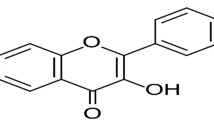
Abstract
Acute pancreatitis (AP) is a common and life-threatening digestive disorder. However, its diagnosis and treatment are still impeded by our limited understanding of its etiology, pathogenesis, and clinical manifestations, as well as by the available detection methods. Fortunately, the progress of microenvironment-targeted nanoplatforms has shown their remarkable potential to change the status quo. The pancreatic inflammatory microenvironment is typically characterized by low pH, abundant reactive oxygen species (ROS) and enzymes, overproduction of inflammatory cells, and hypoxia, which exacerbate the pathological development of AP but also provide potential targeting sites for nanoagents to achieve early diagnosis and treatment. This review elaborates the various potential targets of the inflammatory microenvironment of AP and summarizes in detail the prospects for the development and application of functional nanomaterials for specific targets. Additionally, it presents the challenges and future trends to develop multifunctional targeted nanomaterials for the early diagnosis and effective treatment of AP, providing a valuable reference for future research.
Graphical Abstract

Similar content being viewed by others
Introduction
Acute pancreatitis (AP) is a potentially fatal disease with high morbidity and mortality[1]. The typical clinical symptom is persistent severe pain in the epigastrium with abdominal distension, nausea and vomiting. In recent years, its incidence has increased over time [2, 3]. Gallstones and alcohol are common causes of AP [4], leading to activation of trypsinogen, which further activates other digestive enzymes and causes self-digestion in the pancreas [5]. In terms of the course of the disease, it progresses rapidly and the patient develops moderate AP (MAP) or severe AP (SAP), resulting in infectious necrosis, systemic inflammatory response syndrome (SIRS), and multiple organ dysfunction syndrome (MODS), with a mortality rate of 20–40% [6, 7]. Currently, the diagnosis of AP mainly relies on laboratory tests and imaging examinations, which have lower sensitivity for detecting early AP, leading to a decrease in the diagnostic rate of AP and aggravation of the disease. In terms of treatment, general therapy for AP includes close monitoring of vital signs, fluid balance, pain relief, nutritional support, and infection prevention [7, 8]. However, these methods usually fail to suppress the early response of SIRS and prevent subsequent organ failure, and no effective treatment for AP is currently available [9]. Thus, there is an urgent need for a new strategy for the diagnosis and treatment of AP.
The occurrence of AP is a complex, multifactorial, pathophysiological process. Pathological calcium signaling, mitochondrial dysfunction, impaired unfolded protein response, endoplasmic reticulum (ER) stress, and impaired autophagy are among the multiple factors contributing to the pathophysiological changes in the pancreas [5, 10]. The main pathological changes in the pancreas include the activation of trypsin, aggregation of inflammatory cells, and the excessive release of proinflammatory factors and reactive oxygen species (ROS), along with other factors, producing an abnormal microenvironment. These in turn result in a systemic inflammatory response and extensive pancreatic injury. Therefore, the identification and regulation of relevant indicators of the inflammatory microenvironment may be the key to diagnosing or treating pancreatitis.
In recent years, researchers have made remarkable progress in both the diagnosis and the treatment of AP by developing highly sensitive diagnostic tools and drugs targeting microenvironmental changes. Among these approaches, nanotechnology has attracted widespread attention because of its advantages of high sensitivity, specificity, multimeasurement ability, and targeted therapy [11]. Specifically, nanotechnology can target indicators in the microenvironment for the diagnosis and modulation of a disease. For example, Cheng et al. designed an MMP-13/pH-responsive nanoprobe (CMFn@HCQ) for the diagnosis and treatment of inflammation [12]. It was also reported that ferritin nanocages (CMFn) can be used for fluorescence imaging in response to the overexpression of metalloproteinases (MMP-13), a group of protein hydrolases that are related to the degree of inflammation in the microenvironment. It was also shown that the CMFn@HCQ nanocages could release hydroxychloroquine (HCQ) continuously into an acidic microenvironment, which significantly reduced local inflammation. ROS, as free radicals, are closely related to inflammation. The imaging and regulation of ROS can realize the early diagnosis and treatment of AP. Our group also developed a novel nanotheranostic agent (named TMSN@PM) with the ability to target inflammatory sites. Under acidic conditions featuring excessive ROS, TMSN@PM was shown to degrade and release manganese ions for magnetic resonance imaging (MRI) to assess the severity of inflammation. It was found that the T1-weighted signal was enhanced in the pancreatic region, which peaked 3 h after TMSN@PM injection. TMSN@PM also scavenges excess ROS and reduces JNK and hypoxia-inducible factor-1α (HIF-1α) activation, thereby reducing inflammation. Compared with the findings in an untreated group, ROS in the pancreas decreased significantly after TMSN@PM treatment, which attenuated the damage to pancreatic tissue [13].
As mentioned above, the occurrence and development of AP are determined by changes in the microenvironment. Significant progress has been made in the early detection and treatment of AP by nanoscientists who have proposed various effective strategies targeting indicators in the inflammatory microenvironment in AP. This review summarizes the application of nanotechnology in modulating indicators related to the inflammatory microenvironment of AP for the early diagnosis and treatment of this disease, which has not been reviewed before. It also discusses the advantages and prospects of nanomaterials in the clinical diagnosis and treatment of AP as well as highlight the limitations of research performed to date to provide new ideas for future development directions.
Pathogenesis and inflammatory microenvironment of AP
Factors such as trypsinogen activation, calcium overload, and mitochondrial dysfunction contribute to AP’s complex pathogenesis, and its diverse etiology and clinical manifestations make its traditional diagnosis and treatment difficult. In the pathophysiology of AP, the activation of trypsin is an early intra-acinar event, which leads to the activation of other digestive proteases and early pancreatic injury [5]. Activation of inflammatory cells such as macrophages releases proinflammatory cytokines that exacerbate the progression of pancreatic inflammation [14]. The excessive release of ROS further worsens the pancreatic cell injury [15], producing various damage-related molecules. Multiple signaling pathways including nuclear factor-kappa B (NF-κB) and toll-like receptor (TLR) are activated, triggering an inflammatory cascade response [16, 17]. Additionally, the impairment of pancreatic cell function and the strengthening of glycolysis lead to the decrease of pH. As shown in Fig. 1, in the pathophysiological process of AP, a large number of substances (e.g., H+, digestive enzymes, ROS, and inflammatory cells) are overexpressed and accumulate in the inflammatory site to form an inflammatory microenvironment that has been demonstrated to be significant in disease development [18].
Schematic diagram of microenvironmental targets of pancreatic inflammation. Compared with normal pancreas, AP is characterized by tissue hypoxia and increases in ROS, enzymes, inflammatory cells, H+, and microorganisms, creating an inflammatory microenvironment that exacerbates the pancreatic dysfunction. Therefore, the formation of an inflammatory microenvironment in AP is a potential target for imaging and therapy with the application of nanomaterials
Nanotechnology-based diagnosis and treatment of AP
With the rapid development of nanotechnology, the emergence of integrated nanoprobes for early diagnosis, drug delivery, and targeted therapy has provided a promising approach for the diagnosis and treatment of AP. Recently nanotechnology-based theranostic strategies for the inflammatory microenvironment have been proposed. Aiming at the abnormal indicators in the microenvironment, nanotechnology has led to new theranostic methods for AP by preparing functional nanocarriers and nanoscale drugs.
Nanotechnology refers to science, engineering, and technology conducted at the nanoscale (from 0.1 nm to hundreds of nanometers), in which nanomaterials are made multifunctional by surface modification, encapsulation and controlled release, and the modulation of physical properties [19,20,21]. It is widely used in drug delivery and development [22, 23], imaging [24], anticoagulation and hemostasis [25], phototherapy [24], immunotherapy [26, 27], and multimodal combination therapy [28]. Nanomaterials including inorganic nanoparticles, liposomes, micelles, and dendrimers have been widely reported to exhibit excellent biocompatibility, biodegradability, non-toxicity, and various beneficial physical properties as drug carriers [29,30,31,32].
In terms of diagnosis and treatment of inflammatory diseases, nanomaterials are characterized by their small size and large specific surface area. Nanodrugs and nanocarriers can enhance the bioavailability and effectiveness of drugs by targeting aggregation at inflammatory sites through the effect of extravasation through leaky vasculature and subsequent inflammatory cell-mediated sequestration (ELVIS effect) [33]. Besides, nanocarriers can improve the pharmacokinetics of drugs in the microenvironment or at the cellular level by protecting drugs from degradation, crossing biological barriers, prolonging drug half-life, and targeted controlled release, so as to improve the therapeutic effect [34,35,36,37]. For example, Chuang et al. developed a nanocarrier system that passively targets intestinal M cells, markedly improving the water solubility of curcumin (CUR) and improving the recovery of AP [38]. Owing to the advantages offered by nanotechnology, it is now widely used in diagnosing and treating inflammatory diseases, including AP.
Multiple etiologies contribute to the occurrence of AP, which is characterized by pancreatic inflammation. In the inflamed pancreas, large numbers of ROS, inflammatory cells, enzymes, H+, and other substances form an inflammatory microenvironment that can exacerbate AP progression through various mechanisms. In recent years, a series of drug delivery systems and nanodrugs have been designed based on the microenvironment specific to inflammation to achieve the diagnosis and treatment of inflammatory diseases, including AP. The diagnosis and treatment of AP based on different indicators in the inflammatory microenvironment by using nanotechnology are described below.
Inflammatory cells
There are two main inflammatory cells related to the inflammatory response: macrophages and neutrophils [39]. Neutrophils are the most abundant white blood cells in the human body, which play a vital role in acute inflammation [40]. In the early stage of inflammation, neutrophils are activated and rapidly reach the site of inflammation to eliminate pathogens [41]. Macrophages, as innate immune cells, are involved in cytokine release, tissue damage and repair, and immune disorders [42]. However, excessive activation of macrophages and neutrophils can lead to tissue damage and an inflammatory cascade response, which can result in inflammatory diseases. In the pathogenesis of AP, macrophages and neutrophils have emerged as potential targets in the diagnosis of AP and in the control of systemic inflammatory responses and complications.
As a member of the innate immune system, macrophages play a key role in the recognition of pathogens and defense against them via phagocytosis. Nanomaterials are small in size, so macrophages can internalize nanocontrast agents for the imaging of tissues and organs [43]. As a result, researchers have developed various nanomaterials for applications in macrophage imaging, so as to locate the sites of inflammation [44].
MRI is a common clinical diagnostic modality, which has been used as an important diagnostic tool for AP given its advantages of high soft-tissue contrast, high spatial resolution, and low ionizing radiation. The most common method to diagnose AP with an MRI scanner is to image superparamagnetic iron oxide nanoparticles (SPION). In vivo, SPION can be taken up by macrophages and become a macrophage marker, helping to detect damage to the pancreas and related organs at an early disease stage [45]. Nanotechnology can improve the capabilities of MRI by developing macrophage-targeted-accumulation contrast agents [46]. As reported previously, a novel Gd-containing contrast agent named Gd(III)-dithiolane gold nanoparticles can be phagocytosed by macrophages for targeted accumulation in the pancreas, which showed a very high r1 relaxation rate at both low and high magnetic field strengths for MRI of the pancreas[47]. Decorating nanoparticles with ligands that bind to macrophage surface receptors can improve the targeting of nanoparticles. Since mannose receptors are highly expressed by macrophages, Tian et al. developed novel Gd-DTPA-loaded mannosylated liposomes (named M-Gd-NL) (Fig. 2A). M-Gd-NL can bind to macrophages in a targeted manner in the inflammatory microenvironment and then release Gd-DTPA, resulting in a potent increase in the relaxation rate of Gd-DTPA in macrophages, which substantially enhances the capacity of MRI. This method not only improves the diagnostic capability of MRI, but also enables differentiation between mild and severe AP [48]. Similarly, Long et al. synthesized a P-selectin-targeted, near-infrared fluorescence (NIRF) dye (Cy 5.5)-labeled dual-modal nanoprobe (Gd-DTPA-Cy5.5-PsLmAb) based on the finding that macrophages highly express P-selectin. When PsLmAb of the nanoprobe can bind P-selectin in the microenvironment, the more P-selectin there is, the more Gd-DTPA-Cy5.5-PsLmAb nanoparticles that reside at the site of inflammation, resulting in an enhanced signal in MR/NIRF images (Fig. 2B). This probe can achieve the early diagnosis and treatment of SAP by MR imaging and NIRF imaging, providing a rapid method of visualization for the diagnosis of clinical early-stage SAP [49].
A The preparation procedure of Gd-NL and M-Gd-NL based on lipid film method. Reproduced with permission from reference [48]. Copyright 2017, DOVE Medical Press B Schematic representation of Gd-DTPA-Cy5.5-PsLmAb for NIRF and MR imaging of MAP and SAP. Reproduced with permission from reference [49]. Copyright 2020, American Chemical Society C Schematic illustration of the mechanism of activatable chemiluminescent probes. Reproduced with permission from reference [62]. Copyright 2022, John Wiley and Sons D Fabrication and targeted-therapeutic schematics of ND-MMSNs. Reproduced with permission from reference [63]. Copyright 2018, Springer Nature E Schematic illustration of nanoparticle-encapsulated CQ/TAM combined with MSCs for arresting the increasing severity of AP in mice through iNOS (IDO) signaling. Reproduced with permission from reference [68].
Macrophages also mediate the pathological process of AP through various mechanisms [50, 51]. It has also been reported that macrophages are related to the progression of SAP [52]. During SAP, peritoneal macrophages, alveolar macrophages, and Kupffer cells are activated, which contribute to the damage to various organs. Macrophages can be divided into two subtypes: M1 macrophages and M2 macrophages. M1 macrophages secrete factors related to the proinflammatory stage of AP, while M2 macrophages are mainly involved in pancreatic repair and regeneration [53]. Macrophages can change their phenotype and function spatiotemporally, which is called macrophage polarization. Therefore, regulating the polarization of macrophages is a new direction for the treatment of AP [54, 55]. Kazuaki et al. constructed a nanotechnology-based CO donor (CO-HbV) that can target macrophages and inhibit AP by releasing CO to polarize macrophages toward an M2-like phenotype. CO-HbV was also reported to inhibit neutrophil infiltration in the pancreas and attenuate the subsequent acute lung injury [56]. The degree of severity of AP is related to the number of infiltrating macrophages, which is involved in the development of injuries to the pancreas and multiple other organs. Based on this, researchers have focused particularly on drugs that inhibit macrophage recruitment and deplete macrophages. Tang et al. studied the protective effects of G4.5-COOH and G5-OH on the pancreatic injury of AP mices. It was found that two kinds of dendrimers reduced the inflammatory infiltration of macrophages by inhibiting nuclear translocation of NF-κB in macrophages. Moreover, they also inhibited the expression of proinflammatory cytokines in peritoneal macrophages and significantly decreased the pathological changes of the pancreas [57]. Clodronate liposomes are the most commonly used method to deplete macrophages [58]. Dang et al. loaded liposomes with clodronate and superparamagnetic iron oxide (SPIO), which can be delivered in a targeted manner to macrophages to induce their apoptosis by competing with adenosine triphosphate (ATP), thus inhibiting the release of inflammatory factors and alleviating the renal injury caused by SAP [59]. Different from them, Chen’s team investigated an inflammation-targeted nanoparticle named MU, which was composed of PEG − PLGA and ulinastatin coated by macrophage membrane [60]. In the mouse model of AP, MU can significantly inhibit the secretion of pro-inflammatory cytokines TNF-α and IL-6 by macrophages. In addition, in vitro experiments have proved that MU may play an anti-inflammatory role by reducing the contents of p-IκBα/IκBα and p-p65/p65 through IκBα/NF-κB signaling pathway. Therefore, MU is expected to be an effective targeted drug to inhibit the progress of AP.
Neutrophil infiltration is a hallmark of inflammation. Neutrophils, as a “living” drug delivery carrier, have attracted widespread attention in recent years because of their characteristics of crossing natural barriers, decreasing immune clearance rate and having a long biological half-life [61]. Similar to macrophages, neutrophils can take up nanoparticles [61]. Therefore, researchers have explored various neutrophil tracking probes for disease diagnosis. For example, Huang’s team synthesized three chemiluminescent probes based on benzoazole-phenoxyl-dioxetane for the in vivo imaging of neutrophils in mouse models of peritonitis and psoriasis. These probes activate and prolong chemiluminescence in the presence of neutrophil elastase (NE) (Fig. 2C). In experiments with LPS-induced peritonitis, benzothiazole-phenoxyl-dioxetane (BTPDNE) exhibited more intense brightness and a longer half-life than methyl acrylate-phenoxyl-dioxetane (MPDNE) [62]. Moreover, Wu et al. developed core–shell structured magnetic mesoporous silica nanoparticles (called MMSNs) and constructed a theranostic platform of ND-MMSNs for internalizing MMSNs loaded with doxorubicin (D-MMSNs) by neutrophils [63]. In the inflammatory mouse glioma model, ND-MMSNs are internalized by neutrophils, which can be targeted to accumulate at the inflammatory site of glioma with chemokines. Then, neutrophils release neutrophil extracellular traps (NETs) and D-MMSNs to realize the diagnosis and treatment of residual tumors (Fig. 2D). Similar to the features of the above diseases, there are a large number of neutrophils in the inflammatory microenvironment of AP, and it is expected that nanoparticles used for neutrophil imaging in the future can be used for the diagnosis of AP.
Neutrophils can release ROS to cause tissue damage [64]. Moreover, the production of NETs can speed up the progression of AP [65]. Neutrophils may serve as a target for the treatment of AP because they can mediate local tissue damage in the pancreas and associated damage to other organs when AP occurs [66]. Nanotechnology provides an plausible pathway for neutrophil-related therapeutic intervention. For example, nucleic acid nanoparticles (tFNAs) were recently reported to regulate cell proliferation and migration, and have potent anti-inflammatory and antiapoptotic abilities against AP. Wang et al. found that compared with a saline group, tFNAs significantly decreased neutrophil activity and alleviated pancreatic injury in a treatment group [67]. Additionally, Liu et al. introduced nanoparticle-encapsulated chloroquine/tamoxifen in combination with bone marrow-derived mesenchymal stem cells (BMSCs) that acted synergistically for the treatment of AP. BMSCs prevented the progression of AP by suppressing the recruitment of neutrophils, macrophages, and CD4+ T cells through iNOS signaling (Fig. 2E) [68]. Furthermore, another membrane-encapsulation technology has been applied for targeting inflammation [69, 70]. Membrane-encapsulation technology can confer nanoparticle-derived cell membrane-related functions such as immune evasion [71], crossing barriers [72], and homing to inflammatory sites [73]. Zhou et al. designed neutrophil membrane-coated nanoparticles (NNPs/CLT) that cross the blood-pancreas barrier (BPB), driven to sites of inflammation through chemokine recruitment, which significantly downregulate the level of pancreatic myeloperoxidase and reduce associated lung injuries in AP rats [74].
Macrophages and neutrophils play an important role in the systemic production of inflammatory mediators. Nanodiagnostic and nanotherapeutic agents targeting neutrophils or macrophages can be designed by nanotechnology to assess the severity of AP and suppress overactive inflammatory responses. At present, notable achievements have been made in the research and development of targeted drugs for macrophages and neutrophils. Since macrophages and neutrophils have abundant surface receptors, the development of more nanoparticles targeting these receptors is a promising future strategy.
Oxidative stress and reactive oxygen and nitrogen species
Oxidative stress is an important factor in the progression of AP, and it generates a large number of free radicals including ROS and reactive nitrogen species (RNS), leading to an imbalance between the oxidative and antioxidant systems [75]. ROS, as free radicals, are closely related to inflammation [76]. The existence of ROS not only recruits inflammatory cells to infiltrate and activate inflammatory signal pathways, but also induces oxidative damage, cell apoptosis, and necrosis [77, 78]. In response to ROS, researchers have developed ROS response strategies for the diagnosis and treatment of related diseases. Shen et al. designed theranostic polymeric NPs (named TKCP@DEX nanoprobes) targeting the ROS response in osteoarthritis (OA), which consists of thioketal linkers and cartilage-targeting peptide (TKCP) encapsulated with dexamethasone (DEX) (Fig. 3A). The nanoprobe released Cy5.5 and DEX to target articular cartilage at high levels of ROS, enabling the effective detection and treatment of OA [79]. Similarly, Hong et al. developed ROS-responsive NPs (LFP/PCDPD) for atherosclerosis-targeted diagnosis and bifunctional therapy [80]. LFP/PCDPD released lipid-specific aggregation-induced emission (AIE) fluorescent probe (LFP) and prednisolone in response to ROS and removed lipids, enabling the fluorescent diagnosis and targeted therapy of atherosclerosis. Moreover, our group introduced a therapeutic platform (P311@PEPS) for ROS-responsive micelles to promote the healing of diabetic wounds [81]. P311@PEPS was synthesized by the self-assembly of P311 peptide and ROS-responsive polymer (denoted PEPS) (Fig. 3B). Under conditions with a high level of ROS, P311@PEPS can not only promote cell migration by releasing P311, but also activate the Akt signaling pathway to accelerate the migration of epidermal cells, so as to induce wound re-epithelization. It was also observed that P311@PEPS can improve the bioavailability of P311 by scavenging excessive ROS. Our team also reported a ROS-triggered single-cell nanogel system that scavenges ROS and releases triiodothyronine to induce neural stem cells to differentiate into oligodendrocytes to promote white matter tract regeneration, which showed promising therapeutic effects in white matter after intracerebral hemorrhage [82].
A Schematic illustration of the self-assembly of ROS-responsive nanoparticles for bioimaging and targeted therapy. Reproduced with permission from reference [79]. Copyright 2021, BIOMED CENTRAL B Schematic illustration of the preparation of poly (ethylene glycol)-block-poly (propylene sulfide) (PEPS) and the therapeutic mechanism of action of ROS-sensitive micelles in vivo. Reproduced with permission from reference [81]. Copyright 2022, Elsevier C Schematic illustration of the therapeutic mechanism by which PBzyme prophylactically treats AP by inhibiting activation of the TLR/NF-κB signaling pathway. Reproduced with permission from reference [17]. Copyright 2021, Ivyspring International Publisher D Schematic diagram of the steps of synthesis of MoSe2-PVP NPs and the therapeutic mechanism for alleviating AP by scavenging RONS. Reproduced with permission from reference [89].
Additionally, functional nanocarriers loaded with antioxidant drugs can treat AP by their targeted aggregation at the site of inflammation. Shahin et al. found that CAPE-loaded nanoliposomes (CAPE-loaded NL) may exert antioxidant, anti-inflammatory, and antiapoptotic effects by modulating Nrf2 and NF-κB signaling. CAPE-loaded NL can reduce myeloperoxidase activity, and TNF-α and caspase-3 expression, thus inhibiting neutrophil infiltration, inflammation, and apoptosis [83]. Li et al. prepared a novel self-nanomicellizing system of empagliflozin (RA-EMP) that addressed the poor water solubility and low bioavailability of empagliflozin (EMP). RA-EMP alleviated the severity of AP by inhibiting oxidative stress and inflammatory factors [84].
Moreover, nanomaterials can be used directly as antioxidants. A series of nanomaterials exerting antioxidant functions have been discovered in recent years, which have significant therapeutic effects on AP. It was reported that inorganic nanoparticles can efficiently scavenge ROS because of their excellent antioxidant capacity. Our group has developed ultrasmall copper-based nanoparticles (Cu5.4O USNPs) with enzymatic ROS scavenging capabilities [85, 86]. Compared with other nanomaterials used for treating ROS-related diseases, Cu5.4O USNPs exhibit significant antioxidant efficiency at very low doses for many acute and chronic inflammatory diseases. Khurana et al. found that nanoceria showed potent activities mimicking superoxide dismutase and catalase to scavenge free radicals and attenuate cerulein-induced oxidative stress [87]. In the same year, they reported the therapeutic effect of nanoyttria (NY) in AP. NY reduced not only ROS but also the levels of amylase and lipase in plasma. Surprisingly, NY attenuated mitochondrial stress and ER stress through the Nrf2/NF-κB pathway to alleviate experimental AP [88]. Recently, artificial enzymes have drawn attention for their antioxidant capacity mimicking endogenous enzymes. Xie et al. prepared Prussian blue nanoenzyme (PBzyme), which had good preventive therapeutic effects on AP by inhibiting the TLR/NF-κB signaling pathway and scavenging ROS (Fig. 3C). In a cerulein-induced mouse AP model, PBzyme, as an antioxidant, decreased the level of MDA and increased the levels of SOD and GSH, thus alleviating oxidative stress [17]. Xie et al. also developed artificial enzymes (MoSe2-PVP NPs) with a one-pot hydrothermal strategy that mimics endogenous antioxidant systems for the treatment of free radical-induced injury. In a cerulein-induced AP mouse model, MoSe2-PVP NPs were able to scavenge free radicals to produce strong cytoprotective effects as well as inhibit the release of inflammatory factors, with potent antioxidant and anti-inflammatory effects (Fig. 3D) [89]. Similarly, this group synthesized 2D MoSe2@PVP nanosheets (NSs) [90]. MoSe2@PVP NSs exhibited thermostable multienzyme activity, which could significantly remove overexpressed ROS and RNS and help to treat diseases related to oxidative stress.
In addition to the use of inorganic nanomaterials with antioxidant properties for the treatment of AP, organic drugs with antioxidant functions can be made directly into nanoparticles. For example, nonmetallic inorganic nanoparticles (Nano-Se) improve pancreatic endocrine and exocrine functions through antioxidant and anti-inflammatory effects [91]. Furthermore, Abizaid’s group prepared cinnamic acid nanoparticles (CA-NPs) to evaluate the therapeutic effect on AP. CA-NPs reduced the MDA level and downregulated caspase-3 expression by inhibiting various signaling pathways, thereby reducing oxidative stress, inflammation, and apoptosis [92].
Nanotechnology-based delivery systems not only prevent the pathological progress of pancreatitis by inhibiting oxidative stress and acinar cell damage, but also inhibit ROS-related inflammatory signaling pathways to play an anti-inflammatory role, thus reducing various types of inflammation. Furthermore, nanocarriers can release imaging agents in the microenvironment in response to ROS, improving the diagnosis of diseases. Similar to previously mentioned inflammatory conditions, ROS are overexpressed in the inflammatory microenvironment of AP, which provide a potential target to develop ROS-responsive nanocarriers for the diagnosis and treatment of this disease. These results suggest that ROS in the inflammatory microenvironment can be an important target for the diagnosis and treatment of AP.
Enzymes
The premature activation of trypsin leads to the activation of other pancreatic enzymes when AP occurs, thus increasing the severity of pancreatitis [5, 93]. Therefore, there are numerous digestive enzymes in the AP inflammatory microenvironment and enzymes excessively released by the pancreas can be detected and used as candidate targets for AP diagnosis [94]. However, some of the conventional methods are aimed at determining the concentrations of enzymes in peripheral blood and cannot overcome the limitations caused by the lipid-water interface in lipase assays, making them less sensitive.
In response to the shortcomings of traditional detection methods, nanomaterials make it possible to substantially improve the affinity between the probe and the enzyme and the interfacial catalytic efficiency, thus realizing lipase detection with higher sensitivity and a lower detection limit with the help of the AIE mechanism [95]. Furthermore, enzyme-responsive nanocarriers can be constructed for drug delivery based on the characteristics of AP for diagnosis and even treatment. Zhang et al. synthesized a conditionally activated, gadolinium-containing MRI nanoprobe (named Gd-DTPA-FA) via the conjugation of DTPA-FA ligand and gadolinium acetate. This probe became soluble when conditionally activated by lipase activity, which increased T1 and enhanced MRI signal intensity in early AP. It was found that the signal intensity of the position corresponding to the pancreas in AP mices was significantly higher than that in the control group on T1-weighted images after the intravenous injection of Gd-DTPA-FA nanoparticles, and the highest signal intensity was observed at 6 h (Fig. 4A). These results show that Gd-DTPA-FA can be used for MR imaging of early AP [96]. Furthermore, Yao et al. designed targeted bilirubin-loaded silk fibrin nanoparticles able to selectively deliver to inflammatory lesions in the pancreas and release bilirubin rapidly in an enzymatic reaction to exert antioxidant and anti-inflammatory effects, which significantly reduce the damage to the pancreas and related tissues and organs (Fig. 4B) [97].
A Representative MRI of SD rats before and after the tail vein injection of Gd-DTPA-FA. Reproduced with permission from reference [96]. Copyright 2014, Elsevier B Schematic graph of bilirubin loaded silk fibroin nanoparticles (BRSNPs) for the experimental AP application. Reproduced with permission from reference [97]. Copyright 2020, Elsevier C Schematic representation of MΦ-NP(L&K) designed to inhibit PLA2 during AP progression. Reproduced with permission from reference [99].
Surprisingly, nanocarriers can also carry drugs to directly inhibit the release and activity of enzymes. At present, somatostatin is often used clinically to inhibit the release of pancreatic enzymes. However, its short half-life affects the therapeutic effect. Cervin et al. found that the combination of lipid-based liquid crystalline nanoparticle carrier and somatostatin (SST) can prolong the circulating half-life of SST, which shows remarkable potential for treating AP [98]. It has also been reported that phospholipase A2 (PLA2) is a pathogenic factor of AP, which damages acinar cells and promotes disease progression. Bionic nanotechnology provides another therapeutic direction to treat AP. Recently, Zhang et al. obtained macrophage membrane-coated nanoparticles [MΦ-NP(L&K)] by doping melittin and MJ-33 into macrophage membrane-coated nanoparticles. MΦ-NP(L&K) lures and kills serum PLA2 by leveraging the function of the macrophage membrane, which reduces the level of proinflammatory cytokines (Fig. 4C). In mouse models of mild and severe AP, MΦ-NP(L&K) significantly attenuated alveolar necrosis or immune infiltration and effectively reduced the severity of AP[99].
As biomarkers, digestive enzymes can be used to diagnose AP. Moreover, as important components of the inflammatory microenvironment, they are important to the development of AP and used to regulate the inflammatory microenvironment. Functional composite nanoparticles have multiple roles in the diagnosis and treatment of AP. First, as drug carriers, they can prolong the half-life of drugs; second, they can react with digestive enzymes in the microenvironment to release drugs; finally, they can bind with enzymes covalently or noncovalently to inhibit enzyme activity. Therefore, the design of functional composite nanoparticles targeting the inflammatory microenvironment using the biocatalytic properties of enzymes is extremely promising.
pH
The decrease of pH is one of the characteristics of the inflammatory microenvironment. When AP occurs, enhanced glycolysis of inflamed tissue leads to increased lactate production and a decrease in pH. Impaired endocrine and/or exocrine function of the pancreas in AP patients inhibits bicarbonate secretion by ductal cells, leading to enhanced acidification of the acinar luminal space. Lowering of pH promotes trypsinogen activation by cathepsin B [100], leading to self-digestion of the pancreas. Furthermore, persistent extracellular acidification can disrupt cell junctions and lead to the leakage of zymogen into the interstitial fluid [101]. Thus, extracellular acidification exacerbates the development of AP.
In recent years, pH-responsive drug carriers have achieved good results in the diagnosis and treatment of various diseases including AP by targeting drugs to sites of inflammation and modulating drug release in response to pH stimuli [102, 103]. In terms of diagnosis, Lu et al. synthesized a pH-responsive MRI contrast agent, SPIO@SiO2@MnO2, which can improve the diagnostic accuracy of MRI in an acidic environment by decomposing manganese dioxide (MnO2) into Mn2+ to increase T1- and T2-weighted signals (Fig. 5A) [104]. Experimental results demonstrated that the contrast sensitivity of diseased tissues is about 12.3 times that of normal tissues. As for treatment, Mei et al. developed porous COS@SiO2 nanocomposites that enable the continuous release COSs and maintain the drug at high concentrations in a pH-controlled manner, which helps to reduce the severity of SAP and its associated lung injury [105]. It was found that the release rate of COS was greater at pH 7.4 than at pH 8.0 (Fig. 5B). Yang’s team used chloroquine diphosphate (CQ) for gene transfection to construct Ca-CQ-pDNA-PLGA-NPs that can deliver targeted genes to the site of pancreatitis and protect the pancreas from deterioration based on pH changes. Compared with the findings at pH 7.4 and pH 6.8, the cumulative pDNA release at pH 4.5 exceeded 30% within 24 h and eventually reached 60% within 4 weeks [106]. Similarly, Hassanzadeh et al. prepared a neutrophil membrane-encapsulated nanoformulation (FA-SF-NPs) using silk fibroin (SF) and ferulic acid (FA). FA-SF-NPs released FA with higher kinetics in a low-pH environment compared with the findings at physiological pH, thereby downregulating serum enzymes and oxidative stress-related indicators to reduce the severity of AP [107]. Moreover, with the development of nanotechnology, pH-responsive theranostic nanoplatforms have been successively developed. Dou et al. constructed metal Fe/Ce-doped mesoporous silica nanoparticles (Fe-Ce-MSN) for the treatment of inflammation and oxidative stress-related diseases [108]. In the mildly acidic environment of inflammatory sites, Fe-/Ce-MSN nanoparticles released Fe ions, which enhanced the T2-weighted signals. Additionally, Fe-Ce-MSN NPs not only scavenged overproduced ROS but also controlled the production of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), with significant antioxidant and anti-inflammatory effects (Fig. 5C).
A SPIO@SiO2@MnO2 shows weak T1 and T2 contrast intensity in normal physiological conditions, as the T2 signal of SPIO is quenched by the MnO2 layer. In the acidic environment of a tumor or inflamed tissue, the MnO2 layer will decompose into magnetically active Mn2+ (T1-weighted), and the T1 and T2 signals are sequentially recovered. Reproduced with permission from reference [104]. Copyright 2022, Springer Nature B The cumulative release of COSs from COS@SiO2 at pH 7.4 and pH 8.0. Reproduced with permission from reference [105]. Copyright 2020, Frontiers Media S.A. C Schematic illustration of biodegradation, ROS scavenging effects, and enhanced theranostic functions by Fe/Ce-MSN-PEG NPs. Reproduced with permission from reference [108].
The inflammatory response of AP fosters a pH gradient between inflamed and healthy tissues, which provides a suitable physiological stimulus for pH-responsive drug delivery. pH-responsive drug delivery systems overcome the shortcomings of conventional drug formulations and show advantages in terms of biocompatibility, stability, size, and structural control. Moreover, they can deliver drugs to specific sites in a controlled manner and at predetermined release rates, reducing drug side effects and improving drug efficacy. Therefore, acid-responsive nanocarriers are of high value for the diagnosis and treatment of AP.
As a brief summary, Table 1 shows the strategies of diagnosis and treatment of AP with various nanomaterials.
Microorganisms
AP can be classified as MAP, moderate-to-severe AP (MSAP), and SAP according to the severity [109]. Patients with AP can develop MSAP and SAP, leading to necrotizing pancreatitis (NP), which has a high mortality rate [110]. In the later stage, patients develop intestinal dysfunction and are at risk of the translocation of intestinal flora and secondary infection of necrotic tissue. Most of the bacteria that cause pancreatic tissue necrosis infections are from the intestinal flora [111], mainly including Gram-negative and Gram-positive bacteria. The gut microbiota exists in the inflammatory microenvironment, which is an important mediator during AP and influences the progression of AP.
New imaging strategies have been developed by those researching infectious diseases [112]. For example, Yang’s team introduced an imaging strategy of gold nanoparticles modified by glucose polymer [113], In their study, bacteria ingested nanoparticles through the ATP-binding cassette transporter pathway, and then, under laser irradiation, the nanoparticles aggregated, thereby enhancing the photoacoustic signal. Compared with some optical contrast agents, the nanoparticles can image bacteria in vivo at levels as low as ~ 105 colony-forming units, and have higher sensitivity. Surprisingly, these nanoparticles also have antibacterial activity and an enhanced antibacterial rate. Another photoacoustic contrast agent (AuNPs@P1) not only specifically binds to the cell surface of Staphylococcus aureus in the infected area through an active targeting mechanism, but also induces the targeted aggregation of gold nanoparticles at the site of infection through bacterial overexpression of collagenase IV, which significantly enhances the photoacoustic signal [114]. Compared with traditional AuNPs, AuNPs@P1 has higher sensitivity and specificity.
On the treatment side, the regulation or suppression of intestinal flora may be an effective treatment for AP. Antibiotics are now often used to prevent infection in pancreatic necrosis. However, the long-term use of antibiotics increases the level of bacterial resistance and the incidence of fungal infections [115]. Nanomaterials could be developed to replace biocides and provide new antimicrobial strategies for the treatment of infectious diseases due to its excellent anti-infection ability [116, 117]. It has been reported that metal-based nanomaterials usually have broad-spectrum antibacterial properties with no drug resistance. Silver and photothermal therapy (PTT) are highly effective in killing drug-resistant bacteria. Our group combined these two therapies to develop a bacterial-targeted therapeutic platform (Ag+-GCS-PDA@GNRs) based on polydopamine (PDA)-encapsulated gold nanorods (GNRs) to exert synergistic antibacterial activity [118]. Ag+-GCS-PDA@GNRs release Ag+ under acidic conditions and deliver it to abscess environment to destroy the cell membrane of bacteria and reduce their heat resistance. On the other hand, combined PPT treatment not only kills bacterial cells but also triggers the release of more Ag+, exhibiting higher bactericidal efficiency. Yu and colleagues developed a new strategy to eradicate drug-resistant bacteria by inducing the production of alkyl radicals [119]. An antimicrobial depot (AIBI-GCS-PDA@CG) was synthesized by modifying polydopamine-coated carboxyl graphene with glycol chitosan (GCS) and utilizing an initiator of 2,2-azobis[2-(2-imidazolin-2-yl)propane]dihydrochloride (AIBI) as a source of free radicals. Under the conditions of acidic pus and hypoxia at the infected site, AIBI-GCS-PDA@CG decomposed into alkyl radical by NIR radiation, which destroyed bacterial DNA and led to the death of bacteria. Additionally, it has been demonstrated that AIBI-GCS-PDA@CG has an equivalent therapeutic effect on bacteria under normoxic conditions. Based on the role of macrophage membranes in bacterial recognition, Wang et al. designed a macrophage-membrane-coated gold nanocage that can target bacteria more effectively by improving bacterial adhesion and retaining bacteria at the site of infection [120].
With disease aggravation, AP progresses to NP. Infection is a characteristic of patients with NP. Nanotechnology can be applied to exploit unique targets of the infectious microenvironment to design nanoparticles that target the site of infection, improving diagnostic rates and therapeutic efficacy. Although many studies have reported the use of nanomaterials for the treatment and diagnosis of infectious diseases, few have been reported for acute NP, which has prompted us to shift our research plans toward the use of nanotechnology for the diagnosis and anti-infective treatment of AP.
Hypoxia
Most inflammatory diseases are characterized by hypoxia, and AP is no exception. The inflammatory response in diseased tissues increases metabolic activity and leads to hypoxia, which then activates HIF-1α [121]. It has been reported that HIF-1α is involved in the histopathological progression of AP [122]. Knockdown of HIF-1α reduces the production of ROS to attenuate the necrosis of pancreatic follicular cells [123]. AP with pulmonary dysfunction further aggravates hypoxia, leading to hypoxemia and acute respiratory distress syndrome [124]. The anoxic nature of AP offers the possibility to design anoxia-responsive nanoplatforms. Currently, hypoxia-responsive nanomaterials are widely used in the treatment and diagnosis of tumors. It is known that nitroreductase (NTR) is highly expressed in hypoxic regions. Based on this, Zheng et al. reported a near-infrared off–on fluorescence probe (Cy-NO2) [125]. Cy-NO2 was found to be hypersensitive and highly selective for rapid response to NTR, which is ideal in models of cerebral ischemia and deep vein thrombosis. Zhang et al. proposed hypoxia-responsive nanocarriers (CPs-CPT-Ce6 NPs) combined with photodynamic therapy (PDT) as a strategy to treat tumors [126]. Upon irradiation with a laser, CPs-CPT-Ce6 NPs release ROS, which aggravate tissue hypoxia and lead to the release of camptothecin (CPT) from conjugated polymers (CPs) loaded with photosensitizers and chemotherapeutic agents, enhancing the therapeutic effect. Combined treatment of CPs-CPT-Ce6 NPs with PDT exerts a synergistic effect to enhance the potency of killing PDT-resistant tumor cells. Hypoxia-responsive nanoplatforms that combine diagnosis and treatment have also been developed. For example, Zhou and colleagues constructed an azo-based hypoxia-responsive theranostic agent (AzP1) [127]. Under hypoxic conditions, cleavage of the azo bond in AzP1 releases the drug (SN-38) with enhanced cytotoxicity. Additionally, SN-38 exhibits fluorescence enhancement at λEm = 560 nm and can be used for inflammation-specific imaging. AzP1 is an excellent theranostic system that combines hypoxia response therapy and imaging technology.
Controlling hypoxia may be one way of alleviating the symptoms of AP. In a clinical context, physicians mainly depend on oxygenation to relieve patients’ hypoxia. However, the potential toxicity of an excessive oxygen supply hinders its clinical application [128]. Nanotechnology offers a new approach to oxygen supply. To date, researchers have adopted different strategies to overcome hypoxia and improve the treatment effect. One strategy is to prepare O2 carriers using smart nanomaterials to transport molecular oxygen directly to the hypoxic site. Shi et al. used hemoglobin (Hb) to synthesize a multifunctional nanoprobe (Gd@HbCe6−PEG) based on Gd-based nanostructures [129]. This probe can be loaded with the photosensitizer chlorine e6 (Ce6) and oxygen to alleviate the hypoxic environment of the tumor. Under laser irradiation, Gd@HbCe6−PEG produces oxygen and kills tumor cells. Experiments have shown that Gd@HbCe6−PEG has excellent oxygen-carrying capacity, which can enhance the therapeutic effect of PDT. Another strategy is to generate O2 in situ by H2O2 based on the characteristics of the tumor microenvironment. In recent years, various nanoparticle-based catalysts/enzymes have been constructed to catalyze the decomposition of H2O2 to generate O2 to ameliorate tumor hypoxia. For example, Wang and colleagues designed a multifunctional mesoporous nanoenzyme that reacts with endogenous H2O2 to produce O2 and successfully ameliorate tumor hypoxia [130]. Moreover, Liu et al. created a core–shell nanosystem that uses catalase to catalyze the decomposition of H2O2 into O2 to alleviate inflammatory hypoxia and inhibit HIF-1α expression [131]. However, there is no particularly precise method for the real-time dynamic monitoring and control of local oxygen concentration.
Multitargeting strategies
In the inflammatory microenvironment in AP, excessive digestive enzymes, H+, inflammatory cells, and ROS are major parts of the inflammatory response, which can exacerbate the progression of AP through various mechanisms. Therefore, the use of a single index for diagnosis and treatment has limited efficacy. The coexistence of these inflammation-associated substances provides additional opportunities for the diagnosis and treatment of AP. In recent years, multitargeted drug delivery systems have been developed. In contrast, dual-targeted drug delivery systems can better utilize the microenvironment to achieve efficient drug delivery. Dai et al. constructed a nanosystem with self-amplified drug release for synergistic oxidation-chemotherapy [132]. The system is not only pH-sensitive, enhancing the cellular uptake of drugs by shifting surface charge, but also responds to ROS to release β-lapachone and cephaeline, overcoming multidrug resistance at tumor sites and promoting apoptosis in tumor cells. In another study, Gou et al. designed SF nanoparticles (CS-CUR-NP) surface-functionalized (SF) with chondroitin sulfate (CS). Upon stimulation by pH and ROS, SF can release CUR on demand. Moreover, CS-CUR-NP has the ability to target macrophages and can release drugs at inflammatory sites for the treatment of ulcerative colitis [133]. Unexpectedly, nanoparticles for AP therapy and diagnosis have also been developed. Our group developed a nanotheranostic agent (TMSN@PM) with slight acidic and excessive ROS stimulation. TMSN@PM can be used for MRI and therapy of AP. The development of TMSN@PM provides a precedent for combining diagnostic and therapeutic applications in AP, and it is believed that more multifunctional nanoplatforms will be applied to AP in the future with the joint efforts of researchers.
The complex microenvironmental changes in AP increase the difficulty of diagnosis and treatment, but also provide more targets, as shown in Fig. 6. Compared with a single targeting drug, regulating two or more targets simultaneously improves the effect. Therefore, finding more targets and designing multitargeting nanocarriers are expected to lead to better strategies for the diagnosis and treatment of AP.
Schematic diagram of different modes of interaction between nanoparticles and targets in the inflammatory microenvironment. Nanoparticles are released from blood vessels into the pancreatic inflammatory microenvironment, which not only react with reduced oxygen and excess enzymes, ROS, and H+ to release imaging agents and drugs for diagnostic and therapeutic purposes, but also act directly on these targets to reduce ROS and enzymes and increase oxygen. Additionally, inflammatory cells and bacteria can phagocytose nanoparticles for imaging and exert anti-inflammatory and antibacterial effects. Meanwhile, M1 macrophages were shown to be regulated to M2 macrophages by the action of nanoparticles and changes in the microenvironment. The application of nanotechnology can monitor and reduce the severity of acute pancreatitis
Conclusions and outlooks
AP is a complex inflammatory disease associated with multiple mechanisms, such as Ca2+ overload, mitochondrial dysfunction, trypsinogen activation, impaired autophagy, and ER stress. In the early stage of AP, mild damage to pancreatic tissue occurs, but as the disease progresses it can lead to SIRS and MODS in severe and life-threatening cases. Therefore, early diagnosis and treatment are crucial for patients with AP.
In terms of diagnosis, the development of nanotechnology has addressed the limitations of conventional medical diagnosis and treatment. On the one hand, nanomaterials with a highly selective bioresponse can improve sensitivity and reduce response time, thus increasing the efficiency of various detection techniques [95, 134]. On the other hand, various biosensors and nanoprobes have been developed as indicators of the inflammatory microenvironment. Nanomaterials can enhance their performance and characteristics through surface modification, accumulating in organs in a targeted way and increasing the signal intensity [135]. Therefore, nanotechnology has been widely used in the diagnosis of AP by improving the efficiency of pancreatic enzyme detection and pancreatic tissue imaging, improving the diagnostic rate of AP.
In terms of treatment, in recent years a series of nanodrugs and nanocarrier systems have been developed to restore the damage of AP, which has solved the shortcomings of poor solubility and bioavailability of conventional drugs and improved the efficiency of drug delivery [38]. Furthermore, therapeutic nanomaterials targeting constituents of the microenvironment have been applied to treat AP, exerting anti-inflammatory, antioxidant, and antiapoptotic effects. The inflammatory microenvironment forms when AP occurs, so carriers that target abnormal biochemical indicators (H+, ROS, abundant digestive enzymes, etc.) in the microenvironment can be designed by applying surface functionalization technologies and stimulus-responsive materials to achieve targeted drug delivery to pancreatic tissues and related damaged organs and prolong the residence time at the site of inflammation. Additionally, through the construction of responsive drug delivery systems, the substances overexpressed in the microenvironment are consumed to produce synergistic effects and improve the therapeutic efficacy. Therefore, targeting corresponding indicators in the microenvironment for drug therapy is an attractive strategy and raises the possibility of developing effective treatments of various inflammatory diseases.
Nanotechnology has achieved excellent results in the diagnosis and treatment of AP by targeting potential targets in the inflammatory microenvironment, but there are still many challenges and limitations. Firstly, due to the anatomical features of the pancreas, there exists a unique BPB composed of the capillary endothelial cell layer around the glandular follicles, the basement membrane layer and other structures, which prevents pancreas from pathogenic microorganisms infecting but creates a certain obstacle to the delivery of drugs into the pancreas at the same time, resulting in a limited number of types of drugs that can effectively cross the BPB as well as a low effective concentration and utilization rate [72, 136]. Currently, nanocarriers targeting the inflammatory microenvironment are limited to a single type, resulting in low delivery efficiency. In the future, the advantages of different carriers can be combined to develop carriers with higher delivery efficiency and biosafety. Secondly, the pathogenesis of AP has not been fully clarified, so there are not enough specific targets for AP, resulting in fewer nanodrugs that can specifically enter the pancreatic inflammatory microenvironment. At present, most nanodrugs passively target inflammatory lesions through the ELVIS effect to increase the drug concentration, but there is an off-target effect, which leads to low efficiency of nanodrug delivery [74, 107]. There is an urgent need to develop more active targeted nanodrugs combined with target molecules, instead of passive targeting, and to improve the delivery efficiency. Recently, the emergence of genomics, protein genomics and metabonomics has made it possible to find more specific markers of pancreatitis, thus improving the diagnosis rate and treatment efficiency.
The development and application of functional nanomaterials targeting various potential targets in the inflammatory microenvironment is a future trend in the early diagnosis and treatment of AP, but there are still many concerns that have led to the fact that nanomaterials are not yet widely used in the clinic, and one of the most important issue is the biosafety of nanomaterials. On the one hand, we still know little about the risks and their potential threats of nanomaterials, and the fact of lacking regulatory guidance and uniform standards for the toxicological assessment of nanomaterial-based drug delivery systems worsen this situation [137, 138]. On the other hand, most of the experiments conducted to date have been based on animal models, however, it is difficult for animal models to simulate the absorption, distribution, metabolism and excretion of nanomaterials in the human body as well as their effects on organs and tissues due to the complexity of the human immune system in terms of drug metabolism. Moreover, there are many common methods to establish AP models [97, 99, 107], and forms of AP caused by various different factors have their own specific characteristics. Researchers need to know the pathophysiology and limitations of each model in order to choose the appropriate model according to their own experimental needs. The technology for preparing AP models is not fully mature, and it is difficult for animal models to simulate the pathogenesis of human AP due to the diversity and complexity of its causes. Therefore, it is necessary to establish a unified scientific evaluation system, improve animal models, evaluation indexes and testing methods, and vigorously develop nanotoxicology, so as to promote the clinical translation of nanomaterials.
With the joint efforts of researchers in the future, multicenter and large-scale clinical trials can be realized. Additionally, with continuous technological development, artificial intelligence, big data analysis, 3D printing technology, and other fields have emerged, with which nanotechnology can be combined to generate new innovations and improve the ability to diagnose and treat human diseases, thus bringing a new era in the field of medicine.
Availability of data and materials
Not applicable.
Change history
23 January 2024
A Correction to this paper has been published: https://doi.org/10.1186/s12951-023-02232-3
Abbreviations
- AP:
-
Acute pancreatitis
- ROS:
-
Reactive oxygen species
- MAP:
-
Moderate acute pancreatitis
- SAP:
-
Severe acute pancreatitis
- ER:
-
Endoplasmic reticulum
- SIRS:
-
Systemic inflammatory response syndrome
- MODS:
-
Multiple organ dysfunction syndrome
- HCQ:
-
Hydroxychloroquine
- MRI:
-
Magnetic resonance imaging
- HIF-1α:
-
Hypoxia-inducible factor-1α
- NF-κB:
-
Nuclear factor-kappa B
- TLR:
-
Toll-like receptor
- ELVIS effect:
-
The effect of extravasation through leaky vasculature and subsequent inflammatory cell-mediated sequestration
- CUR:
-
Curcumin
- SPION:
-
Superparamagnetic iron oxide nanoparticles
- NIRF:
-
Near-infrared fluorescence
- SPIO:
-
Superparamagnetic iron oxide
- ATP:
-
Adenosine triphosphate
- NE:
-
Neutrophil elastase
- MMSNs:
-
Magnetic mesoporous silica nanoparticles
- NETs:
-
Neutrophil extracellular traps
- BMSCs:
-
Bone marrow-derived mesenchymal stem cells
- RNS:
-
Reactive nitrogen species
- OA:
-
Osteoarthritis
- DEX:
-
Dexamethasone
- TKCP:
-
Thioketal linkers and cartilage-targeting peptide
- AIE:
-
Aggregation-induced emission
- CAPE-loaded NL:
-
CAPE-loaded nanoliposomes
- EMP:
-
Empagliflozin
- NY:
-
Nanoyttria
- PBzyme:
-
Prussian blue nanoenzyme
- NSs:
-
Nanosheets
- CA-NPs:
-
Cinnamic acid nanoparticles
- SST:
-
Somatostatin
- PLA2:
-
Phospholipase A2
- MnO2 :
-
Manganese dioxide
- CQ:
-
Chloroquine diphosphate
- SF:
-
Silk fibroin
- FA:
-
Ferulic acid
- Fe-Ce-MSN:
-
Fe/Ce-doped mesoporous silica nanoparticles
- TNF-α:
-
Tumor necrosis factor-α
- IL-1β:
-
Interleukin-1β
- MSAP:
-
Moderate-to-severe acute pancreatitis
- NP:
-
Necrotizing pancreatitis
- PTT:
-
Photothermal therapy
- GCS:
-
Glycol chitosan
- GNRs:
-
Gold nanorods
- AIBI:
-
2,2-Azobis[2-(2-imidazolin-2-yl)propane]dihydrochloride
- PDA:
-
Polydopamine
- NTR:
-
Nitroreductase
- PDT:
-
Photodynamic therapy
- CPs:
-
Conjugated polymers
- CPT:
-
Camptothecin
- Hb:
-
Hemoglobin
- Ce6:
-
Chlorine e6
- CS:
-
Chondroitin sulfate
- SF:
-
Surface-functionalized
References
Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology Guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400–15.
Sternby H, Bolado F, Canaval-Zuleta HJ, Marra-Lopez C, Hernando-Alonso AI, Del-Val-Antonana A, et al. Determinants of severity in acute pancreatitis: a nation-wide multicenter prospective cohort study. Ann Surg. 2019;270(2):348–55.
Iannuzzi JP, King JA, Leong JH, Quan J, Windsor JW, Tanyingoh D, et al. Global incidence of acute pancreatitis is increasing over time: a systematic review and meta-analysis. Gastroenterology. 2022;162(1):122–34.
Roberts SE, Morrison-Rees S, John A, Williams JG, Brown TH, Samuel DG. The incidence and aetiology of acute pancreatitis across Europe. Pancreatology. 2017;17(2):155–65.
Saluja A, Dudeja V, Dawra R, Sah RP. Early intra-acinar events in pathogenesis of pancreatitis. Gastroenterology. 2019;156(7):1979–93.
Schepers NJ, Bakker OJ, Besselink MG, Ahmed Ali U, Bollen TL, Gooszen HG, et al. Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut. 2019;68(6):1044–51.
van Dijk SM, Hallensleben NDL, van Santvoort HC, Fockens P, van Goor H, Bruno MJ, et al. Acute pancreatitis: recent advances through randomised trials. Gut. 2017;66(11):2024–32.
Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, et al. Acute pancreatitis. Lancet. 2020;396(10252):726–34.
Kambhampati S, Park W, Habtezion A. Pharmacologic therapy for acute pancreatitis. World J Gastroenterol. 2014;20(45):16868–80.
Biczo G, Vegh ET, Shalbueva N, Mareninova OA, Elperin J, Lotshaw E, et al. Mitochondrial dysfunction, through impaired autophagy, leads to endoplasmic reticulum stress, deregulated lipid metabolism, and pancreatitis in animal models. Gastroenterology. 2018;154(3):689–703.
Han S, Chen C, Chen C, Wu L, Wu X, Lu C, et al. Coupling annealed silver nanoparticles with a porous silicon Bragg mirror SERS substrate and machine learning for rapid non-invasive disease diagnosis. Anal Chim Acta. 2023;1254:341116.
Chen H, Qin Z, Zhao J, He Y, Ren E, Zhu Y, et al. Cartilage-targeting and dual MMP-13/pH responsive theranostic nanoprobes for osteoarthritis imaging and precision therapy. Biomaterials. 2019;225:119520.
Li X, Liu Y, Qi X, Xiao S, Xu Z, Yuan Z, et al. Sensitive activatable nanoprobes for real-time ratiometric magnetic resonance imaging of reactive oxygen species and ameliorating inflammation in vivo. Adv Mater. 2022;34(19):e2109004.
Manohar M, Jones EK, Rubin SJS, Subrahmanyam PB, Swaminathan G, Mikhail D, et al. Novel circulating and tissue monocytes as well as macrophages in pancreatitis and recovery. Gastroenterology. 2021;161(6):2014-29.e14.
Xiang H, Guo F, Tao X, Zhou Q, Xia S, Deng D, et al. Pancreatic ductal deletion of S100A9 alleviates acute pancreatitis by targeting VNN1-mediated ROS release to inhibit NLRP3 activation. Theranostics. 2021;11(9):4467–82.
Sendler M, Weiss F-U, Golchert J, Homuth G, van den Brandt C, Mahajan UM, et al. Cathepsin B-mediated activation of trypsinogen in endocytosing macrophages increases severity of pancreatitis in mice. Gastroenterology. 2018;154(3):704-18.e10.
Xie X, Zhao J, Gao W, Chen J, Hu B, Cai X, et al. Prussian blue nanozyme-mediated nanoscavenger ameliorates acute pancreatitis via inhibiting TLRs/NF-kappaB signaling pathway. Theranostics. 2021;11(7):3213–28.
Zhang G, Ma L, Bai L, Li M, Guo T, Tian B, et al. Inflammatory microenvironment-targeted nanotherapies. J Control Release. 2021;334:114–26.
Ke W, Afonin KA. Exosomes as natural delivery carriers for programmable therapeutic nucleic acid nanoparticles (NANPs). Adv Drug Deliv Rev. 2021;176:113835.
Murugesan S, Scheibel T. Copolymer/clay nanocomposites for biomedical applications. Adv Funct Mater. 2020. https://doi.org/10.1002/adfm.201908101.
Ahmad A, Rashid S, Chaudhary AA, Alawam AS, Alghonaim MI, Raza SS, et al. Nanomedicine as potential cancer therapy via targeting dysregulated transcription factors. Semin Cancer Biol. 2023;89:38–60.
Kumar A, Ahmad A, Ansari MM, Gowd V, Rashid S, Chaudhary AA, et al. Functionalized-DNA nanostructures as potential targeted drug delivery systems for cancer therapy. Semin Cancer Biol. 2022;86:54–68.
Hajinezhad MR, Shahraki S, Nikfarjam Z, Davodabadi F, Mirinejad S, Rahdar A, et al. Development of a new vesicular formulation for delivery of Ifosfamide: evidence from in vitro, in vivo, and in silico experiments. Arab J Chem. 2023;16(9):105086.
Xu XAH, Zhang D, Tao H, Dou Y, Li X, Huang J, Zhang J. A self-illuminating nanoparticle for inflammation imaging and cancer therapy. Sci Adv. 2019;5(1):eaat2953.
Wang Y, Zhai W, Cheng S, Li J, Zhang H. Surface-functionalized design of blood-contacting biomaterials for preventing coagulation and promoting hemostasis. Friction. 2023;11(8):1371–94.
Huang S, Yuan J, Xie Y, Qing K, Shi Z, Chen G, et al. Targeting nano-regulator based on metal–organic frameworks for enhanced immunotherapy of bone metastatic prostate cancer. Cancer Nanotechnol. 2023. https://doi.org/10.1186/s12645-023-00200-y.
Gowd V, Ahmad A, Tarique M, Suhail M, Zughaibi TA, Tabrez S, et al. Advancement of cancer immunotherapy using nanoparticles-based nanomedicine. Semin Cancer Biol. 2022;86:624–44.
Zhang Y, Bo S, Feng T, Qin X, Wan Y, Jiang S, et al. A versatile theranostic nanoemulsion for architecture-dependent multimodal imaging and dually augmented photodynamic therapy. Adv Mater. 2019;31(21):e1806444.
Ghosh B, Biswas S. Polymeric micelles in cancer therapy: state of the art. J Control Release. 2021;332:127–47.
Shah S, Dhawan V, Holm R, Nagarsenker MS, Perrie Y. Liposomes: advancements and innovation in the manufacturing process. Adv Drug Deliv Rev. 2020;154–155:102–22.
Li H, Zha S, Li H, Liu H, Wong KL, All AH. Polymeric dendrimers as nanocarrier vectors for neurotheranostics. Small. 2022. https://doi.org/10.1002/smll.202203629.
Yang G, Phua SZF, Bindra AK, Zhao Y. Degradability and clearance of inorganic nanoparticles for biomedical applications. Adv Mater. 2019;31(10):e1805730.
Pan W, Li Z, Qiu S, Dai C, Wu S, Zheng X, et al. Octahedral Pt-MOF with Au deposition for plasmonic effect and Schottky junction enhanced hydrogenothermal therapy of rheumatoid arthritis. Mater Today Bio. 2022;13:100214.
Wang J, Ni Q, Wang Y, Zhang Y, He H, Gao D, et al. Nanoscale drug delivery systems for controllable drug behaviors by multi-stage barrier penetration. J Control Release. 2021;331:282–95.
van der Koog L, Gandek TB, Nagelkerke A. Liposomes and extracellular vesicles as drug delivery systems: a comparison of composition, pharmacokinetics, and functionalization. Adv Healthc Mater. 2022;11(5):e2100639.
Wu W, Luo L, Wang Y, Wu Q, Dai HB, Li JS, et al. Endogenous pH-responsive nanoparticles with programmable size changes for targeted tumor therapy and imaging applications. Theranostics. 2018;8(11):3038–58.
Narayanaswamy R, Torchilin VP. Hydrogels and their applications in targeted drug delivery. Molecules. 2019;24(3):603.
Chuang EY, Lin KJ, Huang TY, Chen HL, Miao YB, Lin PY, et al. An intestinal “transformers”-like nanocarrier system for enhancing the oral bioavailability of poorly water-soluble drugs. ACS Nano. 2018;12(7):6389–97.
Su Y, Gao J, Kaur P, Wang Z. Neutrophils and macrophages as targets for development of nanotherapeutics in inflammatory diseases. Pharmaceutics. 2020;12(12):1222.
Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–89.
Peiseler M, Kubes P. More friend than foe: the emerging role of neutrophils in tissue repair. J Clin Invest. 2019;129(7):2629–39.
Wang Z, Koenig AL, Lavine KJ, Apte RS. Macrophage plasticity and function in the eye and heart. Trends Immunol. 2019;40(9):825–41.
Cörek ERG, Siegrist S, Einfalt T, Detampel P, Schlepütz CM, Sieber S, Fluder P, Schulz G, Unterweger H, Alexiou C, Müller B, Puchkov M, Huwyler J. Shedding light on metal-based nanoparticles in zebrafish by computed tomography with micrometer resolution. Small. 2020. https://doi.org/10.1002/smll.202000746.
Deng H, Li X, Ju J, Mo X, Ge G, Zhu X. Multifunctional nanoprobes for macrophage imaging. Biomaterials. 2022;290:121824.
Zhang JXDS, Zhang Y, Sha X, Zhang LR, Wei CS, Chen M, Jiang DL. MRI shows clodronate-liposomes attenuating liver injury in rats with severe acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2010;9(2):192–200.
Yang B, Liu Q, Yao X, Zhang D, Dai Z, Cui P, et al. FePt@MnO-based nanotheranostic platform with acidity-triggered dual-ions release for enhanced MR imaging-guided ferroptosis chemodynamic therapy. ACS Appl Mater Interfaces. 2019;11(42):38395–404.
Holbrook RJ, Rammohan N, Rotz MW, MacRenaris KW, Preslar AT, Meade TJ. Gd(III)-dithiolane gold nanoparticles for T1-weighted magnetic resonance imaging of the pancreas. Nano Lett. 2016;16(5):3202–9.
Tian B, Liu R, Chen S, Chen L, Liu F, Jia G, et al. Mannose-coated gadolinium liposomes for improved magnetic resonance imaging in acute pancreatitis. Int J Nanomed. 2017;12:1127–41.
Long L, Deng L, Wang L, Wen S, Luo L, Liang L, et al. P-selectin-based dual-model nanoprobe used for the specific and rapid visualization of early detection toward severe acute pancreatitis in vivo. ACS Biomater Sci Eng. 2020;6(10):5857–65.
Zhao Q, Wei Y, Pandol SJ, Li L, Habtezion A. STING signaling promotes inflammation in experimental acute pancreatitis. Gastroenterology. 2018;154(6):1822-35.e2.
Iyer S, Bava EP, George J, Tarique M, Sahay P, Edwards DB, et al. Mo1380 pirfenidone treatment ameliorates the severity of acute pancreatitis by reducing macrophage infiltration and modulating its polarization. Gastroenterology. 2020;158(6):S-869.
Sendler M, van den Brandt C, Glaubitz J, Wilden A, Golchert J, Weiss FU, et al. NLRP3 inflammasome regulates development of systemic inflammatory response and compensatory anti-inflammatory response syndromes in mice with acute pancreatitis. Gastroenterology. 2020;158(1):253-69.e14.
Wu J, Zhang L, Shi J, He R, Yang W, Habtezion A, et al. Macrophage phenotypic switch orchestrates the inflammation and repair/regeneration following acute pancreatitis injury. EBioMedicine. 2020;58:102920.
Sahay P, Bava EP, Iyer S, Dudeja V. Modulation of macrophage polarity for treatment of acute pancreatitis: are we there yet? EBioMedicine. 2020;60:103002.
He Y, Dai J, Niu M, Li B, Husain SZ, Hu G, et al. Su302 inhibition of nampt protects against acute pancreatitis via modulating macrophage polarization and metabolic alteration. Gastroenterology. 2021;160(6):S-666-S−7.
Taguchi K, Nagao S, Maeda H, Yanagisawa H, Sakai H, Yamasaki K, et al. Biomimetic carbon monoxide delivery based on hemoglobin vesicles ameliorates acute pancreatitis in mice via the regulation of macrophage and neutrophil activity. Drug Deliv. 2018;25(1):1266–74.
Tang Y, Han Y, Liu L, Shen W, Zhang H, Wang Y, et al. Protective effects and mechanisms of G5 PAMAM dendrimers against acute pancreatitis induced by caerulein in mice. Biomacromol. 2015;16(1):174–82.
Haider N, Bosca L, Zandbergen HR, Kovacic JC, Narula N, Gonzalez-Ramos S, et al. Transition of macrophages to fibroblast-like cells in healing myocardial infarction. J Am Coll Cardiol. 2019;74(25):3124–35.
Dang SC, Zeng YH, Wang PJ, Chen BD, Chen RF, Kumar Singh A, et al. Clodronate-superparamagnetic iron oxide-containing liposomes attenuate renal injury in rats with severe acute pancreatitis. J Zhejiang Univ Sci B. 2014;15(6):556–65.
Chen Y, Tao H, Chen R, Pan Y, Wang J, Gao R, et al. Biomimetic nanoparticles loaded with ulinastatin for the targeted treatment of acute pancreatitis. Mol Pharm. 2023;20(8):4108–19.
Chu D, Dong X, Shi X, Zhang C, Wang Z. Neutrophil-based drug delivery systems. Adv Mater. 2018;30(22):e1706245.
Huang J, Cheng P, Xu C, Liew SS, He S, Zhang Y, et al. Chemiluminescent probes with long-lasting high brightness for in vivo imaging of neutrophils. Angew Chem Int Ed Engl. 2022;61(30):e202203235.
Wu M, Zhang H, Tie C, Yan C, Deng Z, Wan Q, et al. MR imaging tracking of inflammation-activatable engineered neutrophils for targeted therapy of surgically treated glioma. Nat Commun. 2018. https://doi.org/10.1038/s41467-018-07250-6.
Lagnado A, Leslie J, Ruchaud-Sparagano MH, Victorelli S, Hirsova P, Ogrodnik M, et al. Neutrophils induce paracrine telomere dysfunction and senescence in ROS-dependent manner. EMBO J. 2021;40(9):e106048.
Merza M, Hartman H, Rahman M, Hwaiz R, Zhang E, Renstrom E, et al. Neutrophil extracellular traps induce trypsin activation, inflammation, and tissue damage in mice with severe acute pancreatitis. Gastroenterology. 2015;149(7):1920-31.e8.
Lei Y, Tang L, Liu S, Hu S, Wu L, Liu Y, et al. Parabacteroides produces acetate to alleviate heparanase-exacerbated acute pancreatitis through reducing neutrophil infiltration. Microbiome. 2021;9(1):115.
Wang Y, Li Y, Gao S, Yu X, Chen Y, Lin Y. Tetrahedral framework nucleic acids can alleviate taurocholate-induced severe acute pancreatitis and its subsequent multiorgan injury in mice. Nano Lett. 2022;22(4):1759–68.
Liu H, Liu S, Song X, Jiang A, Zou Y, Deng Y, et al. Nanoparticle encapsulated CQ/TAM combination harmonizes with MSCs in arresting progression of severity in AP mice through iNOS (IDO) signaling. Mater Today Bio. 2022;14:100226.
Zhang Q, Dehaini D, Zhang Y, Zhou J, Chen X, Zhang L, et al. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat Nanotechnol. 2018;13(12):1182–90.
Wang H, Liu Y, He R, Xu D, Zang J, Weeranoppanant N, et al. Cell membrane biomimetic nanoparticles for inflammation and cancer targeting in drug delivery. Biomater Sci. 2020;8(2):552–68.
Chen HY, Deng J, Wang Y, Wu CQ, Li X, Dai HW. Hybrid cell membrane-coated nanoparticles: a multifunctional biomimetic platform for cancer diagnosis and therapy. Acta Biomater. 2020;112:1–13.
Cao X, Hu Y, Luo S, Wang Y, Gong T, Sun X, et al. Neutrophil-mimicking therapeutic nanoparticles for targeted chemotherapy of pancreatic carcinoma. Acta Pharm Sin B. 2019;9(3):575–89.
Zhao Y-Z, ZhuGe D-L, Tong M-Q, Lin M-T, Zheng Y-W, Jiang X, et al. Ulcerative colitis-specific delivery of keratinocyte growth factor by neutrophils-simulated liposomes facilitates the morphologic and functional recovery of the damaged colon through alleviating the inflammation. J Control Release. 2019;299:90–106.
Zhou X, Cao X, Tu H, Zhang ZR, Deng L. Inflammation-targeted delivery of celastrol via neutrophil membrane-coated nanoparticles in the management of acute pancreatitis. Mol Pharm. 2019;16(3):1397–405.
Perez S, Pereda J, Sabater L, Sastre J. Redox signaling in acute pancreatitis. Redox Biol. 2015;5:1–14.
Wang S, Huang J, Zhu H, Zhu J, Wang Z, Xing Y, et al. Nanomodulators capable of timely scavenging ROS for inflammation and prognosis control following photothermal tumor therapy. Adv Funct Mater. 2023. https://doi.org/10.1002/adfm.202213151.
Cao Y, Wang J, Tian H, Fu GH. Mitochondrial ROS accumulation inhibiting JAK2/STAT3 pathway is a critical modulator of CYT997-induced autophagy and apoptosis in gastric cancer. J Exp Clin Cancer Res. 2020;39(1):119.
Woodby B, Penta K, Pecorelli A, Lila MA, Valacchi G. Skin health from the inside out. Annu Rev Food Sci Technol. 2020;11:235–54.
Shen C, Gao M, Chen H, Zhan Y, Lan Q, Li Z, et al. Reactive oxygen species (ROS)-responsive nanoprobe for bioimaging and targeting therapy of osteoarthritis. J Nanobiotechnol. 2021;19(1):395.
Xu H, She P, Ma B, Zhao Z, Li G, Wang Y. ROS responsive nanoparticles loaded with lipid-specific AIEgen for atherosclerosis-targeted diagnosis and bifunctional therapy. Biomaterials. 2022;288:121734.
Shi R, Li H, Jin X, Huang X, Ou Z, Zhang X, et al. Promoting Re-epithelialization in an oxidative diabetic wound microenvironment using self-assembly of a ROS-responsive polymer and P311 peptide micelles. Acta Biomater. 2022;152:425–39.
Lei XJHQ, Ge HF, Zhang XYRX, Chen YJ, Hu R, Feng H, Deng J, Huang Y, Li WY. A redox-reactive delivery system via neural stem cell nanoencapsulation enhances white matter regeneration in intracerebral hemorrhage mice. Bioeng Transl Med. 2022. https://doi.org/10.1002/btm2.10451.
Shahin NN, Shamma RN, Ahmed IS. A nano-liposomal formulation of caffeic acid phenethyl ester modulates Nrf2 and NF-kappabeta signaling and alleviates experimentally induced acute pancreatitis in a rat model. Antioxidants. 2022;11(8):1536.
Li Q, Cao Q, Yuan Z, Wang M, Chen P, Wu X. A novel self-nanomicellizing system of empagliflozin for oral treatment of acute pancreatitis: an experimental study. Nanomedicine. 2022;42:102534.
Liu T, Xiao B, Xiang F, Tan J, Chen Z, Zhang X, et al. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat Commun. 2020;11(1):2788.
Peng Y, He D, Ge X, Lu Y, Chai Y, Zhang Y, et al. Construction of heparin-based hydrogel incorporated with Cu5.4O ultrasmall nanozymes for wound healing and inflammation inhibition. Bioactive Mater. 2021;6(10):3109–24.
Khurana AAP, Allawadhi P, Kumar V, Sayed N, Packirisamy G, Godugu C. Superoxide dismutase mimetic nanoceria restrains cerulein induced acute pancreatitis. Nanomedicine. 2019;14(14):1805–25.
Khurana A, Anchi P, Allawadhi P, Kumar V, Sayed N, Packirisamy G, et al. Yttrium oxide nanoparticles reduce the severity of acute pancreatitis caused by cerulein hyperstimulation. Nanomedicine. 2019;18:54–65.
Xie P, Zhang L, Shen H, Wu H, Zhao J, Wang S, et al. Biodegradable MoSe(2)-polyvinylpyrrolidone nanoparticles with multi-enzyme activity for ameliorating acute pancreatitis. J Nanobiotechnol. 2022;20(1):113.
Zhang L, Xie P, Wu H, Zhao J, Wang S. 2D MoSe2@PVP nanosheets with multi-enzyme activity alleviate the acute pancreatitis via scavenging the reactive oxygen and nitrogen species. Chem Eng J. 2022;446:136792.
Abdel-Hakeem EA, Abdel-Hamid HA, Abdel Hafez SMN. The possible protective effect of Nano-Selenium on the endocrine and exocrine pancreatic functions in a rat model of acute pancreatitis. J Trace Elem Med Biol. 2020;60:126480.
Abozaid OAR, Moawed FSM, Ahmed ESA, Ibrahim ZA. Cinnamic acid nanoparticles modulate redox signal and inflammatory response in gamma irradiated rats suffering from acute pancreatitis. Biochim Biophys Acta Mol Basis Dis. 2020;1866(11):165904.
Sendler M, Weiss FU, Golchert J, Homuth G, van den Brandt C, Mahajan UM, et al. Cathepsin B-mediated activation of trypsinogen in endocytosing macrophages increases severity of pancreatitis in mice. Gastroenterology. 2018;154(3):704-18.e10.
Hou S, Feng T, Zhao N, Zhang J, Wang H, Liang N, et al. A carbon nanoparticle-peptide fluorescent sensor custom-made for simple and sensitive detection of trypsin. J Pharm Anal. 2020;10(5):482–9.
Shi J, Deng Q, Wan C, Zheng M, Huang F, Tang B. Fluorometric probing of the lipase level as acute pancreatitis biomarkers based on interfacially controlled aggregation-induced emission (AIE). Chem Sci. 2017;8(9):6188–95.
Zhang HW, Wang LQ, Xiang QF, Zhong Q, Chen LM, Xu CX, et al. Specific lipase-responsive polymer-coated gadolinium nanoparticles for MR imaging of early acute pancreatitis. Biomaterials. 2014;35(1):356–67.
Yao Q, Jiang X, Zhai YY, Luo LZ, Xu HL, Xiao J, et al. Protective effects and mechanisms of bilirubin nanomedicine against acute pancreatitis. J Control Release. 2020;322:312–25.
Cervin C, Vandoolaeghe P, Nistor C, Tiberg F, Johnsson M. A combined in vitro and in vivo study on the interactions between somatostatin and lipid-based liquid crystalline drug carriers and bilayers. Eur J Pharm Sci. 2009;36(4–5):377–85.
Zhang Q, Zhou J, Zhou J, Fang RH, Gao W, Zhang L. Lure-and-kill macrophage nanoparticles alleviate the severity of experimental acute pancreatitis. Nat Commun. 2021;12(1):4136.
Bhoomagoud M, Jung T, Atladottir J, Kolodecik TR, Shugrue C, Chaudhuri A, et al. Reducing extracellular pH sensitizes the acinar cell to secretagogue-induced pancreatitis responses in rats. Gastroenterology. 2009;137(3):1083–92.
Behrendorff N, Floetenmeyer M, Schwiening C, Thorn P. Protons released during pancreatic acinar cell secretion acidify the lumen and contribute to pancreatitis in mice. Gastroenterology. 2010;139(5):1711–20, 20.e1-5.
Liu X, Dou G, Li Z, Wang X, Jin R, Liu Y, et al. Hybrid biomaterial initiates refractory wound healing via inducing transiently heightened inflammatory responses. Adv Sci. 2022;9(21):e2105650.
Ding H, Tan P, Fu S, Tian X, Zhang H, Ma X, et al. Preparation and application of pH-responsive drug delivery systems. J Control Release. 2022;348:206–38.
Lu H, Chen A, Zhang X, Wei Z, Cao R, Zhu Y, et al. A pH-responsive T(1)-T(2) dual-modal MRI contrast agent for cancer imaging. Nat Commun. 2022;13(1):7948.
Mei Q, Deng G, Huang Z, Yin Y, Li C, Hu J, et al. Porous COS@SiO(2) nanocomposites ameliorate severe acute pancreatitis and associated lung injury by regulating the Nrf2 signaling pathway in mice. Front Chem. 2020;8:720.
Yang C, Hu T, Cao H, Zhang L, Zhou P, He G, et al. Facile construction of chloroquine containing PLGA-based pDNA delivery system for efficient tumor and pancreatitis targeting in vitro and in vivo. Mol Pharm. 2015;12(6):2167–79.
Hassanzadeh P, Arbabi E, Rostami F. Coating of ferulic acid-loaded silk fibroin nanoparticles with neutrophil membranes: a promising strategy against the acute pancreatitis. Life Sci. 2021;270:119128.
Dou Y, Zhang Y, Lin C, Han R, Wang Y, Wu D, et al. pH-responsive theranostic nanoplatform of ferrite and ceria co-engineered nanoparticles for anti-inflammatory. Front Bioeng Biotechnol. 2022;10:983677.
Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–11.
Garret C, Peron M, Reignier J, Le Thuaut A, Lascarrou JB, Douane F, et al. Risk factors and outcomes of infected pancreatic necrosis: retrospective cohort of 148 patients admitted to the ICU for acute pancreatitis. United Eur Gastroenterol J. 2018;6(6):910–8.
Jiang X, Shi JY, Wang XY, Hu Y, Cui YF. The impacts of infectious complications on outcomes in acute pancreatitis: a retrospective study. Mil Med Res. 2020;7(1):38.
Huang H, Ali A, Liu Y, Xie H, Ullah S, Roy S, et al. Advances in image-guided drug delivery for antibacterial therapy. Adv Drug Deliv Rev. 2023;192:114634.
Yang Y, Chu B, Cheng J, Tang J, Song B, Wang H, et al. Bacteria eat nanoprobes for aggregation-enhanced imaging and killing diverse microorganisms. Nat Commun. 2022;13(1):1255.
Lu SZ, Guo XY, Zou MS, Zheng ZQ, Li YC, Li XD, et al. Bacteria-instructed in situ aggregation of AuNPs with enhanced photoacoustic signal for bacterial infection bioimaging. Adv Healthc Mater. 2020;9(1):e1901229.
Timmerhuis HC, van den Berg FF, Noorda PC, van Dijk SM, van Grinsven J, Sperna Weiland CJ, et al. Over- and misuse of antibiotics and the clinical consequence in necrotizing pancreatitis: an observational multicenter study. Ann Surg. 2023. https://doi.org/10.1097/SLA.0000000000005790.
Fan X, Yang F, Nie C, Ma L, Cheng C, Haag R. Biocatalytic nanomaterials: a new pathway for bacterial disinfection. Adv Mater. 2021;33(33):e2100637.
Wang Y, Li C, Shen B, Zhu L, Zhang Y, Jiang L. Ultra-small Au/Pt NCs@GOX clusterzyme for enhancing cascade catalytic antibiofilm effect against F. nucleatum-induced periodontitis. Chem Eng J. 2023;466:143292.
Liu M, He D, Yang T, Liu W, Mao L, Zhu Y, et al. An efficient antimicrobial depot for infectious site-targeted chemo-photothermal therapy. J Nanobiotechnol. 2018;16(1):23.
Yu X, He D, Zhang X, Zhang H, Song J, Shi D, et al. Surface-adaptive and initiator-loaded graphene as a light-induced generator with free radicals for drug-resistant bacteria eradication. ACS Appl Mater Interfaces. 2019;11(2):1766–81.
Wang C, Wang Y, Zhang L, Miron RJ, Liang J, Shi M, et al. Pretreated macrophage-membrane-coated gold nanocages for precise drug delivery for treatment of bacterial infections. Adv Mater. 2018;30(46):e1804023.
Lee P, Chandel NS, Simon MC. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol. 2020;21(5):268–83.
Lee KE, Spata M, Maduka R, Vonderheide RH, Simon MC. Hif1alpha deletion limits tissue regeneration via aberrant B cell accumulation in experimental pancreatitis. Cell Rep. 2018;23(12):3457–64.
Ji L, Guo X, Lv J, Xiao F, Zhang W, Li J, et al. Hypoxia-inducible factor-1alpha knockdown plus glutamine supplementation attenuates the predominance of necrosis over apoptosis by relieving cellular energy stress in acute pancreatitis. Oxid Med Cell Longev. 2019;2019:4363672.
Zhou M-T. Acute lung injury and ARDS in acute pancreatitis: Mechanisms and potential intervention. World J Gastroenterol. 2010;16(17):2094.
Zheng J, Shen Y, Xu Z, Yuan Z, He Y, Wei C, et al. Near-infrared off-on fluorescence probe activated by NTR for in vivo hypoxia imaging. Biosens Bioelectron. 2018;119:141–8.
Zhang X, Wu M, Li J, Lan S, Zeng Y, Liu X, et al. Light-enhanced hypoxia-response of conjugated polymer nanocarrier for successive synergistic photodynamic and chemo-therapy. ACS Appl Mater Interfaces. 2018;10(26):21909–19.
Zhou Y, Maiti M, Sharma A, Won M, Yu L, Miao LX, et al. Azo-based small molecular hypoxia responsive theranostic for tumor-specific imaging and therapy. J Control Release. 2018;288:14–22.
Baik AH, Jain IH. Turning the oxygen dial: balancing the highs and lows. Trends Cell Biol. 2020;30(7):516–36.
Shi X, Yang W, Ma Q, Lu Y, Xu Y, Bian K, et al. Hemoglobin-mediated biomimetic synthesis of paramagnetic O(2)-evolving theranostic nanoprobes for MR imaging-guided enhanced photodynamic therapy of tumor. Theranostics. 2020;10(25):11607–21.
Wang D, Wu H, Lim WQ, Phua SZF, Xu P, Chen Q, et al. A mesoporous nanoenzyme derived from metal-organic frameworks with endogenous oxygen generation to alleviate tumor hypoxia for significantly enhanced photodynamic therapy. Adv Mater. 2019;31(27):e1901893.
Liu P, Xie X, Shi X, Peng Y, Ding J, Zhou W. Oxygen-self-supplying and HIF-1alpha-inhibiting core-shell nanosystem for hypoxia-resistant photodynamic therapy. ACS Appl Mater Interfaces. 2019;11(51):48261–70.
Dai L, Li X, Duan X, Li M, Niu P, Xu H, et al. A pH/ROS cascade-responsive charge-reversal nanosystem with self-amplified drug release for synergistic oxidation-chemotherapy. Adv Sci. 2019;6(4):1801807.
Gou S, Huang Y, Wan Y, Ma Y, Zhou X, Tong X, et al. Multi-bioresponsive silk fibroin-based nanoparticles with on-demand cytoplasmic drug release capacity for CD44-targeted alleviation of ulcerative colitis. Biomaterials. 2019;212:39–54.
Mandal N, Bhattacharjee M, Chattopadhyay A, Bandyopadhyay D. Point-of-care-testing of alpha-amylase activity in human blood serum. Biosens Bioelectron. 2019;124–125:75–81.
Ramesh A, Kumar S, Brouillard A, Nandi D, Kulkarni A. A nitric oxide (NO) nanoreporter for noninvasive real-time imaging of macrophage immunotherapy. Adv Mater. 2020;32(24):e2000648.
Li J, Zhang J, Fu Y, Sun X, Gong T, Jiang J, et al. Dual pancreas- and lung-targeting therapy for local and systemic complications of acute pancreatitis mediated by a phenolic propanediamine moiety. J Control Release. 2015;212:19–29.
Foulkes R, Man E, Thind J, Yeung S, Joy A, Hoskins C. The regulation of nanomaterials and nanomedicines for clinical application: current and future perspectives. Biomater Sci. 2020;8(17):4653–64.
Halamoda Kenzaoui HB, Van Elk M, Gaitan S, Geertsma R, Gainza E, Lafuente AO, Del Pozo A, Roesslein M, Bremer S. Anticipation of regulatory needs for nanotechnology-enabled health products. Precis Nanomed. 2019. https://doi.org/10.33218/001c.13521.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (82222039, 92168106), the Youth Program of Natural Science Foundation of Sichuan Provincial Department of Science and Technology (23NSFSC6011) and the Affiliated Hospital of North Sichuan Medical College (2022JB001, BS20211116).
Author information
Authors and Affiliations
Contributions
LL and YZ search related documents, draft and modify manuscripts. JD and XL reviewed the paper, proposed amendments and provided financial support.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article has been revised: the table 1 is corrected.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, L., Zhang, Y., Li, X. et al. Microenvironment of pancreatic inflammation: calling for nanotechnology for diagnosis and treatment. J Nanobiotechnol 21, 443 (2023). https://doi.org/10.1186/s12951-023-02200-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-023-02200-x