Abstract
Background
Selection of appropriate trial endpoints and outcome measures is particularly important in rare disease and rapidly progressing disease such as amyotrophic lateral sclerosis (ALS) where the challenges to conducting clinical trials, are substantial: patient and disease heterogeneity, limited understanding of exact disease pathophysiology, and lack of robust and available biomarkers. To address these challenges in ALS, the Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised version (ALSFRS-R) was developed and has become a key primary endpoint in ALS clinical trials to assess functional disability and disease progression, often replacing survival as a primary outcome. However, increased understanding of the ALS disease journey and improvements in assistive technology for ALS patients have exposed issues with the ALSFRS-R, including non-linearity, multidimensionality and floor and ceiling effects that could challenge its continued utility as a primary outcome measure in ALS clinical trials. Recently, other qualitative scale measures of functioning disability have been developed to help address these issues. With this in mind, we conducted a literature search aimed at identifying both established and promising new measures for potential use in clinical trials.
Methods
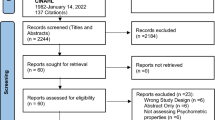
We searched PubMed, Google, Google Scholar, and the reference sections of key studies to identify papers that discussed qualitative measures of functional status for potential use in ALS studies. We also searched clinicaltrials.gov to identify functional status and health-related quality of life (HRQoL) measures that have been used in ALS interventional studies.
Results
In addition to the ALSFRS-R, we identified several newer qualitative scales including ALSFRS-EX, ALS-MITOS, CNS-BFS, DALS-15, MND-DS, and ROADS. Strengths and limitations of each measure were identified and discussed, along with their potential to act as a primary or secondary outcome to assess patient functional status in ALS clinical trials.
Conclusion
This paper serves as a reference guide for researchers deciding which qualitative measures to use as endpoints in their ALS clinical trials to assess functional status. This paper also discusses the importance of including ALS HRQoL and ALS cognitive screens in future clinical trials to assess the value of a new ALS therapy more comprehensively.
Similar content being viewed by others
Background
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder of motor neurons characterized by loss of physical function across various domains (bulbar, arm/leg motor, and respiratory) and average survival of 3–5 years from symptom onset [1]. Given the poor prognosis and dearth of effective treatments, development of new therapeutic approaches is of primary importance for ALS patients.
Historically, the primary endpoint in ALS clinical trials was survival, defined as survival or tracheostomy, necessitating relatively long trial duration particularly in patients with less severe ALS, and imposing difficulties associated with personal preference with regards to end-of-life choices and access to assistive technology and tracheostomy impacting trial outcome. Objective measures such as quantitative muscle testing and handheld dynamometry to assess muscle strength, and vital capacity (VC) to assess ventilatory muscle strength were used to assess ALS functional status. Early clinical ALS rating scales such as the Norris Scale [2] and the Appel Scale [3] combined interview and objective functional assessments. All these methods were lengthy, required clinician time and specialized equipment to administer, and were not feasible if patients were too ill to visit a medical clinic.
In response, the Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS) and its revised form Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (ALSFRS-R) was developed in the 1990s as a qualitative measure of function to evaluate the clinical characteristics of ALS [4, 5]. Since then, the ALSFRS (and later the ALSFRS-R) has become the most widely applied rating scale in ALS in clinical trials as a primary or secondary outcome and is considered the gold standard measure of functional disability and disease progression in ALS patients. It is an accepted primary endpoint measure for Phase 3 ALS clinical trials to monitor functional decline patients over time [6,7,8,9] and recommended as part of the EMA and FDA Guidance for ALS drug development [8, 9], although survival is still often measured as a secondary endpoint and EMA considers it a critical part of assessment of efficacy [8].
Why look at other qualitative measures to assess ALS functional disability?
Almost 30 years after development, the ALSFRS-R is sometimes criticized as being too rudimentary to accurately track disease progression [10]. Reports vary regarding the linearity of the measure over time, with early and late phases of ALS showing the quickest rates of decline [11,12,13,14], while heterogeneity of the disease may affect ALSFRS-R results between ALS clinical subgroups [12, 15, 16].
Rasch analyses of the ALSFRS-R have demonstrated its lack of unidimensionality, meaning it better constitutes a profile of domain scores than a total overall score of disease severity. Rasch analyses also supports three domains instead of four, recommending the collapse of fine and gross motor domains into one, and prescribes a better fit with a 0–2 response instead of 0–4 [17,18,19,20]. Substantial misfit of many ALSFRS-R items, including overlapping response options and disordered thresholds indicating issues with patients’ ability to discriminate between items have been reported, with only the items on the bulbar domain showing good fit [20].
Grade response monitoring (GRM) analysis [19] of the ALSFRS-R using the largest publicly available repository of merged ALS clinical trials data (PRO-ACT; https://nctu.partners.org/ProACT/Data [21]) indicated floor effects (poor discrimination in more severe patients) for the items “dressing and hygiene” and “climbing stairs” on the gross motor domain and ceiling effects (poor discrimination in patients with milder disability) for the items “speech”, “salivation”, and “swallowing” on the bulbar domain and all items on the respiratory domain [19, 21]. This suggests the ALSFRS-R may not adequately assess ALS patients with more severe motor disability, less severe bulbar disability or lesser respiratory severity (or that patients upon first clinical trial visit may self-select or be selected for having minimal respiratory dysfunction) (see Table 1 for the full analysis [19]). GRM analysis [19] does support the ALSFRS-R having 4 domains, although revision to some of the items and the response options is recommended to help clarify their meaning.
These studies suggest that the ALSFRS-R, in its current form may not be the “best” as a single primary outcome measure to track ALS disease progression in a clinical trial. Revising the ALSFRS-R to address some of the issues discussed above could improve its performance; however, any modification will require additional validation of the modified ALSFRS-R measure in accordance with current FDA guidelines for PRO development [22] and in line with the FDA guidelines for ALS [9] suggesting that ALSFRS-R should be supplemented with additional functional measures. Recently developed qualitative functioning scales that have addressed these issues could offer an alternative to the ALSFRS-R, either as a primary outcome or as a supplemental measure to the ALSFRS-R to assess functional disability in clinical trials.
These identified issues with the ALSFRS-R, including non-linearity (potentially leading to incorrect statistical assumptions and spurious associations with the rate of decline) [11], multidimensionality (in that it better constitutes a profile of domain scores than a total overall score representing disease severity) [15, 17, 18] and floor (poor discrimination in more severe patients) and ceiling (poor discrimination in patients with milder disability) effects [19], have challenged its continued utility as a primary outcome measure in ALS clinical trials and driven the development of other qualitative scale measures of functional disability in ALS.
This paper serves as a reference guide for researchers deciding which qualitative measures to use as endpoints in their ALS clinical trials to assess functional status. It provides a targeted overview of the ALSFRS-R and newer qualitative scales along with their potential to act as primary or secondary outcomes in ALS clinical trials. It has also been suggested that different, or at least complementary ways to assess the value of a new therapy would be to include measures of the treatment’s impact on quality of life (QoL) [23, 24] along with cognitive screening measures [25].
With this in mind, we present the results of a search of published and grey literature aimed at identifying both established and promising new measures for potential use in clinical trials. To our knowledge, no papers exist that provide a collected list such as this. This paper further discusses the utility of including ALS health-related quality of life (HRQoL) measures and ALS cognitive screening measures in future clinical trials to more fully assess the patient perceived value of a new therapy and to help determine if cognitive or behavioral impairment has an impact on physical or motor functioning, particularly in more severe or elderly ALS patients.
Methods
Two researchers searched PubMed, Google, Google Scholar, and the reference sections of key studies to identify papers that discussed qualitative measures of functional status for potential use in ALS studies. Qualitative or subjective measures of ALS functional status are explored as opposed to objective measures such as muscle strength, muscle electromyography, vital capacity, walking tests and include disease specific and general instruments. An initial search used terms associated with ‘amyotrophic lateral sclerosis’, ‘qualitative measure’, ‘functional status’, and ‘patient-reported outcomes’. Studies were included if they were English-language and were published from January 2000 through April 2021. Titles and abstracts were first screened by one reviewer to determine whether the study provided information on qualitative measures for ALS functioning. Full-text reviews were then conducted in cooperation between the two researchers to extract relevant information on the use of the measure including strengths and limitations. The two researchers discussed their findings together and agreed which papers were relevant to the research. Data on the measure content, validity, use in published literature, and any noted strengths or weaknesses were extracted in cooperation by two researchers and discussed with all authors.
We also searched clinicaltrials.gov to identify any additional scales that had been used in clinical trials to measure functional ability or quality of life in patients with ALS using keywords such as “ALS”, “scales”, and “functional measures”. Studies had to be registered, industry-sponsored, Phase 2–4, interventional with ‘Not yet recruiting’, ‘Recruiting’, ‘Active/not recruiting’, or ‘Completed’ status. Data on the phase, primary, secondary, and exploratory measures, sponsor, and status were extracted by a single reviewer and discussed with a second researcher.
Results
Qualitative assessment of functional decline in ALS clinical trials
ALSFRS-R
The ALSFRS-R is well validated, reliable, simple, brief, and requires no equipment or special training. It can be completed by the clinician, patient self-report, or proxy caretaker for those with more severe disease [7]. It has 12 items and assesses current disability across 4 domains—bulbar, fine motor, gross motor, and respiratory. Each item has five ordinal response options ranging from 0 (loss of function) to 4 (normal function), with a total score ranging from 0 to 48; higher scores indicating a higher level of functioning.
Developed with clinician input, initial validity was established by documenting that change in ALSFRS scores correlated with change in strength over time, measured by isometric muscle strength (r = 0.60 with fine motor and gross motor domain), and lung function [forced vital capacity (FVC) r = 0.55 with respiratory domain], while total ALSFRS-R baseline scores strongly predicted survival across 9 months in ALS patients (HR: 0.94) [4, 26]. The ALSFRS-R added additional assessments of respiratory dysfunction, including orthopnea, and need for ventilatory support, making the respiratory function equal in weight on overall score to other domains such as fine and gross motor function [5], but retained properties of the original scale, showing strong internal consistency [intraclass correlation (ICC) 0.73 total score], interrater reliability (0.93–0.95) and construct validity with survival, death/tracheostomy [5, 7, 12, 27,28,29], length of hospital stay and survival in ALS patients with acute respiratory failure on mechanical ventilation [30].
The ALSFRS-R was validated for self-administration [31], can be performed in person or via telephone [32], smartphone [33], and videoconference [34], making it particularly useful when patients are unable to attend clinic.
Current ALS clinical trials (from clinicaltrials.gov) often define a clinical response as a rate or slope of change of ALSFRS-R over time. On average, patients with ALS in the community have a decline of an average of -1 point/month [7], but clinical trial populations vary based on inclusion criteria [21]. For example, recently released Phase II results for the ALS CENTAUR trial for AMX0035 reported ALSFRS-R scores for the treated group declined less than the placebo group (1.24 vs. 1.66 points per month) [35]. On the other hand, in the PRO-ACT database of completed ALS clinical trials, average progression was a decline of 0.7 point/month [21]. A survey of 65 clinicians of the Northeast ALS Consortium (NEALS) reported that the majority of clinicians and clinical researchers surveyed believed that a therapy that resulted in a change of 20% or greater in the slope of the ALSFRS-R would be the percentage in which a somewhat clinically significant change starts to be noted [13].
New qualitative measures for the assessment of functional disability in ALS clinical trials
ALS functional rating scale extension (ALSFRS-EX)
Improvements in assistive technology have led to increased survival in ALS, with patients experiencing continued changes in their physical functioning despite having reached the lower bounds of the ALSFRS-R (floor effects). As a result, an online community for people with ALS (PALS) were recruited to construct and pilot new items to add to the ALSFRS-R scale to improve its sensitivity at lower levels of physical function in patients with advanced ALS. Item generation and item reduction processes led to the addition of 3 new items to the ALSFRS-R, (1) ability to use fingers to manipulate devices (fine motor), (2) ability to show emotional expression in the face (bulbar), and (3) ability to get around inside the home (gross motor). Additional items used the same 5-point ordinal scale as the ALSFRS-R where a score of 0 represents a total loss of function and 4 represents normal function [36].
The overall original ALSFRS-R scale scores and extended scale scores were correlated 0.99 over the 1-week re-test. The ALSFRS-EX was able to detect a 3-month change in a group of 20 ALS patients with the lowest functional status (0–12), whereas the original ALSFRS-R did not (t = 2.727 vs t = 1.395) [36].
Additional validation studies in ALS clinical trials (i.e., longitudinal validation in ALS clinical trial populations) are required to assess the utility of the ALSFRS-EX as a possible replacement for the ALSFRS-R in clinical trials, particularly in patients with more advanced disease.
ALS Milano–Torino staging (ALS-MITOS)
The ALS-MITOS staging system [37] was proposed as a novel “one scale measures all” tool to measure the progression of ALS and its ability to serve as a proxy for long-term survival. It was thought that a valid staging system should correlate with ALS disease progression, as well as quality of life QoL and economic burden, and can be derived from the ALSFRS-R. The ALS-MITOS includes 6 stages based on functional ability, based on the 4 key domains from the ALSFRS-R (walking/self-care, swallowing, communicating and breathing). Each domain has a threshold reflecting the loss of function in the specific ALSFRS-R subscores. Values of 0 (below threshold) or 1 (above threshold) are assigned, and the stages are determined as the sum of values across the four domains. Six stages are defined: stage 0 indicates no loss of function in any domain; stages 1–4 represent the loss of independence of function in 1, 2, 3 or 4 domains, and stage five is death. A similar staging system, King’s Staging, is also frequently used, but is not a solely qualitative measure, as it included quantitative assessments. [38]
Studies showed ALS patients progressed to higher stages of disease at 12 months compared with their baseline stage; functional (ALSFRS) and QOL measures were inversely related to disease stage, and health service costs were directly and significantly related to increasing disease stages from 0 to 4 [37, 39]. ALS progression from baseline to 6 months as defined by the ALS-MITOS system predicted death, tracheostomy or > 23-h non-invasive ventilation (NIV) [40]. The ALS-MITOS developers suggest the staging system can reliably predict the course of ALS up to 18 months and can be considered a novel and valid outcome measure in ALS clinical trials; however additional validation studies are required, particularly longitudinal validation in a clinical trial.
Center for neurologic study bulbar function scale (CNS-BFS)
Dysphagia occurs in about 85% of patients at some point during the ALS process and is associated with malnutrition, weight loss, reduced QOL, aspiration pneumonia, and death [41,42,43]. Early detection and consistent monitoring of dysphagia provides the opportunity to improve survival and QOL with timely interventions. The ALSFRS-R has reported poor discrimination in patients with milder disability for the items, “speech”, “salivation”, and “swallowing” on the bulbar domain [19] as well as inadequate diagnostic accuracy of the swallowing item to detect radiographically confirmed swallowing impairment, suggesting the need for alternate measures to assess dysphagia in ALS [44].
The CNS-BFS is a 5-min, 21-question self-administered questionnaire that assesses three domains of bulbar function: speech, swallowing, and salivation. Recall is one week and for each domain, subjects are asked to rate seven items on a scale of 1 (does not apply)–5 (applies all of the time). Scores range from 21 (no symptoms of bulbar dysfunction) to 112. Internal consistency was 0.97, and all three domains were highly correlated with the Global General Impression Scale (r = 0.83 to 0.95) [32] and test–retest reliability over a 2-week screening interval was 0.86. The CNS-BFS total score and ALSFRS-R bulbar subscale were highly predictive of clinician diagnosis of impaired bulbar function [receiver operating characteristic (ROC) area under the curve (AUC), 0.95 and 0.92, respectively] and the CNS-BFS total score was highly and significantly correlated with the bulbar subscale of the ALSFRS-R (r = − 0.90) [45].
When compared to the composite ALSFRS-R, the speech domains of both the CNS-BFS and the ALSFRS-R bulbar scale were sensitive measures of a treatment effect [45]. In contrast, the swallowing and salivation domains of the CNS-BFS were both responsive to treatment (whereas the swallowing and salivation questions on the ALSFRS-R were not. Each of these associations was statistically significant [45].
Dyspnea ALS scale (DALS-15)
Dyspnea occurs in about 80% of ALS patients during the course of disease [46]. The DALS-15 [47, 48] is a 15-item, ALS-specific self-reported questionnaire developed with Rasch methodology to detect and quantify dyspnea. Recall is the past two weeks and response options are never (0), occasionally (1), and often (2). Item thresholds are distributed across the entire spectrum of dyspnea and not clustered, so dyspnea can be estimated with good accuracy over a wide range without a ceiling or floor effect. The sum score can be easily computed by summing the individual item scores to obtain an overall score ranging from 0 to 30 points. Cronbach’s alpha was 0.88. Test–retest reliability was 0.98. Minimally detectable change was 3.21 (10.87%) on the 0–30 scale. The DALS-15 correlated highly with the respiratory subscale of ALSFRS-EX (r = − 0.56), Borg scales (r − 0.52, 0.50) and the SRI (severe respiratory insufficiency) subscale of respiratory complaints (r = − 0.75). The scale was able to detect significant differences between patients with and without NIV. The DALS-15 is considered most valuable for the guidance of patients in later stages when NIV is already introduced, and in patients with severe bulbar dysfunction in whom assessment of respiratory function is difficult due to loss of speech and inability to perform spirometric tests.
Motor neuron disease—dyspnea scale (MND-DS)
The newly developed MND-DS may be a valuable tool for remotely monitoring respiratory function between clinic visits in patients with motor neuron disease (MND) including ALS. Developed in accordance with the FDA 2009 guidance for patient-reported outcomes (PROs) [22], the MND-DS has three self-reported dyspnea symptoms (1) dyspnea while eating/talking, (2) dyspnea while lying flat, and (3) dyspnea during light activity [49]. Each item is scored from 0 to 4, resulting in a possible total score between 0 (no dyspnea) and 12 (severe dyspnea), with an optimal cut-off-score of ≥ 2 with 75% sensitivity. Significant correlation with the ALSFRS-R respiratory domain was observed at 0.6, reliability was adequate with ICC values ranging from 0.66 to 0.90 and the scale was responsive to disease severity with higher MND-DS scores in patients with more severe dyspnea [49]. The MND-DS showed better diagnostic performance than the ALSFRS-R respiratory domain [49], suggesting that the MND-DS may be a preferred option to identify patients with a reduced respiratory function upon entry into clinical trials or as a supplemental measure to the ALSFRS-R in clinical trials where treatment is expected to have the largest impact on respiratory functioning. Validation studies will need to be conducted before the MND-DS can be considered as a key outcome measure in ALS clinical trials.
Rasch overall ALS disability scale (ROADS)
Using Rasch methodology and measure development in accordance with FDA 2009 guidance for PROs and the 2019 FDA guideline for ALS drug development [9], the recently developed ALS disability scale, ROADS, is a 28-item, self-reported questionnaire targeting a broad range of disability levels expected to be more responsive in detecting clinical changes than the ALSFRS-R. Each item is scored as 0 (unable to perform), 1 Abnormal (able to perform but with difficulty compared to before ALS symptoms) or 2 Normal (able to perform without difficulty as before ALS symptoms).
Test–retest reliability for the ROADS was good (ICC = 0.97), construct validity was good with the ALSFRS-R (r = 0.82) and moderate with vital capacity percentage (r = 0.57). ROADS variance explained by the measured construct was 58.2%, considered sufficient for unidimensionality [50]. The ROADS is linearly weighted, meaning that a 1-point change in the overall normed score captures a measurable unit of disability that is consistent across the entire scale, and 2-point changes reflect twice the disability level compared with a 1-point change. The ROADS developers suggest it provides a more consistent and sensitive grading scale than the ALSFRS-R, allowing for better tracking of ALS disease progression. Future studies of the ROADS should examine its longitudinal performance, assess correlation of ROADS with survival, and examine predictive features of the scale. Ongoing studies are also planned to determine test–retest reliability for telephone-administered scales and scales completed by live-in caregivers [51].
Table 2 provides a summary comparison of the measurement properties among the qualitative measurement scales for functioning. Table 3 describes the strengths and weaknesses of the qualitative scales that assess functional disability.
Assessment of HRQoL in ALS clinical trials
HRQoL is a key measure that should be considered as a key outcome along with functional status. This is even more important given that ALS patients are surviving longer than before [24]. Table 4 describes the strengths and weaknesses of the more commonly used qualitative scales that assess HRQoL in patients with ALS.
Amyotrophic lateral sclerosis assessment questionnaires (ALSAQ-40, ALSAQ-5)
The self-reported 40 item ALSAQ-40 is a Rasch modeled instrument designed to evaluate aspects of health considered important to patients with ALS and is frequently listed as a secondary outcome in current ALS clinical trials to assess HRQoL. It was developed in accordance with FDA 2009 guidelines for PROs and covers many of the same items as the ALSFRS-R with the exception of the respiratory domain. The recall period for its five domains—communication, eating/drinking, physical mobility, activities of daily living (ADL) independence and emotional functioning, is 2 weeks and responses are on a Likert scale from 0 to 4 (never true to always true). A measure of global HRQoL impact can be obtained by summing individual domain scores for a total score between 0 (best health) to 100 (worst health) [52, 53].
To minimize patient burden, a 5-item subset of the ALSAQ-40 called the ALSAQ-5 was developed [54] with one item representing each domain. ALSAQ-40 and ALSAQ-5 scores are very highly correlated (ICC) = 0.95 at baseline, and 0.96 at follow up). Scores on the ALSAQ-5 replicated those on the ALSAQ-40 to within one or two points, suggesting the ALSAQ-5 may be a valid alternative in studies where the ALSAQ-40 is impracticable or inappropriate to use, or if HRQoL is an exploratory endpoint.
ALS specific quality of life—short form (ALSSQOL-SF)
The 20-item ALSSQOL-SF questionnaire measures overall QoL in individuals with ALS and is a short-form version of the original 50-item ALSSQOL-R [55]. Developed in accordance with FDA 2009 guidelines for PROs, the final items for each subscale were estimated using Modified Graded Response (MGR) analysis and addresses six domains and subscales (Negative emotion, Interaction with people and environment, Intimacy, Religiosity, Physical symptoms, and Bulbar function). Responses are on a 0–10-point scale from ‘strongly disagree’ to ‘strongly agree.’ Recall is one week, and completion time is between 2 and 4 min. The ALSSQOL-SF has 6 items that are thought to be applicable to ALS patients (pain, fatigue, excessive saliva, problems with speaking, problems with strength and ability to move, problems with sleep).
Internal consistency as measured with Cronbach’s alpha between the ALSSQOL-R and the ALSSQOL-SF ranged from 0.70 (physical symptoms) to 0.89 (religiosity). Correlation of the Physical Symptoms subscale with the ALSFRS-R was significant (r = 0.48). A comparison with ALSFRS-R subscales shows significant correlations among all, but most closely with ALSFRS-R Fine motor (r = 0.37) and ALSFRS-R Gross motor functioning (r = 0.44), and less so with ALSFRS-R Speech (r = 0.17) and Respiratory (r = 0.34) domains [55].
Although well developed and validated, the assessment of HRQoL by the ALSSQOL-SF is more applicable in a clinical setting than a clinical trial, where information about the individual patient’s overall self-perceived well-being is more useful and meaningful. When assessing the impact of a specific therapeutic intervention, global QoL instruments are likely to be insensitive, because the intervention in question targets only one of many factors affecting overall QoL; for ALS, it is physical functioning.
EuroQoL-5D (EQ-5D)
The EQ-5D is a 5-item, self-report measure of health status developed by the EuroQoL Group that provides a simple, generic measure of HRQoL for clinical and economic appraisal. Well validated and commonly used in clinical trials as a secondary outcome, it applies to a wide range of health conditions and treatments and provides a simple descriptive profile and a single index value for health status. It consists of 5 items across 5 domains—mobility, self-care, usual activities, pain/discomfort and anxiety/depression. HRQoL as measured by the EQ-5D visual analog scale (VAS) found worse HRQoL in ALS patients with fatigue and ventilator use during home visits [56], and in ALS patients randomized to placebo vs. oral lithium in the lithium carbonate in the amyotrophic lateral sclerosis trial (LiCALS) [57]. Patients’ HRQoL as assessed by the EQ-5D decreased with increasing severity of ALS disease with patients' mean VAS rating of their own health ranging from 0.74 for stage 1 (early) disease severity, to 0.37 for stage 4 (late stage) disease severity [58].
World Health Organization Quality of Life BREF Scale (WHOQOL-BREF)
The WHOQOL-BREF is a shortened version of the generic 100-item WHOQOL and was recently validated in a large ALS/MND population [59]. It consists of 26 items across 4 domains: Physical health, Psychological, Social relationships and Environment. Responses are on a Likert Scale ranging from 0 to 5 with higher scores indicating better QOL. It can provide a Total score, and independent subscores for the Physical, Psychological and Environmental domains. Reliability across the domains ranged from alpha values of 0.57 (Social) to 0.82 (Physical). Excluding the social domain, the domains on the WHOQOL-BREF showed adequate internal construct validity demonstrating invariance of age, gender and ALS onset type, and acceptable levels of unidimensionality as determined by fit to the Rasch model.
WHOQOL-BREF domains showed a significant difference and strong gradient across most ALSFRS-R levels, with the Physical domain showing significant differences between limb or bulbar onset. The total WHOQOL-BREF score was also shown to correlate with the NRS-QOL (r = 0.6493), Modified Hospital Anxiety and Depression Scale (mHADS) (r = − 0.6787), WHODAS-2.0 (World Health Organization’s Disability Assessment Schedule– 2.0) (r = − 0.6489) and EQ-5D (r = 0.6651) [60]. Further validation is required, particularly longitudinally to assess the scale’s responsiveness across time, and responsiveness to therapy in clinical trials.
Neuro-QoL
Neuro-QoL are brief measures of HRQoL for clinical research in neurology and quantify the physical, mental and social impacts on adults and children living with neurological conditions. Recall for Neuro-QoL measures is 7 days and response options are 5-point Likert scale (never to always; no difficulty to unable to do). In ALS patients, 1-week test–retest reliability ranged from 0.79 to 0.96 and ICCs from 0.48 (social) to 0.92 (upper extremity functioning) [24]. ALS patients who reported a worsening of their physical well-being showed significantly worse upper extremity function scores than those who reported no change (t = − 2.17), and patients who reported a decrease in overall HRQoL also showed significant worsening of upper extremity function (t = − 3.17) and a trend toward increasing fatigue (t = − 1.68) [24, 60].
To mirror ALSFRS-R subscores, the Neuro-QoL measures that assess upper extremity functioning (8 items), lower extremity functioning (8 items), speech difficulties (6 items) and swallowing difficulties (6 items) are recommended as an option to assess HRQoL in these domains. Additional Neuro-QoL measures to assess impact of ALS on fatigue (8 items) and sleep disturbance (8 items) are also recommended [24].
The Neuro-QoL Fatigue score was inversely correlated with the ALSFRS-R score. Higher fatigue correlated significantly with lower function (r = − 0.72) [24]. Ambulatory ALS patients had significantly lower NeuroQoL-fatigue scores than non-ambulatory patients [24].
PROMIS Global Health 10
The PROMIS Global Health has 5 physical health items (GHP) and 5 mental health items (GHM). Recall is 7 days, and response options are on a 5-point Likert scale (excellent health to poor health; ‘completely able to carry out activity’ to ‘not at all able to do activity’). The GHP and GHM scales had internal consistency reliability coefficients of 0.81 and 0.86, respectively. GPH correlated more strongly with the EQ-5D than did GMH (r = 0.74 vs. 0.56). GPH correlated most strongly with pain impact (r = − 0.75); whereas GMH correlated most strongly with depressive symptoms (r = − 0.71) [61].
PROMIS GHP and GHM scores correlated positively with the ALSFRS-R score. Lower physical and mental health correlated with lower functioning (physical: r = 0.85; mental: r = 0.58) and ambulatory ALS patients had significantly higher PROMIS-10 physical health scores than non-ambulatory patients [24].
Both Neuro-QoL and PROMIS instruments were developed following FDA 2009 Guidelines for PRO development [22]. They are well-validated, psychometrically-sound and clinically relevant measures of HRQoL and Global Health for individuals with neurological conditions such as ALS.
Table 4 provides a summary of measures to assess HRQoL and QoL in ALS.
Cognitive screens
By end-stage disease, up to half of ALS patients will develop neuropsychological impairment [62], in some cases reaching a joint diagnosis of ALS and frontotemporal dementia (FTD). Future ALS clinical research should include cognitive screening to help provide evidence of cognitive or behavioral changes that might shorten survival, affect the assessment of ALS disease severity and progression, and potentially confound response to therapy [63]. A recent review article considered the ALS-Cognitive Behavioral Screen (ALS-CBS) and the Edinburgh cognitive and behavioral ALS screen (ECAS) to be the most suitable for detecting cognitive/behavioral changes in ALS [25]. Table 4 outlines the strengths and limitations of these two cognitive screens.
ALS cognitive behavior screen (ALS-CBS)
The ALS-CBS tracks the progression of cognitive/behavioral impairments in ALS. The cognitive section is clinician or care-staff administered and includes eight tasks addressing attention—concentration, tracking/monitoring, and initiation and retrieval. Scores range from 0 to 20. In general, cognitive scores from 17 to 20 do not indicate cognitive impairment. Patients with scores of 11–16 are classified as ALS cognitively impaired. Scores of 10 and below suggests the need for evaluation for ALS frontotemporal (FTD) or other dementia.
The 2-min, 15-item behavioral section rates caregiver-perceived changes in the patient since disease onset and assesses for: apathy, inhibition, empathy, emotional control, frustration tolerance, cognitive flexibility, insight, judgment, decision making, food preferences and language disturbance [64]. Items are scored from 0 to 3, with a total score ranging from 0 to 45. Scores below the cutoff (≤ 32) are classified as possible FTD-behavioral type, those scoring in the impaired range (33–36) are classified as having ALS behavior impairment, and those scoring ≥ 37 are considered ALS behaviorally normal [65].
Correlation of ALS-CBS cognitive scores was 0.7 with FVC and 0.04 with ALSFRS-R. Behavior scores correlated 0.19 with FVC and 0.08 with ALSFR-R. Compared to the gold standard of neuropsychological assessment, mean scores for both cognition and behavior of the ALS-CBS were significantly lower in ALS patients than the control group [64]. Interrater reliability for the behavior section was very high, r = 1.0 [66]. Test–retest reliability has not yet been established for this scale.
Edinburgh cognitive and behavioral ALS screen (ECAS)
The ECAS was launched as a rapid screening test to provide early, ALS-specific identification of cognitive and behavioral changes specific to ALS [67, 68]. The 15–20-min cognitive screen is clinician assessed while a 25-min behavioral interview is administered separately to the caregivers. The cognitive screen includes assessment of fluency, executive functions, language, memory, and visuospatial function. The domains considered specific to ALS disease are executive functions (including social cognition), language and fluency. The ECAS ALS-specific composite score ranges from 0 to 100, while the ECAS total score ranges from 0 to 136 with higher scores indicating less impairment. Reported test–retest reliability was good for the majority of subdomains (ICC > 0.70) [62]. ALS patients with bulbar involvement demonstrated significantly worse ALS-specific and total ECAS scores and impairment in behavior was significantly related to a worse ALSFRS-R score [62]. Validated against an extensive neuropsychological battery, the AUC for the ALS-specific score was 0.94 and 0.91. An ALS-specific score of ≤ 77 and an ECAS total score of ≤ 105 are the cut-off scores for “abnormality” or cognitive impairment due to ALS [69].
Both the ECAS and ALS-CBS take motor problems into consideration, but the ALS-CBS requires shorter administration time. Conversely, the ECAS assesses language and social cognition domains and might be more suitable for screening in ALS patients. Both screen for behavioral problems, which is an added advantage in this population [25].
Both these measures have been included in recent ALS clinical trials, nonetheless, additional validation would offer further insight into the scales’ test characteristics and continued usefulness in clinical trials.
Discussion
Data generated by a PRO can provide a statement of a treatment benefit from the patient perspective. For a treatment benefit to be meaningful, there should be evidence that the PRO under consideration measures the particular concept (e.g., disease construct/attribute) that is studied. Content validity is the extent to which the content of the measure is an adequate reflection of the construct to be measured [70], and content validity is emphasized in both the EMA [71] and FDA guidelines [22] as a requirement when developing and selecting PRO measures for use in a clinical trial and potential labeling purposes. The functional measures as well as the majority of the QoL measures discussed in this paper are disease-specific measures, specific to ALS and will generally have adequate content validity if used in an ALS population similar to the ALS population that the measure was developed in. In addition, all measures have a well-defined conceptual model, and all were developed following FDA guidelines with the exception of the ALSFRS-R which was developed prior to the FDA guidelines.
The selection of appropriate endpoints for ALS clinical trials is particularly important to quantify functional status for ALS where there is no standard measure of disease progression, no single objective measure of overall disability or functional status, and a lack of widely available, well-established candidate biomarkers.
Although there is no universal “best” instrument to measure functional status, almost all ALS clinical trials to date have employed the ALSFRS-R as the primary outcome measure for assessing ALS disease progression and functional disability. There are several advantages of the ALSFRS-R that support its inclusion as a primary endpoint in ALS clinical trials—it is relatively simple, easy to administer, reliable and well-validated, cost-effective and is a proxy for survival. However, issues of non-linearity, multidimensionality and floor and ceiling effects have challenged the ALSFRS-R’s continued utility as a primary outcome measure. Rasch analysis suggests that some functional aspects are especially difficult to capture in the context of the ALSFRS-R. In response, other qualitative instruments to measure functional status in ALS including the ALSFRS-EX, ALS-MITOS, CNS-BFS, DALS-15, MND-DS, and ROADS have been developed. These newer measures could provide alternative or complementary endpoints to the ALSFRS-R, to assess functional status in ALS clinical trials. The inclusion of QOL measures and ALS cognitive screens in future clinical trials is also recommended to assess the impact of new ALS therapies more fully.
The findings from this paper demonstrate several research needs. Specifically, newer measures require additional testing and validation in future ALS clinical trials. Some of these measures are psychometrically more rigorous and sensitive than the ALSFRS-R. Using newer measures requires some willingness to move beyond the commonly used ALSFRS-R, but additional longitudinal validity data for the newer scales may pave the way for them to eventually be used as a primary outcome measure in the assessment of physical functioning in ALS trials. Further exploration of the role of digital devices and wearable technology to assess physical functioning will also play a part in the future of ALS research and with more emphasis placed on the patient experience, or the patient journey, future clinical trials research should include a measure of patient well-being such as a QoL or HRQoL. Approaches beyond functional scales as trial endpoints (e.g. time to next confirmed event) should also be further explored. Additional research also needs to continue towards establishing a simple quantitative “general use” biomarker to assess ALS and ALS progression which can then be supplemented by the qualitative measures discussed herein. Natural history studies should also be encouraged to help provide a more clearly delineated map of ALS progression and its impacts across various subgroups of patients.
An important limitation to this review was its targeted, rather than systematic approach, which may have resulted in a collection of qualitative measures that is not exhaustive of those available in the ALS field. However, we believe our supplemental search of the clinicaltrials.gov database led to the most prominent and promising measures being included herein.
Conclusion
This paper serves as a reference guide for researchers seeking to identify potential qualitative measures of functional status for use in their ALS clinical trials. To our knowledge, this is the first paper that provides a descriptive collection of these measures including information on their strengths and weaknesses and recommendations for their implementation based on the published literature.
How to best quantify disease progression in ALS remains unclear. The measures discussed herein offer alternative or complementary options to the ALSFRS-R, in the context of the currently available tools. Additional research is needed to determine whether any of these qualitative measures of functional status, perhaps combined with a QOL measure will more accurately track and describe ALS disease progression, that could then help accelerate development of effective treatments for ALS.
Availability of data and materials
Not applicable.
Abbreviations
- ADL:
-
Activities of daily living
- ALS:
-
Amyotrophic lateral sclerosis
- ALSAQ:
-
Amyotrophic Lateral Sclerosis Assessment Questionnaires
- ALS-CBS:
-
ALS-Cognitive Behavioral Screen
- ALSFRS-R:
-
Amyotrophic Lateral Sclerosis Functional Rating Scale—Revised version
- ALSFRS-EX:
-
ALS Functional Rating Scale Extension
- ALS-MITOS:
-
ALS Milano–Torino Staging
- ALSSQOL-SF:
-
ALS Specific Quality of Life—Short Form
- CI:
-
Confidence interval
- CNS-BFS:
-
Center for Neurologic Study Bulbar Function Scale
- DALS-15:
-
Dyspnea ALS Scale
- ECAS:
-
Edinburgh cognitive and behavioral ALS screen
- EQ-5D:
-
EuroQoL-5D
- FDA:
-
US Food and Drug Administration
- FTD:
-
Frontotemporal
- FVC:
-
Forced vital capacity
- GHP:
-
Global Health Physical
- GHM:
-
Global Health Mental
- GRM:
-
Grade Response Modeling
- HRQoL:
-
Health-related quality of life
- ICC:
-
Intraclass correlation
- LiCALS:
-
Lithium carbonate in the amyotrophic lateral sclerosis trial
- MGD:
-
Modified Grading Response
- mHADS:
-
Modified Hospital Anxiety and Depression Scale
- MND-DS:
-
Motor Neuron Disease—Dyspnea Scale
- NIV:
-
Non-invasive ventilation
- NRS-QoL:
-
Numerical Rating Scale—Quality of Life
- PALS:
-
People with Amyotrophic Lateral Sclerosis
- PRO:
-
Patient reported outcome
- PROMIS:
-
Patient-Reported Outcomes Measurement Information System
- QoL:
-
Quality of life
- ROADS:
-
Rasch Overall ALS Disability Scale
- ROC AUC:
-
Receiver operating curve area under the curve
- VC:
-
Vital capacity
- WHODAS-2.0:
-
World Health Organization’s Disability Assessment Schedule—2.0
References
Quereshi M, Schoenfeld DA, Paliwal Y, Shui A, Cudkowicz ME. The natural history of ALS is changing: improved survival. Amyotroph Lateral Scler. 2009;10(5–6):324–31.
Norris FH, Calanchini PR, Fallat RJ, Panchari S, Jewett B. The administration of guanidine in amyotrophic lateral sclerosis. Neurology. 1974;24(8):721–8.
Appel V, Stewart S, Smith G, Appel SH. A rating scale for amyotrophic lateral sclerosis: description and preliminary experience. Ann Neurol. 1987;22(3):328–33.
Cedarbaum JM, Stambler N. Performance of the Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS) in multicenter clinical trials. J Neurol Sci. 1997;152(Suppl 1):S1-9.
Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci. 1999;169(1–2):13–21.
ALS Pathways. Assessing ALS function. 2021. https://alspathways.com/assessing-function/. Accessed 16 Mar 2021.
Cedarbaum JM, Mitsumoto H, Pestronk A, Ringel S, Florence J, Sanjak M, et al. The ALSFRS @ 20: Evolution of the ALSFRS-R, history, clinimetric properties and future directions. 2015. https://cytokinetics.com/wp-content/uploads/2015/10/2011ALS_MND_ASLFRS20.pdf. Assessed 16 Mar 2021.
European Medicines Agency (EMA). Guideline on clinical investigation of medicinal products for the treatment of amyotrophic lateral sclerosis (ALS). 19 November 2015 EMA/531686/2015, Corr.1 Committee for Medicinal Product for Human Use (CHMP).
Food and Drug Administration (FDA). Amyotrophic lateral sclerosis: developing drugs for treatment. Guidance for industry. 2019. https://www.fda.gov/drugs/guidance-compliance-regulatory-information/guidances-drugs. Assessed 16 Mar 2021.
ALS Crowd News. Do we need a new scale for measuring ALS? 2020. https://alscrowd.org/do-we-need-a-new-scale-for-measuring-als/. Accessed 19 Mar 2021.
Gordon PH, Cheng B, Salachas F, Pradat PF, Bruneteau G, Corcia P, et al. Progression in ALS is not linear but curvilinear. J Neurol. 2010;257(10):1713–7.
Kollewe K, Mauss U, Krampfl K, Petri S, Dengler R, Mohammadi B. ALSFRS-R score and its ratio: a useful predictor for ALS progression. J Neurol Sci. 2008;275(1–2):69–73.
Castrillo-Viguera C, Grasso DL, Simpson E, Shefner J, Cudkowicz ME. Clinical significance in the change of decline in ALSFRS-R. Amyotroph Lateral Scler. 2010;11(1–2):178–80.
Proudfoot M, Jones A, Talbot K, Al-Chalabi A, Turner MR. The ALSFRS as an outcome measure in therapeutic trials and its relationship to symptom onset. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17(5–6):414–25.
Rooney J, Burke T, Vajda A, Heverin M, Hardiman O. What does the ALSFRS-R really measure? A longitudinal and survival analysis of functional dimension subscores in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2017;88(5):381–5.
Mandrioli J, Biguzzi S, Guidi C, Sette E, Terlizzi E, Ravasio A, et al. Heterogeneity in ALSFRS-R decline and survival: a population-based study in Italy. Neuro Sci. 2015;36(12):2243–52.
Franchignoni F, Mora G, Giordano A, Volanti P, Chiò A. Evidence of multidimensionality in the ALSFRS-R Scale: a critical appraisal on its measurement properties using Rasch analysis. J Neurol Neurosurg Psychiatry. 2013;84(12):1340–5.
Franchignoni F, Mandrioli J, Giordano A, Ferro S. ERRALS Group. A further Rasch study confirms that ALSFRS-R does not conform to fundamental measurement requirements. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(5–6):331–7.
Bacci ED, Staniewska D, Coyne KS, Boyer S, White LA, Zach N, et al. Item response theory analysis of the Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised in the pooled resource open-access ALS clinical trials database. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17(3–4):157–67.
Fournier CN, Bedlack R, Quin C, Russell J, Beckwith D, Kaminski KH, et al. Development and validation of the Rasch–Built Overall Amyotrophic Lateral Sclerosis Disability Scale (ROADS). JAMA Neurol. 2020;77(4):480–8.
Atassi N, Berry J, Shui A, Zach N, Sherman A, Sinani E, et al. The PRO-ACT database: design, initial analyses, and predictive features. Neurology. 2014;83(19):1719–25.
Food and Drug Administration. Guidance for industry patient-reported outcome measures: use in medical product development to support labeling claims. 2009. https://www.fda.gov/media/77832/download. Accessed 30 Mar 2020.
Simmons Z. Patient-perceived outcomes and quality of life in ALS. Neurotherapeutics. 2015;12(2):394–402.
DeMarchi F, Berry JD, Chan J, Caldwell S, Ellrodt A, Scalia J, et al. Patient reported outcome measures (PROMs) in amyotrophic lateral sclerosis. J Neurol. 2020;267(6):1754–9.
Gosselt IK, Tanja C, Nijboer W, Van Es MA. An overview of screening instruments for cognition and behavior in patients with ALS: selecting the appropriate tool for clinical practice. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21(5–6):324–36.
Magnus T, Beck M, Giess R, Puls I, Naumann M, Toyka KV. Disease progression in amyotrophic lateral sclerosis: predictors of survival. Muscle Nerve. 2002;25(5):709–14.
Kaufmann P, Levy G, Thompson JL, DelBene ML, Battista V, Gordon PH, et al. The ALSFRS-R predicts survival time in an ALS population. Neurology. 2005;64(1):38–43.
Kaufmann P, Levy G, Montes J, Buchsbaum R, Barsdorf AI, Battista V, et al. Excellent inter-rater, intra-rater, and telephone-administered reliability of the ALSFRS-R in a multicenter clinical trial. Amyotroph Lateral Scler. 2007;8(1):42–6.
Bakker LA, Shroder CD, Tan HHG, Vugts SMAG, van Eijk RPA, van Es MA. Development and assessment of the inter-rater and intra-rater reproducibility of a self-administered version of the ALSFRS-R. J Neurol Neurosurg Psychiatry. 2020;91(1):75–81.
Lo Coco D, Marchese S, La Bella V, Piccoli T, Lo CA. The Amyotrophic Lateral Sclerosis Functional Rating Scale predicts survival time in amyotrophic lateral sclerosis patients on invasive mechanical ventilation. Chest. 2007;132(1):64–9.
Montes J, Levy G, Albert S, Kaufmann P, Buchsbaum R, Gordon PH, et al. Development and evaluation of a self-administered version of the ALSFRS-R. Neurology. 2006;67(7):1294–6.
Kasarskis EJ, Dempsey-Hall L, Malley Thompson M, Luu LC, Mendiondo M, Kryscio R. Rating the severity of ALS by caregivers over the telephone using the ALSFRS-R. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6(1):50–4.
Berry JD, Paganoni S, Carlson K, Burke K, Weber H, Staples P, et al. Design and results of a smartphone-based digital phenotyping study to quantify ALS progression. Ann Clin Transl Neurol. 2019;6(5):873–81.
Newton J, Jayaprakash K, Glasmacher SA, McEleney A, Bethell A, Fraser E, et al. Excellent reliability of the ALSFRS-R administered via videoconferencing: a study of people with motor neuron disease in Scotland. J Neurol Sci. 2020;416:116991.
Ahlstrom J. Phase II ALS clinical trial produces promising results for AMX0035. ALSCrowd Foundation web site. 2020. https://alscrowd.org/phase-ii-clinical-trial-produces-promising-results-for-amx0035/. Accessed 22 Nov 2020.
Wicks P, Massagli MP, Wolf C, Heywood J. Measuring function in advance ALS: validation of ALSFRS-EX extension items. Eur J Neurol. 2009;16(3):353–9.
Chiò A, Hammond ER, Mora G, Bonito V, Filippini G. Development and evaluation of a clinical staging system for amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2015;86(1):38–44.
Roche JC, Rojas-Garcia R, Scott KM, et al. A proposed staging system for amyotrophic lateral sclerosis. Brain. 2012;135(Pt 3):847–52. https://doi.org/10.1093/brain/awr351.
Filippini G, Bonito V, Chio A, et al. Quality of life in patients with amyotrophic lateral sclerosis: the QuaC-ALS study database. J Neurol. 2003;250(Suppl 2):23.
Tramacere I, Dalla Bella E, Chiò A, Mora G, Filippini G, Lauria G, et al. The MITOS system predicts long-term survival in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2015;86(11):1180–5.
Chen A, Garrett CG. Otolaryngologic presentations of amyotrophic lateral sclerosis. Otolaryngol Head Neck Surg. 2005;132(3):500–4.
Sorenson EJ, Crum B, Stevens JC. Incidence of aspiration pneumonia in ALS in Olmsted County. MN Amyotroph Lateral Scler. 2007;8(2):87–9.
Tabor L, Gaziano J, Watts S, Robison R, Plowman EK. Defining swallowing-related quality of life profiles in individuals with amyotrophic lateral sclerosis. Dysphagia. 2016;31(3):376–82.
Chapin JL, Gray LT, Vasilopoulos T, Anderson A, DiBiase L, York JD, et al. Diagnostic utility of the amyotrophic lateral sclerosis Functional Rating Scale—Revised to detect pharyngeal dysphagia in individuals with amyotrophic lateral sclerosis. PLoS ONE. 2020;15(8):e0236804.
Smith RA, Macklin EA, Myers KL, Pattee GL, Goslin KL, Meekins GD, et al. Assessment of bulbar function in amyotrophic lateral sclerosis: validation of a self-report scale (Center for Neurologic Study Bulbar Function Scale). Eur J Neurol. 2018;25(10):907-e66.
Nicholson K, Murphy A, McDonnell E, Shapiro J, Simpson E, Glass J, et al. Improving symptom management for people with amyotrophic lateral sclerosis. Muscle Nerve. 2018;57(1):20–4.
Vogt S, Petri S, Dengler R, Heinze HJ, Vielhaber S. Dyspnea in amyotrophic lateral sclerosis: Rasch-based development and validation of a patient-reported outcome (DALS-15). J Pain Symptom Manag. 2018;56(5):736–45.
Vogt S, Schreiber S, Heinze HJ, Dengler R, Petri S, Vielhaber S. The Dyspnea-ALS (DALS-15) optimizes individual treatment in patients with amyotrophic lateral sclerosis (ALS) suffering from dyspnea. Health Qual Life Outcomes. 2019;17(1):95.
Helleman J, Kruitwagen-van Reenen ET, Bakers J, Kruithof WJ, van Groenestijn AC, Jaspers Focks RJH, et al. Using patient-reported symptoms of dyspnea for screening reduced respiratory function in patients with motor neuron diseases. J Neurol. 2020;267(11):3310–8.
Linacre JM. Rasch analysis of rank-ordered data. J Appl Meas. 2006;7(1):129–39.
Fournier C, Bedlack R, Quinn C, Russell J, Beckwith D, Kaminski K, et al. The Rasch–Built overall ALS disability scale: ROADS to a better ALS outcome measure (1706). Neurology. 2020;94(15 Supplement):1706.
Jenkinson C, Fitzpatrick R, Brennan C, Bromberg M, Swash M. Development and validation of a short measure of health status for individuals with amyotrophic lateral sclerosis/motor neurone disease: the ALSAQ-40. J Neurol. 1999;246(Suppl 3):16–21.
Jenkinson C, Levvy G, Fitzpatrick R, Garratt A. The Amyotrophic Lateral Sclerosis Assessment Questionnaire (ALSAQ-40): tests of data quality, score reliability and response rate in a survey of patients. J Neurol Sci. 2000;180(1–2):94–100.
Jenkinson C, Fitzpatrick R. A reduced item set for the Amyotrophic Lateral Sclerosis Assessment Questionnaire: development and validation of the ALSAQ-5. J Neurol Neurosurg Psychiatry. 2001;70(1):70–3.
Felgoise SH, Feinberg R, Stephens HE, Barkhaus P, Boylan K, Caress J, et al. Amyotrophic lateral sclerosis—specific quality of life—short form (ALSSQOL-SF): a brief, reliable, and valid version of the ALSSQOL-R. Muscle Nerve. 2018;58(5):646–54.
Sandstedt P, Johannsson S, Ytterberg C, Ingre C, Holmqvist LW, Kierkegaard M. Predictors of health-related quality of life in people with amyotrophic lateral sclerosis. J Neurol Sci. 2016;370:269–73.
UKMND-LiCALS Study Group. Lithium in patients with amyotrophic lateral sclerosis (LiCALS): a phase 3 multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2013;12(4):339–45.
Kiebert GM, Green C, Murphy C, Mitchell JD, O’Brien M, Burrell A, et al. Patients’ health-related quality of life and utilities associated with different stages of amyotrophic lateral sclerosis. J Neurol Sci. 2001;191(1–2):87–93.
Young CA, Mills R, Al-Chalabi A, Burke G, Chandran S, Dick DJ, et al. Measuring quality of life in ALS/MND: validation of the WHOQOL-BREF. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21:1–9.
Cella D, Lai JS, Nowinski CJ, Victorson D, Peterman A, Miller D, et al. Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology. 2012;78(23):1860–7.
Hays RD, Bjorner J, Revicki DA, Spritzer K, Cella D. Development of physical and mental health summary scores from the Patient Reported Outcomes Measurement Information System (PROMIS) global items. Qual Life Res. 2009;18(7):873–80.
Crockford C, Newton J, Lonergan K, Madden C, Mays I, O’Sullivan M, et al. Measuring reliable change in cognition using the Edinburgh Cognitive and Behavioural ALS Screen (ECAS). Amyotroph Lateral Scler Frontotemporal Degener. 2018;19(1–2):65–73.
Mitsumoto H, Brooks BR, Silani V. Clinical trials in amyotrophic lateral sclerosis: why so many negative trials and how can trials be improved? Lancet Neurol. 2014;13(11):1127–38.
Woolley SC. Utility of the Amyotrophic Lateral Sclerosis Cognitive Behavioral Screen (ALS CBSTM). Neurodegener Dis Manag. 2011;1(6):473–9.
Woolley SC, York MK, Moore DH, Strutt AM, Murphy J, Schulz PE, et al. Detecting frontotemporal dysfunction in ALS: utility of the ALS Cognitive Behavioral Screen (ALS-CBS). Amyotroph Lateral Scler. 2010;11(3):303–11.
Murphy J, Factor-Litvak P, Goetz R, Lomen-Hoerth C, Nagy PL, Hupf J, et al. Cognitive-behavioral screening reveals prevalent impairment in a large multicenter ALS cohort. Neurology. 2016;86(9):813–20.
Abrahams S, Newton J, Niven E, Foley J, Bak TH. Screening for cognition and behaviour changes in ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(1–2):9–14.
Niven E, Newton J, Foley J, Colville S, Swingler R, Chandran S, et al. Validation of the Edinburgh Cognitive and Behavioural Amyotrophic Lateral Sclerosis Screen (ECAS): a cognitive tool for motor disorders. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(3–4):172–9.
Motor Neuron Disease Association, UK. The Edinburgh Cognitive and Behavioural ALS Screen (ECAS). Administration and Guidance Notes 2013. English Version. 2013. https://www.encals.eu/wp-content/uploads/2016/09/ECAS-Guidelines-administration-and-translation.pdf. Accessed 28 Oct 2020.
Cappelleri JC, Zou KH, Bushmakin AG, Alvir JM, D Alemayehu D, Symonds T. Patient-Reported Outcomes. Measurement, Implementation and Interpretation. CRC Press; 2014. p. 4, 31.
European Medicines Agency. Committee for Medicinal Products for Human use (CHMP): reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products. 2005. http://www.emea.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003637.pdf. Accessed 17 May 2021.
Acknowledgements
This study was sponsored by Takeda Pharmaceuticals. Molly Aldridge provided medical writing services during the drafting and review of this manuscript. Dakota Pastore provided assistance with the manuscript tables and formatting.
Funding
This study was funded in full by Takeda Pharmaceuticals.
Author information
Authors and Affiliations
Contributions
SLH, TR, SC, and CS were involved in the literature review design, performing the literature review, drafting of the report and manuscript, critical revision and approval of the manuscript. SC, SLH, SH, NZ, VM, and SD were involved in the study concept, literature review design, drafting of the report and manuscript, critical revision and approval of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Takeda Pharmaceuticals contracted with CERobs Consulting, LLC, a consulting firm with focus on real-world evidence, outcomes research, epidemiology and clinical outcome assessments, including patient reported outcomes; S. Hartmaier, T. Rhodes, S. Cook, and C. Schlusser consulted on this project through CERobs Consulting, LLC. C. Chao, S. Han, N Zach, V. Murthy, and S. Dave are/were salaried employees of Takeda Pharmaceuticals at the time the publication was written.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hartmaier, S.L., Rhodes, T., Cook, S.F. et al. Qualitative measures that assess functional disability and quality of life in ALS. Health Qual Life Outcomes 20, 12 (2022). https://doi.org/10.1186/s12955-022-01919-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12955-022-01919-9