Abstract
Background
Allergies caused by pollen from Populus deltoides are common, but the allergic components are still unclear.
Methods
The total proteins in pollen of P. deltoides were analyzed by proteomics, and the potential allergens were identified via the WHO/IUIS database and the allergenOnline database retrieval. One target protein was screened by bioinformatics and expressed in Escherichia coli. The biological activity of the expressed product was verified by animal experiments.
Results
The total of 3929 proteins in pollen of P. deltoides were identified, and 46 potential allergens belonging to 10 protein families were recognized by database retrieval. B9N9W6 protein of Hsp70 family was screened by bioinformatics analysis and expressed successfully. ELISA showed that B9N9W6 can stimulate the immune system to produce specific IgE and promote the generation of IL-4. Flow cytometry showed that B9N9W6 can significantly stimulate the proliferation of CD4+ T cells and promote the polarization of Th2 cells. The pathological sections of mice lung tissues indicated that alveolar destruction was more severe in the B9N9W6 group than that of extract group, and there were more inflammatory cells infiltration, mucus exudation and bleeding.
Conclusion
B9N9W6 is an important antigenic substance in the pollen of P. deltoides. Due to the conserved structure of Hsp70 family, more attention should be paid to the possibility of sensitization when Hsp70 from any pathogenic species is administered.
Similar content being viewed by others
Background
Allergic asthma is a chronic airway inflammation disease, which characterized by chest tightness, shortness of breath, and coughing after exposure to allergens [1]. The incidence of allergic asthma has been increasing in recent years [2]. At least 300 million people suffer from allergic asthma worldwide [3], which is highest among children [4, 5]. The main pathogenesis of allergic asthma is the production of specific IgE antibody, chronic airway inflammation and airway hyperresponsiveness during the immune system responds to allergens in the environment, accompanied by the imbalance of Th1/Th2 cells and other comprehensive factors [6,7,8].
Pollen from plants is an important source of air-borne allergens, which seriously affects the quality of life for people who is susceptible to allergies [9]. During the period of flower opening, pollen grains are released into the air to form biological aerosols; thus, individuals are inevitably exposed to pollen. Populus deltoides is widely cultivated in China due to its urban greening, windbreak, and sand-fixing berm. In May of each year, mature pollen of P. deltoides is densely suspended in the air, which causes a mass of pollen to contact people’s eyes, nostrils, mouth and skin frequently, leading to tears, sneezing, itching and other symptoms. Our recent studies demonstrated that mature pollen of P. deltoides extract contains antigenic substances with strong sensitization. However, allergic components in P. deltoides pollen remain largely unclear.
The identification and purification of pollen allergens is of great significance for pollen allergy disease, especially in diagnosis and allergen immunotherapy (AIT). In recent years, proteomic techniques have become powerful tools for comprehensive analysis of allergen, such as Par h 1 in Parthenium hysterophorus, and Art an 7 in Artemisia vulgaris were identified by this method [10, 11]. In China, proteomics was used to analyze and compare the possible allergens in mutants of P. deltoides [12, 13]. Wang et al. have analyzed the possible allergen components in Populus tomentosa by proteomics [14]. However, a systematic experimental basis is lacking for the identification of allergens in P. deltoides pollen.
Hsp (Heat Shock Protein) 70 has a subtle relationship with allergic diseases. Previous studies have found that Hsp70 is an important mediator to mediate allergic reactions and is capable of binding IgE antibodies in allergic patients [15]. The levels of Hsp70 are significantly elevated in patients with allergic rhinitis [16]. Interestingly, Hsp70 is widely present in plant pollen as a pan-allergen, which could be responsible for a part of the allergenic cross-reactivity between proteins from different pollens and plant food [17]. However, the biological function of Hsp70 remains largely unknown.
To screen and verify P. deltoides pollen allergens, we analyzed the total protein of pollen through proteomics. Then, the sequences of identified protein were compared with the confirmed allergen via relevant allergen database to identify the allergen components in pollen. After that, Hsp70 was expressed in prokaryotic expression system and explored its biological function by animal models. As far as we know, this is the first report of comprehensive allergenic proteins and Hsp70 biological function in pollen of P. deltoides.
Materials and Methods
P. deltoides pollen sample
The pollen samples of P. deltoides used in this study were collected at the Yangtze River embankment (Wuhu, China) from Apr 20 to May 20, 2018, and stored in a refrigerator at -80°C.
Experimental animal
A total of 30 SPF female BALB/c mice (6-weeks) were purchased from Shanghai Slack Laboratory Animal Co., Ltd. (License number: 20170005030182). The animals were kept under Specific Pathogen Free (SPF) laboratory conditions in the Hangzhou Hibio Technology Co. Ltd, with the room temperature at 22-26 °C, light and darkness for 12 hours, respectively. Animals were free to eat and drink.
Protein extraction
Protein preparation for proteomics was as follows: The pollen samples were treated with trichloroacetic acid with a final concentration of 20% at 4 °C for 2 h, centrifuged at 12000 g at 4 °C for 3 min, and the supernatant was discarded. The protein precipitate was washed three times with pre-cooled acetone and reconstituted with 8 M urea. Then, the proteins were analyzed by 12% SDS-PAGE. Protein preparation for animal experiments was as follows: Dried pollen was extracted with PBS (solid-liquid ratio of 1 g: 20 mL) at room temperature (RT) for 24 hours. The extracted materials were precipitated at RT for 8 hours. The crude extracts were filtered through 20 μm filter paper. Then, the extracts were sterilized through a 0.22 μm strainer (Millex GP) and stored at 4°C. Finally, the protein concentration was measured by the BCA kit (Sangon, C503071-0250).
Label-Free Quantitative Proteomics analysis of P. deltoides pollen
Proteomics analysis was performed with reference to relevant study methods [18]. The detailed process was as follows:
The protein solution was reduced with 5 mM dithiothreitol for 30 min at 56 °C and alkylated with 11 mM iodoacetamide for 15 min at room temperature in darkness. Then, the sample was diluted with 100 mM NH4HCO3 to urea concentration less than 2 M. Trypsin was added at 1:50 trypsin-to-protein mass ratio for the first digestion overnight and 1:100 trypsin-to-protein mass ratio for a second 4 h-digestion.
The tryptic peptides were fractionated into fractions by high pH reverse-phase HPLC using Agilent ZORBAX 300Extend C18 column (5 μm particles, 4.6 mm ID, 250 mm length), and were dissolved in solvent A, directly loaded onto a home-made reversed-phase analytical column (15-cm length, 75 μm i.d.). The gradient was comprised of an increase from 7% to 25% solvent B (0.1% formic acid in 98% acetonitrile) over 40 min, 25% to 35% in 12 min and climbing to 80% in 4 min then holding at 80% for the last 4 min, all at a constant flow rate of 700 nl/min on an EASY-nLC 1000 UPLC system. Then, the peptides were subjected to NSI source followed by tandem mass spectrometry (MS/MS) in Q ExactiveTM Plus (Thermo) coupled online to the UPLC. The m/z scan range was 350 to 1800 for full scan, and intact peptides were detected in the Orbitrap at a resolution of 70,000. Peptides were then selected for MS/MS using NCE setting as 28 and the fragments were detected in the Orbitrap at a resolution of 17,500.
Assigning peptide sequences and functional annotation
The results of MS/MS data were processed by Maxquant search engine (v.1.5.2.8). Tandem mass spectra were searched against “Populus deltoides” database concatenated with reverse decoy database. False discovery rate (FDR) was adjusted to < 1%. The obtained MS/MS data were mapped and annotated with Maxquant software (v1.5.2.8), and all the annotated peptide data were classified by R software for gene ontology classification and KEGG signaling pathway enrichment. The thresholds for the identification of total proteins and potential allergens in P. deltoides pollen were determined by relevant literature [19].
Screening of potential allergens in the Hsp70 protein family
The potential allergens of the Hsp70 protein family were screened by following steps. First, we performed T/B cell epitopes prediction for potential allergens of the Hsp70 protein family by the Immune Epitope Database and Analysis Resource (IEDB) (http://www.iedb.org/). Then, Antigenicity of the HSP70 was predicted through Vaxijen v2.0 (http://www.ddgpharmfac.net/vaxijen/VaxiJen/VaxiJen.html) (Threshold 0.4). The formula, molecular weight (MW), stability and isoelectric point (PI) for B9N9W6 were predicted via ExPASy (http://web.expasy.org/protparam/), and its solubility was analyzed by ProtScale (http://web.Expasy.org/protscale/). Thirdly, we performed sequence alignment with Hsp70s through the known allergens reported in the WHO/IUIS database and AllergenOnline database, respectively. Finally, we used Swiss-model for homology modeling to screen out Hsp70 with the highest surface complementarity with IgE antibody.
Prokaryotic expression and purification of Hsp70
Availability of adequate amounts of the pure allergens is essential to further understand their molecular structures, which is a pre-requisite for the development of more efficacious allergen immunotherapy. The amino acid sequences and coding genes of Hsp70 were retrieved from Uniprot database. The gene of Hsp70 was synthesized and cloned into prokaryotic expression vector pET-28a (+). The expression of His-tagged recombinant Hsp70 in transformant E. coli BL21 was induced by IPTG (final concentration = 1 mmol/L), and the expression products were analyzed by SDS-PAGE. Then, the bacteria were collected and disrupted by sonication. Hsp70 was purified by Ni-NTA protein purification kit (Sangon, C600320-0001), and the recombinant protein was eluted with elution buffer (containing 50mM Tris, 300mM NaCl and 500mM imidazole, Ph 8.0). Then, the endotoxin was removed by Endotoxin Removal Spin Columns (Thermo Fisher, NO. 88273). Purified protein was resuspended in 1×phosphate buffered solution (PBS), and analyzed by SDS-PAGE. The protein concentration was measured by the protein concentration assay kit (Sangon, C503071-0250).
Preparation of asthmatic model
All BALB/c mice were randomly divided into 3 groups (10 mice in each group): PBS group, pollen extract group, and Hsp70 group. Mice in extract group and Hsp70 group were injected intraperitoneally with 10 μg pollen extract or Hsp70 [dissolved in 4% (W/V) Al(OH)3 in PBS, endotoxin-free] on 0, 7, and 14 days, respectively, to sensitize. From the 21st day, 0.5 μg/ml pollen extract or Hsp70 suspension was sprayed for excitation for 30 min/time for one week. The PBS group was replaced with PBS containing 4% (W/V) Al(OH)3. Mice in each group were injected intraperitoneally with 0.8 mg BrdU (dissolved in PBS) before the last challenge. After 24 hours, blood was taken from the eye socket, left at 4°C for 2 hours, and centrifuged at 4000 g for 5 minutes. The collected serum was stored at -80°C.
Enzyme-linked immunosorbent assay (ELISA)
The levels of total IgE antibody and IL-4 in serum were measured by ELISA kits from Songon, Inc. and Jianglaibio (Shanghai, China) (Sangon, D721094 \ Jianglaibio, JL20266) according to the manufacturer’s protocol.
Flow cytometry
The spleens of mice in each experimental group were aseptically collected, and splenic cell suspension was prepared. The proliferation of CD4+ T cells and the ratio of Th1/Th2 in spleen cell suspension were detected according to the reported method [20]. In brief, the Th1/Th2 ratio in the splenic cell suspension was detected by the Th1/Th2 flow cytometry kit (Lianke Biotech, No. 70-KTH201) according to the kit’s instructions. Cell suspension was incubated for one hour at RT in the dark after adding Anti-mouse CD4-FITC antibody (Invitrogen, No. 11-0042-85) and Brdu-PE antibody (Invitrogen, No. 12-5071-41), respectively, and then determined by flow cytometry.
Histological analysis
After the mice lung tissues were embedded in paraffin, sectioned (5μm thick), and hydrated with a series of alcohol, stained with hematoxylin and eosin (H&E), and then the changes in lung tissue structure were observed with a Nikon Ni-E microscope. Images were captured with a Nikon DS-Ri2 camera.
Statistical analysis
Data are expressed as mean ± standard deviation (SD). Data analysis was performed with SPSS16.0 statistical software package. One-way analysis of variance (ANOVA) was used for the comparison of multiple sample means, and the least significant difference (LSD) method and Tamhane’s T2 method were used for the comparison between the two samples. The statistical test level α = 0.05.
Results
Proteomics of P. deltoides pollen and its comparison with P. tomentosa
In the present study, we have used workflow (Fig. 1) to classify its allergen families and to characterize new P. deltoides pollen allergen candidates. The GO annotation found that P. deltoides and P. tomentosa [14] have great similarities in biological process, cellular component and molecular function, but great differences in KEGG pathway (Table 1). In terms of the allergens identified, P. deltoides had more allergens (46 vs 27), and more members in the Hsp70 family than P. tomentosa (10 vs 7, Table 2).
Workflow of the study to delineate the P. deltoides pollen proteome and analyze its bioactivity. Protein mass was mainly concentrated within 150kD, and protein sequence coverage was mainly below 40%. Peptides were mainly distributed in the length of 7-27 amino acid residues, and the peptides with length 7-17AA was relatively concentrated
Allergenic protein families and candidate allergenic proteins
Sequence alignment between the WHO/IUIS database and the allergenOnline database identified 46 potential allergens that belonged to 10 protein families (Table 2). The top three allergen families in relative abundance included seed storage proteins (1.63%), proteases (0.603%) and Hsp70 (0.49%). The biological activity of both seed storage proteins and proteases has been studied in-depth [21, 22], but that of the Hsp70 family is still largely unknown. It has been confirmed that Hsp70 is an important mediator to mediate allergic diseases and has the ability to specifically bind to IgE antibody [15]. However, the immunogenicity and antigenicity of Hsp70 in P. deltoides pollen have rarely been reported.
Screening of potential allergens in the Hsp70 protein family
All the Hsp70s were checked, and B9N9W6 was selected as a potential allergen to elicit host immune response due to its physical and chemical characteristics as follows. IEDB epitope prediction showed that B9N9W6 had 3 T-cell epitopes and 4 B-cell epitopes with high affinity (Tables 3 and 4). The formula of B9N9W6 was C3146H5062N880O1001S21, MW was 71 900, PI was 5.34, and antigenicity was 0.5714 (Threshold > 0.4), which was considered as a stable hydrophilic protein. Query through AllergenOline database showed that B9N9W6 had a conserved Hsp70 domain. Protein homology analysis revealed that the Hsp70 family protein sequences were extremely conserved (Fig. 2). Secondary structure of B9N9W6 was dominated by α-helix and β ladder (Fig. 3), and it might be a hetero-dimer according to the crystal structure on the surface (Fig. 4). Compared with B9N9W6, other Hsp70 proteins are defective: B9HV59 is a hydrophobic protein, B9GEL5 is an unstable protein, B9HN74 has low immunogenicity, and B9GJ14, B9HTJ7 and B9NBF4 homologous modeling have low reliability. Although B9HMG2, B9HMG7 and B9HMG8 have high quality in terms of physical and chemical properties and immunogenicity, they have low relative abundances (Table S1). Therefore, B9N9W6 were chosen as the target protein.
Aligning of B9N9W6 with template 3c7n.1.B amino acid sequences and secondary structure. The residues found in all protein polypeptide chains of the model are displayed in an interactive sequence display. Each residue is displayed by its one letter code below a bar chart displaying the QMEAN local quality estimation value. The secondary structure of the protein is displayed above each residue code as a single letter. B: residue in isolated β-bridge; C: loop or irregular; E: extended strand, participates in β ladder; G: 3-helix; H: α-helix; T: hydrogen bonded turn; S: bend
Molecular model diagram of B9N9W6 constructed by SWISS-Model. Template ID: 3c7n.1.B. QMEAN Value -0.86, GMQE Value 0.66 (The Qmean Value range is -4-0, and the closer it is to 0, the better the matching degree between the target protein and the template. The confidence range of GMQE Value is 0-1, the higher the value, the better the quality of the model). A: Ribbon diagram of B9N9W6. B: Surface structure diagram of B9N9W6. C: Ramachandran Plots of B9N9W6. The number of residues in favored (90.11%), in allowed regions (7.61%) and in outlier regions (2.20%), which demonstrates the high quality of the model construction
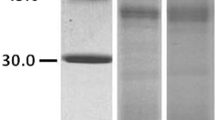
Protein expression and purification of B9N9W6
SDS-PAGE of B9N9W6 showed specific band with a MW at 70KD, and purified product showed a clear band at same position (Fig. 5). The protein purity was more than 90%.
B9N9W6 induced antibody and cytokine responses
In order to explore the effects of B9N9W6 on serum antibodies and cytokines, the levels of IgE and cytokine IL-4 in serum were detected by ELISA kit. The results were showed in Fig. 6. The IgE level in the extract group (13.83 ± 5.59 ng/mL) was significantly higher than that in the PBS group (7.58 ± 2.40 ng/mL, P < 0.05), but significantly lower than that in B9N9W6 group (25.76 ± 11.90) ng/mL, P < 0.01). In terms of IL-4 level, the IL-4 level in the extract group (120.08±36.58 pg/mL) was higher than that in the PBS group (74.69±25.30 pg/mL, P < 0.05), while the IL-4 level in the B9N9W6 group (255.24±81.88 pg/mL) was the highest among all groups (P < 0.01).
B9N9W6 induced CD4+ T cell proliferation and Th2 differentiation
In order to evaluate the ability of B9N9W6 to stimulate the proliferation of CD4+ T cells, we detected the number of proliferating CD4+ T cells and their subsets by flow cytometry. The results showed that compared with the PBS group (12.84 ± 0.53 %) and extract group (18.10 ± 0.58 %), B9N9W6 significantly promoted the proliferation of CD4+ T cells (27.86 ± 1.07 %, P < 0.01) (Fig. 7A, C1). Compared with the PBS group (2.39 ± 0.34), the ratio of Th1/Th2 in extract group (1.75 ± 0.24) and B9N9W6 group (1.69 ± 0.21) had significantly lower (P < 0.01) (Fig. 7B, C2).
B9N9W6 induced allergic inflammation in the lung tissue of mice models
Compared with the PBS group, the extract group showed the number of lung tissue alveoli decreased due to destroyed anatomical structure, and increased mucus in the respiratory bronchus (Fig. 8B). A large number of inflammatory cells were exudated to the lung field. Alveolar septum thickens due to edema while mucus and inflammatory cells appeared in the terminal bronchioles and its periphery (Fig. 8E, H). Compared with the extract group, a mass of red blood cells exudate was found in the B9N9W6 group, and the destruction of alveoli was further aggravated (Fig. 8C); Bullae formed, the collapsed alveolar cavity and exudate of erythrocyte were detected; Mucus and inflammatory cells were abundant in the terminal bronchioles (Fig. 8F, I).
Discussion
In recent years, allergic diseases caused by pollens have attracted much attention, especially in the plant species releasing pollens, specific IgE reactivity and the influence of air pollutants on pollen transmission [23,24,25]. Nevertheless, identification of allergenic proteins and their bioactivity have remained elusive. In this study, we detected 3929 distinct proteins in pollen of P. deltoides by proteomics. We performed systematic bioinformatics analysis of all identified proteins, including protein annotation, functional classification and functional enrichment. Through functional annotation, we found that the total proteins of P. deltoides pollen and P. tomentosa pollen have great difference in function [14]. GO categorization showed that the total proteins of P. deltoides pollen were significantly different from those of its two mutants in biological process, molecular function, and cellular component [12]. KEGG enrichment analysis indicated that these identified proteins were not only involved in organelle composition and biogenesis, but also in biological processes such as metabolism and synthesis. There are great differences in the ingredients and functions of pollen proteins among different species of the same genus, which provides a material basis for antigen screening.
Plant-derived allergens mainly belong to disease-related protein 10 (DRP-10), thomas protein-like protein (TLP), Non-specific lipid transfer proteins (nsLTPs), expansion proteins, calcium binding proteins and profilin protein families [26, 27]. These proteins are called pan-allergen in specialized terms such as profilin, because the same family of proteins has a common antigenic determinant, and they can cause a wide range of cross-reactions [28]. Proteins of the same family share a common domain and are relatively conservative in structure, which caused the common allergen proteins that can be identified in a variety of plants [29,30,31]. In this study, through sequence alignment in the database, we identified 46 potential allergens belonging to 10 protein families. The top three allergen families in relative abundance are the seed storage proteins, protease family and the Hsp70 family. These results indicated that P. deltoides pollen contained abundant protein components. As pan-allergens, these proteins are widely found in pollen and plant fruits, and easily had fruit-vegetable-pollen cross-reactive allergy syndromes.
Hsp represent a family of molecular chaperones that respond to refolding proteins, protein trafficking, and cell signaling processes [32,33,34]. Hsp70 is an important member of the Hsp family involved in stress response, which often used as a potential biomarker, therapeutic target, or modulator of inflammation [35, 36]. Furthermore, Hsp70 is also involved in leaf remodeling, flowering and disease resistance in plant [37,38,39]. A correlation between biological function and allergenic capacity of proteins related to stress response has not been clearly demonstrated. Studies had shown that luminal binding protein of Hsp70 family in hazel pollen is a cross-reactive allergen [17]. C-terminal region of Hsp70 of Echinococcus Granulosus is antigenic molecule inducing both B and T cell responses [15]. Coincidentally, a large number of proteins and potential allergens containing the Hsp70 domain were identified in this study, such as B9N9W6. Epitope prediction suggested that B9N9W6 might have antigenic activity. Sequence alignment showed that B9N9W6 was highly consistent with the amino acid sequences of known allergens Cla h 4, Der f 28, etc. Homology modeling for B9N9W6 found that its 3-dimensional structure was also highly similar which consists of two main useful realms separated by a hinge region (Fig. 4A, and B); which accorded with the structural characteristics of the Hsp70 family [40]. This remarkable conservation of both surface residues and main chain conformations in the Hsp70 family plays an important role in conservation of IgE-binding epitopes, and more beneficial to promote the polarization of Th2 cells [41, 42].
Identification and purification of pollen allergens is of great significance both for the study of cross-allergic reaction and AIT. The immunoreactivity of Hsp70 had been demonstrated in previous studies [17, 41, 43]. In this study, the bioactivity of B9N9W6 was detected by animal model. First, we demonstrated that B9N9W6 can stimulate the immune system to produce high levels of IgE antibodies and promote the production of IL-4 via ELISA. Allergen specific IgE antibody is a major cause of type I allergic diseases, such as asthma [44], In the experiment, we detected specific IgE produced by B9N9W6 via Western blot, and the results suggested that B9N9W6 could produce specific IgE and had the ability to bind it (Fig. S1). IL-4 is an important proinflammatory factor secreted by Th2 cells to mediate allergic airway inflammation [45]. Meanwhile, the significant increase of IL-4 concentration in the B9N9W6 group indicated the imbalance of Th1/Th2 cells and the increase of Th2 cells. Secondly, we detected the proliferation of B9N9W6 to stimulate CD4+ T cells and their subgroup Th1/Th2 cells by flow cytometry. It was found that B9N9W6 could significantly stimulate CD4+ T cell proliferation and promote Th2 cell polarization. These results suggested an immunogenicity of B9N9W6, which were consistent with ELISA. All the results above suggest that B9N9W6 may induce allergic inflammation in the airway of the mice model. To verify our hypothesis, the presence of inflammation was observed through H&E staining sections of the mice lung tissues. More inflammatory cells infiltration and mucus exudation were observed in the lung tissue of B9N9W6 group; the alveolar rupture was the most serious, and even caused pulmonary hemorrhage. Therefore, we consider that under the same concentration, the sensitization of B9N9W6 was stronger than that of extract. There are three possible reasons for this result: 1) B9N9W6 might have a dominant T/B cell epitope, which can strongly stimulate the immune response (Tables 3 and 4). 2) As a member of the Hsp70 family, B9N9W6 could enhance the activity of antibody-presenting cells (APCs) in the process of antigen processing and presentation, which is essential for the initiation and modulation of the asthmatic immune response [46]. 3) Hsp70 is a positive regulator of airway inflammation and goblet cell hyperplasia in allergic airway inflammation [47]; the pathological findings of this study supported this view. Therefore, our findings suggested that B9N9W6 potentially induces allergen and promotes Th2 inflammatory responses.
Conclusions
In summary, this study employed the proteomics method to screen out 46 potential allergens from 10 protein families of P. deltoides pollen. Furthermore, B9N9W6 belongs to Hsp70 family was confirmed to have strong allergen activity and can induce allergic asthma in animal models. Our conclusions not only enrich the theoretical study of Hsp70 family in pollen allergens, but also provide reference for the study of cross-allergic reaction and immunotherapy of allergic diseases.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- Hsp:
-
Heat shock protein
- AIT:
-
Allergen immunotherapy
- DRP-10:
-
Disease-related protein 10
- TLP:
-
Thomas protein-like protein
- nsLTPs:
-
Non-specific lipid transfer proteins
- SPF:
-
Specific pathogen free
- GO:
-
Gene Ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
References
Schatz M, Rosenwasser L. The allergic asthma phenotype. J Allergy Clin Immunol Pract. 2014;2:645–8 quiz 649.
Cevhertas L, Ogulur I, Maurer DJ, Burla D, Ding M, Jansen K, et al. Advances and recent developments in asthma in 2020. Allergy. 2020;75:3124–46.
Stern J, Pier J, Litonjua AA. Asthma epidemiology and risk factors. Semin Immunopathol. 2020;42:5–15.
Pakkasela J, Ilmarinen P, Honkamaki J, Tuomisto LE, Andersen H, Piirila P, et al. Age-specific incidence of allergic and non-allergic asthma. BMC Pulm Med. 2020;20:9.
Poowuttikul P, Saini S, Seth D. Inner-City Asthma in Children. Clin Rev Allergy Immunol. 2019;56:248–68.
Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–54.
Rachmiel M, Bloch O, Bistritzer T, Weintrob N, Ofan R, Koren-Morag N, et al. TH1/TH2 cytokine balance in patients with both type 1 diabetes mellitus and asthma. Cytokine. 2006;34:170–6.
Ying L, Fu Z, Luo J, Zhou C, Chen Y, Wang L, et al. Cytotoxic T lymphocyte antigen 4 immunoglobulin modified dendritic cells attenuate allergic airway inflammation and hyperresponsiveness by regulating the development of T helper type 1 (Th1)/Th2 and Th2/regulatory T cell subsets in a murine model of asthma. Clin Exp Immunol. 2011;165:130–9.
Camacho I, Caeiro E, Nunes C, Morais-Almeida M. Airborne pollen calendar of Portugal: a 15-year survey (2002-2017). Allergol Immunopathol (Madr). 2020;48:194–201.
Pablos I, Eichhorn S, Briza P, Asam C, Gartner U, Wolf M, et al. Proteomic profiling of the weed feverfew, a neglected pollen allergen source. Sci Rep. 2017;7:6049.
Fu W, Gao Z, Gao L, Jin J, Liu M, Sun Y, et al. Identification of a 62-kDa major allergen from Artemisia pollen as a putative galactose oxidase. Allergy. 2018;73:1041–52.
Zhang XL, Zhang J, Guo YH, Sun P, Jia HX, Fan W, et al. Comparative Proteomic Analysis of Mature Pollen in Triploid and Diploid Populus deltoides. Int J Mol Sci. 2016;17:1475.
Zhang J, Wu LS, Fan W, Zhang XL, Jia HX, Li Y, et al. Proteomic analysis and candidate allergenic proteins in Populus deltoides CL. "2KEN8" mature pollen. Front Plant Sci. 2015;6:548.
Wang L, Zhang X, Zhang J, Fan W, Lu M, Hu J. Proteomic Analysis and Identification of Possible Allergenic Proteins in Mature Pollen of Populus tomentosa. Int J Mol Sci. 2018;19:250.
Ortona E, Margutti P, Delunardo F, Vaccari S, Rigano R, Profumo E, et al. Molecular and immunological characterization of the C-terminal region of a new Echinococcus granulosus Heat Shock Protein 70. Parasite Immunol. 2003;25:119–26.
Min HJ, Kim KS, Yoon JH, Kim CH, Cho HJ. T-helper 2 cytokine-induced heat shock protein 70 secretion and its potential association with allergic rhinitis. Int Forum Allergy Rhinol. 2017;7:530–5.
Gruehn S, Suphioglu C, O'Hehir RE, Volkmann D. Molecular cloning and characterization of hazel pollen protein (70 kD) as a luminal binding protein (BiP): a novel cross-reactive plant allergen. Int Arch Allergy Immunol. 2003;131:91–100.
Feng J, Wu Z, Wang X, Zhang Y, Teng N. Analysis of Pollen Allergens in Lily by Transcriptome and Proteome Data. Int J Mol Sci. 2019;20:5892.
San Segundo-Acosta P, Oeo-Santos C, Benede S, de Los RV, Navas A, Ruiz-Leon B, et al. Delineation of the Olive Pollen Proteome and Its Allergenome Unmasks Cyclophilin as a Relevant Cross-Reactive Allergen. J Proteome Res. 2019;18:3052–66.
Xu F, Yu S, Qin M, Mao Y, Jin L, Che N, et al. Hydrogen-Rich Saline Ameliorates Allergic Rhinitis by Reversing the Imbalance of Th1/Th2 and Up-Regulation of CD4+CD25+Foxp3+Regulatory T Cells, Interleukin-10, and Membrane-Bound Transforming Growth Factor-beta in Guinea Pigs. Inflammation. 2018;41:81–92.
Villalta D, Scala E, Mistrello G, Amato S, Asero R. Evidence of Cross-Reactivity between Different Seed Storage Proteins from Hazelnut (Corylus avellana) and Walnut (Juglans regia) Using Recombinant Allergen Proteins. Int Arch Allergy Immunol. 2019;178:89–92.
McKenna OE, Posselt G, Briza P, Lackner P, Schmitt AO, Gadermaier G, et al. Multi-Approach Analysis for the Identification of Proteases within Birch Pollen. Int J Mol Sci. 2017;18:1433.
Wang X, Guo M, Wang H, Wang X. Pollen allergen sensitization feature of seasonal allergic rhinitis in children and adolescents in northern China. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020;34:1005–10.
Scevkova J, Vaskova Z, Sepsiova R, Dusicka J, Kovac J. Relationship between Poaceae pollen and Phl p 5 allergen concentrations and the impact of weather variables and air pollutants on their levels in the atmosphere. Heliyon. 2020;6:e04421.
Sabit M, Wong C, Andaya A, Ramos JD. Pollen allergen skin test and specific IgE reactivity among Filipinos: a community-based study. Allergy Asthma Clin Immunol. 2020;16:74.
Breiteneder H, Ebner C. Molecular and biochemical classification of plant-derived food allergens. J Allergy Clin Immunol. 2000;106:27–36.
Radauer C, Breiteneder H. Pollen allergens are restricted to few protein families and show distinct patterns of species distribution. J Allergy Clin Immunol. 2006;117:141–7.
Sircar G, Bhowmik M, Sarkar RK, Najafi N, Dasgupta A, Focke-Tejkl M, et al. Molecular characterization of a fungal cyclophilin allergen Rhi o 2 and elucidation of antigenic determinants responsible for IgE-cross-reactivity. J Biol Chem. 2020;295:2736–48.
Subbarayal B, Schiller D, Mobs C, de Jong NW, Ebner C, Reider N, et al. Kinetics, cross-reactivity, and specificity of Bet v 1-specific IgG4 antibodies induced by immunotherapy with birch pollen. Allergy. 2013;68:1377–86.
Hofmann C, Scheurer S, Rost K, Graulich E, Jamin A, Foetisch K, et al. Cor a 1-reactive T cells and IgE are predominantly cross-reactive to Bet v 1 in patients with birch pollen-associated food allergy to hazelnut. J Allergy Clin Immunol. 2013;131:1384–92 e1386.
Fernandez Rivas M. Cross-reactivity between fruit and vegetables. Allergol Immunopathol (Madr). 2003;31:141–6.
Zininga T, Ramatsui L, Shonhai A. Heat Shock Proteins as Immunomodulants. Molecules. 2018;23:2846.
Bolhassani A, Agi E. Heat shock proteins in infection. Clin Chim Acta. 2019;498:90–100.
Tukaj S. Heat Shock Protein 70 as a Double Agent Acting Inside and Outside the Cell: Insights into Autoimmunity. Int J Mol Sci. 2020;21:5298.
Elmallah MIY, Cordonnier M, Vautrot V, Chanteloup G, Garrido C, Gobbo J. Membrane-anchored heat-shock protein 70 (Hsp70) in cancer. Cancer Lett. 2020;469:134–41.
Shevtsov M, Huile G, Multhoff G. Membrane heat shock protein 70: a theranostic target for cancer therapy. Philos Trans R Soc Lond B Biol Sci. 2018;373:20160526.
Gorovits R, Moshe A, Ghanim M, Czosnek H. Recruitment of the host plant heat shock protein 70 by Tomato yellow leaf curl virus coat protein is required for virus infection. PLoS One. 2013;8:e70280.
Chen X, Shi L, Chen Y, Zhu L, Zhang D, Xiao S, et al. Arabidopsis HSP70-16 is required for flower opening under normal or mild heat stress temperatures. Plant Cell Environ. 2019;42:1190–204.
Rowarth NM, Dauphinee AN, Denbigh GL, Gunawardena AH. Hsp70 plays a role in programmed cell death during the remodelling of leaves of the lace plant (Aponogeton madagascariensis). J Exp Bot. 2020;71:907–18.
Lu RC, Tan MS, Wang H, Xie AM, Yu JT, Tan L. Heat shock protein 70 in Alzheimer's disease. Biomed Res Int. 2014;2014:435203.
Chiung YM, Lin BL, Yeh CH, Lin CY. Heat shock protein (hsp 70)-related epitopes are common allergenic determinants for barley and corn antigens. Electrophoresis. 2000;21:297–300.
Nakajima S, Kabata H, Kabashima K, Asano K. Anti-TSLP antibodies: Targeting a master regulator of type 2 immune responses. Allergol Int. 2020;69:197–203.
Shen HD, Au LC, Lin WL, Liaw SF, Tsai JJ, Han SH. Molecular cloning and expression of a Penicillium citrinum allergen with sequence homology and antigenic crossreactivity to a hsp 70 human heat shock protein. Clin Exp Allergy. 1997;27:682–90.
Shokouhi Shoormasti R, Fazlollahi MR, Kazemnejad A, Tayebi B, Nadali F, Sharif Shoushtari M, et al. IgE Sensitization to Inhalant Allergens and Its Association with Allergic Diseases in Adults. Iran J Allergy Asthma Immunol. 2018;17:123–33.
Wang X, Xu C, Ji J, Cai Y, Shu Y, Chao Y, et al. IL-4/IL-13 upregulates Sonic hedgehog expression to induce allergic airway epithelial remodeling. Am J Physiol Lung Cell Mol Physiol. 2020;318:L888–99.
Bertorelli G, Bocchino V, Zhuo X, Chetta A, Del Donno M, Foresi A, et al. Heat shock protein 70 upregulation is related to HLA-DR expression in bronchial asthma. Effects of inhaled glucocorticoids. Clin Exp Allergy. 1998;28:551–60.
Yombo DJK, Mentink-Kane MM, Wilson MS, Wynn TA, Madala SK. Heat shock protein 70 is a positive regulator of airway inflammation and goblet cell hyperplasia in a mouse model of allergic airway inflammation. J Biol Chem. 2019;294:15082–94.
Acknowledgement
We would like to thank Hangzhou Hibio Technology Co. Ltd. for providing us with the SPF animal room and flow cytometer for this study.
Funding
This study was supported by National Natural Science Foundation of China, Grant Number 31971562.
Author information
Authors and Affiliations
Contributions
Wei Guo and Yilong Xi conceived the idea and wrote the manuscript. Xiaodong Zhan depicted figures and analyzed data. Feng Jiang contributed for revision. Yilong Xi contributed for overall editing and supervision. All authors approved its submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in strict accordance with the proposals of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and the mouse in this study were reviewed and approved by the Institutional Animal Care and Use Committee of Hangzhou Hibio Technology Co. Ltd. (IACUC protocol number: HBFM3.68-2015). The pollen of P. deltoides used in this study were conducted in strict accordance with the Convention on Biological Diversity and the Convention on the Trade in Endangered Species of Wild Fauna and Flora.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 2: Table S1.
Characteristics of potential allergens in HSP70 family.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guo, W., Zhan, X., Jiang, F. et al. Analysis of allergen components and identification of bioactivity of HSP70 in pollen of Populus deltoides. Proteome Sci 19, 10 (2021). https://doi.org/10.1186/s12953-021-00178-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12953-021-00178-8