Abstract
Mitochondria are crucial organelles responsible for energy generation in eukaryotic cells. Oxidative stress, calcium disorders, and mitochondrial DNA abnormalities can all cause mitochondrial dysfunction. It is now well documented that mitochondrial dysfunction significantly contributes to the pathogenesis of numerous illnesses. Hence, it is vital to investigate innovative treatment methods targeting mitochondrial dysfunction. Extracellular vesicles (EVs) are cell-derived nanovesicles that serve as intercellular messengers and are classified into small EVs (sEVs, < 200 nm) and large EVs (lEVs, > 200 nm) based on their sizes. It is worth noting that certain subtypes of EVs are rich in mitochondrial components (even structurally intact mitochondria) and possess the ability to transfer them or other contents including proteins and nucleic acids to recipient cells to modulate their mitochondrial function. Specifically, EVs can modulate target cell mitochondrial homeostasis as well as mitochondria-controlled apoptosis and ROS generation by delivering relevant substances. In addition, the artificial modification of EVs as delivery carriers for therapeutic goods targeting mitochondria is also a current research hotspot. In this article, we will focus on the ability of EVs to modulate the mitochondrial function of target cells, aiming to offer novel perspectives on therapeutic approaches for diverse conditions linked to mitochondrial dysfunction.
Graphical Abstract

Similar content being viewed by others
Introduction
Mitochondria are ubiquitous in nearly all eukaryotic cells and serve as indispensable organelles for maintaining cellular energy metabolism. They fulfill a vital role in cellular processes by serving as the primary source of ATP synthesis, controlling the creation of reactive oxygen species (ROS), facilitating intracellular Ca2+ cycling, and participating in apoptosis [1]. The occurrence of even a minor disruption in any of the mitochondrial membrane, respiratory chain, enzyme activity, or mitochondrial DNA (mtDNA) can result in the disruption of cellular processes and subsequent mitochondrial dysfunction, which can have direct or indirect implications on the overall normal function of the cell [2]. Therefore, therapeutic modulation by targeting mitochondria could lead to better management of diseases involving mitochondrial dysfunction, such as neurodegenerative diseases, cardiovascular diseases (CVD), and cancer [3]. Currently, mitochondrially targeted antioxidants [4], mitochondrial transplantation [5], and mitochondrial fission inhibitor Mdivi-1 [6] have made progress in experimental and clinical studies. Nevertheless, considering the drawbacks of these medications, such as poor efficacy and weak targeting, there is a need to find more effective strategies for mitochondria-targeted therapy [7].
Extracellular vesicles (EVs) are nanoscale vesicles that cells release into the extracellular space, and these vesicles act as communication vehicles by carrying proteins, lipids, and nucleic acids for intercellular transfer [8]. Under physiological conditions, EVs are crucial for tissue growth, homeostasis maintenance, and tissue repair [9, 10]. More importantly, parental cells and the surrounding environment have a significant impact on the cargo and secretion of EVs. In a diseased state, they obtain pathological content through cellular processes closely related to the pathogenesis of the disease, thus becoming contributors to many diseases. For example, EVs are linked to the spread of neurotoxic proteins in Alzheimer’s disease. Recent investigation has indicated that EVs are also enriched in mitochondria or mitochondrial components [11]. Under pathological conditions, the mitochondrial content of EVs released by donor cells into the circulatory system is altered, making EVs an important target for liquid biopsy [12]. On the other hand, healthy cells-derived EVs can rescue impaired mitochondrial function in recipient cells by transporting functional mitochondria (mitochondrial components) or other contents. By transferring mitochondria, some research showed that EVs enhanced heart function following myocardial infarction [13] or lessened hepatic ischemia-reperfusion (I/R) injury [14]. In addition, another focus of EV research is to utilize them as carriers for delivering mitochondria-targeted therapeutic agents, such as mitochondria-targeted photosensitizers and sonosensitizers [15, 16]. These findings suggest that EVs may become a new and effective treatment approach for mitochondrial dysfunction related diseases. However, some key issues remain elusive, such as the detailed mechanism of integration between mitochondria (mitochondrial components) in EVs and the mitochondrial network in target cells and how different goods in EVs affect the mitochondrial function of target cells, which require further exploration.
In this review, we elucidate the significance of mitochondrial dysfunction in the occurrence and development of various diseases, as well as the evidence for the presence of mitochondrial components in EVs. We also discussed the potential utility of EVs as diagnostic indicators and therapeutic interventions for mitochondrial dysfunction related diseases, providing a theoretical basis for their clinical translation.
Mitochondrial dysfunction
Mitochondrial structure
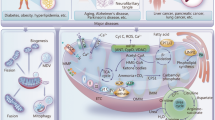
Mitochondria are encapsulated by two membranes and are divided into four distinct regions: the outer mitochondrial membrane (OMM), the intermembrane space (IMS), the inner mitochondrial membrane (IMM), and the mitochondrial matrix. The OMM contains pore proteins involved in material transportation and acts as a communication platform between mitochondria and other organelles [17]. The IMS contains cytochrome C (Cyt C) and apoptosis-inducing factor (AIF), which participate in apoptosis [18]. The IMM is a highly complex region that houses the electronic transport chain (ETC), ATP synthase, and transport proteins [19]. The IMM folds inward to form cristae, increasing the surface area of the IMM and thus maximizing the function of mitochondria in synthesizing bioenergy [20]. Within the mitochondrial matrix, the enzymes connected to the tricarboxylic acid (TCA) cycle and fatty acid oxidation (FAO), as well as mtDNA, are present (Fig. 1).
Mitochondrial structure and function (by Figdraw). Mitochondria are composed of four functional regions: OMM, IMS, IMM, and mitochondrial matrix. Mitochondria are essential for multiple cellular processes, including ATP synthesis; protein, lipid, and nucleotide metabolism; calcium homeostasis maintenance; mitochondrial dynamics; iron-sulfur clusters and heme synthesis; mitochondrial autophagy; apoptosis; formation of mitochondria-associated membranes (MAMs) with endoplasmic reticulum and lysosomes. OMM, outer mitochondrial membrane; IMS, intermembrane space; IMM, inner mitochondrial membrane; MFN1, mitofusin 1; MFN2, mitofusin 2; OPA1, optic atrophy 1; DRP1, dynamin-related protein 1; FIS1, fission 1; MFF, mitochondrial fission factor; MID49, mitochondrial dynamics proteins of 49 kDa; MID51, mitochondrial dynamics proteins of 51 kDa; PINK1, PTEN-induced kinase 1; Ub, ubiquitin; P, phosphorylation; MCU, mitochondrial calcium transporter; NCLX, mitochondrial Na+/Ca2+ exchanger; APAF-1, apoptotic peptidase activating factor 1; mPTP, mitochondrial permeability transition pore; Cyt C, Cytochrome C; AAA, ATP-dependent proteases of the inner membrane; mtDNA, mitochondrial DNA; TCA, tricarboxylic acid; IP3R, inositol 1,4,5-trisphosphate receptor; GRP75, glucose-regulated protein 75; VDAC, voltage-dependent anion-selective channel; IP3R2, type 2 inositol 1,4,5-trisphosphate receptors; FUNDC1, FUN14 domain containing 1; BAP31, B cell receptor associated protein 31; TBC1D15, TBC domain family member 15; FAD, flavin adenine dinucleotide; FADH2, dihydroflavine adenine dinucleotide; NAD+, nicotinamide adenine dinucleotide; NADH, dihydronicotinamide adenine dinucleotide
Mitochondrial function
ATP synthesis
Known as the powerhouse, the principal role of mitochondria is to generate ATP to fulfill the energy requirements of cells for survival. This process involves the ETC, which oxidizes dihydronicotinamide adenine dinucleotide (NADH) and dihydroflavine adenine dinucleotide (FADH2) produced by the TCA cycle and uses the energy thus gained to pump protons from the mitochondrial matrix into the IMS, thereby creating a proton gradient. Subsequently, as protons move back across the gradient into the matrix, the enzyme ATP synthase is activated, catalyzing the synthesis of ATP [21].
Mediating apoptosis
The process of active cell death called apoptosis, which is regulated by particular genes, is crucial for embryonic growth and preserving internal homeostasis [22]. The pathways of apoptosis include intrinsic (or mitochondrial), extrinsic (or death receptor), and intrinsic endoplasmic reticulum pathways [23]. Several cellular stressors, including hypoxia, growth factor deprivation, and damage to DNA, can trigger the mitochondrial pathway of apoptosis. Under stress stimulation, the pro-apoptotic proteins Bax and Bak form oligomers embedded in the OMM, inducing mitochondrial outer membrane permeabilization (MOMP). This process facilitates the release of pro-apoptotic substances into the cytoplasm from the IMS, such as Cyt C [24]. Once in the cytoplasm, Cyt C binds to apoptotic peptidase activating factor 1 and procaspase-9 to form an apoptosome, initiating the caspase cascade reaction and leading to apoptosis [25].
Calcium homeostasis
Ca2+ is an intracellular second messenger, participating in many physiological processes, such as muscle contraction and cellular signal transduction [26]. As an important organelle for intracellular calcium regulation, mitochondria can take up and release Ca2+. The passage of Ca2+ through the IMM is mediated by the mitochondrial calcium transporter, which functions as a highly selective ion channel [27]. There are three ways that Ca2+ can leave the mitochondrial matrix: the mitochondrial permeability transition pore (mPTP), the mitochondrial Na+/Ca2+ exchanger, and the mitochondrial H+/Ca2+ exchanger [28].
ROS generation
Mitochondria are the main source of intracellular ROS. These chemicals result from an incomplete reduction of oxygen during the electron transfer cascade of oxidative phosphorylation (OXPHOS) [29]. Under physiological circumstances, ROS are widely involved in cell signal transduction and life processes. For example, ROS are necessary for skeletal muscle contraction and ovarian cycle regulation [30, 31]. Appropriate concentrations of ROS drive cyclin-dependent kinase 2 phosphorylation to promote cell proliferation [32]. However, high levels of ROS damage cellular lipids, proteins, and nucleic acids, which can ultimately result in apoptotic or necroptotic cell death [33].
Other functions
Apart from the primary functions mentioned above, mitochondria also participate in the metabolism of amino acids, lipids, and nucleic acids, the biosynthesis of heme and iron-sulfur (Fe/S) clusters, and the import and processing of precursor proteins synthesized on cytoplasmic ribosomes [34]. Additionally, the OMM can come into physical contact with other cellular organelles, forming mitochondria-associated membranes [35]. Mitochondria-endoplasmic reticulum contacts provide a platform for various cellular functions, including calcium homeostasis maintenance, autophagy, and lipid metabolism [36]. Similarly, mitochondria-lysosome contacts modulate mitochondrial and lysosomal dynamics and calcium signaling [37, 38].
The mechanism of mitochondrial dysfunction
Oxidative stress
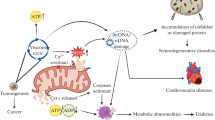
The term “oxidative stress” implies an imbalance between oxidation and anti-oxidation caused by excessive ROS formation that exceeds the body’s ability to scavenge them. Overexposure to ROS causes mPTP to open, which in turn drives mitochondria to enlarge, rupture, and release Cyt C, resulting in mitochondrial malfunction and apoptosis [39]. Elevated ROS also directly harm mtDNA, and the buildup of compromised mtDNA can cause the destruction of mitochondria [40]. Furthermore, ROS induce Ca2+ into mitochondria, disrupting calcium homeostasis, which is another important contributing factor to mitochondrial dysfunction (Fig. 2) [41].
The mechanisms of mitochondrial dysfunction (by Figdraw). Mitochondrial dysfunction can result from a range of mechanisms, primarily encompassing oxidative stress, mtDNA mutation, disturbances in mitochondrial dynamics, defects in autophagy, impaired mitochondrial biogenesis, reduced mitochondrial respiratory chain enzyme activity, calcium overload, and impaired mitochondrial protein homeostasis. ROS, reactive oxygen species; AMPK, AMP-activated protein kinase; PGC-1α, peroxisome proliferator-activated receptor-gamma coactivator-1α; NRF1/2, nuclear respiratory factor 1/2; TFAM, mitochondrial transcription factor A; mtDNA, mitochondrial DNA; LONP, Lon protease; ER, endoplasmic reticulum
mtDNA mutation
Mitochondrial dysfunction also occurs when mtDNA mutations accumulate to a certain threshold [42]. Mitochondria have their own DNA and can independently replicate, transcribe, and translate some of the mitochondrial proteins. The proteins encoded are essential for participating in electron transfer and OXPHOS. Due to its naked nature, absence of histone protection, and direct exposure to high ROS, mtDNA is more prone to mutations than nuclear DNA (nDNA) [43]. The presence of mtDNA deletion mutations impedes OXPHOS, diminishes ATP synthesis, and increases oxygen radical production, all of which impair mitochondrial function [44].
Impaired mitochondrial quality control
Impaired mitochondrial dynamics
Mitochondrial dynamics is the process by which mitochondria continually fuse and fission to preserve the quantity and quality of mitochondria [45]. Mitochondrial fusion promotes the interchange of mtDNA, proteins, and lipids within mitochondria. The fusion of normal and damaged mitochondria helps lessen cellular stress and maintain mitochondrial integrity [46]. Mitochondrial fission generates new mitochondria and isolates damaged mitochondria for degradation through mitophagy [47]. Mitofusin 1 and mitofusin 2 (MFN2) mediate OMM fusion, while IMM fusion is mediated by optic atrophy 1. The primary proteins involved in fission are dynamin-related protein 1 (Drp1) and fission 1. Abnormal fission and fusion lead to excessive mitochondrial fragmentation and elongation, respectively, which upset the dynamic equilibrium of the mitochondria and impair their normal physiological processes [48].
Impaired mitochondrial biogenesis
Peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α) serves as an important regulator of mitochondrial biogenesis and has the capacity to stimulate the expression of genes related to FAO and OXPHOS by activating peroxisome proliferator-activated receptors and nuclear respiratory factor 1/2 [49]. AMP-activated protein kinase (AMPK) plays a pivotal role in upregulating the expression of PGC-1α, thereby facilitating mitochondrial biogenesis and energy production [50]. Decreased expression of PGC-1α leads to a decline in biogenesis and ATP synthesis, causing mitochondrial dysfunction [51].
Impaired mitophagy
Mitophagy, a crucial process for the control of mitochondrial quality, selectively eliminates excess or malfunctioning mitochondria to preserve intracellular and mitochondrial homeostasis [52]. The predominant mechanism for mitophagy is the PINK1/Parkin pathway. When mitochondria are under stress, the entry of PINK into the IMM is hindered, leading to the accumulation of PINK1 at the OMM and the activation and recruitment of Parkin to damaged mitochondria [53]. Activated Parkin ubiquitinates OMM proteins, which are subsequently recognized by autophagy receptors such as p62, and then degrades damaged mitochondria by binding to LC3 to form autophagosomes [54]. The buildup of impaired mitochondria arises from defective mitochondrial autophagy, triggering the generation of ROS and ultimately culminating in cell death.
Mitochondrial dysfunction related diseases
Neurodegenerative diseases
Alzheimer’s disease (AD)
The accumulation of Amyloid β (Aβ) and hyperphosphorylated tau proteins has traditionally been considered the main causes of AD. However, recent findings indicate that mitochondrial dysfunction represents an early stage in the development of AD and could potentially contribute to or exacerbate its progression [55]. Oxidative stress is a key contributor to the development and progression of AD. ROS have the ability to increase the generation of Aβ, thus intensifying the level of oxidative stress within the mitochondria and creating a detrimental feedback loop [56]. In the pathological conditions of AD, mitochondrial ROS (mtROS) and Ca2+ are abnormally elevated, promoting the opening of mPTP. Simultaneous inhibition of mitochondrial Ca2+ overload and mPTP opening effectively alleviated AD neuropathology and rescued cognitive deficits [57]. In addition, the equilibrium between fusion and fission is disrupted under the pathological circumstances of AD. Xie et al. [58] discovered that decreased CEND1 led to the overexpression of Drp1, which caused aberrant mitochondrial fission and accelerated the advancement of AD. Overexpression of CEND1 or the use of Mdivi-1 restored mitochondrial function and improved cognitive impairment.
Parkinson’s disease (PD)
In the substantia nigra of individuals with PD, there was a notable reduction in the activity of mitochondrial complex I and an elevated presence of mtDNA deletion. These findings suggested that mitochondrial dysfunction was implicated in the pathogenesis of PD [59]. Gonzalez-Rodriguez et al. [60] discovered that targeted suppression of the Ndufs2 gene, responsible for encoding the catalytic core subunit of mitochondrial complex I, led to the emergence of motor impairments linked to PD. In addition, mitochondrial dysfunction resulting from mutations in Parkin and PINK1 genes has been strongly linked to PD. In Parkin gene deletion mice, elevated levels of PARIS were observed, along with a decrease in its primary inhibitory factor, PGC-1α [61]. The downregulation of PGC-1α has been connected to changes in mitochondrial dynamics, an increase in ROS production, and a decrease in OXPHOS. The absence of Parkin in PD also destabilized the mitochondria-lysosome contact, and deficiencies of various amino acids in neuronal mitochondria contributed to mitochondrial dysfunction, which exacerbated the pathology of PD [62]. In dopaminergic neurons with PINKI mutations, the ER-mitochondrial contact site was enhanced, and mitochondrial Ca2+ levels were raised, causing mitochondrial enlargement and neuronal death [63].
Cardiovascular disease
Myocardial infarction (MI)
MI is myocardial ischemic-hypoxic necrosis brought on by a reduction in coronary artery blood flow. In the ischemic and hypoxic environments, mitochondrial membrane potential decreases, mitochondrial Ca2+ overload increases, and mtROS are produced excessively, leading to mitochondrial damage, which in turn enlarges the area of MI. Decreased Ndufs1 expression, along with mitochondrial impairment and disorganization of cristae, were evident in the cardiac tissue of the mice following MI [64]. Overexpression of Ndufs1 could restore mitochondrial respiratory capacity and reduce levels of mtROS. In addition, the mitigation of excess ROS has the potential to counteract mitochondrial damage in cases of MI. Zheng et al. [65] developed a ROS-responsive liposomal composite hydrogel that contained elamipretide (SS-31) that directly targeted mitochondria in damaged myocardium, inhibited ROS production, and improved mitochondrial dysfunction, thereby enhancing cardiac performance.
Myocardial ischemia-reperfusion injury (MI/R)
MI/R refers to the phenomenon that the injury aggravates after a certain period of ischemic myocardial tissue restores blood supply. During the process of I/R, the opening of mPTP occurs continuously, causing mitochondrial swelling and OMM rupture, as well as the release of Cyt C and AIF, which induce myocardial cell apoptosis [66]. Li et al. [67] found that S100a8/a9, a key initiator molecule of MI/R, inhibited mitochondrial complex I, resulting in insufficient ATP synthesis and ultimately leading to cardiomyocyte death. Another study demonstrated that during MI/R, MMP2 was activated to catalyze the hydrolysis of MFN2, leading to compromised mitochondrial respiration and subsequent induction of cardiac inflammation [68]. Increased mitochondrial fission is a key contributor to MI/R injury, and the use of Mdivi-1 has been shown to effectively attenuate cardiomyocyte apoptosis, ameliorate myocardial mitochondrial dysfunction, and enhance cardiac function [69].
Cancer
Tumor development is a multifaceted process in which multiple signaling networks interact with and modulate each other. Various forms of mitochondrial dysfunction, such as TCA cycle enzyme defects, mtDNA mutations, and mitochondrial dynamics changes, have been observed across a broad range of cancers. Dysregulation of TCA cycle metabolites caused by deficiency of isocitrate dehydrogenase, succinate dehydrogenase (SDH), and fumarate hydratase (FH) drives the initiation and progression of cancer [70]. SDH mutations are commonly seen in pheochromocytomas, gastrointestinal stromal tumors, and pituitary tumors [71]. A recent study has found a significant link between the lowering of SDHA/B and the advancement and poor prognosis of hepatocellular carcinoma [72]. Mutations in the FH gene can cause hereditary leiomyomatosis and renal cell carcinoma [73]. The excessive fumarate produced by FH mutations induced epigenetic changes in miR200 with anti-tumor metastasis effects, promoting epithelial-mesenchymal transformation (EMT) and the occurrence and spread of cancer [74]. Furthermore, the accumulation of fumarate inhibited the biological activity of PTEN through cysteine succinylation, thereby promoting the malignant progression of type 2 papillary renal cell carcinoma [75]. Additionally, there is a substantial functional association between mtDNA mutations and malignancies, as demonstrated by the confirmation of this relationship in eosinophilic tumors and prostate cancer [76]. Smith et al. [77] found that somatic mtDNA mutations resulted in OXPHOS deficiency, thus promoting metabolic remodeling, which in turn sped up the growth of colorectal tumors.
Lung diseases
Chronic obstructive pulmonary disease (COPD)
COPD is a chronic lung disease typified by persistent airstream limitation, often linked to smoking and genetic determinants. The connection between COPD and mitochondrial dysfunction has been supported by numerous investigations. In a study involving bronchial epithelial cells exposed to aqueous cigarette smoke extract, it was observed that mitochondria were swollen, severely disrupted cristae, mitochondrial depolarized, and increased mtROS, indicating that cigarette smoke-induced damage to mitochondrial structure and function [78]. Furthermore, decreased levels of Parkin in COPD patients confirmed the involvement of impaired mitophagy in the progression of COPD [79]. The relationship between the mitochondrial-iron axis and COPD also has significant importance. In an inducible model of COPD, upregulation of IRP2 caused increased mitochondrial iron loading, which in turn resulted in impaired mitochondrial function and consequent death of epithelial cells [80].
Acute lung injury (ALI)
ALI is acute hypoxic respiratory failure syndrome following severe infection, trauma, or shock. Cen et al. [81] observed that stimulation of lung microvascular endothelial cells with lipopolysaccharides (LPS) resulted in ROS generation and apoptosis, accompanied by mitochondrial swelling, reduced matrix density, cristae loss, and a decrease in mitochondrial membrane potential, suggesting impaired mitochondrial function during ALI. Treatment with the antioxidant mitoQ was found to reverse LPS-induced mitochondrial dysfunction by eliminating excessive ROS and reducing the mitochondrial apoptotic pathway. In another model of LPS-induced ALI, it was reported that alveolar epithelial cells (AECs) had a significantly higher citrate content in response to LPS stimulation. The accumulated citrate drove mitochondrial fission through the recruitment of Drp1 by FUN14 domain containing 1 (FUNDC1), leading to excessive autophagy and causing necroptosis, which ultimately initiated and promoted the development of ALI [82].
Kidney diseases
Chronic kidney disease (CKD)
CKD is characterized by the gradual and ongoing destruction of the structure and function of the kidneys, which can be caused by various factors including diabetes, hypertension, glomerulonephritis, and hereditary or cystic diseases, with a duration of more than three months. Coughlan et al. [83] discovered that aberrant mitochondrial bioenergetics occurred before the onset of albuminuria and renal histologic changes in the development of diabetes to diabetic kidney disease (DKD) in rats, thereby providing evidence in favor of the theory that mitochondrial damage is an important factor in DKD. In individuals with CKD and in mouse models, there was a decrease in the expression of LONP1, a protein responsible for preserving the stability of mitochondrial proteins. This downregulation resulted in the impairment of mitochondrial function, thereby exacerbating the progression of CKD [84].
Acute kidney injury (AKI)
AKI is a clinical condition brought on by an abrupt loss of renal function due to I/R, sepsis, or nephrotoxic substances [85]. Experimental investigations revealed a breakdown between the oxidative and antioxidant systems in the I/R-induced AKI model, where inadequate ROS scavenging by renal tubular mitochondria led to ROS accumulation [86]. An additional study indicated that mtROS contributed to mtDNA deletion and heightened cytokine release by reducing mitochondrial transcription factor A (TFAM) abundance, which consequently caused mitochondrial dysfunction and inflammation [87]. Additionally, mtDNA leakage was observed in the AKI model caused by cisplatin [88], and clinical studies showed a correlation between the severity and prognosis of the illness in AKI patients with elevated circulating mtDNA levels [89].
Liver disease
Alcohol-associated liver disease (ALD)
ALD is a persistent liver condition resulting from prolonged alcohol consumption. In ethanol-fed mice, the expression of Parkin and p62 was reduced, indicating compromised mitophagy [90]. AMPK could minimize the damage induced by alcohol by increasing levels of mitophagy, which might be a promising strategy for treating ALD [91]. In the context of ALD, an increase in ROS was observed, concomitant with a decrease in the levels of superoxidase dismutase (SOD) and glutathione (GSH) [92]. The synthesis of ROS was also found to activate receptor-interacting protein kinase 1, causing necrotic apoptosis [93]. Moreover, mtDNA release into the cytoplasm and activation of the cGAS-STING signaling pathway were triggered by alcohol-induced liver injury, and these events ultimately resulted in liver inflammation [94].
Nonalcoholic fatty liver disease (NAFLD)
Triacylglycerol (TG) buildup in the liver is the hallmark of NAFLD, which affects approximately one-quarter of the world’s population [95]. One important factor in the onset and course of NAFLD has been identified as impaired mitochondrial β-oxidation, leading to dramatically raised ROS levels [96]. Furthermore, studies showed that a marked decrease in proliferator-activated receptor alpha (PPARα) was present in NAFLD patients’ livers, together with impaired mitochondrial oxidative capacity and hepatic steatosis [97]. The overexpression of PPARα has been found to promote mitochondrial biogenesis while reducing TG accumulation. Abnormal mitochondrial fission also encouraged the growth of NAFLD, and targeted inhibition of hepatic Drp1 expression shielded mice from obesity induced by a high-fat diet (HFD) (Fig. 3) [98].
The roles of mitochondrial dysfunction in liver diseases (by Figdraw). In alcohol-associated liver disease and nonalcoholic fatty liver disease, mitochondrial dysfunction, including oxidative stress, mtDNA leakage, reduced FAO, and altered mitochondrial dynamics, exacerbates the progression of liver fibrosis and non-alcoholic steatohepatitis. ROS, reactive oxygen species; SOD, superoxidase dismutase; GSH, glutathione; RIPK1, receptor-interacting protein kinase 1; RIPK3, receptor-interacting protein kinase 3; MLKL, mixed lineage kinase domain-like protein; NLPR3, NOD-like receptor thermal protein domain associated protein 3; MOMP, mitochondrial outer membrane permeabilization; cGAS, cyclic GMP-AMP synthase; STING, stimulator of interferon genes; DRP1, dynamin-related protein 1; MFN1, mitofusin 1; MFN2, mitofusin 2; OPA1, optic atrophy 1; PGC-1α, peroxisome proliferator-activated receptor-gamma coactivator-1α; NRF1/2, nuclear respiratory factor 1/2; PPARα, peroxisome proliferator-activated receptor alpha
Summary of EV biology
Classification and biogenesis of EVs
EVs are lipid bilayer-encapsulated nanoparticles released from cells and are highly heterogeneous [99]. According to the International Society for Extracellular Vesicles (ISEV) position MISEV2018 and MISEV2023, these vesicles can be further categorized into small EVs (sEVs, < 200 nm) and large EVs (lEVs, > 200 nm) based on their particle size [100, 101]. Whereas researchers have typically classified EVs into exosomes (30–150 nm) and microvesicles (MVs, 50–1000 nm) based on their biogenesis in previous studies [102,103,104]. Exosomes originate in the endosomal system and are formed by the fusion of multivesicular bodies (MVBs) with the cytoplasmic membrane to release contained intraluminal vesicles (ILVs) [105]. MVs are produced by budding directly from the plasma membrane [8]. In addition to exosomes and MVs, apoptotic bodies (50–5000 nm) were also one of the most extensively studied subtypes of EVs, formed by bubbling the plasma membrane and enveloping the cytoplasm, organelles (mitochondria), and nucleic acid fragments after cell apoptosis [106]. Moreover, mitochondrial-derived vesicles (MDVs, 70–150 nm) are a specific category of EVs that are generated following minor mitochondrial impairment, which are then released into the extracellular milieu as EVs subsequent to merging with MVBs [107]. The vesicles of mitochondrial origin were initially considered as a quality control mechanism to compensate for the insufficient maintenance of mitochondrial homeostasis when the mitochondrial autophagy system is impaired [108]. The biogenesis of MDVs is slightly complex. Several molecules are involved in the sprouting of damaged mitochondrial fragments to MDVs, including Parkinson’s disease-associated proteins PINK1, Parkin, and the vacuolar sorting protein 35 (Vps35) [109,110,111,112]. Mitochondrial membrane proteins are oxidized under mild oxidative stress. ROS and oxidative stress initiate local activation of PINK1 and Parkin, leading to the budding of oxidized membrane proteins into vesicles [109, 113]. The mutation of Vps35 impaired the formation of MDVs, which proved that Vps35 played an important role in the formation of MDVs [111]. In addition, Matheoud et al. [113] found that Rab9 and sorting nexin 9 (SNX9) need to be recruited for MDV biogenesis, but the specific mechanism remains to be explored. The destinations of MDV transport include lysosomes, peroxisomes, bacterial phagosomes, and EVs. The transport of MDV-containing oxidized protein to the lysosome is mainly affected by the PINK1/Parkin pathway [109, 110, 114, 115]. The transport of MDVs to the peroxisome is related to mitochondrial-anchored protein ligase (MAPL) [116]. In addition, the transport of MDVs to phagosomes containing bacteria can play an antibacterial defense role [117]. However, there are still many unclear aspects of MDVs that need further exploration (Fig. 4). In various literature, researchers have used different names to describe EVs based on specific biogenesis, diameter, or content. However, ISEV recommends using the umbrella term “extracellular vesicles” to encompass all types of membrane-derived vesicles and can be further divided into sEVs and lEVs based on their size [101]. Therefore, in this review, the term EV will be used primarily to refer to all nanoparticles unless a specific name is applied according to the context of the study.
Classification, biogenesis and uptake of EVs (by Figdraw). Exosomes are produced through the endosomal pathway, while vesicles and apoptotic bodies are directly generated by plasma membrane budding. Exosomes and MVs can be taken up by recipient cells through various pathways such as membrane fusion, ligand-receptor binding, and endocytosis, and apoptotic bodies can be phagocytosed by phagocytes. MDVs are mainly released into the extracellular space through the MVB-mediated pathway and the microvesicle pathway. MVB, multivesicular bodies; ILV, intraluminal vesicles. MDVs, mitochondrial-derived vesicles
Contents of EVs
EVs are rich in a variety of biologically active substances, such as proteins, nucleic acids, and lipids. Due to the different plasma membrane origins of the different subtypes of EVs, their protein expression is also different. lEVs express Cav-1, CK18, and GAPDH, whereas sEVs are characterized by biological proteins (Alix, TSG101), tetraspanins (CD9, CD63, and CD81), and heat-shock proteins (HSP70 and HSP90) [118]. In addition, many studies have shown that different mitochondrial contents are enriched in some EV subgroups, and sEVs contain mitochondrial components such as mtDNA, mtRNA, and mitochondrial protein, while lEVs contain structurally intact mitochondria [119]. By shotgun proteomics analysis of EVs derived from human brain endothelial cells (BECs), it was found that lEVs were rich in 89 kinds of mitochondrial proteins (such as SOD2, MRPS22, MRPL13, and ATP5A1), while sEVs contained only one kind of mitochondrial protein [120], indicating that the size and type of EVs may be closely related to the content of mitochondria. In addition, the complete mitochondrial genome was also detected in circulating EVs of patients with hormone therapy-resistant metastatic breast cancer [121]. Abundant data shows that EVs are rich in mitochondrial components, but due to the high heterogeneity of EVs and the limitations of separation technology, the specific relationship between the type and size of EVs and the content of mitochondria is still elusive (Fig. 5).
Components of EVs (by Figdraw). EVs have lipid bilayers, heterogeneous components, and highly expressed tetraspanins (CD9, CD81, and CD63). In addition, large amounts of DNA, RNA, enzymes, and other functional proteins are encapsulated. sEVs may contain mitochondrial components such as mtDNA, mtRNA, and mitochondrial protein, while lEVs may contain structurally intact mitochondria. HSP70, heat shock protein 70; MVB, multivesicular body; Alix, ALG-2-interacting protein X; TSG101, tumor susceptibility gene 101; EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; EVs, extracellular vesicles; sEVs, small extracellular vesicles; lEVs, large extracellular vesicles
Isolation and characterization of EVs
Based on the properties of EV size, density, and surface charge, various separation methods have been developed. Ultracentrifugation is one of the most commonly used methods to isolate EVs. However, this method requires a large amount of cell culture fluid and high-speed centrifugation equipment and is prone to introduce contamination. Other conventional separation methods include density gradient centrifugation, size exclusion chromatography, coprecipitation, and field flow fractionation. The advantages and disadvantages of these separation methods have been well-reviewed [122, 123]. In recent years, to improve separation efficiency and specificity, microfluidic, contactless sorting, immunoaffinity enrichment, and other new EV enrichment technologies have emerged. However, due to the overlap of different EV subtypes in size and biogenetic pathway and the presence of contamination of non-mitochondrial EV content after ultracentrifugation, the existing separation methods cannot meet the specific separation of EVs rich in mitochondrial components. In this context, D’Acunzo et al. [124] have developed a new method to isolate EVs from brain tissue based on iodixanol-based high-resolution density gradient centrifugation. Compared with sucrose gradient centrifugation, it can better separate mitochondrial component-enriched EVs and other sEVs. But this method still needs 0.22 μm filtration to further purify EVs, which may lead to the loss of lEVs containing the entire mitochondria. In addition, a study reported that chromatography on immobilized heparin could separate MDVs from free mitochondria, which could not be achieved by size exclusion chromatography [125]. Combining different separation methods may be a better option for separating EVs.
Conventional characterization methods for EVs include atomic force microscopy, electron microscopy, nanoparticle tracking analysis, and western blotting, and these methods have been well reviewed previously [126, 127]. In addition, flow cytometry has become one of the commonly used characterization methods for EVs due to its ability to characterize multiple parameters on a single particle in a high-throughput manner. However, conventional flow cytometry has a low detection limit, may exclude a large number of smaller EVs present from analysis, and has the potential to detect multiple EVs as a single event, making its applicability in the field of EV research remains controversial [128, 129]. To address the limitations of conventional flow cytometry in EV characterization, many improvements have been made, including replacing photodiodes with photomultiplier tubes on the light-scattering channels and adding reduced wide-angle forward scatter/medium-angle light scatter collection, which has facilitated the development of high-resolution flow cytometers with detection limits lower than those of conventional flow cytometers [130,131,132,133]. Currently, the use of high-resolution flow cytometry has become more and more common in EV research [134, 135]. Similarly, for mitochondrial components in EVs, RT-PCR and western blotting can be used to detect the presence of mtDNA, mtRNA, and mitochondrial proteins [136]. Through the observation of the transmission electron microscope, we can see that there are mitochondria with complete structure in EVs [13]. Integrity EVs can be labeled with calcein, and intact mitochondria can be labeled with MitoTracker. A double-positive event by flow cytometry detection of calcein and MitoTracker is considered to be EVs containing functional mitochondria [137]. In addition, Zhang et al. [135] stained respiring mitochondria in EVs using MitoTracker and examined the presence and activity of functional mitochondria in EVs of different sizes by high-resolution multicolor flow cytometry, and found that the percentage of EVs containing functional mitochondria and the relative activity of respiring mitochondria was highest in lEVs, and lower in sEVs. This result again demonstrates that the mitochondrial content and function within EVs correlate with the size and type of EVs. For mitochondrial function within EVs, Peruzzotti-Jametti et al. [138] assessed the function of mitochondria in the crude EV preparations by examining the activity of ETC in maintaining a mitochondrial transmembrane potential and respiration using a JC1 assay and high-resolution respirometry, respectively. However, further research is needed on more specific, effective, and convenient separation and detection techniques.
The early detection and treatment effects of EVs on mitochondrial dysfunction related diseases
Mitochondrial damage significantly contributes to the pathogenesis of many diseases. Damaged cells or tissues released damaged-mitochondria enriched-EVs can be used as biomarkers for diagnosing diseases. More importantly, EVs can not only directly transport mitochondria (or mitochondrial components) to target cells to restore mitochondrial function but also play a therapeutic role by modulating mitochondrial function in target cells through their unique internal components originating from parental cells. In addition, modifying EVs to load substances targeted for mitochondrial therapy may be one of the future development directions. These important findings motivate our research into EVs and their functional relevance to mitochondria in immune regulation and tissue damage repair and offer novel perspectives on the potential utility of EVs as a tool for the diagnosis and treatment of mitochondrial dysfunction related diseases (Fig. 6).
Clinical application potential of EVs (by Figdraw). (A) EVs are abundant in various bodily fluids, and their content levels reflect the type and activation status of their parent cells. This makes them valuable biomarkers for diagnosing mitochondrial dysfunction related diseases. Moreover, natural EVs contain a variety of bioactive substances that can enter receptor cells through multiple pathways to regulate the mitochondrial function of target cells, making them promising therapeutic agents for mitochondrial dysfunction related diseases. Furthermore, EVs are also high-quality drug nanocarriers that can be engineered to deliver therapeutic drugs in a targeted manner. (B) Different preclinical models have been developed to evaluate the biology of endogenous or injected EVs in vivo. (C) Prospects for clinical applications of EVs
Neurological disorders
EVs as promising non-invasive biomarkers for the diagnosis of neurological disorders
Aging caused by mtDNA mutations and oxidative damage is a key risk factor for neurodegenerative diseases, and mitochondrial damage occurs earlier, which is causally related to the pathogenesis of neurodegenerative diseases [139]. Therefore, EVs rich in mitochondria or mitochondrial components are considered potential biomarkers for detecting and diagnosing neurodegenerative diseases. Yao et al. [140] discovered that ATP synthase and mitochondrial ETC complex I/III/IV were lower in EVs obtained from plasma neurons of AD patients than in normal. Kim et al. [141] found that mtRNA was significantly elevated in plasma EVs in individuals with mild cognitive impairment and AD. This may be because the toxic environment in the AD brain stimulates brain cells to secrete mtRNA-rich EVs across the blood-brain barrier (BBB) into the plasma. It is worth noting that some researchers have developed more novel isolation methods to obtain a specific subgroup of mitochondrial EVs called mitovesicles [124]. D’Acunzo et al. [142] obtained mitochondrial EVs from the extracellular matrix of the brain through high-resolution density gradient separation and demonstrated that brain-derived mitochondrial vesicles contained specific mitochondrial components, and their levels and contents may change in Down syndrome. Furthermore, EVs derived from plasma neurons in patients with multiple sclerosis have higher mitochondrial complex IV activity and lower mitochondrial complex V activity, which may be associated with more rapid whole-brain, brain substructure, and retinal atrophy [143]. These findings prove that mitochondrial content in EVs is strongly associated with neurodegenerative diseases and that its decrease or increase may reflect the extent of mitochondrial damage (Table 1).
The utilization of EVs from various sources for the therapeutic management of neurological disorders
Due to the selective penetration of BBB, according to statistics, only 2% of therapeutic compounds can pass through BBB and achieve their therapeutic goal [157]. Researchers are actively developing safer, more efficient, and more accurate drug delivery systems to promote better treatment for the brain. Compared with other drug delivery systems, EVs, as natural nanovesicles, have the significant advantages of optimal biocompatibility, biodegradability, and low immunogenicity. sEVs can effectively penetrate the physical barrier formed by the tight junction of BBB. In addition, the phospholipid bilayer structure of EVs can protect the goods in EVs from the influence of enzyme barrier in brain parenchyma. More importantly, EVs have been shown to play an important role in brain cell communication. Therefore, EVs derived from brain cells have become excellent brain-targeted cargo delivery carriers because of their natural brain biological effects and the rich parental cell surface proteins loaded during biogenesis. Receptor-mediated transcytosis is the most widely recognized effective way EVs penetrate the BBB. EVs from brain cells attach to BBB cell receptors through surface proteins derived from parental cells. After endocytosis by BECs, they accumulate in endosomes and achieve transcellular trespassing through the formation of MVBs and exocytosis to the brain parenchyma [158]. Then EVs can interact with glial cells and neurons in brain parenchyma.
At present, the main focus of natural EV treatment is mitochondrial transfer. As an illustration, neural stem cell-derived EVs restored mitochondrial dynamics and cellular metabolism by transferring mitochondrial proteins and structurally intact functional mitochondria to the target cell, as well as inhibited the pro-inflammatory state of the cell, and these effects were co-determined by the mitochondrial activity and content of mitochondria released from the parental cell [138]. In addition, endothelial- and macrophage-derived EVs also carried polarized mitochondria, and because of the natural affinity for BECs, endothelial-derived EVs enabled a higher degree of mitochondrial transport, leading to an increase in ATP. Interestingly, it was the transfer of larger EV fractions, rather than the transfer of smaller EVs, that led to increased mitochondrial function [159]. Similarly, Dave et al. [119] found that while BECs-derived sEVs contained mitochondrial proteins, only lEVs contained intact mitochondria and that lEVs, but not sEVs, transferred loaded mitochondria to BECs, increased the relative ATP levels and mitochondrial function, and reduced the size of cerebral infarcts in mice. These results suggest that the sizes and types of EVs may be related to their mitochondrial content and function and that EVs carrying structurally intact mitochondria may have better therapeutic effects.
In addition to delivering mitochondria or mitochondrial components, EVs may also modulate mitochondrial function within target cells by delivering cellular contents originating from their parent cell specifics. For example, although the sEVs secreted by astrocytes in different regions of the nigrostriatal system all could protect the activity of mitochondrial complex I damaged by neurotoxin MPP+, only the sEVs secreted by astrocytes in the ventral midbrain could improve the ATP production of neurons damaged by MPP+, indicating that EVs from different sources may contain specific cellular contents to regulate mitochondrial function, but further research is needed to determine this conclusion [160]. Moreover, there have been multiple reports indicating that EVs derived from adipose stem cells possess the ability to specifically target mitochondrial dysfunction, restore proper mitochondrial function, and mitigate cell apoptosis regulated by mitochondria in neurodegenerative diseases such as amyotrophic lateral sclerosis and Huntington’s disease [161,162,163,164]. Through proteomics, it was found that several molecules in EVs may have the effect of counteracting neurodegenerative mechanisms, including SOD1, ribonuclease RNase 4, the insulin-like growth factors IGF1 and Akt, but the specific mechanism of their role is still unclear [163] (Table 2).
Cardiovascular diseases
The roles of EVs in the diagnosis of cardiovascular disease
Under physiological conditions, intercellular mitochondrial transfer in the cardiovascular system can maintain normal cardiac homeostasis. Under pathological conditions, damaged cells seek help by releasing EVs rich in damaged mitochondria. Therefore, detecting mitochondrial components in EVs may be an effective strategy for diagnosing CVD. An existing study showed that in the plasma EVs of patients with coronary artery disease, the mtRNA (MT-COI) level of patients with new cardiovascular events was lower, which proved that circulating EVs rich in mitochondrial components were a possible predictor of CVD [144]. The specific cargo characteristics and stability of EVs make them a valuable diagnostic tool that needs more research (Table 1).
Therapeutic application of EVs in cardiovascular disease
In ischemic cardiomyopathy and other pathological conditions, damaged cells can use exogenous functional mitochondria to save their mitochondrial network. EVs have great potential as a mitochondrial delivery system, which may contribute to the uptake of mitochondria by recipient cells and improve the vitality and stability of mitochondria [13]. In addition, EVs from different culture conditions and sources are rich in specific contents from parental cells, and these non-mitochondrial cargoes also play a beneficial role in the treatment of CVD. In the long run, EVs can also be modified to transport goods in a highly targeted manner to target mitochondria. Therefore, EVs have great potential as a cell-free strategy for the treatment of CVD. EV-mediated treatment of CVD has a variety of mechanisms, including the following points. (1) Improvement of mitochondrial biogenesis. Mitochondria-rich EVs from human-induced pluripotent stem cells (iPSCs)-derived cardiomyocytes (iCMs) (M-EVs), which could restore intracellular ATP production and improve the contractile properties of iCMs by transporting mitochondria to hypoxic damaged iCMs. In addition, non-mitochondrial cargo (mRNA promoting mitochondrial biosynthesis) in M-EVs could also enhance intracellular bioenergetics by activating mitochondrial biosynthesis [13]. (2) Enhancement of antioxidant capacity. Hypoxia-conditioned human mesenchymal stem cells (hMSCs)-derived EVs, which were abundant in Parkinson disease protein 7, exhibited a more potent inhibitory impact on myocardial hypertrophy compared to normoxia-conditioned hMSC-derived EVs. They could serve as antioxidants to alleviate mitochondrial dysfunction and excessive production of mtROS. The potential mechanism underlying the observed phenomenon involves the inhibition of proteasome subunit beta type 10 activity by Parkinson disease protein 7 through direct physical interactions to reduce the ubiquitination degradation of AT1R-related protein to inhibit the signaling pathway mediated by angiotensin II type 1 receptor leading to myocardial hypertrophy [165]. (3) Reduction of apoptosis. Hypoxic/serum-deprivation stimulated neonatal mice cardiomyocytes treated with EVs derived from bone marrow mesenchymal stem cells (BMSCs) overexpressing macrophage migration inhibitory factor (MIF) showed reduced mitochondrial fragmentation and cell apoptosis [166] (Table 2).
Cancers
EVs as attractive candidates for diagnosing of various types of cancers
Timely detection of cancer is of paramount importance to achieve favorable treatment outcomes in the ongoing fight against this ailment. The transfer of mitochondria (or mitochondrial components) mediated by EVs can occur in tumor cells or between tumor cells and other cell types, and this effect is involved in regulating tumor differentiation, tumor microenvironment, tumor drug resistance, and tumor invasion [121, 147, 167,168,169,170] (Fig. 7). Furthermore, EVs exhibit remarkable stability while present in various bodily fluids, therefore indicating their considerable promise as biomarkers for cancer detection. For instance, previous research has demonstrated a notable rise in the copy number of plasma EV mtDNA in individuals diagnosed with ovarian cancer [145]. The mtDNA levels within plasma EVs of patients with oral squamous cell carcinoma are higher than those of healthy subjects and positively correlate with circulating IFN-γ and PD-L1 levels, which can be used to assess the efficiency of clinical anti-PD-L1 therapy [147]. Similarly, patients with early pancreatic ductal adenocarcinoma (PDAC) exhibited a higher frequency of mtDNA mutations in serum EVs than healthy subjects, and these mutations were unique, demonstrating that EVs carrying mitochondrial cargoes can be used as a reliable tool for diagnosing PDAC [148]. Intriguingly, in addition to detecting changes in mtDNA in EVs, Rasuleva et al. [149] also developed a rapid method combining immunoprecipitation and qPCR quantification (EvIPqPCR) for the detection of mtDNA/nDNA ratios in serum EVs of tumor origin as a cell-specific indicator of mitochondrial enrichment characteristics, and elevated mtDNA/nDNA ratios can be used as a biomarker for pancreatic cancer. Moreover, Jang et al. [146] also discovered that melanoma tissue-derived EVs exhibited a higher abundance of mitochondrial membrane proteins when compared to EVs derived from non-melanoma sources. These studies suggest that EVs enriched with mitochondrial cargoes could be a complementary approach to cancer screening, but their clinical translational applications require further validation in large cohorts (Table 1).
The roles of tumor cell-derived EVs in tumor (by Figdraw). Tumor cell-derived EVs are loaded with a variety of substances, which are involved in the processes of tumor development, invasion, and metastasis through diverse mechanisms. mtDNA, mitochondrial DNA; DLL4, Delta-like 4; ECM, extracellular matrix; TGF-β, transforming growth factor beta; MMPs, matrix metalloproteinases; TME, tumor mircroenvirment; EMT, epithelial-mesenchymal transition; EpCAM, epithelial cell adhesion molecule
EVs target mitochondrial therapy for cancers
Dysfunction in cancer cell energy is a hallmark of cancer, and mitochondrial dysfunction is related to multiple aspects of cancer progression. Moreover, considering the significance of mitochondrial reprogramming in tumor survival by promoting metabolic adaptation and anti-apoptotic properties, the implementation of targeted mitochondrial therapy emerges as a potentially effective strategy for cancer treatment. EVs, as natural nanocarriers, can deliver molecules specifically targeting mitochondria. EVs originating from neoplastic cells have a substantial abundance of biological constituents, akin to those found in their corresponding parental cancer cells. EVs can be internalized by parental cells, hence facilitating the identification of the specific cell type from which they originate. This particular attribute provides significant advantages within the realm of molecular-targeted therapy. For instance, EVs originating from breast cancer cells exhibited the capacity to selectively deliver lipophilic triphenylphosphonium (TPP)-modified therapeutic recombinant P53 proteins (TPP/P53) to the mitochondria of breast cancer cells and induced death in both P53 mutant cancer cells and P53 wild-type cells through P53-mediated endogenous mechanisms, presenting a promising avenue for breast cancer treatment in the future [171]. Furthermore, other tactics involving EVs center on the reprogramming of mitochondria through the regulation of the Bcl-2 family, resulting in the induction of cellular apoptosis [172, 173].
Currently, drug resistance in cancer cells is also one of the main reasons for cancer treatment failure. It has been found that EVs can transport mitochondria with mutated DNA to sensitive cancer cells to make them chemoresistant, and blocking this process may be one of the strategies for cancer treatment [174]. Meanwhile, tumor cell-derived EVs, as naturally occurring nanoparticles that can bypass and evade the exocytosis system and can achieve tumor targeting without the need for surface modification, also have great potential for overcoming drug resistance and improving targeting. Tumor-derived sEVs loaded with pro-oxidants can bypass the efflux pump and deliver pro-oxidants to the cancer cell cytoplasm, depleting GSH to induce oxidative stress, inhibiting ATP production, and inducing mitochondrial dysfunction and thus apoptosis in cancer cells [175].
Photodynamic therapy and sonodynamic therapy have demonstrated significant advantages over traditional radiotherapy, and the use of nanotechnology in conjunction with them has led to the development of new photosensitizers and sonosensitizers, as well as improved efficacy in combination with other therapies. Mitochondria-targeted photosensitizers were transported into glioblastomas via brain endothelium-derived EVs, and light conditions triggered the production of cytotoxic ROS by the mitochondria-targeted photosensitizers to selectively kill infiltrating malignant brain tumors [176]. Similarly, Nguyen Cao et al. [16] recently developed bioreducible EVs of loaded mitochondrial targeted sonosensitizers and glycolysis inhibitors that exhibited GSH/US dual response drug release, which effectively damaged mitochondria and promoted cell apoptosis. Furthermore, Cao et al. [177] constructed dendritic cells (DCs)-derived sEVs (DEVs)-mimicking aggregation-induced emission (AIE) nanoparticles, which, in conjunction with photodynamic therapy, exhibited the potential to enhance the immune response against tumors by promoting mitochondrial targeted immunogenic cell death, highlighting the promising prospects of developing clinical nano-vaccines with anticancer properties. These studies suggest that using EVs as a cross-cutting natural nano-platform for cancer mitochondrial targeting is one of the major directions for future cancer therapy (Table 2).
Lung diseases
EVs may be potential biomarkers for diagnosing lung diseases
Streptococcus pneumoniae (Spn) infection may cause serious complications, including ALI and acute respiratory distress syndrome. It has been shown that treatment of A549 cells with pneumolysin (PLY), a major virulence factor of Spn, resulted in a decrease in the mitochondrial content of EVs released by the cells, which might be attributed to the inhibition of mtROS due to PLY stimulation, which interfered with the packaging of mitochondria within EVs [150]. This study suggests that mitochondrial content within EVs may be a potential biomarker of lung disease, but its clinical translational potential does need to be demonstrated by additional trials and innovative results (Table 1).
EVs treat lung disease by transferring mitochondria or mitochondrial components
Mesenchymal stem cell (MSC) therapy has shown a good therapeutic effect in lung injury disease models by secreting EVs to transfer mitochondria or mitochondrial components. On the one hand, the therapeutic effect of MSC-EVs is mainly through regulating the metabolic state and phenotype of macrophages. For example, MSC-EVs enhanced OXPHOS, promoted phagocytosis of macrophages, and inhibited the secretion of proinflammatory cytokines by transferring functional mitochondria to macrophages [178]. Similarly, adipose-derived MSCs (AdMSCs)-derived EVs transferred mitochondrial components (mtDNA) to alveolar macrophages, resulting in improved mitochondrial integrity and OXPHOS levels in macrophages [179]. In addition, the delivery of mitochondria by MSC-EVs in vitro could also improve the oxygen consumption rate in alveolar macrophages [180].
On the other hand, MSC-EVs regulate the metabolic state of cells in other lung tissues by transferring functional mitochondria. MSC-EVs could transport mitochondria to AECs to restore ATP production and LPS-induced alveolar leukocytosis and protein leakage [181]. In addition, Dutra Silva et al. [182] found that the mitochondria of MSC-EVs could be integrated into the mitochondrial network of recipient cells, thereby restoring normal levels of mitochondrial autophagy and mitochondrial biosynthesis and restoring significant damage to the integrity of the alveolar-capillary barrier. At present, relevant studies mainly focus on the role of MSC-EVs. Whether EVs from other cells, especially lung tissue cells, can also play a role in treating lung diseases by transferring mitochondria or mitochondrial components remains a big research space. EVs derived from homotypic healthy cells of injured lung tissue may have better-targeted therapeutic effects (Table 2).
Kidney injury
Opportunities and challenges of EVs in the diagnosis of kidney injury
The kidney, being a highly energy-consuming organ, is ranked second in mitochondrial mass and oxygen consumption, following only the heart [183]. The involvement of mitochondrial damage is of considerable importance in the pathogenesis of AKI and CKD. In the state of disease, EVs carrying damaged mitochondria are released from donor cells into the extracellular space and enter the circulatory system. Meanwhile, during the progression of DKD, aberrant mitochondrial bioenergetics occurred before proteinuria and renal histological changes. Therefore, the content of mitochondrial cargo in circulating EVs may be an early diagnostic marker of kidney disease, but there is no relevant research at present.
EVs play a therapeutic role by regulating mitochondrial homeostasis in multiple pathways
Previous studies have shown that EVs can play a therapeutic role in kidney injury by maintaining the quality of functional mitochondria in the receptor cells and restoring mitochondrial homeostasis, but the specific way in which EVs affect mitochondria has not been evaluated [184,185,186]. Recent studies have shown that healthy mitochondria can be packaged in EVs and transferred to recipient cells to play a therapeutic role. For example, renal scattered tubular-like cells-derived EVs carried mitochondria and transferred them to injured tubular epithelial cells (TECs) to inhibit Drp1 activity, thus partially restoring mitochondrial function and alleviating kidney injury [187]. MSC-EVs carried mtDNA and ETC proteins to restore TFAM signal transduction and mtDNA stability in damaged TECs [136]. In addition, EVs could also regulate the mitochondrial bioenergetics, biogenesis, mitochondrial autophagy, and kinetics of target cells through the non-mitochondrial cargo carried by EVs. BMSC-derived EVs directly targeted NLRP3 via miR-223-3p to inhibit inflammasome activation and enhanced mitochondrial autophagy to alleviate renal I/R injury [188]. Similarly, MSC-EVs played an anti-apoptotic role by inhibiting Drp1 activation and mitochondrial fission through miR-30 [189]. EVs are enriched with a variety of miRNAs that may be key mediators for rescuing mitochondria. Recently, EVs have attracted strong research interest as natural drug delivery systems. EVs loaded with IL-10 not only improved the stability of IL-10 but also effectively targeted kidney injury, inhibited mammalian target of rapamycin (mTOR) activity, and thus promoted mitochondrial autophagy to maintain mitochondrial health in TECs [190]. Therefore, an EV-based drug delivery system may be a strong candidate for the treatment of kidney injury in the future (Table 2).
Liver injury
EVs as a biomarker in liver injury
Liver biopsy is still the gold standard diagnostic tool for evaluating the staging of liver disease. The lack of non-invasive diagnostic methods is a major obstacle in the clinical management of liver disease. Circulating EVs carry information from parent cells or tissues and become a new target for liquid biopsy. Long-term excessive alcohol intake could promote the release of inflammatory mtDNA-enriched EVs from hepatocytes [151]. Similarly, hepatocytes exposed to alcohol released more EVs containing mitochondrial double-stranded RNA (mtdsRNA) [152]. The changes in mitochondrial content in EVs may reflect the severity of the disease, which provides a new way to predict the progress of liver disease (Table 1).
The application of EVs in liver injury
At present, the implementation of immunomodulatory strategies aimed at reducing neutrophil extracellular traps (NETs) is widely regarded as a primary therapeutic avenue for addressing hepatic I/R injury and may hold the potential to improve the prognosis of individuals undergoing liver transplantation [191]. MSC-EVs, as nanoparticles, can emulate the abilities of MSCs in modulating diverse immune cells [192]. Lu et al. [14] found that MSC-EVs could inhibit the formation of NETs in liver tissue and subsequently ameliorate I/R injury in the liver through transferring functional mitochondria to intrahepatic neutrophils and repairing their mitochondrial function. The utilization of EV-based therapy presents a compelling strategy for immunomodulation in the context of liver injury (Table 2).
Additional diseases
Osteoarthritis
Mitochondrial dysfunction is one of the main characteristics of osteoarthritis (OA). Proteomic analysis showed that, compared with the normal primary chondrocyte EVs, the decrease in the number and score of mitochondrial proteins in the EVs of chondrocytes after treatment with IL-1β indicated that this may be a potential direction for the detection of OA [154].
OA predominantly features wear in articular cartilage, synovial inflammation, and joint space constriction, all of which contribute to compromised joint mobility [193]. More and more evidence showed that EVs might play an important and complex role in the pathogenesis, diagnosis, and treatment of OA. Zheng et al. [154] found that primary chondrocyte EVs cultured in a normal environment restored mitochondrial dysfunction, thereby repairing damaged chondrocytes. This effect may be related to the rich mitochondrial proteins in EVs. In addition, BMSCs-derived EVs have also been reported to significantly ameliorate osteoarthritis, and larger EV fractions had a greater impact on enhancing the mitochondrial function of chondrocytes, potentially due to their ability to transport intact and healthy mitochondria [194]. The type and mitochondrial content of EVs may affect their therapeutic efficacy, and EVs carrying intact mitochondria have better therapeutic effects (Table 2).
Sepsis
Sepsis is a critical medical condition characterized by a systemic inflammatory response, posing a significant threat to the patient’s life. Despite ongoing research efforts, the development of a definitive and effective therapy for sepsis remains an unresolved challenge. In a model of sepsis, circulating neutrophils transmitted mitochondria-rich EVs, which could prevent the accumulation of endothelial ROS and alleviate endothelial dysfunction after LPS attack through superoxide dismutase 2 contained in mitochondria [195]. This research provides new directions for treating mitochondrial dysfunction in critical diseases such as sepsis (Table 2).
Obesity
There has been a significant increase in the occurrence of obesity and its related ailments, making it a serious global public health issue. Remarkable progress has recently been made by researchers in treating obesity by using brown adipose tissue transplants [196], but two primary challenges persist: the challenge of procuring donor tissue and the issue of immune rejection. Hence, it is crucial to explore alternative approaches for addressing obesity-related ailments that do not involve transplantation. Mitochondrial components were significantly enriched in brown adipose tissue-derived EVs and were effective in alleviating metabolic syndrome in obese mice, including hyperglycemia and hepatic lipid accumulation, at least in part, by transferring mitochondria-associated functional proteins to the liver and facilitating energy expenditure [197]. EVs have shown potential as a viable approach for addressing the challenges associated with obesity and its associated disorders (Table 2).
Conclusions and perspectives
Notably, EVs have been shown their involvement in the regulation of several mitochondrial dysfunction related diseases and have displayed considerable promise as both diagnostic and therapeutic agents for such conditions. EVs not only deliver mitochondria or mitochondrial constituents to the target cells to repair impaired mitochondrial function but also modulate mitochondrial function by transferring specific cellular contents that are representative of their parent cells. It is worth noting that the sizes and types of EVs may relate to their mitochondria contents (full unit or partial) and function. EVs carrying structurally intact mitochondria may have better therapeutic effects. Moreover, the transportation of mitochondria through EVs can take place in both physiologic and pathologic states. Therefore, blocking the transfer of damaged mitochondria carried by EVs in pathological states might be a promising therapeutic strategy for a wide range of diseases, but there are currently no specific inhibitors of EVs for in vivo use. Another way to inhibit the spread of EV-mediated diseases is to block the uptake of EVs by recipient cells, but the strategy remains to be thoroughly investigated due to the diversity and complexity of the mechanisms by which EVs are taken up. Furthermore, the therapeutic potential of EVs can be increased by loading therapeutic agents or surface modifications to enhance mitochondrial targeting. Considering the aforementioned benefits, EVs possess the potential to serve as effective nanotherapeutics for mitochondrial dysfunction related diseases.
Some studies on EVs are now in clinical trials, such as DEVs as an alternative to DC vaccines are in clinical phase I and phase II trials, but challenges remain in meeting clinical requirements. Due to the limitations of EV separation methods, most current research focuses on the functions of mixed EV subgroups. Therefore, it is difficult to confirm which EV subgroups are rich in mitochondrial components and the impact of their types on mitochondrial function. In addition, it is still unclear whether other mechanisms affect the mitochondrial content in EVs, in addition to the selective elimination of damaged mitochondria through the release of EVs through mitochondrial quality control. In fact, the limited comprehension of EVs, the absence of standardized quality management protocols, the variability in EVs derived from different tissue sources, donor cells, and preparation techniques, and the lack of consensus on optimal concentrations and intervention strategies for EVs in various diseases pose significant obstacles to the successful translation of EVs in research and practical applications. Moreover, despite the promise of EVs as biomarkers for various diseases, there are few relevant research reports at present, and further clinical evidence is needed to determine whether their changes are indeed related to disease progression and prognosis.
Despite the challenges, EVs have made useful achievements in several fields in recent years. The ongoing advancements in isolation, purification, and characterization techniques have led to a growing interest in investigating the therapeutic applications of EVs as drugs or drug delivery vehicles, and EVs hold great promise as potential diagnostic and therapeutic candidates for clinical mitochondrial dysfunction related diseases.
References
Li Y, Ma Y, Dang QY, Fan XR, Han CT, Xu SZ, et al. Assessment of mitochondrial dysfunction and implications in cardiovascular disorders. Life Sci. 2022;306:120834.
Wen R, Banik B, Pathak RK, Kumar A, Kolishetti N, Dhar S. Nanotechnology inspired tools for mitochondrial dysfunction related diseases. Adv Drug Deliv Rev. 2016;99(Pt A):52–69.
Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discovery. 2010;9(6):447–64.
Hou L, Zhang J, Liu Y, Fang H, Liao L, Wang Z, et al. MitoQ alleviates LPS-mediated acute lung injury through regulating Nrf2/Drp1 pathway. Free Radic Biol Med. 2021;165:219–28.
Hayashida K, Takegawa R, Shoaib M, Aoki T, Choudhary RC, Kuschner CE, et al. Mitochondrial transplantation therapy for ischemia reperfusion injury: a systematic review of animal and human studies. J Transl Med. 2021;19(1):214.
Deng Y, Li S, Chen Z, Wang W, Geng B, Cai J. Mdivi-1, a mitochondrial fission inhibitor, reduces angiotensin-II- induced hypertension by mediating VSMC phenotypic switch. Biomed Pharmacother. 2021;140:111689.
Huang T, Zhang T, Jiang X, Li A, Su Y, Bian Q, et al. Iron oxide nanoparticles augment the intercellular mitochondrial transfer-mediated therapy. Sci Adv. 2021;7(40):eabj0534.
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–28.
Zhu J, Wang S, Yang D, Xu W, Qian H. Extracellular vesicles: emerging roles, biomarkers and therapeutic strategies in fibrotic diseases. J Nanobiotechnol. 2023;21(1):164.
Wu P, Zhang B, Han X, Sun Y, Sun Z, Li L, et al. HucMSC exosome-delivered 14-3-3zeta alleviates ultraviolet radiation-induced photodamage via SIRT1 pathway modulation. Aging. 2021;13(8):11542–63.
Rai A, Fang H, Claridge B, Simpson RJ, Greening DW. Proteomic dissection of large extracellular vesicle surfaceome unravels interactive surface platform. J Extracell Vesicles. 2021;10(13):e12164.
Vikramdeo KS, Anand S, Khan MA, Khushman M, Heslin MJ, Singh S, et al. Detection of mitochondrial DNA mutations in circulating mitochondria-originated extracellular vesicles for potential diagnostic applications in pancreatic adenocarcinoma. Sci Rep. 2022;12(1):18455.
Ikeda G, Santoso MR, Tada Y, Li AM, Vaskova E, Jung JH, et al. Mitochondria-Rich Extracellular vesicles from autologous stem cell-derived cardiomyocytes restore energetics of ischemic myocardium. J Am Coll Cardiol. 2021;77(8):1073–88.
Lu T, Zhang J, Cai J, Xiao J, Sui X, Yuan X, et al. Extracellular vesicles derived from mesenchymal stromal cells as nanotherapeutics for liver ischaemia-reperfusion injury by transferring mitochondria to modulate the formation of neutrophil extracellular traps. Biomaterials. 2022;284:121486.
Nguyen Cao TG, Kang JH, Kang SJ, Truong Hoang Q, Kang HC, Rhee WJ, et al. Brain endothelial cell-derived extracellular vesicles with a mitochondria-targeting photosensitizer effectively treat glioblastoma by hijacking the blood–brain barrier. Acta Pharm Sin B. 2023;13(9):3834–48.
Nguyen Cao TG, Truong Hoang Q, Kang JH, Kang SJ, Ravichandran V, Rhee WJ, et al. Bioreducible exosomes encapsulating glycolysis inhibitors potentiate mitochondria-targeted sonodynamic cancer therapy via cancer-targeted drug release and cellular energy depletion. Biomaterials. 2023;301:122242.
Ellenrieder L, Rampelt H, Becker T. Connection of Protein Transport and Organelle Contact Sites in Mitochondria. J Mol Biol. 2017;429(14):2148–60.
Flores-Romero H, Hohorst L, John M, Albert MC, King LE, Beckmann L, et al. BCL-2-family protein tBID can act as a BAX-like effector of apoptosis. EMBO J. 2022;41(2):e108690.
Lee SY, Kang MG, Shin S, Kwak C, Kwon T, Seo JK, et al. Architecture Mapping of the inner mitochondrial membrane proteome by Chemical Tools in Live cells. J Am Chem Soc. 2017;139(10):3651–62.
van der Laan M, Horvath SE, Pfanner N. Mitochondrial contact site and cristae organizing system. Curr Opin Cell Biol. 2016;41:33–42.
Mnatsakanyan N, Jonas EA. The new role of F(1)F(o) ATP synthase in mitochondria-mediated neurodegeneration and neuroprotection. Exp Neurol. 2020;332:113400.
Tuzlak S, Kaufmann T, Villunger A. Interrogating the relevance of mitochondrial apoptosis for vertebrate development and postnatal tissue homeostasis. Genes Dev. 2016;30(19):2133–51.
Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30(1):87.
Bock FJ, Tait SWG. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. 2020;21(2):85–100.
Dorstyn L, Akey CW, Kumar S. New insights into apoptosome structure and function. Cell Death Differ. 2018;25(7):1194–208.
Giorgi C, Marchi S, Pinton P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol. 2018;19(11):713–30.
Fan M, Zhang J, Tsai CW, Orlando BJ, Rodriguez M, Xu Y, et al. Structure and mechanism of the mitochondrial ca(2+) uniporter holocomplex. Nature. 2020;582(7810):129–33.
Naumova N, Sachl R. Regulation of cell death by Mitochondrial Transport Systems of Calcium and Bcl-2 proteins. Membr (Basel). 2020;10(10).
Yang B, Chen Y, Shi J. Reactive oxygen species (ROS)-Based nanomedicine. Chem Rev. 2019;119(8):4881–985.
Ferreira LF, Laitano O. Regulation of NADPH oxidases in skeletal muscle. Free Radic Biol Med. 2016;98:18–28.
Liang J, Gao Y, Feng Z, Zhang B, Na Z, Li D. Reactive oxygen species and ovarian diseases: antioxidant strategies. Redox Biol. 2023;62:102659.
Kirova DG, Judasova K, Vorhauser J, Zerjatke T, Leung JK, Glauche I, et al. A ROS-dependent mechanism promotes CDK2 phosphorylation to drive progression through S phase. Dev Cell. 2022;57(14):1712–e279.
Zhou B, Zhang JY, Liu XS, Chen HZ, Ai YL, Cheng K, et al. Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell Res. 2018;28(12):1171–85.
Pfanner N, Warscheid B, Wiedemann N. Mitochondrial proteins: from biogenesis to functional networks. Nat Rev Mol Cell Biol. 2019;20(5):267–84.
Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148(6):1145–59.
Missiroli S, Patergnani S, Caroccia N, Pedriali G, Perrone M, Previati M, et al. Mitochondria-associated membranes (MAMs) and inflammation. Cell Death Dis. 2018;9(3):329.
Wong YC, Ysselstein D, Krainc D. Mitochondria-lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature. 2018;554(7692):382–6.
Peng W, Wong YC, Krainc D. Mitochondria-lysosome contacts regulate mitochondrial ca(2+) dynamics via lysosomal TRPML1. Proc Natl Acad Sci U S A. 2020;117(32):19266–75.
Gordan R, Fefelova N, Gwathmey JK, Xie LH. Iron overload, oxidative stress and calcium mishandling in Cardiomyocytes: role of the mitochondrial permeability transition pore. Antioxid (Basel). 2020;9(8).
Shu L, Hu C, Xu M, Yu J, He H, Lin J, et al. ATAD3B is a mitophagy receptor mediating clearance of oxidative stress-induced damaged mitochondrial DNA. EMBO J. 2021;40(8):e106283.
Hamilton S, Terentyeva R, Perger F, Hernandez Orengo B, Martin B, Gorr MW, et al. MCU overexpression evokes disparate dose-dependent effects on mito-ROS and spontaneous ca(2+) release in hypertrophic rat cardiomyocytes. Am J Physiol Heart Circ Physiol. 2021;321(4):H615–32.
Stewart JB, Chinnery PF. The dynamics of mitochondrial DNA heteroplasmy: implications for human health and disease. Nat Rev Genet. 2015;16(9):530–42.
Yoshinaga N, Numata K. Rational designs at the forefront of Mitochondria-targeted gene delivery: recent progress and future perspectives. ACS Biomater Sci Eng. 2022;8(2):348–59.
Hahn A, Zuryn S. Mitochondrial genome (mtDNA) mutations that generate reactive oxygen species. Antioxid (Basel). 2019;8(9).
Tilokani L, Nagashima S, Paupe V, Prudent J. Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem. 2018;62(3):341–60.
Wai T, Langer T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol Metab. 2016;27(2):105–17.
Burman JL, Pickles S, Wang C, Sekine S, Vargas JNS, Zhang Z, et al. Mitochondrial fission facilitates the selective mitophagy of protein aggregates. J Cell Biol. 2017;216(10):3231–47.
Guo Y, Zhang H, Yan C, Shen B, Zhang Y, Guo X, et al. Small molecule agonist of mitochondrial fusion repairs mitochondrial dysfunction. Nat Chem Biol. 2023;19(4):468–77.
Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88(2):611–38.
Wang D, Cao L, Zhou X, Wang G, Ma Y, Hao X, et al. Mitigation of honokiol on fluoride-induced mitochondrial oxidative stress, mitochondrial dysfunction, and cognitive deficits through activating AMPK/PGC-1alpha/Sirt3. J Hazard Mater. 2022;437:129381.
Wang J, Frohlich H, Torres FB, Silva RL, Poschet G, Agarwal A et al. Mitochondrial dysfunction and oxidative stress contribute to cognitive and motor impairment in FOXP1 syndrome. Proc Natl Acad Sci U S A. 2022;119(8).
Zhang T, Liu Q, Gao W, Sehgal SA, Wu H. The multifaceted regulation of mitophagy by endogenous metabolites. Autophagy. 2022;18(6):1216–39.
Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189(2):211–21.
Zhang L, Dai L, Li D. Mitophagy in neurological disorders. J Neuroinflammation. 2021;18(1):297.
Swerdlow RH. Mitochondria and mitochondrial cascades in Alzheimer’s Disease. J Alzheimers Dis. 2018;62(3):1403–16.
Cai Q, Tammineni P. Mitochondrial aspects of synaptic dysfunction in Alzheimer’s Disease. J Alzheimers Dis. 2017;57(4):1087–103.
Zhang Q, Song Q, Yu R, Wang A, Jiang G, Huang Y, et al. Nano-Brake halts mitochondrial dysfunction Cascade to Alleviate Neuropathology and Rescue Alzheimer’s cognitive deficits. Adv Sci (Weinh). 2023;10(7):e2204596.
Xie W, Guo D, Li J, Yue L, Kang Q, Chen G, et al. CEND1 deficiency induces mitochondrial dysfunction and cognitive impairment in Alzheimer’s disease. Cell Death Differ. 2022;29(12):2417–28.
Flones IH, Fernandez-Vizarra E, Lykouri M, Brakedal B, Skeie GO, Miletic H, et al. Neuronal complex I deficiency occurs throughout the Parkinson’s disease brain, but is not associated with neurodegeneration or mitochondrial DNA damage. Acta Neuropathol. 2018;135(3):409–25.
Gonzalez-Rodriguez P, Zampese E, Stout KA, Guzman JN, Ilijic E, Yang B, et al. Disruption of mitochondrial complex I induces progressive parkinsonism. Nature. 2021;599(7886):650–6.
Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, et al. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson’s disease. Cell. 2011;144(5):689–702.
Peng W, Schroder LF, Song P, Wong YC, Krainc D. Parkin regulates amino acid homeostasis at mitochondria-lysosome (M/L) contact sites in Parkinson’s disease. Sci Adv. 2023;9(29):eadh3347.
Lee KS, Huh S, Lee S, Wu Z, Kim AK, Kang HY, et al. Altered ER-mitochondria contact impacts mitochondria calcium homeostasis and contributes to neurodegeneration in vivo in disease models. Proc Natl Acad Sci U S A. 2018;115(38):E8844–53.
Qi B, Song L, Hu L, Guo D, Ren G, Peng T, et al. Cardiac-specific overexpression of Ndufs1 ameliorates cardiac dysfunction after myocardial infarction by alleviating mitochondrial dysfunction and apoptosis. Exp Mol Med. 2022;54(7):946–60.
Zheng Z, Lei C, Liu H, Jiang M, Zhou Z, Zhao Y, et al. A ROS-Responsive liposomal composite hydrogel integrating improved mitochondrial function and pro-angiogenesis for efficient treatment of myocardial infarction. Adv Healthc Mater. 2022;11(19):e2200990.
Zhang CX, Cheng Y, Liu DZ, Liu M, Cui H, Zhang BL, et al. Mitochondria-targeted cyclosporin A delivery system to treat myocardial ischemia reperfusion injury of rats. J Nanobiotechnol. 2019;17(1):18.
Li Y, Chen B, Yang X, Zhang C, Jiao Y, Li P, et al. S100a8/a9 signaling causes mitochondrial dysfunction and Cardiomyocyte Death in response to Ischemic/Reperfusion Injury. Circulation. 2019;140(9):751–64.
Bassiouni W, Valencia R, Mahmud Z, Seubert JM, Schulz R. Matrix metalloproteinase-2 proteolyzes mitofusin-2 and impairs mitochondrial function during myocardial ischemia-reperfusion injury. Basic Res Cardiol. 2023;118(1):29.
Maneechote C, Palee S, Kerdphoo S, Jaiwongkam T, Chattipakorn SC, Chattipakorn N. Differential temporal inhibition of mitochondrial fission by Mdivi-1 exerts effective cardioprotection in cardiac ischemia/reperfusion injury. Clin Sci (Lond). 2018;132(15):1669–83.
Luo Y, Ma J, Lu W. The significance of mitochondrial dysfunction in Cancer. Int J Mol Sci. 2020;21(16).
Gill AJ. Succinate dehydrogenase (SDH)-deficient neoplasia. Histopathology. 2018;72(1):106–16.
Yuan T, Zhou T, Qian M, Du J, Liu Y, Wang J, et al. SDHA/B reduction promotes hepatocellular carcinoma by facilitating the deNEDDylation of cullin1 and stabilizing YAP/TAZ. Hepatology. 2023;78(1):103–19.
Ooi A. Advances in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) research. Semin Cancer Biol. 2020;61:158–66.
Sciacovelli M, Goncalves E, Johnson TI, Zecchini VR, da Costa AS, Gaude E, et al. Fumarate is an epigenetic modifier that elicits epithelial-to-mesenchymal transition. Nature. 2016;537(7621):544–7.
Ge X, Li M, Yin J, Shi Z, Fu Y, Zhao N, et al. Fumarate inhibits PTEN to promote tumorigenesis and therapeutic resistance of type2 papillary renal cell carcinoma. Mol Cell. 2022;82(7):1249–60. e7.
Kopinski PK, Singh LN, Zhang S, Lott MT, Wallace DC. Mitochondrial DNA variation and cancer. Nat Rev Cancer. 2021;21(7):431–45.
Smith AL, Whitehall JC, Bradshaw C, Gay D, Robertson F, Blain AP, et al. Age-associated mitochondrial DNA mutations cause metabolic remodelling that contributes to accelerated intestinal tumorigenesis. Nat Cancer. 2020;1(10):976–89.
Mizumura K, Cloonan SM, Nakahira K, Bhashyam AR, Cervo M, Kitada T, et al. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J Clin Invest. 2014;124(9):3987–4003.
Araya J, Tsubouchi K, Sato N, Ito S, Minagawa S, Hara H, et al. PRKN-regulated mitophagy and cellular senescence during COPD pathogenesis. Autophagy. 2019;15(3):510–26.
Cloonan SM, Glass K, Laucho-Contreras ME, Bhashyam AR, Cervo M, Pabon MA, et al. Mitochondrial iron chelation ameliorates cigarette smoke-induced bronchitis and emphysema in mice. Nat Med. 2016;22(2):163–74.
Cen M, Ouyang W, Zhang W, Yang L, Lin X, Dai M, et al. MitoQ protects against hyperpermeability of endothelium barrier in acute lung injury via a Nrf2-dependent mechanism. Redox Biol. 2021;41:101936.
He B, Yu H, Liu S, Wan H, Fu S, Liu S, et al. Mitochondrial cristae architecture protects against mtDNA release and inflammation. Cell Rep. 2022;41(10):111774.
Coughlan MT, Nguyen TV, Penfold SA, Higgins GC, Thallas-Bonke V, Tan SM, et al. Mapping time-course mitochondrial adaptations in the kidney in experimental diabetes. Clin Sci (Lond). 2016;130(9):711–20.
Bai M, Wu M, Jiang M, He J, Deng X, Xu S, et al. LONP1 targets HMGCS2 to protect mitochondrial function and attenuate chronic kidney disease. EMBO Mol Med. 2023;15(2):e16581.
Jin C, Wu P, Li L, Xu W, Qian H. Exosomes: emerging therapy delivery tools and biomarkers for kidney diseases. Stem Cells Int. 2021;2021:7844455.
Tang C, Cai J, Yin XM, Weinberg JM, Venkatachalam MA, Dong Z. Mitochondrial quality control in kidney injury and repair. Nat Rev Nephrol. 2021;17(5):299–318.
Zhao M, Wang Y, Li L, Liu S, Wang C, Yuan Y, et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics. 2021;11(4):1845–63.
Maekawa H, Inoue T, Ouchi H, Jao TM, Inoue R, Nishi H, et al. Mitochondrial damage causes inflammation via cGAS-STING signaling in Acute kidney Injury. Cell Rep. 2019;29(5):1261–e736.
Hu Q, Ren J, Wu J, Li G, Wu X, Liu S, et al. Urinary mitochondrial DNA levels identify acute kidney Injury in Surgical critical illness patients. Shock. 2017;48(1):11–7.
Li R, Dai Z, Liu X, Wang C, Huang J, Xin T, et al. Interaction between dual specificity phosphatase-1 and cullin-1 attenuates alcohol-related liver disease by restoring p62-mediated mitophagy. Int J Biol Sci. 2023;19(6):1831–45.
Lu X, Xuan W, Li J, Yao H, Huang C, Li J. AMPK protects against alcohol-induced liver injury through UQCRC2 to up-regulate mitophagy. Autophagy. 2021;17(11):3622–43.
Abdelhamid AM, Elsheakh AR, Abdelaziz RR, Suddek GM. Empagliflozin ameliorates ethanol-induced liver injury by modulating NF-kappaB/Nrf-2/PPAR-gamma interplay in mice. Life Sci. 2020;256:117908.
Zhou Y, Wu R, Wang X, Bao X, Lu C. Roles of necroptosis in alcoholic liver disease and hepatic pathogenesis. Cell Prolif. 2022;55(3):e13193.
Ma X, Chen A, Melo L, Clemente-Sanchez A, Chao X, Ahmadi AR, et al. Loss of hepatic DRP1 exacerbates alcoholic hepatitis by inducing megamitochondria and mitochondrial maladaptation. Hepatology. 2023;77(1):159–75.
Younossi ZM, Marchesini G, Pinto-Cortez H, Petta S. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: implications for liver transplantation. Transplantation. 2019;103(1):22–7.
Ramanathan R, Ali AH, Ibdah JA. Mitochondrial dysfunction plays central role in nonalcoholic fatty liver disease. Int J Mol Sci. 2022;23(13).
Wan J, Wu X, Chen H, Xia X, Song X, Chen S, et al. Aging-induced aberrant RAGE/PPARalpha axis promotes hepatic steatosis via dysfunctional mitochondrial beta oxidation. Aging Cell. 2020;19(10):e13238.
Wang L, Ishihara T, Ibayashi Y, Tatsushima K, Setoyama D, Hanada Y, et al. Disruption of mitochondrial fission in the liver protects mice from diet-induced obesity and metabolic deterioration. Diabetologia. 2015;58(10):2371–80.
Jeppesen DK, Zhang Q, Franklin JL, Coffey RJ. Extracellular vesicles and nanoparticles: emerging complexities. Trends Cell Biol. 2023;33(8):667–81.
Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750.
Welsh JA, Goberdhan DCI, O’Driscoll L, Buzas EI, Blenkiron C, Bussolati B, et al. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J Extracell Vesicles. 2024;13(2):e12404.
van de Wakker SI, Meijers FM, Sluijter JPG, Vader P. Extracellular vesicle heterogeneity and its impact for Regenerative Medicine Applications. Pharmacol Rev. 2023;75(5):1043–61.
Dyball LE, Smales CM. Exosomes: Biogenesis, targeting, characterization and their potential as Plug & Play vaccine platforms. Biotechnol J. 2022;17(11):e2100646.
Ashoub MH, Salavatipour MS, Kasgari FH, Valandani HM, Khalilabadi RM. Extracellular microvesicles: biologic properties, biogenesis, and applications in leukemia. Mol Cell Biochem. 2024;479(2):419–30.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478).
Gustafson D, Veitch S, Fish JE. Extracellular vesicles as protagonists of Diabetic Cardiovascular Pathology. Front Cardiovasc Med. 2017;4:71.
Deus CM, Tavares H, Beatriz M, Mota S, Lopes C. Mitochondrial damage-Associated molecular patterns content in Extracellular vesicles promotes early inflammation in neurodegenerative disorders. Cells. 2022;11:15.
Choong CJ, Okuno T, Ikenaka K, Baba K, Hayakawa H, Koike M, et al. Alternative mitochondrial quality control mediated by extracellular release. Autophagy. 2021;17(10):2962–74.
McLelland GL, Soubannier V, Chen CX, McBride HM, Fon EA. Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J. 2014;33(4):282–95.
Sugiura A, McLelland GL, Fon EA, McBride HM. A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J. 2014;33(19):2142–56.
Braschi E, Goyon V, Zunino R, Mohanty A, Xu L, McBride HM. Vps35 mediates vesicle transport between the mitochondria and peroxisomes. Curr Biol. 2010;20(14):1310–5.
Roberts RF, Fon EA. Presenting mitochondrial antigens: PINK1, Parkin and MDVs steal the show. Cell Res. 2016;26(11):1180–1.
Matheoud D, Sugiura A, Bellemare-Pelletier A, Laplante A, Rondeau C, Chemali M, et al. Parkinson’s Disease-related proteins PINK1 and Parkin Repress Mitochondrial Antigen Presentation. Cell. 2016;166(2):314–27.
Soubannier V, Rippstein P, Kaufman BA, Shoubridge EA, McBride HM. Reconstitution of mitochondria derived vesicle formation demonstrates selective enrichment of oxidized cargo. PLoS ONE. 2012;7(12):e52830.
Roberts RF, Bayne AN, Goiran T, Levesque D, Boisvert FM, Trempe JF, et al. Proteomic profiling of mitochondrial-derived vesicles in Brain reveals Enrichment of Respiratory Complex sub-assemblies and small TIM chaperones. J Proteome Res. 2021;20(1):506–17.
Mohanty A, Zunino R, Soubannier V, Dilipkumar S. A new functional role of mitochondria-anchored protein ligase in peroxisome morphology in mammalian cells. J Cell Biochem. 2021;122(11):1686–700.
Abuaita BH, Schultz TL, O’Riordan MX. Mitochondria-Derived vesicles deliver antimicrobial reactive oxygen species to Control Phagosome-localized Staphylococcus aureus. Cell Host Microbe. 2018;24(5):625–36. e5.
Lee Y, Ni J, Beretov J, Wasinger VC, Graham P, Li Y. Recent advances of small extracellular vesicle biomarkers in breast cancer diagnosis and prognosis. Mol Cancer. 2023;22(1):33.
Dave KM, Stolz DB, Venna VR, Quaicoe VA, Maniskas ME, Reynolds MJ, et al. Mitochondria-containing extracellular vesicles (EV) reduce mouse brain infarct sizes and EV/HSP27 protect ischemic brain endothelial cultures. J Control Release. 2023;354:368–93.
Dozio V, Sanchez JC. Characterisation of extracellular vesicle-subsets derived from brain endothelial cells and analysis of their protein cargo modulation after TNF exposure. J Extracell Vesicles. 2017;6(1):1302705.
Sansone P, Savini C, Kurelac I, Chang Q, Amato LB, Strillacci A, et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc Natl Acad Sci U S A. 2017;114(43):E9066–75.
Lou P, Liu S, Xu X, Pan C, Lu Y, Liu J. Extracellular vesicle-based therapeutics for the regeneration of chronic wounds: current knowledge and future perspectives. Acta Biomater. 2021;119:42–56.
Sidhom K, Obi PO, Saleem A. A review of Exosomal isolation methods: is size Exclusion Chromatography the best option? Int J Mol Sci. 2020;21:18.
D’Acunzo P, Kim Y, Ungania JM, Perez-Gonzalez R, Goulbourne CN, Levy E. Isolation of mitochondria-derived mitovesicles and subpopulations of microvesicles and exosomes from brain tissues. Nat Protoc. 2022;17(11):2517–49.
Nguyen A, Turko IV. Isolation protocols and mitochondrial content for plasma extracellular vesicles. Anal Bioanal Chem. 2023;415(7):1299–304.
Crescitelli R, Lasser C, Lotvall J. Isolation and characterization of extracellular vesicle subpopulations from tissues. Nat Protoc. 2021;16(3):1548–80.
Lai JJ, Chau ZL, Chen SY, Hill JJ, Korpany KV, Liang NW, et al. Exosome Processing and characterization approaches for Research and Technology Development. Adv Sci (Weinh). 2022;9(15):e2103222.
van der Pol E, Coumans FA, Grootemaat AE, Gardiner C, Sargent IL, Harrison P, et al. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J Thromb Haemost. 2014;12(7):1182–92.
Arraud N, Gounou C, Linares R, Brisson AR. A simple flow cytometry method improves the detection of phosphatidylserine-exposing extracellular vesicles. J Thromb Haemost. 2015;13(2):237–47.
van der Vlist EJ, Nolte-‘t Hoen EN, Stoorvogel W, Arkesteijn GJ, Wauben MH. Fluorescent labeling of nano-sized vesicles released by cells and subsequent quantitative and qualitative analysis by high-resolution flow cytometry. Nat Protoc. 2012;7(7):1311–26.
Groot Kormelink T, Arkesteijn GJ, Nauwelaers FA, van den Engh G, Nolte-‘t Hoen EN, Wauben MH. Prerequisites for the analysis and sorting of extracellular vesicle subpopulations by high-resolution flow cytometry. Cytometry A. 2016;89(2):135–47.
Stoner SA, Duggan E, Condello D, Guerrero A, Turk JR, Narayanan PK, et al. High sensitivity flow cytometry of membrane vesicles. Cytometry A. 2016;89(2):196–206.
Zhu S, Ma L, Wang S, Chen C, Zhang W, Yang L, et al. Light-scattering detection below the level of single fluorescent molecules for high-resolution characterization of functional nanoparticles. ACS Nano. 2014;8(10):10998–1006.
Zhang X, Hsueh MF, Huebner JL, Kraus VB. TNF-alpha carried by plasma extracellular vesicles predicts knee osteoarthritis progression. Front Immunol. 2021;12:758386.
Zhang X, Hubal MJ, Kraus VB. Immune cell extracellular vesicles and their mitochondrial content decline with ageing. Immun Ageing. 2020;17:1.
Zhao M, Liu S, Wang C, Wang Y, Wan M, Liu F, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate mitochondrial damage and inflammation by stabilizing mitochondrial DNA. ACS Nano. 2021;15(1):1519–38.
Thomas MA, Fahey MJ, Pugliese BR, Irwin RM, Antonyak MA, Delco ML. Human mesenchymal stromal cells release functional mitochondria in extracellular vesicles. Front Bioeng Biotechnol. 2022;10:870193.
Peruzzotti-Jametti L, Bernstock JD, Willis CM, Manferrari G, Rogall R, Fernandez-Vizarra E, et al. Neural stem cells traffic functional mitochondria via extracellular vesicles. PLoS Biol. 2021;19(4):e3001166.
Manickam DS. Delivery of mitochondria via extracellular vesicles – a new horizon in drug delivery. J Controlled Release. 2022;343:400–7.
Yao PJ, Eren E, Goetzl EJ, Kapogiannis D. Mitochondrial Electron Transport Chain Protein Abnormalities Detected in Plasma Extracellular Vesicles in Alzheimer’s Disease. Biomedicines. 2021;9(11).
Kim KM, Meng Q, Perez de Acha O, Mustapic M, Cheng A, Eren E et al. Mitochondrial RNA in Alzheimer’s Disease circulating Extracellular vesicles. Front Cell Dev Biology. 2020;8.
D’Acunzo P, Perez-Gonzalez R, Kim Y, Hargash T, Miller C, Alldred MJ et al. Mitovesicles are a novel population of extracellular vesicles of mitochondrial origin altered in Down syndrome. Sci Adv. 2021;7(7).
Ladakis DC, Yao PJ, Vreones M, Blommer J, Kalaitzidis G, Sotirchos ES, et al. Mitochondrial measures in neuronally enriched extracellular vesicles predict brain and retinal atrophy in multiple sclerosis. Multiple Scler J. 2022;28(13):2020–6.
Holvoet P, Vanhaverbeke M, Bloch K, Baatsen P, Sinnaeve P, Janssens S. Low MT-CO1 in Monocytes and Microvesicles is Associated with Outcome in patients with coronary artery disease. J Am Heart Assoc. 2016;5(12).
Keseru JS, Soltesz B, Lukacs J, Marton E, Szilagyi-Bonizs M, Penyige A, et al. Detection of cell-free, exosomal and whole blood mitochondrial DNA copy number in plasma or whole blood of patients with serous epithelial ovarian cancer. J Biotechnol. 2019;298:76–81.
Jang SC, Crescitelli R, Cvjetkovic A, Belgrano V, Olofsson Bagge R, Sundfeldt K, et al. Mitochondrial protein enriched extracellular vesicles discovered in human melanoma tissues can be detected in patient plasma. J Extracell Vesicles. 2019;8(1):1635420.
Cheng AN, Cheng L-C, Kuo C-L, Lo YK, Chou H-Y, Chen C-H et al. Mitochondrial lon-induced mtDNA leakage contributes to PD-L1–mediated immunoescape via STING-IFN signaling and extracellular vesicles. J Immunother Cancer. 2020;8(2).
Vikramdeo KS, Anand S, Khan MA, Khushman Md, Heslin MJ, Singh S et al. Detection of mitochondrial DNA mutations in circulating mitochondria-originated extracellular vesicles for potential diagnostic applications in pancreatic adenocarcinoma. Sci Rep. 2022;12(1).
Rasuleva K, Jangili KP, Akinlalu A, Guo A, Borowicz P, Li C-z, et al. EvIPqPCR, Target circulating Tumorous Extracellular vesicles for detection of pancreatic Cancer. Anal Chem. 2023;95(27):10353–61.
Letsiou E, Teixeira Alves LG, Fatykhova D, Felten M, Mitchell TJ, Müller-Redetzky HC et al. Microvesicles released from pneumolysin-stimulated lung epithelial cells carry mitochondrial cargo and suppress neutrophil oxidative burst. Sci Rep. 2021;11(1).
Ma J, Cao H, Rodrigues RM, Xu M, Ren T, He Y et al. Chronic-plus-binge alcohol intake induces production of proinflammatory mtDNA-enriched extracellular vesicles and steatohepatitis via ASK1/p38MAPKalpha-dependent mechanisms. JCI Insight. 2020;5(14).
Lee JH, Shim YR, Seo W, Kim MH, Choi WM, Kim HH, et al. Mitochondrial double-stranded RNA in Exosome promotes Interleukin-17 production through toll-like receptor 3 in Alcohol-associated Liver Injury. Hepatology. 2020;72(2):609–25.
Li YJ, Liu RP, Ding MN, Zheng Q, Wu JZ, Xue XY, et al. Tetramethylpyrazine prevents liver fibrotic injury in mice by targeting hepatocyte-derived and mitochondrial DNA-enriched extracellular vesicles. Acta Pharmacol Sin. 2022;43(8):2026–41.
Zheng L, Wang Y, Qiu P, Xia C, Fang Y, Mei S, et al. Primary chondrocyte exosomes mediate osteoarthritis progression by regulating mitochondrion and immune reactivity. Nanomed (Lond). 2019;14(24):3193–212.
Picca A, Beli R, Calvani R, Coelho-Junior HJ, Landi F, Bernabei R et al. Older adults with physical Frailty and Sarcopenia Show increased levels of circulating small Extracellular vesicles with a specific mitochondrial signature. Cells. 2020;9(4).
Lazo S, Noren Hooten N, Green J, Eitan E, Mode NA, Liu QR, et al. Mitochondrial DNA in extracellular vesicles declines with age. Aging Cell. 2021;20(1):e13283.
Rehman FU, Liu Y, Zheng M, Shi B. Exosomes based strategies for brain drug delivery. Biomaterials. 2023;293:121949.
Chen CC, Liu L, Ma F, Wong CW, Guo XE, Chacko JV, et al. Elucidation of Exosome Migration across the blood-brain barrier model in Vitro. Cell Mol Bioeng. 2016;9(4):509–29.
D’Souza A, Burch A, Dave KM, Sreeram A, Reynolds MJ, Dobbins DX, et al. Microvesicles transfer mitochondria and increase mitochondrial function in brain endothelial cells. J Control Release. 2021;338:505–26.
Leggio L, L’Episcopo F, Magri A, Ulloa-Navas MJ, Paterno G, Vivarelli S, et al. Small extracellular vesicles secreted by Nigrostriatal Astrocytes Rescue Cell Death and preserve mitochondrial function in Parkinson’s Disease. Adv Healthc Mater. 2022;11(20):e2201203.
Calabria E, Scambi I, Bonafede R, Schiaffino L, Peroni D, Potrich V, et al. ASCs-Exosomes recover Coupling Efficiency and mitochondrial membrane potential in an in vitro model of ALS. Front NeuroSci. 2019;13:1070.
Lee M, Ban JJ, Kim KY, Jeon GS, Im W, Sung JJ, et al. Adipose-derived stem cell exosomes alleviate pathology of amyotrophic lateral sclerosis in vitro. Biochem Biophys Res Commun. 2016;479(3):434–9.
Bonafede R, Brandi J, Manfredi M, Scambi I, Schiaffino L, Merigo F et al. The anti-apoptotic effect of ASC-Exosomes in an in vitro ALS Model and their proteomic analysis. Cells. 2019;8(9).
Lee M, Liu T, Im W, Kim M. Exosomes from adipose-derived stem cells ameliorate phenotype of Huntington’s disease in vitro model. Eur J Neurosci. 2016;44(4):2114–9.
Lu Y, Zhang J, Han B, Yu Y, Zhao W, Wu T, et al. Extracellular vesicles DJ-1 derived from hypoxia-conditioned hMSCs alleviate cardiac hypertrophy by suppressing mitochondria dysfunction and preventing ATRAP degradation. Pharmacol Res. 2023;187:106607.
Liu X, Li X, Zhu W, Zhang Y, Hong Y, Liang X, et al. Exosomes from mesenchymal stem cells overexpressing MIF enhance myocardial repair. J Cell Physiol. 2020;235(11):8010–22.
Zhao F, Sun L, Yang N, Zheng W, Shen P, Huang Y, et al. Increased release of microvesicles containing mitochondria is associated with the myeloid differentiation of AML-M5 leukaemia cells. Exp Cell Res. 2020;395(2):112213.
Abad E, Lyakhovich A. Movement of Mitochondria with Mutant DNA through Extracellular vesicles helps Cancer cells acquire Chemoresistance. ChemMedChem. 2022;17(4):e202100642.
Salaud C, Alvarez-Arenas A, Geraldo F, Belmonte-Beitia J, Calvo GF, Gratas C, et al. Mitochondria transfer from tumor-activated stromal cells (TASC) to primary Glioblastoma cells. Biochem Biophys Res Commun. 2020;533(1):139–47.
Rabas N, Palmer S, Mitchell L, Ismail S, Gohlke A, Riley JS et al. PINK1 drives production of mtDNA-containing extracellular vesicles to promote invasiveness. J Cell Biol. 2021;220(12).
Jiao Y, Tang Y, Li Y, Liu C, He J, Zhang LK, et al. Tumor cell-derived extracellular vesicles for breast cancer specific delivery of therapeutic P53. J Control Release. 2022;349:606–16.
Vakhshiteh F, Rahmani S, Ostad SN, Madjd Z, Dinarvand R, Atyabi F. Exosomes derived from miR-34a-overexpressing mesenchymal stem cells inhibit in vitro tumor growth: a new approach for drug delivery. Life Sci. 2021;266:118871.
Xu H, Liao C, Liang S, Ye BC. A novel peptide-equipped exosomes platform for delivery of antisense oligonucleotides. ACS Appl Mater Interfaces. 2021;13(9):10760–7.
Abad E, Lyakhovich A. Movement of Mitochondria with Mutant DNA through Extracellular vesicles helps Cancer cells acquire Chemoresistance. ChemMedChem. 2021;17(4).
Kang C, Ren X, Lee D, Ramesh R, Nimmo S, Yang-Hartwich Y, et al. Harnessing small extracellular vesicles for pro-oxidant delivery: novel approach for drug-sensitive and resistant cancer therapy. J Controlled Release. 2024;365:286–300.
Nguyen Cao TG, Kang JH, Kang SJ, Truong Hoang Q, Kang HC, Rhee WJ, et al. Brain endothelial cell-derived extracellular vesicles with a mitochondria-targeting photosensitizer effectively treat glioblastoma by hijacking the blood–brain barrier. Acta Pharm Sinica B. 2023;13(9):3834–48.
Cao H, Gao H, Wang L, Cheng Y, Wu X, Shen X, et al. Biosynthetic Dendritic Cell-Exocytosed Aggregation-Induced Emission nanoparticles for synergistic photodynamic immunotherapy. ACS Nano. 2022;16(9):13992–4006.
Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O’Kane CM, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant Lung Injury models by Extracellular Vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196(10):1275–86.
Xia L, Zhang C, Lv N, Liang Z, Ma T, Cheng H, et al. AdMSC-derived exosomes alleviate acute lung injury via transferring mitochondrial component to improve homeostasis of alveolar macrophages. Theranostics. 2022;12(6):2928–47.
Antunes MA, Braga CL, Oliveira TB, Kitoko JZ, Castro LL, Xisto DG, et al. Mesenchymal stromal cells from Emphysematous donors and their extracellular vesicles are unable to reverse cardiorespiratory dysfunction in experimental severe Emphysema. Front Cell Dev Biol. 2021;9:661385.
Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759–65.
Dutra Silva J, Su Y, Calfee CS, Delucchi KL, Weiss D, McAuley DF et al. Mesenchymal stromal cell extracellular vesicles rescue mitochondrial dysfunction and improve barrier integrity in clinically relevant models of ARDS. Eur Respir J. 2021;58(1).
Bhargava P, Schnellmann RG. Mitochondrial energetics in the kidney. Nat Rev Nephrol. 2017;13(10):629–46.
Collino F, Lopes JA, Tapparo M, Tortelote GG, Kasai-Brunswick TH, Lopes GMC et al. Extracellular vesicles derived from Induced Pluripotent stem cells promote renoprotection in Acute kidney Injury Model. Cells. 2020;9(2).
Lopes JA, Collino F, Rodrigues-Ferreira C, Sampaio LDS, Costa-Sarmento G, Wendt CHC et al. Early effects of Extracellular vesicles secreted by adipose tissue mesenchymal cells in renal ischemia followed by reperfusion: mechanisms rely on a decrease in mitochondrial anion superoxide production. Int J Mol Sci. 2022;23(6).
Gao Z, Zhang C, Peng F, Chen Q, Zhao Y, Chen L, et al. Hypoxic mesenchymal stem cell-derived extracellular vesicles ameliorate renal fibrosis after ischemia-reperfusion injure by restoring CPT1A mediated fatty acid oxidation. Stem Cell Res Ther. 2022;13(1):191.
Zou X, Kwon SH, Jiang K, Ferguson CM, Puranik AS, Zhu X, et al. Renal scattered tubular-like cells confer protective effects in the stenotic murine kidney mediated by release of extracellular vesicles. Sci Rep. 2018;8(1):1263.
Sun Z, Gao Z, Wu J, Zheng X, Jing S, Wang W. MSC-Derived Extracellular vesicles Activate Mitophagy to Alleviate Renal Ischemia/Reperfusion Injury via the miR-223-3p/NLRP3 Axis. Stem Cells Int. 2022;2022:6852661.
Gu D, Zou X, Ju G, Zhang G, Bao E, Zhu Y. Mesenchymal stromal cells derived extracellular vesicles ameliorate Acute Renal Ischemia Reperfusion Injury by Inhibition of mitochondrial fission through miR-30. Stem Cells Int. 2016;2016:2093940.
Tang TT, Wang B, Wu M, Li ZL, Feng Y, Cao JY, et al. Extracellular vesicle-encapsulated IL-10 as novel nanotherapeutics against ischemic AKI. Sci Adv. 2020;6(33):eaaz0748.
Huang H, Tohme S, Al-Khafaji AB, Tai S, Loughran P, Chen L, et al. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology. 2015;62(2):600–14.
Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived Extracellular vesicles: toward cell-free therapeutic applications. Mol Therapy: J Am Soc Gene Therapy. 2015;23(5):812–23.
Ni S, Yi N, Yuan H, Li D, Chen X, Zhuang C. Angelica Sinensis polysaccharide improves mitochondrial metabolism of osteoarthritis chondrocytes through PPARgamma/SOD2/ROS pathways. Phytother Res. 2023.
Yu M, Wang D, Chen X, Zhong D, Luo J. BMSCs-derived Mitochondria Improve Osteoarthritis by ameliorating mitochondrial dysfunction and promoting mitochondrial Biogenesis in Chondrocytes. Stem Cell Rev Rep. 2022;18(8):3092–111.
Bao W, Xing H, Cao S, Long X, Liu H, Ma J, et al. Neutrophils restrain sepsis associated coagulopathy via extracellular vesicles carrying superoxide dismutase 2 in a murine model of lipopolysaccharide induced sepsis. Nat Commun. 2022;13(1):4583.
Liu X, Zheng Z, Zhu X, Meng M, Li L, Shen Y, et al. Brown adipose tissue transplantation improves whole-body energy metabolism. Cell Res. 2013;23(6):851–4.
Zhou X, Li Z, Qi M, Zhao P, Duan Y, Yang G, et al. Brown adipose tissue-derived exosomes mitigate the metabolic syndrome in high fat diet mice. Theranostics. 2020;10(18):8197–210.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [82003379], the Postgraduate Research & Practice Innovation Program of Jiangsu Province [KYCX23_3767], and Zhenjiang Key Laboratory of High Technology Research on Exosomes Foundation and Transformation Application [SS2018003].
Author information
Authors and Affiliations
Contributions
ZXS conceived and revised the paper. JLL and TRW drafted the manuscript and prepared the figures and tables. XMH, YL, JXZ, WHB, and HQ performed manuscript reviewing and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, J., Wang, T., Hou, X. et al. Extracellular vesicles: opening up a new perspective for the diagnosis and treatment of mitochondrial dysfunction. J Nanobiotechnol 22, 487 (2024). https://doi.org/10.1186/s12951-024-02750-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-024-02750-8