Abstract
Neurodegenerative diseases are characterized by extensive loss of function or death of brain cells, hampering the life quality of patients. Brain-targeted drug delivery is challenging, with a low success rate this far. Therefore, the application of targeting ligands in drug vehicles, such as lipid-based and polymeric nanoparticles, holds the promise to overcome the blood-brain barrier (BBB) and direct therapies to the brain, in addition to protect their cargo from degradation and metabolization. In this review, we discuss the barriers to brain delivery and the different types of brain-targeting ligands currently in use in brain-targeted nanoparticles, such as peptides, proteins, aptamers, small molecules, and antibodies. Moreover, we present a detailed review of the different targeting ligands used to direct nanoparticles to specific brain cells, like neurons (C4-3 aptamer, neurotensin, Tet-1, RVG, and IKRG peptides), astrocytes (Aquaporin-4, D4, and Bradykinin B2 antibodies), oligodendrocytes (NG-2 antibody and the biotinylated DNA aptamer conjugated to a streptavidin core Myaptavin-3064), microglia (CD11b antibody), neural stem cells (QTRFLLH, VPTQSSG, and NFL-TBS.40–63 peptides), and to endothelial cells of the BBB (transferrin and insulin proteins, and choline). Reports demonstrated enhanced brain-targeted delivery with improved transport to the specific cell type targeted with the conjugation of these ligands to nanoparticles. Hence, this strategy allows the implementation of high-precision medicine, with reduced side effects or unwanted therapy clearance from the body. Nevertheless, the accumulation of some of these nanoparticles in peripheral organs has been reported indicating that there are still factors to be improved to achieve higher levels of brain targeting. This review is a collection of studies exploring targeting ligands for the delivery of nanoparticles to the brain and we highlight the advantages and limitations of this type of approach in precision therapies.
Similar content being viewed by others
Background
The World Health Organization (WHO) estimates that 1 in every 5 humans suffers from Central Nervous System (CNS) diseases [1]. Neurodegenerative diseases, such as Alzheimer’s disease (AD) or Parkinson’s disease (PD), are becoming more prevalent in today’s increasingly aged societies and are a social and financial burden worldwide [2,3,4]. Despite the increasing awareness for this problem and the efforts of the scientific community to develop therapeutic strategies, this research field has the poorest success rates in terms of effective drug development [5].
The complex physiology of the human brain, the Blood-Brain Barrier (BBB), and the substantial limitations of most animal models used to study human CNS diseases [6] play an important role in the lack of success in the development of new therapies to treat brain diseases. Considering these hurdles, the rational design of nanoparticles (NPs) prone to be administered in minimally invasive ways (e.g. intravenous administration [IV]) can be a promising approach to overcome some of these limitations [7, 8].
NPs comprise materials with size in the nanoscale in at least one dimension [9, 10]. Such nanomaterials can be part of Nanomedicines that, according to the European Commission’s recommendation, are between 1 and 100 nm in size for at least 50% of the particles [11]. NPs can load a great variety of drugs (small molecules, proteins, nucleic acids, etc.), protecting them from metabolization and elimination from the body, and increasing their half-life in the systemic circulation, raising the probability of drugs to reach their target tissue/organ [7, 8, 12, 13]. The materials to be used in the NPs composition must be, whenever possible, biocompatible and biodegradable in order to reduce immunogenicity and toxicity [14]. Furthermore, NPs’ charge, size, and surface chemistry can be manipulated to improve biodistribution [15, 16]. An important functionalization of NPs is the attachment of hydrophilic polymers to their surface, such as polyethylene glycol (PEG). This hydrophilic polymer creates a “cloud” of water molecules on the surface of the NPs, reducing the opsonization effect and the consequent NPs elimination from bloodstream, increasing their time in blood circulation [17, 18] and their ability to efficiently reach the target cells after IV administration. Additionally, the functionalization of NPs surface by adding targeting ligands makes it possible to direct them to a specific cell type or tissue, increasing the accumulation of the NPs in the tissue/cells and reducing the off-target effects [19, 20].
The identification of brain-specific ligands that might be employed in the development of brain-targeted NPs is also a critical aspect. Such ligands might specifically direct the NPs to the brain tissue, avoiding unspecific interactions in other compartments, reducing off-target effect and peripheral drug elimination, and consequently enhancing the bioavailability in the brain of the delivered drug. There are different types of targeting ligands that may be employed in the development of NPs (Fig. 1), such as proteins, antibodies, peptides, small molecules, and aptamers, and each of them presents advantages and disadvantages (Table 1) [21,22,23].
Types of targeting ligands. Several different types of molecules have been employed to achieve specific cellular targeting depending on the characteristics of the NPs used, the goal of the delivery, cost-benefit, and the characteristics of the targeting ligands. Such targeting ligands include antibodies, small molecules, aptamers (RNA/DNA sequences that recognize proteins and receptors with affinity and specificity), proteins, and peptides
Blood-brain barrier composition and crossing
BBB comprises endothelial cells, pericytes, and astrocytes, building a tight barrier that selectively limits the entry of molecules into the CNS (Fig. 2) [49]. Furthermore, this barrier is characterized by (1) the absence of fenestrations and (2) the presence of tight junctions between endothelial cells and the brain microvasculature formed by claudin, occludin, and junction adhesion molecules [49]. The presence of these molecular tight junctions results in a high transendothelial electrical resistance (1500 Ω/cm2 in in vivo measurements [50, 51]), limiting the entry of pathogens and undesired molecules and cells from peripheral circulation into the CNS. However crucial for the maintenance of brain homeostasis, this barrier also hampers the effectiveness of therapies to the brain by limiting their entrance [52, 53]. Less than 1% of the macromolecules and no more than 2% of small molecules are able to cross the BBB by paracellular diffusion [54]. Small hydrophilic and hydrophobic molecules need to have a molecular mass inferior to 150 Da and 400–600 Da, respectively, to be able to cross the BBB by passive diffusion. Consequently, most molecules enter the BBB endothelial cells by endocytosis [55]. After endocytosis, the molecules accumulate in late endosomes, which eventually fuse with lysosomes (forming the phagolysosome), where they can be destroyed by the low pH and hydrolytic enzymes [56]. Thus, the endosomal escape is a key step in the success of therapies that reach the CNS by crossing the BBB [57].
Cellular structure of the Blood-Brain Barrier (BBB). The endothelial cells (red cells) that compose the brain microvasculature are attached to each other by Tight Junctions that bring these cells close together, limiting the passage of unspecific molecules between them. Pericytes (purple cells) are important regulatory cells that involve the endothelial cells. Finally, the endfeet of astrocytes (yellow/orange cells) also involve this structure, providing regulatory support. The BBB strongly suppresses the entry of unwanted pathogens and cells into the brain parenchyma, protecting the resident cells from insults
Furthermore, the “enzymatic BBB”, which is a complex set of enzymes from brain endothelial cells, promotes chemical compounds degradation [55]. Another key issue regarding the transcytosis of the BBB is the presence of highly efficient efflux pumps in these cells. These efflux pumps, mediated by p-glycoprotein, are responsible for the recognition of molecules that are unnecessary for the brain and transport them back to the vascular lumen, preventing their entry into the brain parenchyma [58]. Accordingly, some studies indicate that the concentration of several drugs is increased in the CNS upon blockage of these efflux transporters [59, 60]. The paracellular aqueous and the transcellular lipophilic pathways allow the passage of very small molecules in between the endothelial cells of the BBB or through them, respectively. Besides these mechanisms, there are other pathways required for large macromolecules to enter the CNS, such as the proteins that enter via receptor-mediated or adsorptive transcytosis (Fig. 3) [61, 62].
Pathways for molecular transport across the BBB. The cellular and molecular structure of the BBB makes this barrier highly restrictive and selective to molecules that can only cross the BBB through specific mechanisms. Small molecules like glucose are able to enter the brain using for example the glucose transporter Glut-1 as carrier in a Carrier-Mediated Transport. Small lipophilic molecules are able to overcome the BBB via passive diffusion in the Transcellular Lipophilic Pathway. Small hydrophilic molecules, unable to cross through the endothelial cells, are small enough to pass through the Tight Junctions into the brain parenchyma by the Paracellular Pathway. Some cationic molecules are able to interact with the negative charges on the surface of the endothelial cells and cross this barrier in a low capacity and non-specific mechanism called Adsorptive Transcytosis. Finally, large molecules, such as transferrin and insulin, enter the brain parenchyma via specific receptors expressed on the surface of endothelial cells in a mechanism called Receptor-Mediated Transcytosis
In Carrier-Mediated Transport, macromolecules such as glucose, essential fatty acids, and aminoacids, take advantage of transport proteins inserted in the endothelium and use them to transpose the BBB along or against concentration rates. While in receptor-mediated transcytosis, macromolecules such as insulin, epidermal growth factor, LDL, and transferrin bind to specific receptors on the surface of endothelial cells, which activates their endocytosis in the basolateral side of the cells [61, 62]. Finally, in adsorptive transcytosis (non-specific), positively charged ligands interact with the negatively charged cell surface and this interaction promotes endocytosis (Fig. 3).
Overcoming the blood-brain barrier (BBB)
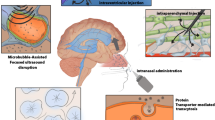
The most direct way to surpass the BBB is by intraventricular, intrathecal, or intraparenchymal injection of the drugs in the brain or intranasal administration. Several publications demonstrated the successful use of these administration routes when aiming at the delivery of molecular therapies to the brain, which are reviewed elsewhere [63, 64]. However, some of these approaches, namely intraventricular, intraparenchymal, and intrathecal, are highly invasive, requiring very delicate brain surgeries and can cause complications such as spinal cord lesions, seizures, encephalopathy, meningitis, cerebral infection, or subdural empyema [65,66,67]. In particular, intraventricular injection is associated with a bulk flow of CSF from the ventricles to the subarachnoid space (where major arteries are located), thus causing fast clearance of the injected therapies from the brain [68, 69]. This fast clearance results in the need of frequent dosing, which may impair patient compliance and tolerance to the treatment [63]. The limited drug penetration from CSF to the brain parenchyma, especially for macromolecules is another handicap of this approach [63]. These limitations, and complications related to the devices, namely severe infections, leakage, and immune system activation (presence of white cells in the CSF), have reduced the use of this strategy for brain therapies [63, 70]. As for intraparenchymal administration, the distribution of the therapies in the brain is frequently limited to the site of injection, constraining the therapeutic effect [63, 64], and complications associated with such an invasive surgery have been described [64, 71]. Intrathecal (IT) administration allows access through the perivascular spaces but this approach is highly dependent on the size of the therapy administered [72], and serious adverse effects have been reported related to blood and lymphatic system disorders due to malfunction of port devices for IT which need to be imbedded in the patients for repeated administration [73]. Intranasal administration is a less invasive approach (and more patient-friendly) that allows access to the brain through the nasal epithelium at the level of the cribriform plate, bypassing the BBB, with minimal serum clearance and peripheral metabolism [63, 64]. This promising administration route to deliver therapies into the brain is challenging due to the physicochemical proprieties of the therapies to be delivered that determine their ability to efficiently cross the nasal epithelium and avoid systemic distribution, and the design of the administration device which is crucial to access the specific location in the cribriform plate and allow a controlled administration to both nostrils [63, 64]. A second approach is the use of strategies that transiently promote BBB leakage using compounds to biochemically modulate tight junctions (such as cereport, mannitol, or borneol) or physical methods like hyperosmotic arabinose solutions, electroconvulsive stimulation, laser-induced thermal therapy, or focused ultrasound [5, 74,75,76,77]. Nevertheless, this approach carries the risk of brain edema and it also facilitates the invasion of pathogens from the bloodstream [78]. In a third approach, the receptors overexpressed in the BBB have been explored as an entrance gate for the brain, by developing brain-targeting NPs incorporating ligands that target these overexpressed receptors [79], such as the Transferrin receptor (TfR) and the Low-Density Lipoprotein Receptor (LDLR) (Table 2).
The TfR is a glycoprotein widely expressed in several cell types including the BBB endothelial cells, which, although lacks cell-specificity, has been extensively used to target NPs to the brain, especially in cancer [30, 80, 81, 122], given the overexpression of this receptor by cancer cells. Despite the straightforward use of this receptor to target NPs, the high levels of circulating transferrin, which will compete for the TfR, may hamper the targeting of NPs to the BBB. In order to overcome this issue, monoclonal antibodies against TfR, such as OX26, 8D3, and RI7217, were developed to deliver drugs into the brain [82, 83].
Low-Density Lipoprotein Receptor (LDLR) has been tested for both direct- and indirect-brain targeting. Regarding indirect-brain targeting, Kreuter and colleagues observed that coating poly(butyl cyanoacrylate)-NPs, encapsulating loperamide or dalargin (drugs with analgesic properties), with polysorbate 80 enables the adsorption of apolipoprotein E (ApoE) from circulation in their surface, allowing these NPs to target LDLR on the BBB and cross it via receptor-mediated transcytosis [85]. For the direct brain-targeting approach, ApoE was covalently bound to human serum albumin NPs (ApoE-NPs) and IV-injected into SV 129 mice. After 15 and 30 min the animals were sacrificed, their brains removed and evaluated by transmission electron microscopy. Interestingly, only ApoE-NPs were observed inside the brain parenchyma and associated with neurons, while unbound NPs were undetected, demonstrating the targeted delivery of NPs using ApoE [86]. Angiopep-2 is a 19 amino acid peptide that has been shown to target LDLR and to improve brain uptake [87, 88]. Angiopep-2 was conjugated with 3 molecules of the anti-cancer drug paclitaxel and this system tested for breast cancer brain metastasis targeting, since this receptor is overexpressed both in the BBB and brain tumors. The Angiopep-2-conjugated paclitaxel and free drug was tested in mice by IV administration. A 161-fold increase in the brain accumulation and a 12-fold increase in the brain metastasis accumulation of the Angiopep-2-conjugated drug were reported. These results suggest an improved brain and brain metastasis delivery of the drug conjugated with Angiopep-2, compared with free drug [89].
Insulin and monoclonal antibodies targeting the insulin receptor have also been used to direct NPs into the brain. Ulbrich and colleagues prepared human serum albumin NPs covalently bound to insulin or to the anti-insulin receptor monoclonal antibody 29B4 to deliver loperamide (an opiate receptor agonist unable to cross the BBB) into the brain after IV administration in mice [90]. The targeted NPs loaded with loperamide were able to induce significant nociceptive effects in mice evaluated by the tail flick test, as compared with NPs attached to an unspecific IgG. Moreover, a pre-injection of free 29B4 anti-insulin receptor antibody, 30 min prior to insulin-targeted NPs administration, inhibited the antinociceptive effects previously observed with these NPs [90]. Thus, data showed that the use of ligands targeting the insulin receptor enables crossing of the BBB.
The high expression of the choline transporter in the BBB has also been explored for brain targeting. Choline is an essential amino acid and a precursor of the neurotransmitter acetylcholine produced by cholinergic neurons that play an important role in learning and memory [123]. Choline is able to transpose the BBB through the choline transporter present on the surface of brain microvascular endothelial cells [123]. Li and colleagues took advantage of the high expression of Choline transporter in the BBB and glioma cells to achieve a dual targeting with a single ligand [91]. Authors complexed a plasmid encoding for human tumor necrosis factor-related apoptosis-inducing ligand (Trail) and the chemotherapeutic drug doxorubicin (DOX) with dendrigraft poly-L-lysine to establish NPs capable to mediate gene therapy and chemotherapy to tackle glioma. Moreover, a choline derivate ligand, designed with the bis-quaternary ammonium compound isoquinoline that has demonstrated high affinity to the choline transporter in the BBB [92], was used as targeting ligand to overcome the BBB. The higher cellular uptake and therapeutic efficiency of the choline transporter-targeted NPs, compared to the non-targeted NPs, was demonstrated in the U87 MG glioma cell line. U87 MG glioma cells were injected in the right striatum of male Balb/c nude mice, and the choline transporter-targeted and non-targeted NPs were intravenously injected 18 days after the cells’ implantation. NIR images, taken 2 h after NPs administration, demonstrated a preferential accumulation of the choline transporter-targeted NPs in the brain, as compared to non-targeted NPs. However, both types of NPs revealed high accumulation in peripheral organs, especially in the liver and spleen [91].
Heparin-binding epidermal growth factor-like growth factor (HB-EGF) is another membrane bound receptor widely expressed in the cerebral blood vessel endothelia, neurons, and glial cells [124]. It has been demonstrated that the carrier protein CRM197 is able to mediate the BBB-targeted delivery using receptor-mediated endocytosis via HB-EGF [125]. CRM197 is a mutated form of the diphtheria toxin produced by the bacteria Corynebaterium diphtheriae that when released in the bloodstream may cause neuritis [126]. CRM197 targeting ligand has been used with success [93, 127]. For example, using an in vitro BBB model composed of human brain-microvascular endothelial cells (HBMEC) seeded on the top (Polyester membrane) of a transwell and human astrocytes seeded on the bottom, Kuo and colleagues investigated the ability of polybutylcyanoacrylate (PBCA) NPs conjugated with CRM197 to deliver zidovudine (AZT). The NPs were loaded with dextran-FITC and their uptake in HBMEC was demonstrated by fluorescent microscopy [93]. Similarly, the ability of CRM197 to deliver polymeric poly-lactide (PLGA) NPs to the brain of CD1 wild-type mice after IV administration was assessed [127]. CRM197-targeted NPs loaded with the rhodamine B dye were administered to the mice, which were sacrificed 30 and 60 min after the administration. For both time points, red spots were observed in whole brain parenchyma, indicating the presence of the NPs. It was also reported significant accumulation of the CRM197-NPs in the liver and spleen and limited uptake in the kidneys and lungs. The cellular tropism of the CRM197-NPs was evaluated 30 min, 6 and 48 h after administration. A preferential accumulation in NeuN-positive cells (neurons) was detected. Additionally, over time there was an increased accumulation of these NPs, being reported that 40%, 48%, and 63% of the cells co-localized with the NPs for each time point, respectively. GFAP-positive cells (astrocytes) presented 35% of co-localization with NPs at 30 min, but their presence was decreased to 15% and 2% for 6 and 48 h, respectively. Furthermore, CRM197-NPs loaded with loperamide were intravenously injected in mice to test their ability to trigger nociceptive effects. Five hours post administration, the analgesic effect reached 35% and remained high for 2 days. Whereas, the control groups, namely free loperamide and unloaded CRM197-NPs, were unable to trigger analgesic effect. The untargeted loperamide-loaded NPs showed reduced analgesic activity with maximum possible effect (MPE) values between 5 and 10% [127].
Brain inflammation is a critical condition observed in most neurodegenerative diseases [128,129,130], promotes significant alterations in the BBB, including enhanced leakage of this structure, further increasing neuroinflammation and brain edema [95, 131, 132]. Some studies explored this inflammatory status to target therapies to the brain, namely by targeting specific markers of inflammation in the endothelium. In particular, Marcos-Conteras and colleagues developed NPs loaded with mRNA of thrombomodulin (a factor produced by endothelial cells that is responsible for inhibiting thrombosis, vascular leakage, and inflammation) using as targeting ligand an antibody to vascular adhesion molecule 1 (anti-VCAM-1) and compared their delivery capacity to TfR- and anti-intracellular adhesion molecule 1 (anti-ICAM1)-targeted liposomes [95]. ICAM1 is expressed in endothelial cells, including vascular endothelial cells, as a surface receptor and its expression is described to be enhanced in pathological conditions [133]. Regarding VCAM-1, this receptor is specifically expressed on the surface of vascular endothelial cells and was described as overexpressed in neuroinflammation, serving as one of the initial players to this process [134]. The delivery capacity of the NPs was tested in C57Bl/6 mice with acute brain inflammation induced by microinjection of TNFα in the striatum. The brain accumulation of liposomes using anti-VCAM-1 as targeting ligand was 27- and 8-fold enhanced compared to liposomes with anti-TfR and anti-ICAM1, respectively. Additionally, lipid NPs conjugated with anti-VCAM-1 and loaded with mRNA of thrombomodulin selectively accumulated in the inflamed brain and the de novo expression of the cargo mRNA resulted in alleviation of TNFα-induced brain edema [95]. Additionally, to improve the targeted delivery, after overcoming the BBB it is important to direct NPs to specific cells in the brain parenchyma. In this regard, several strategies exploring the specific recognition by targeting ligands of the different resident cells in the brain, namely neurons, astrocytes, microglia, oligodendrocytes, and neural stem cells, (Fig. 4) have been developed and will be discussed in the following sections.
Cell specific targeting. The presence of specific receptors or overexpression of certain receptors on the cell surface may be explored to promote a targeted delivery of the NPs to such cells. NPs formulated with a specific targeting ligand are unable to enter cells lacking the specific receptor for the targeting ligand as illustrated by the purple cell. On the other hand, the NPs are able to specifically deliver its cargo to the cells expressing the receptor specific for the targeting ligand, illustrated by the gray cell
Targeting brain tumors
The most common primary malignancy in the CNS is glioma, which, due to its infiltrative growth and difficulty to be removed surgically, is associated with poor prognosis and short survival rates [135, 136]. In this regard, extensive work has been done aiming at the development of anti-cancer medicines capable to overcome the BBB and target glioma using NPs as drug carriers [137,138,139,140]. Interestingly, TfR and LDLR are described to be overexpressed in glioma cells and in endothelial cells of the BBB, marking them attractive targets in the development of such therapies [141,142,143,144,145]. Beside the challenge to overcome the BBB, glioma therapy also faces the hurdle to penetrate the tumor. As so, Zhu and colleagues developed docetaxel-loaded nanomicelles coupled with two targeting ligands, Angiopep-2 and TAT [146]. As discussed above, Angiopep-2 is a peptide that targets LDLR, while TAT is a cell penetrating peptide (CPP). TAT was linked to a short PEG2000, shielded by a longer PEG6000 to avoid unspecific cell penetration during circulation in the bloodstream. Authors argue that after coupling of Angiopep-2 to its target receptor, the close contact between NPs and endothelial cells triggers the effect of TAT, enhancing the crossing of the BBB and further accumulation in the glioma [146]. Several different ratios of the two ligands in the NPs were tested and the combination of 20 mol% of Angiopep-2 with 10 mol% of TAT resulted in higher cell uptake of the NPs compared to single targeted Angiopep-2 micelles and non-targeted micelles. To study the pharmacokinetics of the NPs, authors labeled the docetaxel-loaded micelles with Cy-5 and injected them into Balb/C mice. Comparing to free drug, all micelles (non-, double- or single-targeted) presented over 10-fold higher circulation times. Moreover, the double-targeted NPs exhibited more pronounced drug delivery to the brain. Importantly, the accumulation observed in peripheral organs for double-targeted NPs was relatively low, indicating that the shielding of TAT with PEG was successful. Regarding antitumor efficacy of the double-targeted NPs, the formulation was injected in Balb/C nude mice bearing an orthotopic U87MG glioma. The docetaxel loaded double-targeted NPs were more efficient in inhibiting tumor growth, resulting in pronounced reduction of body weight loss, and increase in survival time up to 2-fold, with residual damage of peripheral organs [146].
Zhu and colleagues also established a formulation based in reduction-sensitive Polycaprolactone (PCL) micelles, functionalized with cyclic RGD peptide, to deliver DOX to U87MG glioma xenografts [147]. cRGD has high affinity for αv β3 integrins, which are described to be highly expressed on malignant tumor cells like U87MG [148, 149]. Beside the lack of a targeted approach, the slow drug release from their vehicle also causes poor efficacy of antitumor therapy [150,151,152]. Hence, the authors took advantage of the reductive environment in cancer cells [153, 154], to develop micelles with a S-S (disulfide) linker between PCL and PEG in order to enhance the NPs destabilization once inside the cancer cells and consequently promote DOX release. DOX release in U87MG cells was 2.3- and 4-fold increased for cRGD/PEG-SS-PCL micelles compared to non-targeted PEG-SS-PCL and reduction insensitive cRGD/PEG-PCL micelles, respectively [147]. In nude mice xenotransplanted with U87MG cells, cRGD/PEG-SS-PCL and cRGD/PEG-PCL micelles exhibited 2.2-fold increase accumulation in the tumor site compared to non-targeted PEG-SS-PCL micelles (4.38% ID/g and 4.12% ID/g VS 1.99% ID/g, respectively), with lower DOX accumulation in liver and heart. Moreover, the DOX signal at the tumor site for cRGD/PEG-PCL micelles was weaker than the signal for cRGD/PEG-SS-PCL, indicating an enhanced DOX release promoted by the latter micelles. Regarding tumor growth, cRGD/PEG-SS-PCL significantly inhibited tumor growth by 50% compared to cRGD/PEG-PCL and PEG-SS-PCL micelles [147], demonstrating the therapeutic efficiency of DOX delivered by the cRGD/PEG-SS-PCL micelles.
The dysregulation of gene expression in glioblastoma cells, namely of microRNAs like miR-21, has been associated with tumor development and progression [155]. As so, modulation of these miRNAs with oligonucleotides (ODNs) has been demonstrated to reduce migration and proliferation of glioblastoma cells and increase the cytotoxic effect of anticancer drugs [156, 157]. With this in mind, Costa and colleagues developed stable nucleic acid lipid particles (SNALPs) loaded with anti-miR-21 ODNs and using chlorotoxin (CTX) as targeting ligand [158]. CTX is reported to bind to matrix metalloproteinase 2 (MMP-2), which is considerably overexpressed in glioblastoma compared to normal tissues [159]. Using FAM-labeled anti-miR-21 ODNs in CTX-targeted and non-targeted SNALPs, the authors observed an almost 10-fold increase in fluorescence signal for CTX-SNALPs compared to non-targeted NPs, indicating that CTX significantly increases the internalization of SNALPs by U87MG cells. Furthermore, CTX-SNALPs promoted a 5-fold reduction in miR-21 expression in these cells compared to non-targeted SNALPs, which had no effect on miR-21 expression. Interestingly, miR-21 silencing resulted in increased expression of PTEN and PDCD4, two tumor suppressors modulated by miR-21 [160, 161]. Moreover, a reduction in the antiapoptotic effect, by a 2-fold increase in caspase 3/7 activity, was also observed. For in vivo experiments, CTX- and non-targeted SNALPs were administered into a glioblastoma mouse model, established through GL261 cell (mouse glioblastoma cell line) injection in the mice brain. A 2-fold accumulation of CTX-SNALPs compared to non-targeted particles was observed in the transplanted glioblastoma cells [158].
Up to 20% of cancer patients will develop brain metastases, leading to poor prognosis and reduced survival rates with current state-of-the-art treatments [162,163,164]. Pharmacological access to these brain metastases is a major hurdle, with reported drug concentrations 10 times lower in brain metastases compared to other metastases, which is explained in part by the presence of the BBB [165, 166]. Prostate-specific membrane antigen (PSMA) is a receptor described to be overexpressed in BBB endothelial cells of newly formed vasculature feeding the brain metastases, while PSMA detection on regular endothelial cells of the BBB is residual [167, 168]. Taking advantage of this different PSMA expression, Ni and colleagues developed PLGA-NPs employing a double-targeting system approach. Thus, NPs were conjugated to the small molecule ACUPA, which has been described as an efficient targeting ligand for PSMA [169, 170], to target the brain metastases endothelial vasculature, and the peptide cyclic TT1 (cTT1) which has demonstrated tumor-targeting abilities [162, 171]. The in vivo evaluation of the NPs was performed in mice bearing breast cancer cell metastases (BCBM), induced by intracardiac injection of 231Br cells (human breast cancer cell line). The NPs were loaded with DOX or Lapatinib (LAP); both types of NPs were co-injected to achieve synergistic activity between both drugs. After injection in BCBM mice, ACUPA (A)-NPs and A-NPs-cTT1 enhanced brain accumulation, while no significant accumulation was observed in peripheral organs. Moreover, treatment with DOX and LAP loaded A-NPs-cTT1 led to tumor growth reduction compared to free drug and non-targeted NPs. Finally, animals treated with A-NPs-cTT1 had an extended median survival time (44 days) compared to saline (25 days), free combination (29 days), non-targeted NPs (29 days), A-NPs (33 days), and NPs-cTT1 (32 days) [162].
Targeting neurons
Neurons are specialized brain cells responsible to process and transmit information to other cells via electrical and chemical signals [172]. Therapies that specifically target these cells are particularly important since they are the major cell type affected in neurodegenerative diseases [173, 174]. Typically, neurodegenerative diseases affect one specific subset of neurons, leading to the dysfunction of specific brain regions [174]. For example, neurons from the hippocampus and the cerebral cortex, which mostly express M1 and M2 muscarinic acetylcholine receptors, are the most affected in AD [175], while neurons from the striatum, which in turn express more M4 muscarinic acetylcholine receptors, are more affected in PD [176]. Given these differences between neurons of different brain regions, it is important to select an appropriate ligand that is able to target the specific cells in the brain aimed to be treated [20]. Although challenging, some work has been done in order to develop NPs that specifically target neurons in the context of several neurodegenerative diseases [20, 177,178,179].
Neurotensin neuropeptide has been demonstrated to be specifically internalized by neurons via receptor-mediated uptake [96]. To target neurons, Hsieh and colleagues coupled Neurotensin to graphene oxide NPs, functionalized with polyethyleneimine (PEI) in order to obtain positively charged NPs [97]. Taking advantage of external destabilization of the cellular membrane using near-infrared (NIR) laser irradiation, the mentioned NPs were used for plasmid DNA (pDNA) delivery specifically into neurons. In vitro, the described system was able to deliver pDNA in PC-12 cells differentiated into neuron-like cells. Upon intracerebral injection in the caudate nucleus of C57Bl/6 mice, the NPs not coupled to neurotensin transfected mostly glial cells. Whereas, neurotensin-coupled NPs transfected mostly neurons [97].
Park and colleagues compared PEGylated neurotensin-coated PEI NPs (NT-PEI) with Tet-1-coated NPs [98]. Tet-1 is a peptide with the binding characteristics of the tetanus toxin, which interacts specifically with motor neurons and has the ability to undertake retrograde transport to the cell soma [98]. The NPs (NT-PEI, Tet1-PEI, and PEI (control)) labeled with the YOYO-1 fluorophore were added to neuron-like differentiated PC-12 cells. Flow cytometry analysis revealed that the PEI-treated cells had a similar fluorescence profile as untreated cells (0.6% of cells). While cells treated with the targeted NT-PEI and Tet1-PEI NPs presented 12.7% and 16.3% higher fluorescence levels, respectively. Furthermore, as the Tet1-PEI NPs revealed higher binding affinity to neuron-like cells, it was also demonstrated, through confocal microscopy, that neuronal cultures internalize the Tet1-PEI NPs [98].
The Tropomyosin receptor kinase B (TrkB) is a receptor abundantly expressed by neurons, being activated by BDNF and internalized upon activation. This receptor is key to neuronal survival, plasticity, and neuroregeneration [180]. Therefore, it might be an interesting entrance gate in neurons. Accordingly, Huang and associates developed a screening platform for aptamers that target this receptor [99]. The C4-3 aptamer was identified as an agonist for TrkB and was tested in primary cultures of embryonic rat cortical neurons. Data revealed an increase in phosphorylated TrkB (p-TrkB) (the activated form of this receptor), as well as increased neuroprotection when the cells were deprived of supplements in their culture media [99]. To test the agonist activity of C4-3 in vivo, this aptamer or a scrambled (control) aptamer were injected into the hippocampus of adult mice. Increased p-TrkB levels were observed in the hippocampus of C4-3-injected mice, which was not detected in mice injected with the scrambled aptamer, demonstrating the agonist activity of C4-3 in vivo [99]. In line with this work, Xu and colleagues developed IKRG, a tetra peptide that mimics BDNF function and interacts with TrkB promoting its internalization, to be used as a targeting ligand for neurons in polymeric polycaprolactone (PCL) NPs functionalized with PEG [100]. In a proof-of-concept study, the authors started to evaluate the uptake of PEG-PCL NPs functionalized with IKRG to selectively target TrkB. The ability of these NPs to be internalized by TrkB-expressing (PC-12) and non-expressing (HeLa) cells was tested. Data indicated that IKRG-NPs were only internalized by TrkB-expressing cells. Furthermore, the authors evaluated the ability of these NPs to deliver VO-OHpic, an inhibitor of PTEN (Phosphatase and tension homolog deleted on chromosome 10), in order to promote neuroregeneration in peripheral neuropathies. For this, the NPs were tested in primary cell cultures obtained from the dorsal root ganglion of C57Bl/6 mice, composed of neurons, Schwann cells, fibroblasts, and glial cells. Successful and preferential internalization of the IKRG-NPs in neurons was reported, as demonstrated by the 2-fold increase in the co-labeling of NPs with TUJ-1 (a neuron-specific marker), compared to untargeted NPs [100].
Lopes and colleagues tested a non-toxic carboxylic fragment of the tetanus neurotoxin heavy chain with 54 kDa and neurotropic properties, which is able to undergo active retrograde transport after peripheral administration [101,102,103]. In this work, the authors took advantage of the neuron-targeting properties of this fragment to direct polymeric NPs composed by thiolated trimethyl chitosan, loaded with pDNA encoding for BDNF. These NPs were tested in a mouse model of peripheral nerve injury, in order to restore enervation and neuroregeneration after intramuscular administration [103]. This delivery system promoted a significant expression of BDNF in neurons, compared to vehicle or non-targeted NPs, followed by neuroregeneration and functional recovery after injury. Additionally, data revealed an increase in the expression of neurofilament heavy chain (associated with neuroregeneration) and GAP-43 (a protein associated with axonal growth) proteins in the site of injury, a significantly higher density of myelinated axons, increased pAKT expression, and enhanced neurite outgrowth and density [103], demonstrating the targeted delivery potential of this fragment of the tetanus neurotoxin.
Cell-penetrating peptides (CPP) are small, relatively non-toxic peptides (with less than 30 aminoacids) that were discovered 30 years ago and since then have been used to deliver different kinds of cargo to cells, including pDNA, small interfering RNA (siRNA), viruses, small molecules, and even therapeutic proteins and NPs [181]. These peptides can be derived from natural proteins (such as viral and antimicrobial proteins), chimeric, or completely synthetic [181]. The exact mechanism of how CPP are able to enter the cell is still a matter of debate. Endocytosis and direct penetration of the cell membrane are the two more likely cell entry mechanisms for CPP and are highly dependent on the type of CPP, its concentration, cargo, and the cell type [181]. For example, one of the first CPP to demonstrate the ability to enter differentiated neurons was a DNA-binding peptide, a 60 aminoacids region of the antennapedia homeobox protein (pAntp) from Drosophila [182]. Moreover, Santos Rodrigues and co-workers tested the ability of liposomes functionalized with transferrin and CPP to accumulate in different cell types (endothelial cells, astrocytes, and neurons) [104]. In this experiment, three different CPPs, Mellitin (Mel), Kaposi fibroblast growth factor (kFGF), and a conjugation of the penetration accelerating sequence (Pas) with the arginine-rich peptide R8 (PasR8), were tested together or not with transferrin. Mel is a 26 aminoacids cationic peptide derived from bee venom, which causes the rearrangement of the cell’s plasma membrane to form pores upon contact, facilitating the entry of cargo into the cell [183, 184]. kFGF is a hydrophobic peptide with the ability to non-covalently bind to DNA by complexation, protecting the cargo from nucleases, and successfully delivering it to cells [185, 186]. Pas is also a hydrophobic peptide (FFLIPKG) that when added to the arginine-rich R8 peptide forms a hybrid peptide with enhanced carrier abilities and capacity to evade lysosomes [187, 188]. The ability of the functionalized liposomes to efficiently deliver pDNA encoding for green fluorescent protein (GFP) to neurons isolated from newborn rats was evaluated 48 h after incubation. Interestingly, liposomes with dual functionalization (conjugated with two ligands) transfected more cells than single-functionalized liposomes conjugated with one of the CPPs. Neurons displayed 5% of transfection after incubation with non-functionalized liposomes vs. 7%, 18%, 8%, 20%, 6%, and 10% of transfection with liposomes functionalized with Mel, Mel + Tf, kFGF, kFGF + Tf, PasR8, and PasR8 + Tf, respectively. Furthermore, liposomes were loaded with lissamine rhodamine, administered in the tail vein of C56Bl/6 mice, and biodistribution was evaluated through the relative fluorescence intensity measured using near-infrared (NIR) imaging. The fluorescence in the brain of the mice injected with the liposomes functionalized with kFGF + Tf was increased. The latter NPs resulted in higher brain accumulation (5.7% of the injected dose/g [ID/g]), as compared with 2.3%, 2.7%, 3.2%, 2.1%, and 3.7% of brain accumulation obtained for liposomes conjugated with kFGF, Mel, Mel + Tf, PasR8, and PasR8 + Tf, respectively. Despite these encouraging results, a significant accumulation of the NPs in the liver (14.6% ID/g), kidneys and lungs (4.8–10.4%), hearth (5.4%), and spleen (3.2%) was also reported [104]. These authors also tested the conjugation of liposomes with Tf and other CPPs, such as the vascular endothelial-cadherin-derived peptide (pVec), the pentapeptide QLPVM (QL), and the HIV-1 trans-activating protein (TAT) [105]. pVec is an 18 aminoacids amphipathic peptide, which presents a hydrophilic end that interacts with the cell membrane and another hydrophobic end that destabilizes the membrane allowing the entry of the CPP into the cell [189, 190]. QL is a hydrophobic pentapeptide derived from the Bax-binding domain of the Ku-70 protein that has cell permeability ability [191, 192]. TAT is a cationic peptide that was the first CPP to be characterized [193]. TAT owes its cell-penetrating capacity to its positive charges that interact with the negative charges of glycosaminoglycans present at the cell surface [194]. Using the same methodology, it was evaluated the transfection ability of single-functionalized (with one of the 3 CCPs) and dual-functionalized (Tf and one of the 3 CPPs) liposomes loaded with pDNA encoding for GFP. As previously observed, the dual-functionalized liposomes outperformed their single-functionalized counterparts. Moreover, TAT (single and dual) functionalized liposomes demonstrated the best delivery capacity. The number of transfected neurons was 4% for non-functionalized liposomes compared with 7%, 10%, 6%, 8%, 9%, and 13% for liposomes functionalized with pVec, pVec + Tf, QL, QL + Tf, TAT, and TAT + Tf, respectively [105]. In vivo, a brain accumulation of 7.7% for TAT + Tf-liposomes and 3.1% for TAT-liposomes was reported. Additionally, the authors also reported a considerable accumulation of these liposomes in the liver and kidneys, and TAT-liposomes were also found accumulated in the lungs [105]. Despite interesting, the poor tissue specificity observed when applying CPPs in delivery systems [195] raises concerns regarding accumulation in off-target cells.
The glycopeptide 7 (g7) has brain-targeting ability. This peptide was engineered from the opioid peptide MMP-2200 through the replacement of the aminoacid Tyr (responsible for the opioid effect) by Phe [107, 196]. Thus, g7 peptide conjugated in PLGA NPs was tested to overcome the BBB and accumulate in neurons [106]. These NPs were injected, via intraperitoneal (i.p.) administration, in C57Bl/6 mice and a brain accumulation of up to 10% of the injected dose was reported. Furthermore, neurons were the main cell type targeted by the NPs, although affinity to microglia and minor co-localization of the NPs with astrocytes was also detected. Interestingly, region-specific brain accumulation of the NPs was reported, namely into some subtypes of neurons, such as neuropeptide Y (NPY) and glutamic acid decarboxylase (GAD) positive interneurons. Moreover, interaction studies revealed a clathrin-dependent internalization mechanism in the NPs’ internalization by the neurons [106].
The rabies virus glycoprotein (RVG) is the glycoprotein responsible for the neurotrophic nature of the rabies virus [197]. The receptor of the nervous system responsible for the interaction with RVG is still a matter of debate; nevertheless, the nicotinic acetylcholine receptor (nAChR) was the first receptor identified to play a key role in this interaction [198]. Derivates of the RVG have been explored to target NPs to the brain. These peptides are shorter versions of the original RVG, which retain the capacity to target and be internalized by neurons. For example, in the context of Machado-Joseph disease (MJD), a neurodegenerative disease presenting extensive neuronal death caused by the mutant ataxin-3 presence in neurons, our group developed RVG-9r-conjugated liposomes encapsulating siRNA to silence mutant ataxin-3 [108]. RVG-9r, a ligand derived from RVG with 9 arginine residues, was used as a brain-targeting ligand that enables the BBB transpose. The biodistribution data of RVG-9r-liposomes (encapsulating the near-infrared dye (NIR) indocyanine green (ICG)) IV injected in mice showed that the RVG-9r targeting ligand increased by 20% the brain accumulation of liposomes, compared to a control ligand. The RVG-9r targeting ligand also led to 25% and 30% decrease in liposomes accumulation in the heart and lungs, respectively, compared to the control ligand. Furthermore, the administration of RVG-9r-liposomes encapsulating siRNA for mutant ataxin-3 silencing, in an MJD transgenic mouse model [199], resulted in 30% reduction in mutant ATXN3 mRNA, as compared with RVG-9r-liposomes encapsulating a control siRNA [108]. These data indicate that RVG-9r-mediated delivery of liposomes encapsulating gene silencing therapies is an efficient approach to silence mutant ataxin-3 in MJD. Moreover, a peptide derived from RVG with 29 amino-acids (RVG-29) was used by Chen and colleagues to target human serum albumin NPs loaded with the antifungal drug itraconazole (ITZ) to treat brain fungal infections [109]. NPs conjugated or not with RVG-29 were injected into the caudal vein of adult mice. Significantly increased levels of ITZ in the group of animals injected with RVG29-conjugated NPs, as compared to control/untargeted liposomes, was reported. Namely, 2 h post-injection, 100 ng of ITZ/g of brain tissue was detected for the untargeted NPs. Whereas, 200 ng of ITZ/g of brain tissue was detected for the RVG-29-conjugated NPs. These data showed that RVG-29-conjugated NPs could be exploited as a brain delivery system [109].
Despite the encouraging data reported for brain-targeted NPs, these reports also highlight the need to develop more specific brain-targeting ligands and/or NPs to avoid their accumulation in peripheral organs, which results in loss of NPs in undesired sites and also to potential off-target effects (Table 3).
Targeting astrocytes
Astrocytes have key functions in neurotrophic, physical, and metabolic maintenance to neurons, and are indispensable in neurotransmission, namely in supporting and modulating synapses [200,201,202,203,204]. Additionally, astrocytes contribute to immune surveillance in the brain becoming activated in insults, infections, and brain diseases, releasing inflammatory mediators [205, 206]. Several neurodegenerative diseases, such as AD, PD, Huntington’s disease (HD), and Amyotrophic Lateral Sclerosis (ALS), affect astrocytes (reviewed in [207]), requiring their treatment and consequently drug targeting. The most employed delivery system targeted to astrocytes are viral vectors, since virus can be engineered to have pseudo-tropism for astrocytes and astrocyte-specific promoters can be used to guarantee the gene expression in these cells [208, 209]. Although the development of drug-delivery NPs that specifically target astrocytes is still limited, astrocytes present a rich repertoire of receptors, which may be used to specifically target drugs and NPs to them.
Aquaporin 4 (AQP4) is a water channel preferentially expressed on astrocytes and displays a wide range of functions, namely, regulation of potassium and calcium concentrations, osmotic pressure, waste clearance, neuroinflammation, and cell migration and synaptic plasticity [210, 211]. Interestingly, AQP4 is strongly expressed on the surface of astrocytes in the context of neurodegeneration [212]. Taking advantage of the preferential expression of this water channel on astrocytes, an anti-AQP4 antibody was conjugated with polymeric poly(glycidyl methacrylate) (PGMA) NPs to deliver the anti-oxidant resveratrol to tackle oxidative stress in the context of neurodegenerative diseases [110]. Resveratrol has shown poor bioavailability and rapid metabolization in vivo [213, 214]. Thus, the authors reported the accumulation of the AQP4-targeted NPs loaded with rhodamine B in GFAP-positive astrocytes, demonstrating the anti-AQP4 antibody targeting to astrocytes. AQP4-targeted NPs loaded with resveratrol were then administered in situ after optic nerve injury induction in adult female Piebald Viral Glaxo rats. The AQP4-targeted NPs were found to accumulate inside astrocytes and to effectively deliver resveratrol when administered to the site of injury. Furthermore, the targeted NPs were also able to rescue oxidative damage in the site of injury, as demonstrated by the reduction of immunoreactivity of 8-hydroxy-2’-deoxyguanosine (8OHdG) (a hallmark of oxidative damage in nuclear and mitochondrial DNA), as compared to non-targeted or non-loaded NPs [110]. Therefore, this study demonstrates the ability of the anti-AQP4 antibody to target NPs to astrocytes.
In another approach, the D4 monoclonal antibody that recognizes the GFAP protein preferentially expressed by astrocytes [215, 216], was linked to PEGylated liposomes [111]. The DiI fluorescent dye was integrated into the liposome’s bilayer allowing the visualization of the targeted NPs interaction with the astrocytes in vitro, through fluorescence microscopy. The specificity of the D4 antibody-conjugated liposomes to specifically interact with astrocytes was confirmed, since non-targeted or liposomes conjugated with a Control (non-specific) antibody were not visualized in the astrocytes. However, when administered to male Wistar rats by IV administration in the femoral vein, these NPs were unable to reach CNS astrocytes, mainly due to their inability to cross the BBB [111]. This work opens the avenue to speculate that these NPs may be useful in the context of diseases that present a weakened BBB or, furthermore, to functionalize these NPs with a second targeting ligand to allow their BBB crossing. In line with the former example, chitosan NPs functionalized with two commercially available antibodies, one targeting the transferrin receptor (widely expressed on BBB endothelial cells) and another targeting the bradykinin B2 receptor. Bradykinin B2 receptor (B2R) is associated with vasodilatation, neuroinflammation, and glucose uptake [217, 218]. B2R is not exclusive to astrocytes but is highly expressed in these cells [219, 220]. Therefore, an antibody anti-BR2, which is rapidly internalized after binding with a specific ligand, was employed in combination with transferrin in chitosan NPs to aid in overcoming the BBB [112]. These double-targeted chitosan NPs were tested in a BBB in vitro model to deliver siRNA to inhibit HIV-1 replication in astrocytes. SiRNA anti-SART3 and -hCycT1 genes, both important for HIV-1 replication in astrocytes, were employed. It was reported that the dual-targeted NPs penetrated across the human cerebral microvascular endothelial cells (hMCEC/D3) and accumulated in the human astrocytoma cells (U138-MG). This cell targeting resulted in a 6 times higher accumulation of siRNA in U138-MG cells, as compared to non-targeted NPs. Furthermore, the presence of the siRNA in these cells resulted in a gene knockdown of 81% and 67% for SART3 and hCycT1 mRNA, respectively [112].
Considering the small development of NPs specifically targeting astrocytes, it is of great interest to further explore more receptors that are exclusively or preferentially expressed by astrocytes in order to use them in NPs. For example, the N-acetylaspartylglutamate (NAAG) receptor, also known as metabotropic glutamate receptor 3 (mGluR3), is expressed in both neurons and astrocytes but their expression is enriched in astrocytes [221]. This receptor is activated by the neurotransmitter NAAG peptidase released by stimulated neurons [222] and its activation is believed to influence neuron and neurovascular stimulation in the context of schizophrenia and other neuropathies [222]. Moreover, a recent review highlighted the importance of some astrocyte receptors and transporters in the context of AD [223]. In particular, the excitatory aminoacid transporters EAAT1 and EAAT2, which although not exclusively expressed by astrocytes are in much larger amount in these cells [224]. In fact, EAAT are more active on astrocytes since they are responsible for 80% of the glutamate uptake [225]. Targeting these receptors would not only be promising to direct NPs to astrocytes but represents as well an opportunity to treat excitotoxicity in the context of neurodegenerative diseases [223, 226, 227]. Furthermore, the protein S100β is a calcium-binding protein abundantly expressed by mature astrocytes with the ability to be internalized [228, 229]. Thus, the coupling of targeting ligands for S100β to NPs may also present a capable strategy to target astrocytes for drug delivery. Finally, the active targeting of the cannabinoid receptors CB1 and CB2 present in glial cells, such as astrocytes and microglia, may help to control the neuroinflammation characteristic of several neurodegenerative diseases, by modulating the expression of inflammatory cytokines in these cells and their migration [230]. However interesting, NPs with targeting ligands that direct them to these receptors are yet to be explored.
Targeting microglia
Despite being CNS resident immune cells, microglia do not develop from the neuroectoderm like other neural cells. They are derived from the yolk sac primitive macrophages and migrate to the CNS during embryonic development [231], representing 5 to 12% of all cells in the healthy CNS [232]. Physiologically, microglia have surveillance phenotype characterized by a ramified morphology and are the first line of defense against pathogens, promoting brain homeostasis and repair [233, 234]. Moreover, these cells have key functions in several processes such as neurogenesis, neural circuits refinement, and mediation of neurotransmission and synaptic pruning [235, 236]. However, in situations where the homeostasis in the brain is compromised, such as neurodegeneration or sustained inflammation, microglia changes their phenotype to an ameboid-like structure and alters their secretome, upregulating the expression of several cytokines, interleukins, and complement factors, enhancing and perpetuating neuroinflammation [232, 237]. Considering the characteristics of microglia as first responders to changes in brain homeostasis and their role in neuroinflammation, they appear as an interesting target for brain therapies. Indeed, several publications demonstrate a high internalization ability of activated microglia compared to non-activated [238,239,240]. Nonetheless, given the intrinsic phagocytic nature of microglia, concerns have been raised considering the specificity of microglial uptake of NPs, since NPs may just be recognized as pathogens [241, 242].
Microglia present a wide range of receptors, due to their surveillance function, so NPs can be tailored to take advantage of these receptors. Innate immune cells, such as microglia, have Pattern Recognition Receptors (PRRs) that have been used to target them [243]. These include Toll-Like Receptors (TLR), Receptors for Advanced Glycation Endproducts (RAGE), and Scavenger Receptors [244,245,246,247].
Choi and colleagues designed ceria-zirconia NPs (composed of Cerium and Zirconium) that specifically targeted microglia by conjugation with antibodies anti-CD11b (a receptor expressed on the surface of microglia and macrophages [248, 249]). In this work, the authors hypothesized that oxidative stress and inflammatory activation of microglia plays a role in neuropathic pain by sensitizing neurons, and tackled this by taking advantage of the anti-oxidant proprieties of ceria, particularly Ce3+ [250]. CD11b-targeted and non-targeted NPs labeled with FITC were incubated with microglia cells isolated from C57Bl/6 pups. Authors reported a higher percentage of FITC-positive cells with targeted NPs compared with non-targeted NPs, 80% and 40%, respectively. Regarding the induction of oxidative stress in microglia using tert-butyl hydroperoxide, a more pronounced reduction of ROS was observed when the CD11b-targeted NPs were added to the culture medium as compared to non-targeted NPs. Additionally, in cells pre-treated with lipoteichoic acid to induce the expression of iNOS, IL-6, and IL-1β (related to oxidative stress and inflammation) the treatment with CD11b-targeted NP, led to a 95%, 86%, and 91%, respectively, reduction in the mRNA levels of these genes. While the treatment with non-targeted NPs was only able to achieve reduction levels of 82%, 63%, and 71%, respectively. Moreover, CD11b-targeted and non-targeted NPs were administered using intrathecal injection in a neuropathic pain C57Bl/6 mouse model (spinal nerve transection). It was described a strong correlation between the FITC signal and the microglia-specific marker Iba-1, with co-localization observed in 84% of cells. Whereas, co-localization with the astrocyte marker GFAP and the neuron marker MAP2 was only detected in 26% and 11% of cells, respectively. Finally, the authors also observed a reduction in the hypersensitivity of these animals after treatment with CD11b-targeted NPs, compared with animals treated with non-targeted NPs [113], demonstrating the targeting ability of these NPs to microglia.
Despite these promising results, more targeting receptors and proteins specific to microglia are required to be explored in NPs development. For example, scavenger receptors are receptors present in cells of the immune system, having a wide range of functions, such as cargo transport inside the cell, lipid transport, recognition and removal of altered lipoproteins, and pathogen clearance [251]. Examples of scavenger receptors expressed by microglia are SR-A1 and CD36, which are used by microglia to bind and clear β-amyloid fibrils in the context of Alzheimer´s disease [252, 253]. However, these receptors are not fully specific of microglia since they are also expressed in macrophages, platelets, and endothelial cells. Therefore, careful consideration must be done when considering these receptors as targets for NPs targeting [254, 255].
Other interesting target is the transmembrane lectin sialic acid-binding immunoglobulin-like lectin H (Siglec-H) that in mice is able to discriminate microglia from CNS-bound macrophages and monocytes more accurately than CD11b or Iba-1 [256]. Further characterization (e.g. binding ligands and specificity, internalization mechanisms, etc.) and the discovery of a human homolog of this receptor may create the opportunity to design NPs to deliver therapies specifically to microglia [256]. Another receptor widely characterized and acknowledged to be microglia specific is the Cx3Cr1 receptor, also known as fractalkine receptor or G-protein coupled receptor 13 (GPR13) [257]. This receptor binds to the chemokine CX3CL1, also known as neurotactin or fractalkine. Moreover, the receptor P2 × 4 is also an interesting potential target, since it is widely expressed in microglia and neurons but has a 3-fold increased expression in microglia under pathological conditions, such as neuroinflammation, hypoxia, and neuropathic pain [258, 259]. Although widely expressed in microglia, to this day there are no NPs developed to specifically target these receptors in these cells.
Targeting oligodendrocytes
Oligodendrocytes are specialized cells of the CNS responsible for the myelination of neurons [260, 261]. The myelin sheath is a highly complex structure composed of 80% lipids and 20% proteins [261, 262] that provides insulating properties to neuronal axons which facilitate electrical signals transmission [261].
Given their unique characteristics, oligodendrocytes are among the most vulnerable cells in the CNS, and demyelination of axons is one of the hallmarks of neurodegeneration [260, 262]. As so, one potential therapeutic approach is to promote remyelination by inducing oligodendrocyte progenitor cells (OPC) to mature into oligodendrocytes and remyelinate the axons [263]. In order to promote remyelination by targeting OPC, Rittchen and colleagues developed PLGA NPs loaded with leukemia inhibitory factor (LIF), a robust pro-remyelination factor [114]. To achieve targeted delivery of the NPs to OPC, the authors used as targeting moiety antibodies anti-NG-2 chondroitin sulfate proteoglycan, a proteoglycan predominately expressed in OPC [264]. Three days after a 24 h treatment with PLGA-LIF NPs targeted to NG-2, rat OPC cultures presented a 33% increase in cells expressing myelin basic protein (MBP), a marker of mature oligodendrocytes, compared to non-targeted PLGA-LIF NPs. The remyelination potential of these NPs in vivo was tested in a mouse model of focal demyelinating lesion, in which the myelin toxin lysophosphatidylcholine (LPC) was administered to the corpus callosum by stereotaxic injection [114]. Eight days after the lesion, NG-2-targeted and non-targeted PLGA-LIF NPs were injected in the animals, and the effects were assessed 10- and 17-days post-administration. Using electron microscopy, a significant increase in the percentage of myelinated fibers per lesion and significantly thicker myelin sheaths were observed in animals treated with NG-2 targeted PLGA-LIF NPs compared to animals that received non-targeted NPs [114].
Interestingly, immunoglobulin M antibodies demonstrated the ability to target reactive oligodendrocytes and promote remyelination in a multiple sclerosis (MS) mouse model [265]. Inspired by this work, Tuerk and colleagues tried to identify DNA aptamers with the same binding affinity to myelin as the immunoglobulin M antibodies [266]. Authors identified a 40-nucleotide guanosine-rich DNA aptamer with anti-myelin proprieties when in a G-quadruplex structure (LJM-3064) [267]. In order to obtain the G-quadruplex structure, the biotinylated DNA aptamer was conjugated to a streptavidin core [268], resulting in a structure the authors called Myaptavin-3064 [267]. The capacity of this structure to promote remyelination in a mouse model of MS was demonstrated, but the specific interaction with oligodendrocytes was not tested [267]. In a recent work, the same group tested the affinity of Myaptavin-3064 to a human oligodendroglioma cell line (HOG) and mature oligodendrocytes differentiated from HOG cells [115]. Flow-cytometry data demonstrated that the binding of Myaptavin-3064 to HOG was increased upon differentiation with almost 90% of differentiated oligodendrocytes positive for Myaptavin-3064, while only 50% of HOG cells bound to Myaptavin-3064 with the same dose. The specificity of Myaptavin-3064 for oligodendrocytes was further confirmed with lung (L2) and kidney (BHK) cells, since flow-cytometry results indicated a residual affinity to these cells. Moreover, in primary cultures of adult rat cortical tissue, the authors identified that 97% of cells positive for the O4, an oligodendrocytes marker, were also positive for anti-streptavidin when co-cultured with Myaptavin-3064, while the co-staining was residual after culture with a control conjugate with a non-specific aptamer (LJM-3060) [115].
Another group linked the same aptamer (LJM-3064) to the surface of mouse mesenchymal stem cell-derived Exosomes to deliver cargo to oligodendrocytes [116]. In this work, LJM-3064 was employed not only as a targeting ligand for oligodendrocytes but also for the remyelinating capacity that it had demonstrated before as well [267]. The binding affinity of the exosome-aptamer conjugate (Exo-APT) was demonstrated in vitro in an oligodendrocytes cell line (OLN93). Exosomes, either targeted or untargeted with the aptamer, were then labeled with ATTO647N. Through flow cytometry analysis an increase in cell fluorescence was observed after incubation with Exo-APT compared to untargeted exosomes. Moreover, Exo-APT also promoted a significant increase in OLN93 proliferation compared to untargeted exosomes, assessed by BrdU cell proliferation assay [116]. Exo-APT or untargeted exosomes were administrated intravenously in mice before the induction of autoimmune encephalomyelitis (a mouse model commonly used to study MS [269]). A strong reduction in demyelination, a robust suppression in inflammation, and a reduction in the disease severity in animals administered with the Exo-APT were reported [116].
Taken together, despite promising, the work done so far to specifically target NPs to oligodendrocytes to treat brain diseases is still very scarce.
Targeting neural stem cells
The loss of neurons is a major hallmark of neurodegenerative diseases; thus, an approach to tackle these diseases is the replacement of dead or impaired neurons. This can be achieved by stimulating neurogenesis, a process in which new mature neural cells are produced from neural stem cells (NSC) present in endogenous niches or engrafted by cell transplantation [270, 271]. The adult brain presents regions where NSC reside, the so-called neurogenic niches. The subgranular zone (SGZ) of the dentate gyrus and the subventricular zone (SVZ) of the lateral ventricles are two well-studied niches of NSC. The activity of these niches is crucial for neuroplasticity and learning. However, studies suggest that with aging a reduction of the proliferative, migratory, and integrative capacity of NSC takes place, which severely hampers neuroplasticity [271, 272]. Therefore, targeting endogenous NSC with drugs that promote their ability to proliferate, differentiate, migrate, and integrate may be advantageous to promote the replacement of the lost neural cells [273, 274]. However, NPs targeting the neurogenic niches and NSC is a field of research poorly explored and there is a demand to find targeting ligands that specifically direct drugs to NSC.
Schmidt and associates identified ligands by phage display technology with the ability to target neural progenitor cells (NPC) [118]. In this study, the ability of random peptides from a 7mer phage library commercially available to bind and be internalized by neurosphere cultures derived from the hippocampus of adult C57Bl/6 mice was evaluated. The authors tested 130 candidates for their binding efficiency for Nestin-positive cells in vitro. QTRFLLH and VPTQSSG peptides showed 10 to 20-fold increased binding to NPC compared with other peptides. Moreover, regarding cell specificity, QTRFLLH binding to NPC was significantly higher compared to Pan02 (pancreatic cancer cells), NIH3T3 (fibroblasts), H1299 (lung cancer cells), and HEK293 (human embryonic kidney cells). As for VPTQSSG, it exhibited lower binding affinity to NPC but higher cell specificity compared to QTRFLLH, with binding affinities 10 times lower to Pan02 and NIH3T3 and residual binding to H1299 and HEK293. QTRFLLH and VPTQSSG also revealed strong uptake by NPC. As adenoviruses present low infection efficiency of NPC [117], QTRFLLH and VPTQSSG were covalently linked to an adenoviral vector (wild-type capsid) expressing red fluorescent protein (RFP) to improve the viral delivery to NPC. Through immunofluorescence microscopy, it was observed the expression of RFP inside the NPC, supporting the hypothesis that these ligands can mediate adenovirus binding and uptake by NPC. Then, these viral vectors coding for RFP and linked with either peptide were injected into the hippocampus of a transgenic mouse model expressing GFP in Nestin-positive cells (pNestin-GFP) [275]. A strong specific co-localization of GFP and RFP was detected, suggesting that the peptides are efficient in guiding the adenovirus to Nestin-positive cells; whereas, the same adenovirus but linked to an unspecific peptide, led to almost no co-localization of RFP and GFP. The percentage of cells with RFP and GFP co-localization was 83.5% for the QTRFLLH peptide and 85.6% for the VPTQSSG peptide, whereas this percentage was 15.5% for the wild-type vector without any peptide and 8.6% for the adenovirus with the unspecific peptide [118]. Thus, these data indicate that these peptides mediate specific targeting to Nestin-expressing NPC.
The neurofilament light subunit (NFL) is known to present a strong interaction with NSC of the SVZ, showing a preferential accumulation in these cells in vivo after intra-lateral ventricular injections and the ability to induce their differentiation in vitro [276, 277]. Accordingly, it was demonstrated that the tubulin-binding site of the NFL (NFL-TBS.40–63), adsorbed to the surface of lipid nanocapsules (NFL-LNC), is able to guide lipid nanocapsules specifically to NSC in the SVZ [120].
Interestingly, besides being used to target the BBB, transferrin has as well been used to target NSC. In the work by Praca and colleagues, gold nanoparticles and gold nanorods were functionalized with medium density of transferrin peptides (between 169 and 230 transferrin peptides per NP) to direct the particles to the NSC [121]. Gold-NPs, with and without transferrin functionalization, were injected in the tail vein of adult (8 weeks old) C57Bl/6 mice. Irradiation with near-infrared light (NIR) was applied 1 h after administration to transiently open the BBB. Then, the animals were sacrificed 2 h after the NPs administration and their presence in the different brain regions was analyzed using mass spectrometry. As expected, the gold-NPs functionalized with transferrin preferentially accumulated in the brain compared to non-functionalized gold-NPs. Interestingly, after NIR irradiation, gold-NPs functionalized with transferrin were significantly accumulated in the SVZ (almost 0.2% of the injected dose). Without radiation, the percentage of these NPs accumulated in the SVZ was less than 0.1% of the injected dose and the NPs were more scattered in the brain and found preferentially in non-neurogenic areas. Gold-NPs without functionalization were found only residually in some non-neurogenic regions [121].
Altogether, these data demonstrate that although interesting, the targeting of NPs to NSC and NPC is still a very unexplored field.
Limitations, successes, and strategies of brain-targeted NPs development for drug delivery
Despite the great potential of brain-targeted NPs to deliver therapeutic molecules to the brain, there is a need to better study the limitations and challenges of this strategy. In this regard, the concept of critical quality attributes (CQAs) has been established by the regulatory authorities to guide their development, characterization, and stability [278, 279]. The lipid composition of the NPs is a critical parameter to determine their proprieties and safety [280]. Therefore, the implementation of biocompatible and biodegradable materials [280,281,282] to develop safe NPs to be used in long-term therapies is key. The best composition of NPs is highly dependent on the intended use and, especially, on the cargo drug to be encapsulated [283, 284]. Physical characteristics, such as morphology, size, size distribution, surface-to-size ratio, and zeta potential are of the utmost importance to their safety and efficiency as delivery vehicles. Nonetheless, these characteristics are also highly dependent on their intended application, cargo drug, and composition. In general, the regulatory advice is that NPs should have a size lower to 100 nm [11]. In fact, smaller NPs are more easily eliminated by the kidneys, while larger NPs tend to be trapped in the lungs [282]. Regarding the size distribution, a polydispersity index (PDI) of 0.3 or below is considered adequate and reflective of a homogenous NPs population [285]. A large surface-to-size ratio (small size and a very large surface area) may lead to problems like limited drug loading, particle aggregation or friction, and a high clearance ratio [281]. The large surface area also increases their chemical reactivity, which may cause toxicity, namely through increased reactive oxygen species (ROS) production, neuroinflammation, and DNA damage [281, 286]. The zeta potential of NPs is determined by the presence of ionic lipids and/or charged surface ligands in their composition, which influences particle repulsion, aggregation tendency, and biodistribution [287]. Values between − 30 mV and + 30 mV are considered to keep stable particles suspension and enough inter-particle repulsion [278, 279]. Finally, the physical stability over time, namely particle fusion or aggregation, drug leakage, and chemical degradation of the lipids and their cargo, are also critical parameters that must be clearly evaluated several months post formulation [278, 279].
Alongside these issues, another limitation is the lack of specific targeting ligands to be used in NPs. In fact, although a preferential accumulation of the targeted NPs exists in the intended cells, some non-specific and potentially toxic accumulation in peripheral organs persists, especially in metabolizing organs such as the liver, lungs, and kidneys (Table 3). This may cause off-target adverse effects and hinder the therapeutic efficacy of the targeted therapies. The use of targets that are ubiquitously expressed through the body enhances this non-specific targeting. Ideally, a targeted drug-delivery approach for the brain using NPs should be able to overcome the BBB, specifically recognize the target cells in the brain aimed for treatment, enable endosomal escape after internalization, and release the cargo drug [288]. Thus, future research needs to focus on identifying more tissue and cell specific markers to be implemented in targeted therapies. An interesting approach to overcome this non-specificity may be the use of a double-targeting strategy. As seen in some reports targeting cancer cells [146, 162], the coupling of two different targeting-ligands to NPs can enhance their active targeting and improve their cargo delivery. Such dual targeting, where a targeting ligand is used to overcome the BBB and a second ligand to deliver the NPs to a specific cell population in the brain, can drastically improve therapeutic efficacy. Zhang and colleagues applied this strategy in the context of AD, using a peptide to target the BBB (TGN) and a second peptide (QSH) that binds to Aβ1-42 in poly(lactic acid) PLA NPs. Authors reported increased brain concentration and distribution in the Aβ1-42 plaques using the dual-targeted NPs [94]. These encouraging results, indicate that a dual targeting strategy to tackle brain diseases is a promising strategy. Nonetheless, whether it is the best therapeutic option or not it will have to be assessed on a case-by-case basis, as in some situations it will not be necessary, particularly given that some ligands may trigger this double targeting.
Furthermore, the production of targeted NPs with defined targeting, high quality and adequate for translational reproducibility remains a challenge. One of the key advantages of NPs is that their surface is highly tunable from a chemistry standpoint. In this regard the conjugation of the targeting ligand to the surface of the NPs becomes a critical aspect in the formulation [289, 290]. Two main strategies exist for the addition of targeting moieties to NPs, one-pot assembly and post-insertion through surface modifications [289]. In the one-pot assembly strategy, the targeting ligand is directly added to the lipid mixture prior to NPs formation. This is only feasible for targeting moieties able to endure exposure to organic solvents and high temperatures used during NPs production. Despite quite simple, this strategy presents a major hurdle, the orientation and density of the targeting ligand is completely unpredictable, resulting in a high percentage of ligand in the inside surface of the NPs. In the post-insertion strategy, the NPs are firstly formed and then a surface modification is performed by conjugation of the targeting ligand [289, 290]. This conjugation requires a chemical modification on the surface of the NPs by addition of functional groups that will react with reactive groups on the targeting ligands [290]. An important limitation of this strategy is the frequently observed low insertion yields of ligands in NPs [289]. Such low yields present a scalability problem since very high amounts of ligand are required to produce small amounts of targeted NPs, resulting in a very expensive manufacturing process.
Targeting ligand density in NPs defines much of the targeting abilities of the NPs since, if there is a too high ligand density, it may result in off-target binding in tissues with lower expression levels of the target receptor. On the other hand, if the density is too low, target cell uptake may be limited. Hence, targeting ligand density in NPs should match the receptor density in target cells [105]. Additionally, in vivo stability of the targeting ligands must also be addressed during NPs development [290,291,292]. In vivo, targeting ligands are subjected to a hostile environment promoted by degrading enzymes, pH, hypoxia, redox, and temperature variations. These are very important factors that might significantly impact targeted NPs therapeutic success [289].
To be successfully marketed, targeted NPs formulations need to have a large-scale manufacturing process. In addition to the issues related to ligand-NPs conjugation mentioned above, batch-to-batch variations in ligand density and stability, the choice of raw materials, synthesis processes, batch sizes, stability analyses, and documentation needs to be carefully considered [293].
The translation of a new therapy from a pre-clinical to a clinical investigation setting is always challenging [294]. For example, the most popular liposome production method is the lipid film hydration method, but the scaling up of this method from milliliters to liters batches, maintaining formulations with similar physicochemical proprieties is demanding [295]. Other methods like ethanol injection or reverse-phase evaporation are more easily adapted to an industrial setting; nevertheless, these methods face other challenges, such as optimization of particles size reduction, formulation homogeneity, and the removal of organic solvents and detergents [294, 295]. Attention must be drawn to several aspects in the early development stages to facilitate transition to an industrial setting. These include use of affordable and high grade raw materials; avoid low-yield and long synthetic reactions; avoid difficult to remove solvents and catalysts; use automation and closed circuit systems for improved safety, cost reduction, and evading errors; establish rigorous and adequate in-process and end-product quality controls; consider production risk assessment for hazardous batch contaminations and interference with the NPs formulation; use adequate methods for stability and shelf-life estimation; give special attention to formulations with a biological product, such as antibodies or proteins; and a cost-effective industrial production [293,294,295]. Overall, a multiparameter evaluation of the targeted NPs production process is needed to achieve successful scale-up manufacturing [293, 296], based in adequate in-process and final quality controls to ensure homogenous characteristics between batches and cost-effectiveness.
Conclusions
Nanovesicles hold the promise to efficiently and precisely deliver diverse therapies into the brain to tackle neurodegenerative diseases. Nonetheless, these nanoparticles need to be specifically and efficiently delivered to the brain in order to potentiate their therapeutic outcomes without causing major side effects due to their accumulation in peripheral tissues. In this review, we summarized the different targeting ligands identified to deliver nanoparticles to specific cells in the brain. The research done so far in the development of brain-targeted NPs shows promising results in the targeted delivery and treatment of brain cells. In the future, this may result in high precision medicine, with reduced adverse side effects or unwanted therapy clearance from the body. However, much room for improvement still exists for these therapies to reach their full potential in the context of neurodegenerative diseases. For example, the identification of specific cell receptors expressed exclusively by each one of the different cell types would certainly prompt this field to the desired targeted drug delivery. Hence, we believe that it is important to keep focusing research endeavors on the screening of brain cell-specific receptors and in the design of high-affinity targeting ligands to be employed in the development of brain-targeted NPs carrying therapeutic molecules.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- AD:
-
Alzheimer’s disease
- ALS:
-
Amyotrophic Lateral Sclerosis
- ApoE:
-
apolipoprotein E
- AQP4:
-
Aquaporin 4
- AZT:
-
zidovudine
- B2R:
-
Bradykinin B2 receptor
- BBB:
-
blood-brain barrier
- BDNF:
-
brain-derived neurotrophic factor
- CB:
-
cannabinoid receptors
- CNS:
-
central nervous system
- CPP:
-
Cell-penetrating peptides
- EAAT:
-
excitatory aminoacid transporters
- g7:
-
glycopeptide 7
- GAD:
-
glutamic acid decarboxylase
- GFP:
-
green fluorescent protein
- HB-EGF:
-
Heparin-binding epidermal growth factor-like growth factor
- HD:
-
Huntington’s disease
- HIV:
-
human immunodeficiency virus
- hMCEC/D3:
-
immortalized human cerebral microvascular endothelial cell line
- HOG:
-
human oligodendroglioma cell line
- i.p.:
-
intraperitoneal
- ICG:
-
indocyanine green
- ITZ:
-
itraconazole
- IV:
-
intravenous administration
- kFGF:
-
kaposi fibroblast growth factor
- LDL:
-
low density lipoprotein
- LDLR:
-
Low-Density Lipoprotein Receptor
- LIF:
-
leukaemia inhibitory factor
- LPC:
-
lysophosphatidylcholine
- MBP:
-
myelin basic protein
- Mel:
-
Mellitin
- mGluR3:
-
metabotropic glutamate receptor 3
- MJD:
-
Machado-Joseph disease
- MS:
-
multiple sclerosis
- N.A.:
-
not applicable
- N.T.:
-
not tested
- NAAG:
-
N-acetylaspartylglutamate
- nAChR:
-
nicotinic acetylcholine receptor
- NFL:
-
neurofilament light subunit
- NIR:
-
near infrared
- NPC:
-
neural progenitor cells
- NPs:
-
nanoparticles
- NPY:
-
neuropeptide Y
- NSC:
-
neural stem cells
- OLN93:
-
oligodendrocytes cell line
- OPC:
-
oligodendrocyte progenitor cells
- Pas:
-
penetration accelerating sequence
- PBCA:
-
poly(n-butyl cyanoacrylate)
- PCL:
-
Polycaprolactone
- PD:
-
Parkinson’s disease
- PEG:
-
polyethylene glycol
- PEI:
-
polyethyleneimine
- PGMA:
-
poly(glycidyl methacrylate)
- PLGA:
-
poly(lactic-co-glycolic acid)
- PRRs:
-
Pattern Recognition Receptors
- pVec:
-
vascular endothelial-cadherin-derived peptide
- QL:
-
pentapeptide QLPVM
- R8:
-
arginine-rich peptide
- RAGE:
-
Receptors for Advanced Glycation Endproducts
- RFP:
-
red fluorescent protein
- ROS:
-
reactive oxygen species
- RVG:
-
rabies virus glycoprotein
- SGZ:
-
subgranular zone
- Siglec-H:
-
transmembrane lectin sialic acid-binding immunoglobulin-like lectin H
- SVZ:
-
subventricular zone
- TAT:
-
HIV-1 trans-activating protein
- Tf:
-
transferrin
- TfR:
-
Transferrin receptor
- TLR:
-
Toll-Like Receptors
- TrkB:
-
Tropomyosin receptor kinase B
- U138-MG:
-
human astrocytoma cell line
- WHO:
-
world health organization
References
Hartl N, Adams F, Merkel OM. From adsorption to covalent bonding: apolipoprotein E functionalization of polymeric nanoparticles for drug delivery across the blood-brain barrier. Adv Ther (Weinh). 2021;4(1).
Raz L, Knoefel J, Bhaskar K. The neuropathology and cerebrovascular mechanisms of dementia. J Cereb Blood Flow Metab. 2016;36(1):172–86.
Racette BA, Willis AW. Time to change the blind men and the elephant approach to Parkinson disease? Neurology. 2015;85(2):190–6.
Frahm-Falkenberg S, Ibsen R, Kjellberg J, Jennum P. Health, social and economic consequences of dementias: a comparative national cohort study. Eur J Neurol. 2016;23(9):1400–7.
Dong X. Current strategies for Brain Drug Delivery. Theranostics. 2018;8(6):1481–93.
Mendonca LS, Onofre I, Miranda CO, Perfeito R, Nobrega C, de Almeida LP. Stem cell-based therapies for Polyglutamine diseases. Adv Exp Med Biol. 2018;1049:439–66.
Alam MI, Beg S, Samad A, Baboota S, Kohli K, Ali J, et al. Strategy for effective brain drug delivery. Eur J Pharm Sci. 2010;40(5):385–403.
Hoshyar N, Gray S, Han H, Bao G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomed (Lond). 2016;11(6):673–92.
Murthy SK. Nanoparticles in modern medicine: state of the art and future challenges. Int J Nanomed. 2007;2(2):129–41.
Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3(1):16–20.
Soares S, Sousa J, Pais A, Vitorino C. Nanomedicine: principles, properties, and Regulatory issues. Front Chem. 2018;6:360.
Boado RJ, Pardridge WM. The trojan horse Liposome Technology for Nonviral Gene transfer across the blood-brain barrier. J Drug Deliv. 2011;2011:296151.
Mendonça LS, De Pedroso MC, Simöes S. Targeted lipid-based systems for siRNA delivery. J Drug Deliv Sci Technol. 2012;22(1):65–73.
Su S, Kang PM. Systemic review of biodegradable nanomaterials in Nanomedicine. Nanomaterials (Basel). 2020;10(4).
Jo DH, Kim JH, Lee TG, Kim JH. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomedicine. 2015;11(7):1603–11.
Kang JH, Jang WY, Ko YT. The Effect of Surface charges on the Cellular Uptake of liposomes investigated by live cell imaging. Pharm Res. 2017;34(4):704–17.
Xu W, Ling P, Zhang T. Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J Drug Deliv. 2013;2013:340315.
Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99(Pt A):28–51.
Khan AR, Yang X, Fu M, Zhai G. Recent progress of drug nanoformulations targeting to brain. J Control Release. 2018;291:37–64.
Zhang F, Lin YA, Kannan S, Kannan RM. Targeting specific cells in the brain with nanomedicines for CNS therapies. J Control Release. 2016;240:212–26.
Attia MF, Anton N, Wallyn J, Omran Z, Vandamme TF. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J Pharm Pharmacol. 2019;71(8):1185–98.
Yoo J, Park C, Yi G, Lee D, Koo H. Active targeting strategies using Biological ligands for Nanoparticle Drug Delivery systems. Cancers (Basel). 2019;11(5).
Zhao Z, Ukidve A, Kim J, Mitragotri S. Targeting strategies for tissue-specific drug delivery. Cell. 2020;181(1):151–67.
Juan A, Cimas FJ, Bravo I, Pandiella A, Ocana A, Alonso-Moreno C. Antibody conjugation of nanoparticles as therapeutics for breast Cancer Treatment. Int J Mol Sci. 2020;21(17).
Pietersz GA, Wang X, Yap ML, Lim B, Peter K. Therapeutic targeting in nanomedicine: the future lies in recombinant antibodies. Nanomed (Lond). 2017;12(15):1873–89.
Juan A, Cimas FJ, Bravo I, Pandiella A, Ocana A, Alonso-Moreno C. An overview of antibody conjugated polymeric nanoparticles for breast Cancer therapy. Pharmaceutics. 2020;12(9).
Alibakhshi A, Abarghooi Kahaki F, Ahangarzadeh S, Yaghoobi H, Yarian F, Arezumand R, et al. Targeted cancer therapy through antibody fragments-decorated nanomedicines. J Control Release. 2017;268:323–34.
Kholodenko RV, Kalinovsky DV, Doronin II, Ponomarev ED, Kholodenko IV. Antibody fragments as potential biopharmaceuticals for Cancer Therapy: Success and limitations. Curr Med Chem. 2019;26(3):396–426.
Eloy JO, Petrilli R, Trevizan LNF, Chorilli M, Immunoliposomes. A review on functionalization strategies and targets for drug delivery. Colloids Surf B Biointerfaces. 2017;159:454–67.
Clark AJ, Davis ME. Increased brain uptake of targeted nanoparticles by adding an acid-cleavable linkage between transferrin and the nanoparticle core. Proc Natl Acad Sci U S A. 2015;112(40):12486–91.
Choi CH, Alabi CA, Webster P, Davis ME. Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc Natl Acad Sci U S A. 2010;107(3):1235–40.
Daniels TR, Bernabeu E, Rodriguez JA, Patel S, Kozman M, Chiappetta DA, et al. The transferrin receptor and the targeted delivery of therapeutic agents against cancer. Biochim Biophys Acta. 2012;1820(3):291–317.
Zhou J, Li M, Lim WQ, Luo Z, Phua SZF, Huo R, et al. A transferrin-conjugated Hollow Nanoplatform for Redox-controlled and targeted chemotherapy of Tumor with reduced inflammatory reactions. Theranostics. 2018;8(2):518–32.
Deshpande P, Jhaveri A, Pattni B, Biswas S, Torchilin V. Transferrin and octaarginine modified dual-functional liposomes with improved cancer cell targeting and enhanced intracellular delivery for the treatment of ovarian cancer. Drug Deliv. 2018;25(1):517–32.
Chi L, Na MH, Jung HK, Vadevoo SM, Kim CW, Padmanaban G, et al. Enhanced delivery of liposomes to lung tumor through targeting interleukin-4 receptor on both tumor cells and tumor endothelial cells. J Control Release. 2015;209:327–36.
Lu Z, Long Y, Cun X, Wang X, Li J, Mei L, et al. A size-shrinkable nanoparticle-based combined anti-tumor and anti-inflammatory strategy for enhanced cancer therapy. Nanoscale. 2018;10(21):9957–70.
Liu M, Fang X, Yang Y, Wang C. Peptide-enabled targeted Delivery systems for therapeutic applications. Front Bioeng Biotechnol. 2021;9:701504.
Accardo A, Aloj L, Aurilio M, Morelli G, Tesauro D. Receptor binding peptides for target-selective delivery of nanoparticles encapsulated drugs. Int J Nanomed. 2014;9:1537–57.
Guan B, Zhang X. Aptamers as versatile ligands for Biomedical and Pharmaceutical Applications. Int J Nanomed. 2020;15:1059–71.
Stein CA, Castanotto D. FDA-Approved Oligonucleotide therapies in 2017. Mol Ther. 2017;25(5):1069–75.
Parashar A. Aptamers in therapeutics. J Clin Diagn Res. 2016;10(6):BE01–6.
Keefe AD, Pai S, Ellington A. Aptamers as therapeutics. Nat Rev Drug Discov. 2010;9(7):537–50.
Eilers A, Witt S, Walter J. Aptamer-modified nanoparticles in Medical Applications. Adv Biochem Eng Biotechnol. 2020;174:161–93.
Catuogno S, Esposito CL, de Franciscis V. Aptamer-mediated targeted delivery of therapeutics: an update. Pharmaceuticals (Basel). 2016;9(4).
Ledermann JA, Canevari S, Thigpen T. Targeting the folate receptor: diagnostic and therapeutic approaches to personalize cancer treatments. Ann Oncol. 2015;26(10):2034–43.
Lv Y, Cao Y, Li P, Liu J, Chen H, Hu W et al. Ultrasound-Triggered Destruction of Folate-Functionalized Mesoporous silica nanoparticle-loaded Microbubble for targeted Tumor Therapy. Adv Healthc Mater. 2017;6(18).
Srinivasarao M, Low PS. Ligand-targeted drug delivery. Chem Rev. 2017;117(19):12133–64.
Li H, Li Y, Ao H, Bi D, Han M, Guo Y, et al. Folate-targeting annonaceous acetogenins nanosuspensions: significantly enhanced antitumor efficacy in HeLa tumor-bearing mice. Drug Deliv. 2018;25(1):880–7.
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25.
Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol. 1990;429:47–62.
Butt AM, Jones HC. Effect of histamine and antagonists on electrical resistance across the blood-brain barrier in rat brain-surface microvessels. Brain Res. 1992;569(1):100–5.
Urayama A. [The blood-brain barrier and neurodegenerative lysosomal storage diseases]. Brain Nerve. 2013;65(2):153–63.
Warren KE. Beyond the blood:Brain Barrier: the importance of Central Nervous System (CNS) pharmacokinetics for the Treatment of CNS Tumors, including diffuse intrinsic pontine glioma. Front Oncol. 2018;8:239.
van Tellingen O, Yetkin-Arik B, de Gooijer MC, Wesseling P, Wurdinger T, de Vries HE. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat. 2015;19:1–12.
Perez-Martinez FC, Guerra J, Posadas I, Cena V. Barriers to non-viral vector-mediated gene delivery in the nervous system. Pharm Res. 2011;28(8):1843–58.
Swanson JA, Baer SC. Phagocytosis by zippers and triggers. Trends Cell Biol. 1995;5(3):89–93.
Suk JS, Suh J, Choy K, Lai SK, Fu J, Hanes J. Gene delivery to differentiated neurotypic cells with RGD and HIV Tat peptide functionalized polymeric nanoparticles. Biomaterials. 2006;27(29):5143–50.
Misra A, Ganesh S, Shahiwala A, Shah SP. Drug delivery to the central nervous system: a review. J Pharm Pharm Sci. 2003;6(2):252–73.
Shalgunov V, Xiong M, L’Estrade ET, Raval NR, Andersen IV, Edgar FG, et al. Blocking of efflux transporters in rats improves translational validation of brain radioligands. EJNMMI Res. 2020;10(1):124.
Batrakova EV, Li S, Vinogradov SV, Alakhov VY, Miller DW, Kabanov AV. Mechanism of pluronic effect on P-glycoprotein efflux system in blood-brain barrier: contributions of energy depletion and membrane fluidization. J Pharmacol Exp Ther. 2001;299(2):483–93.
Wong AD, Ye M, Levy AF, Rothstein JD, Bergles DE, Searson PC. The blood-brain barrier: an engineering perspective. Front Neuroeng. 2013;6:7.
Strazielle N, Ghersi-Egea JF. Physiology of blood-brain interfaces in relation to brain disposition of small compounds and macromolecules. Mol Pharm. 2013;10(5):1473–91.
Yue W, Shen J. Local delivery strategies for peptides and proteins into the CNS: Status Quo, challenges, and future perspectives. Pharmaceuticals (Basel). 2023;16(6).
Yi X, Manickam DS, Brynskikh A, Kabanov AV. Agile delivery of protein therapeutics to CNS. J Control Release. 2014;190:637–63.
Kwong YL, Yeung DY, Chan JC. Intrathecal chemotherapy for hematologic malignancies: drugs and toxicities. Ann Hematol. 2009;88(3):193–201.
Glascock JJ, Osman EY, Coady TH, Rose FF, Shababi M, Lorson CL. Delivery of therapeutic agents through intracerebroventricular (ICV) and intravenous (IV) injection in mice. J Vis Exp. 2011(56).
Shofty B, Neuberger A, Naffaa ME, Binawi T, Babitch T, Rappaport ZH, et al. Intrathecal or intraventricular therapy for post-neurosurgical gram-negative meningitis: matched cohort study. Clin Microbiol Infect. 2016;22(1):66–70.
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4(147):147ra11.
Noguchi Y, Kato M, Ozeki K, Ishigai M. Pharmacokinetics of an intracerebroventricularly administered antibody in rats. MAbs. 2017;9(7):1210–5.
Schulz A, Ajayi T, Specchio N, de Los Reyes E, Gissen P, Ballon D, et al. Study of Intraventricular Cerliponase Alfa for CLN2 disease. N Engl J Med. 2018;378(20):1898–907.
Sampson JH, Akabani G, Archer GE, Berger MS, Coleman RE, Friedman AH, et al. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro Oncol. 2008;10(3):320–9.
Pizzo ME, Wolak DJ, Kumar NN, Brunette E, Brunnquell CL, Hannocks MJ, et al. Intrathecal antibody distribution in the rat brain: surface diffusion, perivascular transport and osmotic enhancement of delivery. J Physiol. 2018;596(3):445–75.
C ID, Sevin C, Krageloh-Mann I, Giugliani R, Sakai N, Wu J, et al. Safety of intrathecal delivery of recombinant human arylsulfatase A in children with metachromatic leukodystrophy: results from a phase 1/2 clinical trial. Mol Genet Metab. 2020;131(1–2):235–44.
Dorovini-Zis K, Bowman PD, Betz AL, Goldstein GW. Hyperosmotic arabinose solutions open the tight junctions between brain capillary endothelial cells in tissue culture. Brain Res. 1984;302(2):383–6.
Ito M, Bolati K, Kinjo T, Ichimura K, Furuta A, McLoughlin DM, et al. Electroconvulsive stimulation transiently enhances the permeability of the rat blood-brain barrier and induces astrocytic changes. Brain Res Bull. 2017;128:92–7.
Zhang S, Gong P, Zhang J, Mao X, Zhao Y, Wang H, et al. Specific frequency electroacupuncture stimulation transiently enhances the permeability of the blood-brain barrier and induces tight Junction Changes. Front Neurosci. 2020;14:582324.
Luo H, Shusta EV. Blood-brain barrier modulation to improve Glioma Drug Delivery. Pharmaceutics. 2020;12(11).
Jahnke K, Kraemer DF, Knight KR, Fortin D, Bell S, Doolittle ND, et al. Intraarterial chemotherapy and osmotic blood-brain barrier disruption for patients with embryonal and germ cell tumors of the central nervous system. Cancer. 2008;112(3):581–8.
Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1–13.
Lalani J, Raichandani Y, Mathur R, Lalan M, Chutani K, Mishra AK, et al. Comparative receptor based brain delivery of tramadol-loaded poly(lactic-co-glycolic acid) nanoparticles. J Biomed Nanotechnol. 2012;8(6):918–27.
Mendonca LS, Firmino F, Moreira JN, Pedroso de Lima MC, Simoes S. Transferrin receptor-targeted liposomes encapsulating anti-BCR-ABL siRNA or asODN for chronic myeloid leukemia treatment. Bioconjug Chem. 2010;21(1):157–68.
Fornaguera C, Dols-Perez A, Caldero G, Garcia-Celma MJ, Camarasa J, Solans C. PLGA nanoparticles prepared by nano-emulsion templating using low-energy methods as efficient nanocarriers for drug delivery across the blood-brain barrier. J Control Release. 2015;211:134–43.
Ulbrich K, Hekmatara T, Herbert E, Kreuter J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood-brain barrier (BBB). Eur J Pharm Biopharm. 2009;71(2):251–6.
Abo-Krysha N, Rashed L. The role of iron dysregulation in the pathogenesis of multiple sclerosis: an Egyptian study. Mult Scler. 2008;14(5):602–8.
Kreuter J, Shamenkov D, Petrov V, Ramge P, Cychutek K, Koch-Brandt C, et al. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J Drug Target. 2002;10(4):317–25.
Zensi A, Begley D, Pontikis C, Legros C, Mihoreanu L, Wagner S, et al. Albumin nanoparticles targeted with apo E enter the CNS by transcytosis and are delivered to neurones. J Control Release. 2009;137(1):78–86.
Demeule M, Currie JC, Bertrand Y, Che C, Nguyen T, Regina A, et al. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector angiopep-2. J Neurochem. 2008;106(4):1534–44.
Demeule M, Regina A, Che C, Poirier J, Nguyen T, Gabathuler R, et al. Identification and design of peptides as a new drug delivery system for the brain. J Pharmacol Exp Ther. 2008;324(3):1064–72.
Thomas FC, Taskar K, Rudraraju V, Goda S, Thorsheim HR, Gaasch JA, et al. Uptake of ANG1005, a novel paclitaxel derivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharm Res. 2009;26(11):2486–94.
Ulbrich K, Knobloch T, Kreuter J. Targeting the insulin receptor: nanoparticles for drug delivery across the blood-brain barrier (BBB). J Drug Target. 2011;19(2):125–32.
Li J, Guo Y, Kuang Y, An S, Ma H, Jiang C. Choline transporter-targeting and co-delivery system for glioma therapy. Biomaterials. 2013;34(36):9142–8.
Li J, Zhou L, Ye D, Huang S, Shao K, Huang R, et al. Choline-derivate-modified nanoparticles for brain-targeting gene delivery. Adv Mater. 2011;23(39):4516–20.
Kuo YC, Chung CY. Transcytosis of CRM197-grafted polybutylcyanoacrylate nanoparticles for delivering zidovudine across human brain-microvascular endothelial cells. Colloids Surf B Biointerfaces. 2012;91:242–9.
Zhang C, Zheng X, Wan X, Shao X, Liu Q, Zhang Z, et al. The potential use of H102 peptide-loaded dual-functional nanoparticles in the treatment of Alzheimer’s disease. J Control Release. 2014;192:317–24.
Marcos-Contreras OA, Greineder CF, Kiseleva RY, Parhiz H, Walsh LR, Zuluaga-Ramirez V, et al. Selective targeting of nanomedicine to inflamed cerebral vasculature to enhance the blood-brain barrier. Proc Natl Acad Sci U S A. 2020;117(7):3405–14.
Faure MP, Alonso A, Nouel D, Gaudriault G, Dennis M, Vincent JP, et al. Somatodendritic internalization and perinuclear targeting of neurotensin in the mammalian brain. J Neurosci. 1995;15(6):4140–7.
Hsieh TY, Huang WC, Kang YD, Chu CY, Liao WL, Chen YY, et al. Neurotensin-conjugated reduced Graphene Oxide with Multi-stage Near-Infrared-triggered synergic targeted neuron gene transfection in Vitro and in vivo for neurodegenerative Disease Therapy. Adv Healthc Mater. 2016;5(23):3016–26.
Park IK, Lasiene J, Chou SH, Horner PJ, Pun SH. Neuron-specific delivery of nucleic acids mediated by Tet1-modified poly(ethylenimine). J Gene Med. 2007;9(8):691–702.
Huang YZ, Hernandez FJ, Gu B, Stockdale KR, Nanapaneni K, Scheetz TE, et al. RNA aptamer-based functional ligands of the neurotrophin receptor, TrkB. Mol Pharmacol. 2012;82(4):623–35.
Xu J, Chau Y. Polymeric nanoparticles decorated with BDNF-derived peptide for neuron-targeted delivery of PTEN inhibitor. Eur J Pharm Sci. 2018;124:37–45.
Lopes CD, Oliveira H, Estevao I, Pires LR, Pego AP. In vivo targeted gene delivery to peripheral neurons mediated by neurotropic poly(ethylene imine)-based nanoparticles. Int J Nanomed. 2016;11:2675–83.
Lopes CD, Gomes CP, Neto E, Sampaio P, Aguiar P, Pego AP. Microfluidic-based platform to mimic the in vivo peripheral administration of neurotropic nanoparticles. Nanomed (Lond). 2016;11(24):3205–21.
Lopes CDF, Goncalves NP, Gomes CP, Saraiva MJ, Pego AP. BDNF gene delivery mediated by neuron-targeted nanoparticles is neuroprotective in peripheral nerve injury. Biomaterials. 2017;121:83–96.
Dos Santos Rodrigues B, Lakkadwala S, Kanekiyo T, Singh J. Dual-modified liposome for targeted and enhanced gene delivery into mice Brain. J Pharmacol Exp Ther. 2020;374(3):354–65.
Dos Santos Rodrigues B, Lakkadwala S, Kanekiyo T, Singh J. Development and screening of brain-targeted lipid-based nanoparticles with enhanced cell penetration and gene delivery properties. Int J Nanomed. 2019;14:6497–517.
Vilella A, Tosi G, Grabrucker AM, Ruozi B, Belletti D, Vandelli MA, et al. Insight on the fate of CNS-targeted nanoparticles. Part I: Rab5-dependent cell-specific uptake and distribution. J Control Release. 2014;174:195–201.
Tosi G, Fano RA, Bondioli L, Badiali L, Benassi R, Rivasi F, et al. Investigation on mechanisms of glycopeptide nanoparticles for drug delivery across the blood-brain barrier. Nanomed (Lond). 2011;6(3):423–36.
Conceicao M, Mendonca L, Nobrega C, Gomes C, Costa P, Hirai H, et al. Intravenous administration of brain-targeted stable nucleic acid lipid particles alleviates Machado-Joseph disease neurological phenotype. Biomaterials. 2016;82:124–37.
Chen W, Zhan C, Gu B, Meng Q, Wang H, Lu W, et al. Targeted brain delivery of itraconazole via RVG29 anchored nanoparticles. J Drug Target. 2011;19(3):228–34.
Lozic I, Hartz RV, Bartlett CA, Shaw JA, Archer M, Naidu PS, et al. Enabling dual cellular destinations of polymeric nanoparticles for treatment following partial injury to the central nervous system. Biomaterials. 2016;74:200–16.
Chekhonin VP, Zhirkov YA, Gurina OI, Ryabukhin IA, Lebedev SV, Kashparov IA, et al. PEGylated immunoliposomes directed against brain astrocytes. Drug Deliv. 2005;12(1):1–6.
Gu J, Al-Bayati K, Ho EA. Development of antibody-modified chitosan nanoparticles for the targeted delivery of siRNA across the blood-brain barrier as a strategy for inhibiting HIV replication in astrocytes. Drug Deliv Transl Res. 2017;7(4):497–506.
Choi B, Soh M, Manandhar Y, Kim D, Han SI, Baik S, et al. Highly selective microglial uptake of ceria-zirconia nanoparticles for enhanced analgesic treatment of neuropathic pain. Nanoscale. 2019;11(41):19437–47.
Rittchen S, Boyd A, Burns A, Park J, Fahmy TM, Metcalfe S, et al. Myelin repair in vivo is increased by targeting oligodendrocyte precursor cells with nanoparticles encapsulating leukaemia inhibitory factor (LIF). Biomaterials. 2015;56:78–85.
Fereidan-Esfahani M, Yue WY, Wilbanks B, Johnson AJ, Warrington AE, Howe CL et al. Remyelination-promoting DNA aptamer conjugate Myaptavin-3064 binds to adult oligodendrocytes in Vitro. Pharmaceuticals (Basel). 2020;13(11).
Hosseini Shamili F, Alibolandi M, Rafatpanah H, Abnous K, Mahmoudi M, Kalantari M, et al. Immunomodulatory properties of MSC-derived exosomes armed with high affinity aptamer toward mylein as a platform for reducing multiple sclerosis clinical score. J Control Release. 2019;299:149–64.
Schmidt A, Bockmann M, Stoll A, Racek T, Putzer BM. Analysis of adenovirus gene transfer into adult neural stem cells. Virus Res. 2005;114(1–2):45–53.
Schmidt A, Haas SJ, Hildebrandt S, Scheibe J, Eckhoff B, Racek T, et al. Selective targeting of adenoviral vectors to neural precursor cells in the hippocampus of adult mice: new prospects for in situ gene therapy. Stem Cells. 2007;25(11):2910–8.
Carradori D, Saulnier P, Preat V, des Rieux A, Eyer J. NFL-lipid nanocapsules for brain neural stem cell targeting in vitro and in vivo. J Control Release. 2016;238:253–62.
Carradori D, Dos Santos AG, Masquelier J, Paquot A, Saulnier P, Eyer J, et al. The origin of neural stem cells impacts their interactions with targeted-lipid nanocapsules: potential role of plasma membrane lipid composition and fluidity. J Control Release. 2018;292:248–55.
Praca C, Rai A, Santos T, Cristovao AC, Pinho SL, Cecchelli R, et al. A nanoformulation for the preferential accumulation in adult neurogenic niches. J Control Release. 2018;284:57–72.
Mendonca LS, Moreira JN, de Lima MC, Simoes S. Co-encapsulation of anti-BCR-ABL siRNA and imatinib mesylate in transferrin receptor-targeted sterically stabilized liposomes for chronic myeloid leukemia treatment. Biotechnol Bioeng. 2010;107(5):884–93.
Inazu M. Functional expression of Choline transporters in the blood-brain barrier. Nutrients. 2019;11(10).
Raab G, Klagsbrun M. Heparin-binding EGF-like growth factor. Biochim Biophys Acta. 1997;1333(3):F179–99.
Gaillard PJ, de Boer AG. A novel opportunity for targeted drug delivery to the brain. J Control Release. 2006;116(2):e60–2.
O’Brien P, Wong RW. Optic neuritis following diphtheria, tetanus, pertussis, and inactivated poliovirus combined vaccination: a case report. J Med Case Rep. 2018;12(1):356.
Tosi G, Vilella A, Veratti P, Belletti D, Pederzoli F, Ruozi B, et al. Exploiting bacterial pathways for BBB crossing with PLGA nanoparticles modified with a mutated form of Diphtheria Toxin (CRM197): in vivo experiments. Mol Pharm. 2015;12(10):3672–84.
Singh J, Habean ML, Panicker N. Inflammasome assembly in neurodegenerative diseases. Trends Neurosci. 2023;46(10):814–31.
Calsolaro V, Edison P. Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimers Dement. 2016;12(6):719–32.
Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, Ma D. Neuroinflammation: the role and consequences. Neurosci Res. 2014;79:1–12.
Candelario-Jalil E, Dijkhuizen RM, Magnus T, Neuroinflammation. Stroke, blood-brain barrier dysfunction, and Imaging modalities. Stroke. 2022;53(5):1473–86.
Yang C, Hawkins KE, Dore S, Candelario-Jalil E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am J Physiol Cell Physiol. 2019;316(2):C135–53.
Hsu J, Rappaport J, Muro S. Specific binding, uptake, and transport of ICAM-1-targeted nanocarriers across endothelial and subendothelial cell components of the blood-brain barrier. Pharm Res. 2014;31(7):1855–66.
Ailuno G, Zuccari G, Baldassari S, Lai F, Caviglioli G. Anti-vascular cell adhesion Molecule-1 nanosystems: a Promising Strategy against Inflammatory Based diseases. J Nanosci Nanotechnol. 2021;21(5):2793–807.
Cuddapah VA, Robel S, Watkins S, Sontheimer H. A neurocentric perspective on glioma invasion. Nat Rev Neurosci. 2014;15(7):455–65.
Huse JT, Holland EC. Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer. 2010;10(5):319–31.
Munir MU. Nanomedicine Penetration to Tumor: challenges, and Advanced strategies to Tackle this issue. Cancers (Basel). 2022;14(12).
Zhao M, van Straten D, Broekman MLD, Preat V, Schiffelers RM. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics. 2020;10(3):1355–72.
Tang W, Fan W, Lau J, Deng L, Shen Z, Chen X. Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem Soc Rev. 2019;48(11):2967–3014.
Lee C, Hwang HS, Lee S, Kim B, Kim JO, Oh KT et al. Rabies virus-inspired silica-coated gold nanorods as a Photothermal Therapeutic platform for treating brain tumors. Adv Mater. 2017;29(13).
Mojarad-Jabali S, Farshbaf M, Hemmati S, Sarfraz M, Motasadizadeh H, Shahbazi Mojarrad J, et al. Comparison of three synthetic transferrin mimetic small peptides to promote the blood-brain barrier penetration of vincristine liposomes for improved glioma targeted therapy. Int J Pharm. 2022;613:121395.
Liu C, Zhao Z, Gao H, Rostami I, You Q, Jia X, et al. Enhanced blood-brain-barrier penetrability and tumor-targeting efficiency by peptide-functionalized poly(amidoamine) dendrimer for the therapy of gliomas. Nanotheranostics. 2019;3(4):311–30.
Choudhury H, Pandey M, Chin PX, Phang YL, Cheah JY, Ooi SC, et al. Transferrin receptors-targeting nanocarriers for efficient targeted delivery and transcytosis of drugs into the brain tumors: a review of recent advancements and emerging trends. Drug Deliv Transl Res. 2018;8(5):1545–63.
Cui Y, Xu Q, Chow PK, Wang D, Wang CH. Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment. Biomaterials. 2013;34(33):8511–20.
Xin H, Sha X, Jiang X, Zhang W, Chen L, Fang X. Anti-glioblastoma efficacy and safety of paclitaxel-loading angiopep-conjugated dual targeting PEG-PCL nanoparticles. Biomaterials. 2012;33(32):8167–76.
Zhu Y, Jiang Y, Meng F, Deng C, Cheng R, Zhang J, et al. Highly efficacious and specific anti-glioma chemotherapy by tandem nanomicelles co-functionalized with brain tumor-targeting and cell-penetrating peptides. J Control Release. 2018;278:1–8.
Zhu Y, Zhang J, Meng F, Deng C, Cheng R, Feijen J, et al. cRGD-functionalized reduction-sensitive shell-sheddable biodegradable micelles mediate enhanced doxorubicin delivery to human glioma xenografts in vivo. J Control Release. 2016;233:29–38.
Zhong Y, Wang C, Cheng R, Cheng L, Meng F, Liu Z, et al. cRGD-directed, NIR-responsive and robust AuNR/PEG-PCL hybrid nanoparticles for targeted chemotherapy of glioblastoma in vivo. J Control Release. 2014;195:63–71.
Miura Y, Takenaka T, Toh K, Wu S, Nishihara H, Kano MR, et al. Cyclic RGD-linked polymeric micelles for targeted delivery of platinum anticancer drugs to glioblastoma through the blood-brain tumor barrier. ACS Nano. 2013;7(10):8583–92.
Talelli M, Barz M, Rijcken CJ, Kiessling F, Hennink WE, Lammers T. Core-Crosslinked Polymeric micelles: principles, Preparation, Biomedical Applications and clinical translation. Nano Today. 2015;10(1):93–117.
Venditto VJ, Szoka FC Jr. Cancer nanomedicines: so many papers and so few drugs! Adv Drug Deliv Rev. 2013;65(1):80–8.
Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12(11):991–1003.
Sun H, Meng F, Cheng R, Deng C, Zhong Z. Reduction-sensitive degradable micellar nanoparticles as smart and intuitive delivery systems for cancer chemotherapy. Expert Opin Drug Deliv. 2013;10(8):1109–22.
Meng F, Hennink WE, Zhong Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials. 2009;30(12):2180–98.
Novakova J, Slaby O, Vyzula R, Michalek J. MicroRNA involvement in glioblastoma pathogenesis. Biochem Biophys Res Commun. 2009;386(1):1–5.
Costa PM, Cardoso AL, Nobrega C, Pereira de Almeida LF, Bruce JN, Canoll P, et al. MicroRNA-21 silencing enhances the cytotoxic effect of the antiangiogenic drug sunitinib in glioblastoma. Hum Mol Genet. 2013;22(5):904–18.
Dong CG, Wu WK, Feng SY, Wang XJ, Shao JF, Qiao J. Co-inhibition of microRNA-10b and microRNA-21 exerts synergistic inhibition on the proliferation and invasion of human glioma cells. Int J Oncol. 2012;41(3):1005–12.
Costa PM, Cardoso AL, Mendonca LS, Serani A, Custodia C, Conceicao M, et al. Tumor-targeted chlorotoxin-coupled nanoparticles for nucleic acid delivery to Glioblastoma cells: a Promising System for Glioblastoma Treatment. Mol Ther Nucleic Acids. 2013;2(6):e100.
Deshane J, Garner CC, Sontheimer H. Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J Biol Chem. 2003;278(6):4135–44.
Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133(2):647–58.
Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH, et al. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 2008;27(31):4373–9.
Ni J, Miao T, Su M, Khan NU, Ju X, Chen H, et al. PSMA-targeted nanoparticles for specific penetration of blood-brain tumor barrier and combined therapy of brain metastases. J Control Release. 2021;329:934–47.
Achrol AS, Rennert RC, Anders C, Soffietti R, Ahluwalia MS, Nayak L, et al. Brain metastases. Nat Rev Dis Primers. 2019;5(1):5.
Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28(20):3271–7.
Taskar KS, Rudraraju V, Mittapalli RK, Samala R, Thorsheim HR, Lockman J, et al. Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer. Pharm Res. 2012;29(3):770–81.
Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16(23):5664–78.
Kasoha M, Unger C, Solomayer EF, Bohle RM, Zaharia C, Khreich F, et al. Prostate-specific membrane antigen (PSMA) expression in breast cancer and its metastases. Clin Exp Metastasis. 2017;34(8):479–90.
Nomura N, Pastorino S, Jiang P, Lambert G, Crawford JR, Gymnopoulos M, et al. Prostate specific membrane antigen (PSMA) expression in primary gliomas and breast cancer brain metastases. Cancer Cell Int. 2014;14(1):26.
Meher N, VanBrocklin HF, Wilson DM, Flavell RR. PSMA-Targeted nanotheranostics for imaging and radiotherapy of prostate Cancer. Pharmaceuticals (Basel). 2023;16(2).
Xu X, Wu J, Liu Y, Saw PE, Tao W, Yu M, et al. Multifunctional envelope-type siRNA delivery nanoparticle platform for prostate Cancer therapy. ACS Nano. 2017;11(3):2618–27.
Paasonen L, Sharma S, Braun GB, Kotamraju VR, Chung TD, She ZG, et al. New p32/gC1qR ligands for targeted Tumor Drug Delivery. ChemBioChem. 2016;17(7):570–5.
Lovinger DM. Communication networks in the brain: neurons, receptors, neurotransmitters, and alcohol. Alcohol Res Health. 2008;31(3):196–214.
Gorman AM. Neuronal cell death in neurodegenerative diseases: recurring themes around protein handling. J Cell Mol Med. 2008;12(6A):2263–80.
Saxena S, Caroni P. Selective neuronal vulnerability in neurodegenerative diseases: from Stressor thresholds to degeneration. Neuron. 2011;71(1):35–48.
Fisher A. Cholinergic modulation of amyloid precursor protein processing with emphasis on M1 muscarinic receptor: perspectives and challenges in treatment of Alzheimer’s disease. J Neurochem. 2012;120(Suppl 1):22–33.
Ztaou S, Maurice N, Camon J, Guiraudie-Capraz G, Kerkerian-Le Goff L, Beurrier C, et al. Involvement of Striatal Cholinergic Interneurons and M1 and M4 muscarinic receptors in motor symptoms of Parkinson’s Disease. J Neurosci. 2016;36(35):9161–72.
Garcia-Chica J, WK DP, Tanabe S, Serra D, Herrero L, Casals N, et al. An overview of nanomedicines for neuron targeting. Nanomed (Lond). 2020;15(16):1617–36.
Babazadeh A, Mohammadi Vahed F, Jafari SM. Nanocarrier-mediated brain delivery of bioactives for treatment/prevention of neurodegenerative diseases. J Control Release. 2020;321:211–21.
Hernando S, Gartziandia O, Herran E, Pedraz JL, Igartua M, Hernandez RM. Advances in nanomedicine for the treatment of Alzheimer’s and Parkinson’s diseases. Nanomed (Lond). 2016;11(10):1267–85.
Numakawa T, Suzuki S, Kumamaru E, Adachi N, Richards M, Kunugi H. BDNF function and intracellular signaling in neurons. Histol Histopathol. 2010;25(2):237–58.
Ramsey JD, Flynn NH. Cell-penetrating peptides transport therapeutics into cells. Pharmacol Ther. 2015;154:78–86.
Joliot A, Pernelle C, Deagostini-Bazin H, Prochiantz A. Antennapedia homeobox peptide regulates neural morphogenesis. Proc Natl Acad Sci U S A. 1991;88(5):1864–8.
Pino-Angeles A, Lazaridis T. Effects of peptide charge, orientation, and concentration on Melittin Transmembrane pores. Biophys J. 2018;114(12):2865–74.
Qian S, Heller WT. Melittin-induced cholesterol reorganization in lipid bilayer membranes. Biochim Biophys Acta. 2015;1848(10 Pt A):2253–60.
Upadhya A, Sangave PC. Hydrophobic and electrostatic interactions between cell penetrating peptides and plasmid DNA are important for stable non-covalent complexation and intracellular delivery. J Pept Sci. 2016;22(10):647–59.
Bolhassani A, Jafarzade BS, Mardani G. In vitro and in vivo delivery of therapeutic proteins using cell penetrating peptides. Peptides. 2017;87:50–63.
Takayama K, Nakase I, Michiue H, Takeuchi T, Tomizawa K, Matsui H, et al. Enhanced intracellular delivery using arginine-rich peptides by the addition of penetration accelerating sequences (pas). J Control Release. 2009;138(2):128–33.
Takayama K, Hirose H, Tanaka G, Pujals S, Katayama S, Nakase I, et al. Effect of the attachment of a penetration accelerating sequence and the influence of hydrophobicity on octaarginine-mediated intracellular delivery. Mol Pharm. 2012;9(5):1222–30.
Guidotti G, Brambilla L, Rossi D. Cell-penetrating peptides: from Basic Research to clinics. Trends Pharmacol Sci. 2017;38(4):406–24.
Milletti F. Cell-penetrating peptides: classes, origin, and current landscape. Drug Discov Today. 2012;17(15–16):850–60.
Yoshida T, Tomioka I, Nagahara T, Holyst T, Sawada M, Hayes P, et al. Bax-inhibiting peptide derived from mouse and rat Ku70. Biochem Biophys Res Commun. 2004;321(4):961–6.
Gomez JA, Gama V, Yoshida T, Sun W, Hayes P, Leskov K, et al. Bax-inhibiting peptides derived from Ku70 and cell-penetrating pentapeptides. Biochem Soc Trans. 2007;35(Pt 4):797–801.
Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55(6):1189–93.
Simon MJ, Gao S, Kang WH, Banta S, Morrison B. 3rd. TAT-mediated intracellular protein delivery to primary brain cells is dependent on glycosaminoglycan expression. Biotechnol Bioeng. 2009;104(1):10–9.
Kristensen M, Birch D, Morck Nielsen H. Applications and challenges for Use of cell-penetrating peptides as delivery vectors for peptide and protein cargos. Int J Mol Sci 2016;17(2).
Tosi G, Ruozi B, Belletti D, Vilella A, Zoli M, Vandelli MA, et al. Brain-targeted polymeric nanoparticles: in vivo evidence of different routes of administration in rodents. Nanomed (Lond). 2013;8(9):1373–83.
Yan X, Mohankumar PS, Dietzschold B, Schnell MJ, Fu ZF. The Rabies virus glycoprotein determines the distribution of different rabies virus strains in the brain. J Neurovirol. 2002;8(4):345–52.
Lafon M. Rabies virus receptors. J Neurovirol. 2005;11(1):82–7.
Torashima T, Koyama C, Iizuka A, Mitsumura K, Takayama K, Yanagi S, et al. Lentivector-mediated rescue from cerebellar ataxia in a mouse model of spinocerebellar ataxia. EMBO Rep. 2008;9(4):393–9.
Verkhratsky A, Nedergaard M. Physiology of Astroglia. Physiol Rev. 2018;98(1):239–389.
Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci. 2010;11(4):227–38.
Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22(5):208–15.
McCall MA, Gregg RG, Behringer RR, Brenner M, Delaney CL, Galbreath EJ, et al. Targeted deletion in astrocyte intermediate filament (gfap) alters neuronal physiology. Proc Natl Acad Sci U S A. 1996;93(13):6361–6.
Araque A, Carmignoto G, Haydon PG. Dynamic signaling between astrocytes and neurons. Annu Rev Physiol. 2001;63:795–813.
Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50(4):427–34.
Pekny M, Wilhelmsson U, Pekna M. The dual role of astrocyte activation and reactive gliosis. Neurosci Lett. 2014;565:30–8.
Phatnani H, Maniatis T. Astrocytes in neurodegenerative disease. Cold Spring Harb Perspect Biol. 2015;7(6).
Colin A, Faideau M, Dufour N, Auregan G, Hassig R, Andrieu T, et al. Engineered lentiviral vector targeting astrocytes in vivo. Glia. 2009;57(6):667–79.
Delzor A, Escartin C, Deglon N. Lentiviral vectors: a powerful tool to target astrocytes in vivo. Curr Drug Targets. 2013;14(11):1336–46.
Nagelhus EA, Ottersen OP. Physiological roles of aquaporin-4 in brain. Physiol Rev. 2013;93(4):1543–62.
Hubbard JA, Szu JI, Binder DK. The role of aquaporin-4 in synaptic plasticity, memory and disease. Brain Res Bull. 2018;136:118–29.
Wells J, Kilburn MR, Shaw JA, Bartlett CA, Harvey AR, Dunlop SA, et al. Early in vivo changes in calcium ions, oxidative stress markers, and ion channel immunoreactivity following partial injury to the optic nerve. J Neurosci Res. 2012;90(3):606–18.
Goldberg DM, Yan J, Soleas GJ. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin Biochem. 2003;36(1):79–87.
Walle T, Hsieh F, DeLegge MH, Oatis JE Jr., Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32(12):1377–82.
Zhang Z, Ma Z, Zou W, Guo H, Liu M, Ma Y, et al. The appropriate marker for astrocytes: comparing the distribution and expression of three astrocytic markers in different mouse cerebral regions. Biomed Res Int. 2019;2019:9605265.
Li D, Liu X, Liu T, Liu H, Tong L, Jia S, et al. Neurochemical regulation of the expression and function of glial fibrillary acidic protein in astrocytes. Glia. 2020;68(5):878–97.
Jean M, Gera L, Charest-Morin X, Marceau F, Bachelard H. In vivo effects of Bradykinin B2 receptor agonists with varying susceptibility to Peptidases. Front Pharmacol. 2015;6:306.
Gregnani MF, Hungaro TG, Martins-Silva L, Bader M, Araujo RC. Bradykinin B2 receptor signaling increases glucose uptake and oxidation: evidence and open questions. Front Pharmacol. 2020;11:1162.
Cholewinski AJ, Stevens G, McDermott AM, Wilkin GP. Identification of B2 bradykinin binding sites on cultured cortical astrocytes. J Neurochem. 1991;57(4):1456–8.
Stephens GJ, Cholewinski AJ, Wilkin GP, Djamgoz MB. Calcium-mobilizing and electrophysiological effects of bradykinin on cortical astrocyte subtypes in culture. Glia. 1993;9(4):269–79.
Sun W, McConnell E, Pare JF, Xu Q, Chen M, Peng W, et al. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science. 2013;339(6116):197–200.
Baslow MH. The astrocyte surface NAAG receptor and NAAG peptidase signaling complex as a therapeutic target. Drug News Perspect. 2008;21(5):251–7.
Zhang X, Lao K, Qiu Z, Rahman MS, Zhang Y, Gou X. Potential astrocytic receptors and transporters in the pathogenesis of Alzheimer’s Disease. J Alzheimers Dis. 2019;67(4):1109–22.
Liang J, Takeuchi H, Doi Y, Kawanokuchi J, Sonobe Y, Jin S, et al. Excitatory amino acid transporter expression by astrocytes is neuroprotective against microglial excitotoxicity. Brain Res. 2008;1210:11–9.
Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105.
Rudy CC, Hunsberger HC, Weitzner DS, Reed MN. The role of the tripartite glutamatergic synapse in the pathophysiology of Alzheimer’s disease. Aging Dis. 2015;6(2):131–48.
Dezsi L, Tuka B, Martos D, Vecsei L. Alzheimer’s disease, astrocytes and kynurenines. Curr Alzheimer Res. 2015;12(5):462–80.
Rickmann M, Wolff JR. S100 protein expression in subpopulations of neurons of rat brain. Neuroscience. 1995;67(4):977–91.
Zhang Y, Zhu J, Xu H, Yi Q, Yan L, Ye L, et al. Time-Dependent internalization of S100B by mesenchymal stem cells via the pathways of Clathrin- and lipid raft-mediated endocytosis. Front Cell Dev Biol. 2021;9:674995.
Walter L, Stella N. Cannabinoids and neuroinflammation. Br J Pharmacol. 2004;141(5):775–85.
Tay TL, Savage JC, Hui CW, Bisht K, Tremblay ME. Microglia across the lifespan: from origin to function in brain development, plasticity and cognition. J Physiol. 2017;595(6):1929–45.
Szepesi Z, Manouchehrian O, Bachiller S, Deierborg T. Bidirectional microglia-neuron communication in Health and Disease. Front Cell Neurosci. 2018;12:323.
Fan Y, Xie L, Chung CY. Signaling pathways Controlling Microglia Chemotaxis. Mol Cells. 2017;40(3):163–8.
Xu Y, Jin MZ, Yang ZY, Jin WL. Microglia in neurodegenerative diseases. Neural Regen Res. 2021;16(2):270–80.
Presumey J, Bialas AR, Carroll MC. Complement system in neural synapse elimination in Development and Disease. Adv Immunol. 2017;135:53–79.
Borst K, Schwabenland M, Prinz M. Microglia metabolism in health and disease. Neurochem Int. 2019;130:104331.
Hickman S, Izzy S, Sen P, Morsett L, El Khoury J. Microglia in neurodegeneration. Nat Neurosci. 2018;21(10):1359–69.
Zhang F, Mastorakos P, Mishra MK, Mangraviti A, Hwang L, Zhou J, et al. Uniform brain tumor distribution and tumor associated macrophage targeting of systemically administered dendrimers. Biomaterials. 2015;52:507–16.
Papa S, Ferrari R, De Paola M, Rossi F, Mariani A, Caron I, et al. Polymeric nanoparticle system to target activated microglia/macrophages in spinal cord injury. J Control Release. 2014;174:15–26.
Nance E, Porambo M, Zhang F, Mishra MK, Buelow M, Getzenberg R, et al. Systemic dendrimer-drug treatment of ischemia-induced neonatal white matter injury. J Control Release. 2015;214:112–20.
Duffy CM, Ahmed S, Yuan C, Mavanji V, Nixon JP, Butterick T. Microglia as a surrogate Biosensor to determine nanoparticle neurotoxicity. J Vis Exp 2016(116).
Yang Z, Liu ZW, Allaker RP, Reip P, Oxford J, Ahmad Z, et al. A review of nanoparticle functionality and toxicity on the central nervous system. J R Soc Interface. 2010;7(Suppl 4):S411–22.
Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91(2):461–553.
Wilkinson K, El Khoury J. Microglial scavenger receptors and their roles in the pathogenesis of Alzheimer’s disease. Int J Alzheimers Dis. 2012;2012:489456.
Lue LF, Walker DG, Brachova L, Beach TG, Rogers J, Schmidt AM, et al. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer’s disease: identification of a cellular activation mechanism. Exp Neurol. 2001;171(1):29–45.
Teismann P, Sathe K, Bierhaus A, Leng L, Martin HL, Bucala R, et al. Receptor for advanced glycation endproducts (RAGE) deficiency protects against MPTP toxicity. Neurobiol Aging. 2012;33(10):2478–90.
Fiebich BL, Batista CRA, Saliba SW, Yousif NM, de Oliveira ACP. Role of Microglia TLRs in Neurodegeneration. Front Cell Neurosci. 2018;12:329.
Honarpisheh P, Lee J, Banerjee A, Blasco-Conesa MP, Honarpisheh P, d’Aigle J, et al. Potential caveats of putative microglia-specific markers for assessment of age-related cerebrovascular neuroinflammation. J Neuroinflammation. 2020;17(1):366.
Jurga AM, Paleczna M, Kuter KZ. Overview of General and discriminating markers of Differential Microglia phenotypes. Front Cell Neurosci. 2020;14:198.
Soh M, Kang DW, Jeong HG, Kim D, Kim DY, Yang W, et al. Ceria-Zirconia nanoparticles as an enhanced multi-antioxidant for Sepsis Treatment. Angew Chem Int Ed Engl. 2017;56(38):11399–403.
Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol. 2013;13(9):621–34.
Chung H, Brazil MI, Irizarry MC, Hyman BT, Maxfield FR. Uptake of fibrillar beta-amyloid by microglia isolated from MSR-A (type I and type II) knockout mice. NeuroReport. 2001;12(6):1151–4.
El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, et al. CD36 mediates the innate host response to beta-amyloid. J Exp Med. 2003;197(12):1657–66.
Shannahan JH, Bai W, Brown JM. Implications of scavenger receptors in the safe development of nanotherapeutics. Receptors Clin Investig. 2015;2(3):e811.
Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2(72):re3.
Konishi H, Kobayashi M, Kunisawa T, Imai K, Sayo A, Malissen B, et al. Siglec-H is a microglia-specific marker that discriminates microglia from CNS-associated macrophages and CNS-infiltrating monocytes. Glia. 2017;65(12):1927–43.
Murai N, Mitalipova M, Jaenisch R. Functional analysis of CX3CR1 in human induced pluripotent stem (iPS) cell-derived microglia-like cells. Eur J Neurosci. 2020;52(7):3667–78.
Duveau A, Bertin E, Boue-Grabot E. Implication of Neuronal Versus Microglial P2X4 Receptors in Central Nervous System Disorders. Neurosci Bull. 2020;36(11):1327–43.
Zabala A, Vazquez-Villoldo N, Rissiek B, Gejo J, Martin A, Palomino A et al. P2X4 receptor controls microglia activation and favors remyelination in autoimmune encephalitis. EMBO Mol Med. 2018;10(8).
Kuhn S, Gritti L, Crooks D, Dombrowski Y. Oligodendrocytes in development, myelin Generation and Beyond. Cells. 2019;8(11).
Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):37–53.
Jin GZ, Chakraborty A, Lee JH, Knowles JC, Kim HW. Targeting with nanoparticles for the therapeutic treatment of brain diseases. J Tissue Eng. 2020;11:2041731419897460.
Munzel EJ, Williams A. Promoting remyelination in multiple sclerosis-recent advances. Drugs. 2013;73(18):2017–29.
Somkuwar SS, Staples MC, Galinato MH, Fannon MJ, Mandyam CD. Role of NG2 expressing cells in addiction: a new approach for an old problem. Front Pharmacol. 2014;5:279.
Warrington AE, Asakura K, Bieber AJ, Ciric B, Van Keulen V, Kaveri SV, et al. Human monoclonal antibodies reactive to oligodendrocytes promote remyelination in a model of multiple sclerosis. Proc Natl Acad Sci U S A. 2000;97(12):6820–5.
Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249(4968):505–10.
Nastasijevic B, Wright BR, Smestad J, Warrington AE, Rodriguez M, Maher LJ. 3rd. Remyelination induced by a DNA aptamer in a mouse model of multiple sclerosis. PLoS ONE. 2012;7(6):e39595.
Sedlak SM, Schendel LC, Gaub HE, Bernardi RC. Streptavidin/biotin: tethering geometry defines unbinding mechanics. Sci Adv. 2020;6(13):eaay5999.
Constantinescu CS, Farooqi N, O’Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol. 2011;164(4):1079–106.
Goncalves JT, Schafer ST, Gage FH. Adult neurogenesis in the Hippocampus: from stem cells to Behavior. Cell. 2016;167(4):897–914.
Valero J, Bernardino L, Cardoso FL, Silva AP, Fontes-Ribeiro C, Ambrosio AF, et al. Impact of Neuroinflammation on hippocampal neurogenesis: relevance to aging and Alzheimer’s Disease. J Alzheimers Dis. 2017;60(s1):S161–8.
Luo J, Daniels SB, Lennington JB, Notti RQ, Conover JC. The aging neurogenic subventricular zone. Aging Cell. 2006;5(2):139–52.
Zhu SZ, Szeto V, Bao MH, Sun HS, Feng ZP. Pharmacological approaches promoting stem cell-based therapy following ischemic stroke insults. Acta Pharmacol Sin. 2018;39(5):695–712.
Vukovic J, Blackmore DG, Jhaveri D, Bartlett PF. Activation of neural precursors in the adult neurogenic niches. Neurochem Int. 2011;59(3):341–6.
Yamaguchi M, Saito H, Suzuki M, Mori K. Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. NeuroReport. 2000;11(9):1991–6.
Lepinoux-Chambaud C, Barreau K, Eyer J. The neurofilament-derived peptide NFL-TBS.40–63 targets neural stem cells and affects their Properties. Stem Cells Transl Med. 2016;5(7):901–13.
Lepinoux-Chambaud C, Eyer J. The NFL-TBS.40–63 anti-glioblastoma peptide enters selectively in glioma cells by endocytosis. Int J Pharm. 2013;454(2):738–47.
Fan Y, Marioli M, Zhang K. Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. J Pharm Biomed Anal. 2021;192:113642.
Peltonen L. Practical guidelines for the characterization and quality control of pure drug nanoparticles and nano-cocrystals in the pharmaceutical industry. Adv Drug Deliv Rev. 2018;131:101–15.
Vinod C, Jena S, Nano-Neurotheranostics. Impact of nanoparticles on neural dysfunctions and strategies to reduce toxicity for Improved Efficacy. Front Pharmacol. 2021;12:612692.
Naqvi S, Panghal A, Flora SJS. Nanotechnology: a Promising Approach for Delivery of neuroprotective drugs. Front Neurosci. 2020;14:494.
Gao H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sin B. 2016;6(4):268–86.
Kelly IB 3rd, Fletcher RB, McBride JR, Weiss SM, Duvall CL. Tuning composition of polymer and porous Silicon Composite nanoparticles for early endosome escape of Anti-microRNA peptide nucleic acids. ACS Appl Mater Interfaces. 2020;12(35):39602–11.
Rodenak-Kladniew B, Islan GA, de Bravo MG, Duran N, Castro GR. Design, characterization and in vitro evaluation of linalool-loaded solid lipid nanoparticles as potent tool in cancer therapy. Colloids Surf B Biointerfaces. 2017;154:123–32.
Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A et al. Impact of particle size and Polydispersity Index on the clinical applications of Lipidic Nanocarrier systems. Pharmaceutics. 2018;10(2).
Vega-Villa KR, Takemoto JK, Yanez JA, Remsberg CM, Forrest ML, Davies NM. Clinical toxicities of nanocarrier systems. Adv Drug Deliv Rev. 2008;60(8):929–38.
Smith MC, Crist RM, Clogston JD, McNeil SE. Zeta potential: a case study of cationic, anionic, and neutral liposomes. Anal Bioanal Chem. 2017;409(24):5779–87.
Luo Y, Yang H, Zhou YF, Hu B. Dual and multi-targeted nanoparticles for site-specific brain drug delivery. J Control Release. 2020;317:195–215.
Marques-Gallego P, de Kroon AI. Ligation strategies for targeting liposomal nanocarriers. Biomed Res Int. 2014;2014:129458.
Friedman AD, Claypool SE, Liu R. The smart targeting of nanoparticles. Curr Pharm Des. 2013;19(35):6315–29.
Choi Y, Cho BK, Seok SH, Kim C, Ryu JH, Kwon IC. Controlled spatial characteristics of ligands on nanoparticles: determinant of cellular functions. J Control Release. 2023;360:672–86.
Rana S, Yeh YC, Rotello VM. Engineering the nanoparticle-protein interface: applications and possibilities. Curr Opin Chem Biol. 2010;14(6):828–34.
Paliwal R, Babu RJ, Palakurthi S. Nanomedicine scale-up technologies: feasibilities and challenges. AAPS PharmSciTech. 2014;15(6):1527–34.
Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, et al. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8(1):102.
Wagner A, Vorauer-Uhl K. Liposome technology for industrial purposes. J Drug Deliv. 2011;2011:591325.
Kwon HJ, Shin K, Soh M, Chang H, Kim J, Lee J, et al. Large-scale synthesis and medical applications of uniform-sized metal oxide nanoparticles. Adv Mater. 2018;30(42):e1704290.
Acknowledgements
Not applicable.
Funding
This work was funded by the European Regional Development Fund (ERDF) through the Centro 2020 Regional Operational Programme under BrainHealth2020 projects (CENTRO-01-0145-FEDER-000008), through the COMPETE 2020 - Operational Programme for Competitiveness and Internationalization and Portuguese national funds via FCT – Fundação para a Ciência e a Tecnologia, under projects - UIDB/04539/2020 and UIDP/04539/2020, POCI-01-0145-FEDER-030737 (NeuroStemForMJD, PTDC/BTM-ORG/30737/2017), CEECIND/04242/2017, and PhD Scholarship 2020.04751.BD.
Author information
Authors and Affiliations
Contributions
Manuscript writing and editing: RM; manuscript revision: CN, LPA, and LM; funding acquisition: LPA and LM; corresponding author: LM. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors gave their consent for the publication of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Moreira, R., Nóbrega, C., de Almeida, L.P. et al. Brain-targeted drug delivery - nanovesicles directed to specific brain cells by brain-targeting ligands. J Nanobiotechnol 22, 260 (2024). https://doi.org/10.1186/s12951-024-02511-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-024-02511-7