detected by FDG-PET but could be detected by contrast-enhanced computed tomography (CT).
Case presentation
A 69-year-old woman was referred to our institute for further evaluation of a tongue mass. She had a 5-year history of pain involving the right lateral tongue edge, and the lesion had been diagnosed as lichen planus by incisional biopsy performed by her dentist. The disease was stable for a long time; however, the tongue pain suddenly worsened. Physical examination revealed an elastic, hard, 1.2 × 0.7 cm mass of the right tongue (Fig. 1). There was no palpable lymphadenopathy in the head and neck area. The patient was being medically treated for hypertension and diabetes mellitus, both of which were well controlled. She denied using tobacco or alcohol but had been exposed to smoke from her family. She had no history of exposure to ionizing radiation or having undergone cancer chemotherapy. Regarding her family history, her younger brother had been treated for rectal cancer and liver metastasis.
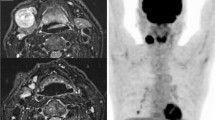
Contrast-enhanced CT scans of the head and neck, chest, and liver were performed as a routine procedure in patients with oral cavity cancer. CT showed no lesions in the tongue, cervical lymph nodes, lungs, bone, or liver; however, three masses were detected in both breasts and the right kidney. Whole-body FDG-PET was performed to identify any other lesions; no other lesions were found. An upper gastrointestinal examination, indirect laryngoscopic examination, and bone scintigraphy revealed no abnormalities. The patient had no breast- or kidney-related signs or symptoms before we identified the masses. Fine-needle aspiration cytology was performed to confirm the diagnosis of the bilateral breast masses and revealed malignancy on both sides. Renal cancer was suspected based on the CT findings (Fig. 2, arrow). All four lesions were diagnosed as early-stage cancers at the initial presentation.
We removed the four tumors over three surgical procedures because the patient had diabetes mellitus. The patient underwent wide local excision of the tongue tumor. Histopathological examination revealed a moderately differentiated SCC. The bilateral breast lesions were then resected, and histopathological examination revealed an invasive ductal carcinoma (positive for estrogen receptor and human epidermal growth factor receptor 2, negative for progesterone receptor and MIB-1; 7–8 %) of the right breast representing a scirrhous growth with one metastatic axillary lymph node. An intraductal carcinoma of the left breast (MIB-1, 7–8 %) was also found. After resection of these lesions, laparoscopically assisted partial resection of the right kidney tumor was performed. Histopathological examination revealed a chromophobe RCC with positive cytoplasmic Hale’s colloidal iron staining. Immunohistochemistry, cytokeratin 7, CD10, and E-cadherin were positive.
In summary, we resected four synchronous MPCs including a moderately differentiated SCC of the mobile tongue, invasive ductal carcinoma of the right breast, intraductal carcinoma of the left breast, and chromophobe RCC of the right kidney. The patient received adjuvant therapy (radiation therapy and anastrozole) for both breast cancers. After a 5-year follow-up period, the patient was alive and well with no clinical or radiologic signs of recurrent or metastatic disease.
This case illustrates two important clinical issues. This combination of MPCs, namely that involving the tongue, bilateral breasts, and kidney, has not been reported to date. To avoid overlooking kidney MPC, we suggest that contrast-enhanced CT should cover an extended region ranging from the liver to the inferior margin of the kidney in patients with oral cavity cancer.
First, regarding the fact that this combination of MPCs has not been reported to date, we searched PubMed and the Japan Medical Abstracts Society databases but found no case reports of the same combination of cancers. The current case was defined as MPCs according to the Warren and Gates criteria [9]; namely, each cancer must be distinct, and the probability of one being a metastasis of the other must be excluded. Using this definition, all four neoplasms were determined to be primary cancers. Furthermore, we defined these cancers as synchronous cancers based on Moertel’s definition; that is, a primary tumor recognized within or after 6 months of diagnosis of another primary tumor is defined as synchronous or metachronous, respectively [1]. We thus diagnosed the present case as synchronous quadruple MPCs. We believe that the patient had three risk factors that contributed to this combination of MPCs. First, the patient had a previous diagnosis of diabetes mellitus, which may increase the risk of cancer [10, 11]. Second, the patient’s family members were chronic, heavy cigarette smokers [12]. Third, the patient’s younger brother had colon cancer [13]. Synchronous quadruple MPCs are extremely rare, and all such cancers can be found in the early stage. MPCs in patients with oral cavity cancer mainly occur in the HNLE region [6]. In contrast, the incidence of non-HNLE cancer, including cancer of the kidney or breast, is relatively low in patients with oral cavity cancer [6]. The 1973–2000 SEER Cancer Registries indicates that of 26,984 patients with oral cavity cancer, the rate of second primary kidney cancer and breast cancer occurring within 1 year after oral cavity cancer diagnosis was only about 0.03 and 0.11 %, respectively [14]. There are few reports of oral-cavity–kidney combinations of MPCs [1, 2, 15, 16]. We found no other English-language literature describing synchronous MPCs as oral-cavity–kidney combinations [9, 17–19].
The second important clinical issue illustrated by this case report is that contrast-enhanced CT should cover an extended region ranging from the liver to the inferior margin of the kidney in patients with oral cavity cancer to avoid overlooking kidney MPC. We usually perform neck, chest, and liver CT scans to detect MPCs or distant metastases in patients with oral cavity cancer. In these cases, we must evaluate the MPCs or distant metastases with nothing but contrast-enhanced CT. We performed a liver CT scan containing the kidneys and incidentally noticed an early-stage mass of the right kidney. In patients with oral cavity cancer, the probability of detecting kidney cancer is low according to the 1973–2000 SEER Cancer Registries [14]. However, recent reports have indicated that the lifetime risk for developing kidney cancer is about 1.6 % [20] and that the incidence of RCC has continued to increase [21]. Furthermore, early-stage RCC can remain clinically silent for a long time, and the clinical symptomatology is generally expressed in patients with advanced disease [22]. Advanced or metastatic RCC is associated with a much lower 5-year disease-specific survival rate than is early-stage RCC [23]. According to the lack of symptoms and the invisibility with FDG-PET, hidden synchronous renal cancer in patients with oral cavity cancer may be much more frequently present than previously thought. Therefore, clinicians should detect the kidney cancer in the early stage using an extended CT scan with consideration of both the benefits and risks, the latter of which include high cost and radiation exposure. The risk of development of secondary kidney or breast cancer after oral cavity cancer is not particularly higher than the risk of after other HNLE or skin (non-melanoma) cancers [14, 24–27]. Therefore, in patients with not only oral cavity cancers but also other HNLE or skin cancers, hidden kidney cancer may be detected by CT covering an extended region ranging from each routine region to the inferior margin of the kidney.
In general, the performance of several types of examinations can help to prevent overlooking synchronous MPCs. FDG-PET is usually used to identify synchronous MPCs; however, FDG-PET sometimes identifies false-positive lesions. In the current case, we could not detect the kidney mass by FDG-PET. The application of FDG-PET to the urinary tract is relatively limited because this tract is the major excretion route for FDG, and background activity may thus obscure the presence of lesions [28]. Additionally, FDG-PET is generally expensive; therefore, some patients with oral cavity cancer are denied this examination before cancer therapy.
Conclusions
The combination of MPCs described herein has not been reported to date. To avoid overlooking kidney MPC, we suggest that contrast-enhanced CT extends to the inferior margin of the kidney in patients with oral cavity cancer. Early-stage RCC can remain clinically silent for a long time; therefore, this kidney cancer in patients with oral cavity cancer may be overlooked. Further reports should be accumulated to make a new protocol for the CT scan range in patients with oral cavity cancer to detect hidden synchronous renal cancer, which may be much more frequently present than previously thought.
Importantly, we followed up this patient for 5 years after surgery. The patient was alive and well with no clinical or radiologic signs of recurrent or metastatic disease at the time of writing.
Consent
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review from the Editor-in-Chief of this journal.
Abbreviations
- CT:
-
computed tomography
- FDG-PET:
-
2-[18F]-fluoro-2-deoxy-d-glucose positron emission tomography
- HNLE:
-
head and neck, lung, and esophagus
- MPCs:
-
multiple primary cancers
- RCC:
-
renal cell carcinoma
- SCC:
-
squamous cell carcinoma
References
Moertel CG, Dockerty MB, Baggenstoss AH. Multiple primary malignant neoplasms. I. Introduction and presentation of data. Cancer. 1961;14:221–30.
Okajima E, Ozono S, Nagayoshi J, Uemura H, Hirao Y, Nakajima Y, et al. A case report of synchronous triple cancer resected simultaneously. Jpn J Clin Oncol. 1994;24:166–70.
Linz C, Müller-Richter UD, Kircher S, Lapa C, Bluemel C. Value of FDG PET/CT in staging of oral cancer: four simultaneous primary malignancies. Clin Nucl Med. 2015;40:455–7.
Kim JS, Chung CY, Park HC, Myung DS, Cho SB, Lee WS, et al. Synchronous quadruple primary tumors of thyroid, breast, pancreas, and stomach: a case report. Anticancer Res. 2013;33:2135–8.
Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–8.
Jain KS, Sikora AG, Baxi SS, Morris LG. Synchronous cancers in patients with head and neck cancer: risks in the era of human papillomavirus-associated oropharyngeal cancer. Cancer. 2013;119:1832–7.
Leong PM, Lin M, Fowler AR. Three synchronous tumours identified by FDG-PET/CT. Med J Aust. 2009;191:275.
Miyazaki T, Sohda M, Higuchi T, Tanaka N, Suzuki S, Sakai M, et al. Effectiveness of FDG-PET in screening of synchronous cancer of other organs in patients with esophageal cancer. Anticancer Res. 2014;34:283–7.
Warren S, Gates O. Multiple primary malignant tumors: a survey of the literature and a statistical study. Am J Cancer. 1932;16:1358–414.
Bosetti C, Rosato V, Polesel J, Levi F, Talamini R, Montella M, et al. Diabetes mellitus and cancer risk in a network of case–control studies. Nutr Cancer. 2012;64:643–51.
Shikata K, Ninomiya T, Kiyohara Y. Diabetes mellitus and cancer risk: review of the epidemiological evidence. Cancer Sci. 2013;104:9–14.
Kotnis A, Namkung J, Kannan S, Jayakrupakar N, Park T, Sarin R, et al. Multiple pathway-based genetic variations associated with tobacco related multiple primary neoplasms. PLoS One. 2012;7, e30013.
Forrest J, Slaney G, Crocker J, Hallam J, Taylor AM. Multiple malignancy with a familial tendency. Clin Oncol. 1981;7:357–64.
Curtis RE, Freedman DM, Ron E, Ries LAG, Hacker DG, Edwards BK, et al. New malignancies among cancer survivors: SEER Cancer Registries, 1973–2000. National Cancer Institute. http://www.seer.cancer.gov/archive/publications/mpmono/MPMonograph_complete.pdf. Accessed 27 Jul 2015.
Martin-Granizo R, Naval L, Castro P, Goizueta C, Muñoz M. Quintuple cancers: report of a case with triple cancers in the head and neck. J Craniomaxillofac Surg. 1997;25:153–7.
Mukaiyama Y, Suzuki M, Morikawa T, Mori Y, Takeshima Y, Fujimura T, et al. Multiple primary malignant neoplasms of the glottis, renal pelvis, urinary bladder, oral floor, prostate, and esophagus in a Japanese male patient: a case report. World J Surg Oncol. 2014;12:294.
Beisland C, Talleraas O, Bakke A, Norstein J. Multiple primary malignancies in patients with renal cell carcinoma: a national population-based cohort study. BJU Int. 2006;97:698–702.
Antonelli A, Calza S, Arrighi N, Zani D, Corti S, Cozzoli A, et al. Clinical features and prognosis of patients with renal cancer and a second malignancy. Urol Oncol. 2012;30:294–300.
Koutsopoulos AV, Dambaki KI, Datseris G, Giannikaki E, Froudarakis M, Stathopoulos E. A novel combination of multiple primary carcinomas: urinary bladder transitional cell carcinoma, prostate adenocarcinoma and small cell lung carcinoma--report of a case and review of the literature. World J Surg Oncol. 2005;3:51.
Kidney Cancer (Adult) - Renal cell carcinoma. In: American Cancer Society. 2015. http://www.cancer.org/acs/groups/cid/documents/webcontent/003107-pdf.pdf. Accessed 27 Jul 2015.
Wang C, Hu J, Lu M, Gu H, Zhou X, Chen X, et al. A panel of five serum miRNAs as a potential diagnostic tool for early-stage renal cell carcinoma. Sci Rep. 2015; doi:10.1038/srep07610
Piccinini L, Luppi G, Zoboli A, Torricelli P. Occasional diagnosis of synchronous renal cell carcinoma during staging of other primary tumors. Tumori. 1996;82:488–90.
Escudier B, Eisen T, Porta C, Patard JJ, Khoo V, Algaba F, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23:vii65–71.
Tabuchi T, Ito Y, Ioka A, Miyashiro I, Tsukuma H. Incidence of metachronous second primary cancers in Osaka, Japan: update of analyses using population-based cancer registry data. Cancer Sci. 2012;103:1111–20.
Nugent Z, Demers AA, Wiseman MC, Mihalcioiu C, Kliewer EV. Risk of second primary cancer and death following a diagnosis of nonmelanoma skin cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2584–90.
Kaneko S, Yamaguchi N. Epidemiological analysis of site relationships of synchronous and metachronous multiple primary cancers in the National Cancer Center, Japan, 1962–1996. Jpn J Clin Oncol. 1999;29:96–105.
Coyte A, Morrison DS, McLoone P. Second primary cancer risk - the impact of applying different definitions of multiple primaries: results from a retrospective population-based cancer registry study. BMC Cancer. 2014;14:272.
Wang HY, Ding HJ, Chen JH, Chao CH, Lu YY, Lin WY, et al. Meta-analysis of the diagnostic performance of [18F]FDG-PET and PET/CT in renal cell carcinoma. Cancer Imaging. 2012;12:464–74.
Acknowledgements
No funding source supported this case report. We thank Prof. Seiichi Saito, Department of Urology, Graduate School of Medicine, University of the Ryukyus (Japan), and Dr. Kouichi Kuninaka, Division of Digestive and General Surgery, Graduate School of Medicine, University of the Ryukyus; both of whom contributed to patient treatment and care.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TM collected the data, images, and clinical information. TN, NM, and AM searched the relevant literature. TM drafted the manuscript. AA conceived the idea for the case report, helped prepare the manuscript, and edited the manuscript. All authors approved the final manuscript.
Authors’ information
TM, TN, NM, AM, and AA are dentists. TM is a doctoral student, TN is an assistant professor, NM is a medical staff member, AM is a research associate, and AA is a professor.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Maruyama, T., Nakasone, T., Maruyama, N. et al. Synchronous quadruple multiple primary cancers of the tongue, bilateral breasts, and kidney in a female patient with a disease-free survival time of more than 5 years: a case report. World J Surg Onc 13, 263 (2015). https://doi.org/10.1186/s12957-015-0684-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-015-0684-5