Abstract
Exosomes are extracellular vesicles with the diameter of 30 ~ 150 nm, and are widely involved in intercellular communication, disease diagnosis and drug delivery carriers for targeted disease therapy. Therapeutic application of exosomes as drug carriers is limited due to the lack of sources and methods for obtaining adequate exosomes. Milk contains abundant exosomes, several studies have shown that milk-derived exosomes play crucial roles in preventing and treating intestinal diseases. In this review, we summarized the biogenesis, secretion and structure, current novel methods used for the extraction and identification of exosomes, as well as discussed the role of milk-derived exosomes in treating intestinal diseases, such as inflammatory bowel disease, necrotizing enterocolitis, colorectal cancer, and intestinal ischemia and reperfusion injury by regulating intestinal immune homeostasis, restoring gut microbiota composition and improving intestinal structure and integrity, alleviating conditions such as oxidative stress, cell apoptosis and inflammation, and reducing mitochondrial reactive oxygen species (ROS) and lysosome accumulation in both humans and animals. In addition, we discussed future prospects for the standardization of milk exosome production platform to obtain higher concentration and purity, and complete exosomes derived from milk. Several in vivo clinical studies are needed to establish milk-derived exosomes as an effective and efficient drug delivery system, and promote its application in the treatment of various diseases in both humans and animals.
Similar content being viewed by others
Introduction
Intestinal diseases such as inflammatory bowel diseases (IBD), necrotizing enterocolitis (NEC), colorectal cancer (CRC), intestinal ischemia and reperfusion injury (IR) are generally characterized by clinical symptoms, including intestinal dysfunction and injury, intestinal inflammation, intestinal mucosal immune disorder, and microbiome imbalance [1,2,3,4]. Exosomes are cell-derived vesicles which are widely involved in the progression of intestinal diseases as well as play an important role in disease diagnosis and also serve as drug carriers [1,2,3,4]. Exosome is obtained via merging multivesicular bodies and is the latest family member of bioactive vesicles that play functional roles in promoting cell-cell communication [5]. In addition, exosomes were reported to be originated from endosome, in consequence contained many biomolecular elements based on their cell of origin, hence, they are described as a ‘‘fingerprint’’ of the origin of the cell [6].
Biogenesis, secretion and structure of exosomes
Attention on exosome research has broaden due to their description in antigen-presenting cells as well as the reports that they play active role in enhancing immune response in vivo [7]. Exosomes are membranous vesicles with a diameter ranging from 30 ~ 150 nm. They are released outside the cell after the cellular polyvesicles fused with the cell membrane originating from the endocytic pathway through the inward budding of the plasma membrane [8, 9]. This process generates the early endosome, which by a subsequent inward budding process creates the multivesicular bodies (MVB) characterized by the presence of vesicles in their lumen (intraluminal vesicles, ILV). The MVB is responsible for releasing exosomes by the transport and fusion of MVB with the plasma membrane, thus, the ILV are released into the extracellular space and then referred to as “exosomes” [10,11,12]. The exosomes are coated with bilayer phospholipid membranes and contain high levels of cholesterol, sphingomyelin, ceramides, and short/long chain saturated fatty acids [10, 13]. In addition, the exosomes contain cell-specific proteins, lipids, and nucleic acids (nucleic acids, namely mRNA, noncoding RNA species, and DNA) [14, 15]. The exosome biogenesis is the mechanism for protein quality control. Once the exosomes are released, they are involved in several activities such as extracellular matrix remodeling, as well as serving as signaling molecule to other target cells, thereby altering their functions [16, 17], however, their effects on target cells vary due to the differences in the expression profile of receptors on the cell surface. Such functional heterogeneity cause exosomes to modulate cell survival induction, apoptosis, and immune regulation in different target cell types [11]. In addition, exosome heterogeneity increases the functional diversity and complexity of exosomes in an intercellular communication. Exosomes originating from different cell types may have different compositions, however, they possesses similar conserved proteins such as CD63, CD81, CD9, etc. [18] (Fig. 1). In general, exosomes are found in a variety of living cells including dendritic cells (DCs), lymphocytes, epithelial cells, endothelial cells, etc. They are also found in the body fluids of eukaryotes, such as blood, urine, saliva, cerebrospinal fluid and emulsion [19, 20]. Studies have reported that exosomes are also involved in progress of diseases, such as neurodegenerative diseases [21,22,23], obesity and diabetes [24,25,26,27], cancer [28,29,30], etc., as well as play important role in disease diagnosis and also serve as a drug carrier [31,32,33,34,35,36].
Current methods for the extraction of milk-derived exosomes
Milk obtained from animals or humans is a complex, heterogeneous fluid containing a non-nutritive, bioactive extracellular vesicle known as exosome. Milk-derived exosomes (MDEs) are very difficult to characterize because of the lack of effective and efficient standardized methods used for milk pre-processing, storage, and exosome segregation [37]. Several techniques such as ultracentrifugation, size exclusion chromatography (SEC), and density gradient centrifugation (DGC) are currently available for separating exosomes from milk [38, 39]. Among these exosome isolation methods, ultracentrifugation (differential centrifugation) is the most standard, and with this procedure, raw milk can be centrifuged at approximately 2,000×g to remove fat globules, dead cells, and bulky apoptotic debris. Thereafter the exosomes are precipitated approximately at the speed of 100,000 to 150,000×g [37, 40,41,42]. Then the exosome pellets are loaded on a SEC column to get four fractions of exosomes for further characterization and analysis [42]. SEC is the method used to extract milk exosomes according to the size of the exosomes. In present studies, the extraction of milk exosomes by the SEC method is mostly combined with the ultracentrifugation method [42, 43]. Studies have extracted milk exosomes by using ultracentrifugation method combined with the SEC and DGC methods [44, 45]. In our previous study, we successfully separated bovine milk-derived exosomes using the ultracentrifugation method combined with SEC method (Fig. 2). At present, isolating exosomes from milk using these methods may be superior compared to the single method. For the DGC method, samples are added into an inert gradient medium for centrifugal sedimentation [46]. Various ingredients of the sample will settle on their respective isodensity zone under a centrifugal force, after which the exosomes will be separated from each other. In addition, the sucrose gradient centrifugation could effectively avoid the co-precipitation of nucleosomal fragments, apoptotic bodies, or protein aggregates [47] to achieve greater separation efficiency than the conventional method, thereby providing exosomes with high purity [48].
Furthermore, some receptor molecules such as CD9/63/81 on the membrane surface can be utilized to isolate exosomes by employing immuno-affinity capture method [49, 50]. The most commonly used immunocapture kits are enzyme-linked immunosorbent assay (ELISA), and in recent years, immunomagnetic beads are also becoming popular [51]. Microfluidic technology is a recently developed technique which is specifically used for high demanding separation tasks. At present, microfluidic technology is mainly divided into three categories: these are separation based on size, separation based on immunoaffinity, and dynamic separation [52]. To isolate exosomes from various milk fractions, other studies have introduced a novel approach based on natural nanosolid cellulose nanofibers (CNFs) and short time low gravity centrifugation, as well as encasing exosomes with flexible and entangled network of CNFs forms nanoporous [53].
The isolation process of bovine milk-derived exosome. ①: Dairy cow; ②: Raw milk; ③: Divide milk into EP tubes; ④: Centrifuge at 12,000×g, 4 ℃ for 30 min to remove the remaining fat and cell debris; ⑤: Skimmed milk; ⑥: Skimmed milk was transferred into an ultracentrifugation tubes; ⑦: Centrifuge at 150,000×g, 4 ℃ for 2 h; ⑧: The exosome pellet was collected and transferred into a low binding tube and resuspended in phosphate buffer solution (PBS) to 500 µL; ⑨: the suspension sample was loaded on a qEV original 35 nm SEC column; ⑩ After 3 mL of void volume, 4 fractions (A, B, C, D) of each 500 µL were immediately collected
Methods and markers for the identification of exosomes
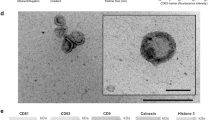
Generally, exosomes are characterized using nanoparticle tracking analysis (NTA) [54,55,56,57,58], transmission electron microscopy (TEM) [54,55,56,57,58], western blot (WB) [54, 55, 57, 58], flow cytometry (FCM) [56,57,58], and PKH67 fluorescent labeling [59, 60]. The size distribution and concentration of particles in exosomes are analyzed by NanoSight instrument. The fractions are diluted to 1:25 − 1:1000 fold with PBS to keep the number of particles in the field between 50 and 200/frame. For TEM analysis, a total of 50 µL purified exosome are pipetted on Parafilm® and immediately adsorbed to an Athene old 400 mesh copper grid coated with 1% Piolofom® in chloroform (w/v), and then incubated for 5 min at RT. The grid will then be carefully washed twice with distilled water and negatively stained with 50 µL of 2% uranyl acetate (w/v). Then the samples can be viewed using the Zeiss EM 109 TEM. The WB can also be performed to verify exosomal markers, such as CD9/63/81 [61,62,63,64,65], tumor susceptibility gene 101 (Tsg101) [63, 66,67,68,69], heat shock 70 kDa protein (HSP70) [70,71,72], Alix [73,74,75], and Flotillin 1 [74, 76, 77]. FCM is mostly used for exosome characterization. In brief, the freshly isolated exosomes are diluted in the 0.22 μm-filtered PBS and then stained under sterile dark conditions with green-RNA-binding, a liposoluble fluorophore SYTO that can penetrate the exosomal membrane. Before the samples can be loaded into the flow cytometer CytoFlex S, they are vortexed and bathed at 37 ℃ in the dark for 30 min, and then can be visualized using the CytExpert software [78]. PKH67 is a novel dye that can fluorescently label exosomes by binding to lipid molecules in the exosomal membrane structure [60]. Several studies on cellular uptake have co-cultivated cells with PKH67-labeled exosomes [79,80,81], and the results showed that PKH67-labelled breast milk exosomes can be taken up by macrophages [82] and IECs [80]. In our previous study, bovine milk-derived exosome was characterized by methods such as NTA, TEM, and WB (Fig. 3).
Therapeutic effects of milk-derived exosomes on intestinal Diseases
The intestinal tract plays major role in nutrient digestion, absorption, as well as serves as an immune and endocrine organ. The intestinal tract is the main immune defense barrier which is composed of the mucosal immune system and intestinal epithelial cells (IECs). Exosomes has key physiological and pathological implications on gut-related diseases, such as Inflammatory Bowel Disease (IBD) [83,84,85,86], colitis [87, 88], Colorectal Cancer (CRC) [89,90,91,92,93], Necrotizing enterocolitis (NEC) [94,95,96,97], and intestinal ischemia and reperfusion (IR) injury [98,99,100]. Mammalian milk is rich in exosomes, which play key roles in intestinal development, prevention of excessive inflammation, maintenance of intestinal epithelium integrity, and can also be used for disease treatment [101,102,103,104,105]. Mammalian breast milk exosomes transport proteins and nucleic acids to the neonatal intestinal system, thereby protecting them from acidity degradation and digestion, and also promote their intestinal structural integrity and absorption, hence, milk-derived exosome promotes intestinal development [105]. A study analyzed the expression of miRNAs in the human breast milk and reported high expression levels of immune-related miRNAs in the first 6 months of lactation, which regulated the development of intestinal immune system in the infants [106]. Other studies have shown that milk derived exosomes are rich in transforming growth factor β (TGF-β), which perform significant role in the development of intestinal barrier function, the production of immunoglobulin A (IgA) and mucosal immunity during the infancy period [107]. Reports have indicated that breast milk-derived exosomal circRNAs bind to related miRNAs promote IEC proliferation and migration through the vascular endothelial-derived growth factor (VEGF) signaling pathway, thereby promoting the development of intestinal tract [108].
In addition, studies have revealed that porcine milk-derived exosomes promote proliferation and intestinal development of porcine’s small intestinal cells, improve the expressions of caudal-related homeobox transcription factor 2 (CDX2), proliferate cell nuclear antigen (PCNA) and type 1 insulin-like growth factor receptor (IGF-1R) genes in porcine small intestine cells, as well as inhibit the expression of tumor suppressor gene p53 [109]. CDX2 is a gut specific transcription factor that is directly involved in the intestinal development and maintenance of intestinal phenotypes [110]. It was reported that porcine milk-derived exosomal circ-XPO4 plays a crucial role in the intestinal acquired immunity and mucosal homeostasis via inhibiting the expression of miR-221-5p, promoting the expression of polymeric immunoglobulin receptors and the production of intestinal IgA [111]. Porcine milk-derived exosomal miRNAs were found to alleviate deoxynivalenol (DON) induced intestinal mucosal damage in mice by promoting cell proliferation and inhibiting apoptosis [112]. Specifically, porcine milk-derived exosomal miR-4334, miR-219 and miR-338 attenuate lipopolysaccharide (LPS)-induced intestinal cell inflammation and apoptosis, and relieve intestinal damage, as well as maintain the intestinal epithelial integrity via inhibiting the activation of Toll-like receptor 4 (TLR4)/ NF-kappaB (NF-κB) and p53 signaling pathways [113].
Several studies have shown that bovine milk-derived exosomes escape the absorption in the digestive tract, and induce changes in the intestinal microbial community, leading to the enrichment of the polymorphisms and mutations of the rectal bacteria in mice [114], improve the atrophy of the intestinal villus in mice [115], and also increase the production of the intestinal mucus and enhanced tight junction protein expression via miRNAs and TGF-β to aid in the restoration of the intestinal barrier function induced by diseases [104]. Bovine milk-derived exosomes change the intestinal microbial community of mice and promoted the communication between the host and bacteria [114]. It was also reported that bovine milk-derived exosomal miRNAs are involved in immune response, growth and development, which is beneficial to dairy cows and the maturation of the intestinal structure of the neonate [116]. Another study have indicated that the oral administration of bovine milk-derived exosomes cause senescence of the primary intestinal tumors and accelerate cancer metastasis in mice [117], in addition, yak milk-derived exosomes were reported to promote proliferation and survival of IECs under hypoxic environment [72]. Goat milk-derived exosomes can be used as a natural probe to detect inflammatory process. Injection of goat milk-derived exosomes in peritonitis mice significantly increased the exosomal content of the intestine [118]. Rat milk-derived exosomes also significantly increased the expression of PCNA and leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5) genes, as well as enhance the activity of IECs [119]. Other studies have shown that giant panda milk-derived exosomes promote the development of the intestinal immune system and absorption in newborn cubs [120]. Exosomes play significant physiological and pathological role on proper functioning of the intestine. The exosomes affect the progression of intestinal inflammatory response following the beginning of related pathologies. The existence of many uptake exosomal mechanisms of the intestine promotes the alleviation of pathological conditions of the intestine [102, 121]. Cells communicate with each other by releasing exosomes that transfer their composition, such as nucleic acids, proteins, and lipids, to the nearby cells, hence play important function in several pathophysiological processes [122, 123]. For instance, during pathogenic bacteria infection, exosomes are secreted by infected cells to affect the innate immune responses of the neighbouring cells to the infection. These vesicles can release different biological fluids to allow changes in the content of the exosome to help in the discovery of non-invasive biomarkers related to inflammatory bowel disease and infectious diseases [122, 123]. Studies also indicated that exosomes could be utilized as a vaccine for boosting the immune system to get rid of various pathogenic bacteria and to attenuate intestinal damage [122, 123].
Inflammatory bowel Disease
Inflammatory Bowel Disease (IBD) is a recurrent and lifelong disease that includes Ulcerative colitis (UC) and Crohn’s disease (CD) characterized by chronic, recurrent, and nonspecific intestinal inflammation [124,125,126]. The clinical manifestations of IBD are persistent or recurrent abdominal pain, diarrhea, fever, rectal bleeding and other symptoms. The diagnosis and treatment of IBD are complicated [127, 128]. Currently, the pathogenesis of IBD is related to several factors such as genetics, intestinal mucosal barrier damage, intestinal inflammation, gut dysbiosis and intestinal mucosal immune disorder [129,130,131,132]. Several studies have shown that milk-derived exosomes play crucial roles in the prevention and treatment of IBD by participating in the interaction and communication of IECs-immune cell-intestinal flora to regulate the immune response and intestinal homeostasis, as well as attenuate intestinal inflammation [133,134,135].
Studies have also shown that bovine milk-derived exosomes alleviate UC by reducing inflammatory response through inhibition the production of pro-inflammatory factors via TLR4-NF-κB signaling pathway and the activation of nod-like receptor family pyrin domain containing 3 (NLRP3) inflammatories, attenuating cytokine production disorder and restoring the balance between the interleukin-10+Foxp3+ regulatory T (Treg) cells and T helper type 17 (Th17) cells in the inflamed colon, and also restoring the α-diversity of gut microbiota effectively, as well as regulating intestinal immune homeostasis [136]. Bovine milk-derived exosomes were also reported to alleviate dextran sodium sulfate (DSS)-induced IBD in mice by restoring the intestinal impermeability and promoting mucin secretion by regulating the intestinal microbial flora, reducing inflammation by down-regulating the expression of colitis related miR-125b, increasing the expression of anti-inflammatory protein such as TNF-alpha-induced protein 3 (TNFAIP3, A20), reducing the production and release of pro-inflammatory cytokines and increasing the production of anti-inflammatory cytokines to restore the structure and integrity of the colon [137]. A study investigated the therapeutic effect of cow and human milk derived exosomes on colitis mice, and they have found that the oral administration of cow and human milk-derived exosomes play an anti-inflammatory and therapeutic role to reduce the severity of DSS-induced UC in mice by down-regulating the expression of pro-inflammatory cytokines tumour necrosis factor alpha (TNF-α) and interleukin 6 (IL-6), and also up-regulates the expression of TGF-β [138]. Oral administration of bovine milk-derived exosomes alleviates clinical symptoms and colon damage in mice with UC induced by DSS by attenuating oxidative stress, as well as reducing the expression of inflammatory cytokines and chemokines in the colon [139]. In addition, bovine milk-derived exosomes can attenuate DSS-induced UC in the mice by remodeling and optimizing the abundance of intestinal flora, regulating intestinal gene expression, and restoring the structure and integrity of the intestinal surface epithelium [140]. Moreover, bovine milk-derived exosome was reported to help in the restoration of metabolic abnormalities induced by DSS-induced UC in the mice, and also prevent intestinal inflammation by regulating lipid and amino acid metabolism, thereby providing new insights into the identification and utilization of lactation-derived exosomes as potential regulators for the prevention and treatment of IBD [141]. Goat milk-derived exosomes were also reported to show anti-inflammatory and immunomodulatory effects, hence can reduce LPS-induced inflammation of the porcine jejunal epithelial cells (IPEC-J2 cells) and also restore cellular homeostasis by decreasing the level of expressions of IL18, IL12p40, matrix metalloproteinase 9 (MMP9) and nitric oxide synthase (NOS2), but increase the level of expressions of mucin 2 (MUC2), epstein-barr virus-induced gene 3 (EBI3), and IL-8 [142].
Necrotizing enterocolitis (NEC)
Necrotizing enterocolitis (NEC) is one of the most devastating diseases of premature infants, characterized by high morbidity and mortality rates [143, 144]. Therefore, it is urgent to develop effective treatments for this devastating condition. Breast milk, which has been known for decades to have health benefits, contains large amounts of exosomes and has the potential to treat NEC diseases [145]. Breast milk has been shown to reduce the incidence of NEC, however, NEC condition is rare in infants whose diets contain breast milk [146]. Compared with formula milk, breast milk feeding reduces the risk of NEC [147]. Various studies have shown that the activation of TLR4 induced-inflammation inhibits IEC proliferation, reduces intestinal microcirculation, and promotes the occurrence and progression of NEC [148, 149], however, other studies have reported that epidermal growth factor in breast milk inhibits TLR4 signaling, protects IECs from apoptosis, promote intestinal cell proliferation, and inhibit the occurrence of NEC [150]. Breast milk-derived exosomes have been shown to prevent NEC in premature infants [151]. In vitro and in vivo studies have demonstrated that peptides highly enriched in milk-derived exosomes can reduce ileal damage by promoting the intestinal cell proliferation and migration, which may be an effective preventive method for NEC [152]. Human breast milk-derived exosomes were found to protect the intestinal stem cells from oxidative stress damage via the Wnt/β-catenin signaling pathway to prevent and treat the development of NEC [153]. Reports indicated that the incidence of NEC is 0% in breastfed pups, and human breast milk-derived exosomes significantly increased the IEC proliferation and also inhibited apoptosis, as well as reduced the incidence and severity of NEC [154]. Other studies have reported that human breast milk-derived exosomes exert protective effect on IECs, and also promote cell viability by alleviating oxidative stress, thereby preventing the occurrence of NEC and intestinal injury [155]. Human milk-derived exosomal lncRNAs and mRNAs prevent the occurrence of NEC by promoting intestinal tissue proliferation and development, reducing intestinal tissue necrosis and epithelial injury, as well as reducing the severity of NEC through the JAK-STAT and adenosine monophosphate-activated protein kinase (AMPK) signaling pathways [95]. Human milk-derived exosomal lipids reduce the severity of NEC through the extracellular signal-regulated protein kinase/mitogen activated protein kinase (ERK/MAPK) pathway to rescue the apoptosis and migration inhibition of IECs induced by LPS [156]. In other studies, human milk-derived exosomes were reported to alleviate hypoxia and LPS-induced NEC inflammation, mucosal damage, and mucus production [103]. It was also established that human milk-derived exosomal miR-148a-3p prevents NEC by promoting Sirtuin 1 and inhibiting p53 and NF-κB expression [94]. Moreover, a study revealed that human milk-derived exosomes play a beneficial role in the prevention of NEC by reducing inflammation and injury of LPS-induced NEC of the intestinal epithelium, and also protect the integrity of the intestinal epithelial barrier, and also promote cell proliferation, as well as reduce the level of pro-inflammatory cytokines, and also increase the expression of the intestinal tight junction proteins [157]. A recent study indicated that human breast milk derived exosomes alleviate NEC associated intestinal injury and NEC ileal inflammation by reducing the NEC scores, restoring the number of damaged ileal crypts, and also inhibit the inflammatory responses of IECs [96], in addition, the human breast milk-derived exosomes prevent the development of NEC by reducing the expression of inflammatory cytokines such as TNFα and TLR4, as well as protecting the intestinal tract from epithelial inflammatory damage induced by LPS [158]. Furthermore, studies have established that porcine milk-derived exosomal miRNAs promote cell proliferation, inhibit the formation of tight junction proteins (TJs), and protect IECs from intestinal mucosal damage induced by DON [112]. Porcine milk-derived exosomal miRNAs such as miR-4334, miR-219, and miR-338 were reported to protect IEC damage induced by LPS by inhibiting apoptosis and inflammation via the p53 and TLR4/NF-κB pathways [113]. In other study, it was reported that bovine milk-derived exosomes enhance goblet cell activity and prevent the development of experimental NEC [159], furthermore, rat milk-derived exosomes exert several biological functions such as enhancing IEC activity, promoting cell proliferation, stimulating intestinal stem cell activity, and preventing the development of NEC in infants with breastfeeding intolerance [119].
Colorectal cancer (CRC)
Colorectal cancer (CRC) is the third most common malignancy in the world, with an average of one person diagnosed with colorectal cancer every 1.5 min, resulting in nearly 900,000 deaths annually. With the process of urbanization and the aging population, the incidence and mortality cause by colorectal cancer is on the rise, therefore, developing ways to control and prevent the colorectal cancer disease is urgently needed. This is because, the symptoms of this disease only appear in advanced stages. Hence, several countries worldwide promote screening programs with the aim of increasing early detection rates of colorectal cancer in order to reduce morbidity and mortality [160,161,162,163]. Recent studies have reported that exosomes can be used as delivery vectors in vivo, to deliver valuable genetic cargo, containing biomarkers and load drugs for delivery to specific tissues, attracting an increasing interest because exosomes exert no adverse immune responses as well as prevent tumor formation [164, 165], hence, exosomes can be employed as potential biomarkers and target therapies for colorectal cancer [166]. Studies have shown that exosomal delivery of miR-128-3p is a novel strategy to enhance CRC chemical sensitivity through negative regulation of Bmi1 and MRP5 [90]. Exosomal delivered circRNAs promote glycolysis and chemotherapy resistance in CRC via the miR-122/PKM2 axis [167]. Exosomal circPACRGL promotes colorectal cancer proliferation and metastasis through the miR-142-3p/miR-506-3p-TGF-β1 axis [92]. Mesenchymal stem cells (MSCs)-derived exosomes contain tumor-inhibiting miRNAs (miR-3940-5p/miR-22-3p/miR-16-5p), which inhibits the proliferation, migration and invasion of CRC cells by regulating Ras-associated protein B2 (RAP2B)/phosphoinositide 3-kinase (PI3K)/AKT signaling pathway and integrin alpha 2/6 (ITGA2/6), thereby showing therapeutic potential in the UC and CRC [168]. Adipocyte derived exosomal microsomal triglyceride transfer protein (MTTP) inhibits ferroptosis and promotes chemotherapy resistance in CRC [2]. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to enhance liver metastasis of CRC [169].
Due to its potential in preventing and treating CRC, milk is receiving increasing attention due to the abundance of exosomes it contains. Milk exosomes have been widely reported to exert direct antitumor effects on colorectal cancer. For instance, bovine milk-derived exosomes were reported to exhibit intrinsic antitumor activity by inhibiting the growth and activity of CRC cells, providing an effective alternative to oral administration for the treatment of CRC [170]. In addition, human milk-derived exosomes were shown to increase the expression level of miR-148a in the CRC cells but decrease the expression of its target gene DNA methyltransferase1 (DNMT1) to reduce the occurrence of CRC [171, 172]. It was also revealed in other studies that human milk-derived exosomes alter the miRNA expression profile of colon epithelial cells and also promote the proliferation of healthy colon epithelial cells without affecting the growth of CRC cells [173]. Furthermore, bovine milk-derived exosomes were also reported to attenuate the primary CRC by decreasing the number of CRC cell colonies as well as increase the cell death [117]. In goats, the milk-derived exosomes showed potential biological functions such as anti-inflammation, tumor retention, and increase production performance and high biosafety, and also act as ideal nanocarriers for the construction of CRC comprehensive diagnosis and treatment. The nanoprobes designed by goat milk-derived exosomes are used to trigger anti-tumor immune and inflammatory responses to enhance their potential in CRC therapy [174]. High levels of miR-27b in buffalo milk-derived exosomes exert their anti-CRC activity in vitro through the promotion of apoptosis of CRC cells, and increasing the accumulation of lysosome and mitochondrial reactive oxygen species (ROS), as well as aggravating the endoplasmic reticulum (ER) stress-mediated CRC cell death via protein kinase RNA-like ER kinase (PERK)/inositol-requiring enzyme 1 (IRE1)/X-box binding protein 1 (XBP1) and CHOP protein pathways [175].
Intestinal ischemia and reperfusion injury (I/R)
Intestinal Ischemia/reperfusion (I/R) injury is a common clinical event caused by acute mesenteric ischemia, intestinal obstruction, intestinal transplantation and other pathophysiological factors, which cause micro vascular injury, mitochondrial oxidative damage and cell apoptosis [176, 177]. Due to the hidden onset and lack of effective treatment of I/R, the morbidity and mortality are high. Exploring strategies to reduce intestinal I/R injury is of great significance for improving organ recovery and patient survival [178, 179]. NLRX1/FUNDC1/NIPSNAP1-2 axis mediated mitophagy [180], live kinase B1 (LKB1)/AMPK mediated autophagy [181], and mtDNA-STING pathway [182] were reported as key mechanisms in the pathogenesis of intestinal I/R injury. Bone marrow mesenchymal stem cell-derived exosomes were found to ameliorate the intestinal I/R via the miR-144-3p-mediated oxidative stress and the phosphatase and tensin homolog (PTEN)/Akt/nuclear factor E2-related factor 2 (Nrf2) pathway [98], and also regulate the immune responses and attenuate neuronal apoptosis [183] and intestinal I/R injury-induced lung injury via the TLR4/NF-κB pathway [184]. During the intestinal I/R injury, gut-derived exosomes induce liver injury by promoting hepatic M1 macrophage polarization [185], mediate memory impairment by activating microglia [186]. In addition, the inhibition of the secretion of gut-derived exosome may be a therapeutic target for the prevention of hepatic impairment and memory impairment after the intestinal I/R. Human breast milk provides neonates with the protective and therapeutic for intestinal IR injury and NEC through deceasing the IL-1β-induced activation of NF-κB pathway [187]. Milk exosomes have the potential to cross physiological boundaries and cell membrane [188], however, exert no systemic toxic effects or anaphylaxis [189]. Human breast milk-derived exosomes alleviate intestinal damage in IR rats by reducing the intestinal hyperplasia and decrease the expression of an inflammatory cytokine TNFα [190].
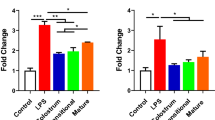
In general, it is increasingly clear that milk-derived exosomes are significantly involved in alleviating intestinal diseases, such as IBD, NEC, CRC, and intestinal IR injury, via regulating gut microbiota intestinal immune homeostasis, oxidative stress, inflammatory response, and proliferation and apoptosis (Fig. 4 and Table 1). The application of the exosomes based on their properties such as stability, transportability, and bioavailability, milk derived exosomes may be used as drug carriers for the transportation of drugs used for the treatment of targeted diseases.
Milk exosome-based drug delivery systems for Disease therapy
Presently, drug delivery system is a novel area that many researchers are experimenting. This research area is rooted in the difficulty of treating some diseases with traditional therapeutic drugs and several drug delivery methods. Interestingly, exosomes can act as clinical drug carriers and they are also immune compatible. However, due to the lack of sources and methods for obtaining adequate exosomes, the therapeutic application of exosomes as drug carriers is limited. Milk-derived exosomes have several advantages such as higher yield, additional therapeutic benefits and oral delivery characteristic compared with other delivery vectors [191]. Milk-derived exosomes are highly biocompatible and remain intact after absorption in the gastrointestinal tract, indicating good stability. These properties make lacto-derived exosomes suitable drug carriers, but these lacto-derived exosomes already have substantial immunomodulatory functions on their own, and these vesicles can be used as therapeutic agents even when they are not loaded. However, milk exosomes show cross-species tolerance, no adverse immune and inflammatory responses, and further, milk exosomes are good drug deliverers, carrying cargo with tumor targeted therapy capabilities [192]. Multifunctional lacto-derived exosomes provide solutions to the challenges posed by the oral drug delivery, thus providing new insights into the development of oral drug delivery nanocarriers for natural equipment [193] [135]. Milk-derived exosome-loaded insulin (MDEI) elicited a more excellent and sustained hypoglycemic effect, the excellent oral delivery ability of MDEI attributed to versatile effects include high biocompatibility and bioavailability, active multi-targeting uptake, nutrient assimilation related ERK1/2 and p38 MAPK signal pathway activation, and intestinal mucus penetration, which is simple and cost-effective approach for the preparation of MDEI contributed to their large-scale production [193]. Studies have indicated that milk-derived exosomes serve as nanocarriers to deliver curcumin and resveratrol to breast tissues and enhance their anticancer activities [194], loaded with curcumin to improve the cell uptake and intestinal permeability of curcumin [195], and also act as agents for anticancer drug delivery [196], as well as have higher mucus penetration to improve the efficacy of the oral administration in the treatment of the intestinal bacterial infection. Natural flavonoid such as alpha-mangosteen was loaded into the milk exosomes and it was observed that it has eliminated approximately 99% of the bacteria in the macrophages [197], hence, milk-derived exosomes can be used as stable oral drug delivery carriers. Curcumin encapsulated in milk exosomes can resist human digestion and has enhanced in vitro intestinal permeability, and effectively penetrate the intestinal barrier [198]. Oral chemotherapy drug paclitaxel encapsulated in milk exosomes replaces conventional intravenous therapy to improve the efficacy and also reduce toxicity, thereby inhibiting the effect on tumor growth [199].
Recently, milk-derived exosomes have attracted attention as vehicles for delivering RNA therapeutics to cancers [200]. Milk-derived exosomes act as a novel system for the delivery of miR-31-5p, and also successfully encapsulated miR-31-5p mimics into milk exosomes through electroporation dramatically to improve the endothelial cell functions in vitro and promote the angiogenesis and also enhance the diabetic wound healing in vivo [201]. Bovine milk is a cost-effective source of potential exosomes which can be used as nanocarriers of functional drug delivery vehicle for miRNA-based therapy, exosome-transported miR-148a-3p can be delivered and taken up by cells in-vitro, and exert a biological effect through the modulation of gene expression [202]. Milk-derived exosomes can be used as a natural nanoparticles for novel small interfering RNA (siRNA) delivery system, and can enhance mucus penetrability and penetrated multiple biological barriers for oral drug delivery of siRNA [203, 204], and delivered endogenous RNA payloads into the recipient cells, and loaded siRNA against specific genes such as KRAS which represents a viable natural nano-carrier for the delivery of siRNA for the therapeutic application against cancer [205]. Milk-derived exosomes carrying siRNA-KEAP1 promote diabetic wound healing by alleviating oxidative stress [206].
Milk-derived exosomes have high concentration and diversity of cargos, which cross the blood-brain barrier and are absorbed and accumulated in tissues following oral administrations to deliver drugs to the diseased tissues [207]. Milk-derived exosome as an oral drug delivery system with a great application potential improve drug safety, bioavailability, and effectiveness in the delivery of the oral preparations [208]. Milk-derived exosomes encapsulated doxorubicin can penetrate the tumor and delivery to triple-negative breast cancer cells would be effective in reducing triple-negative breast cancer cells’ survival [209]. Hyaluronic acid-coated bovine milk exosomes for tumor-specific delivery of miR-204 showed an excellent biocompatibility and exert no significant systemic toxicity, but significantly increased antitumor efficacy both in vitro and in vivo. Both hyaluronic acid and bovine milk-derived exosomes are low-cost and highly accessible biogenic materials with broad biomedical applications. The hyaluronic acid-decorated bovine milk-derived exosomes are proven as practical drug delivery system of RNA drugs for targeted cancer therapy [210]. An in vitro experiment indicated that doxorubicin-loaded milk-derived exosomes with hyaluronic acid triggers tumor cell death, and therefore, demonstrates its potential use for tumor cell-specific drug delivery and feasible for targeted cancer therapy [211]. A study by Zhang et al. proved that milk-derived exosomes-based drug delivery system showed controlled drug-release and biocompatibility, hence, they are effective in treating oral squamous cell carcinomas [212]. In addition, milk-derived exosomes encapsulation of hydrophilic biomacromolecule drugs could significantly improve the transepithelial transport and bioavailability of the oral drugs [213]. Milk-derived exosomes encapsulated with forsythiaside A combats liver fibrosis via regulating NLRP3-mediated pyroptosis [214]. This shows that milk-derived exosomes exert several advantages, such as no adverse immune and inflammatory responses, and have great application potential in the treatment of targeted diseases by clinical drug delivery systems.
Conclusions and future perspectives
Exosome is widely involved in the progression of various diseases, and plays an important role in disease diagnosis and also act as a drug carrier. In this comprehensive review, we summarized the biogenesis, secretion and structure, current methods for the extraction, and identification methods and markers of exosomes, and further highlighted the biological roles of the milk-derived exosomes in preventing and treatment of intestinal diseases, such as inflammatory bowel disease, necrotizing enterocolitis, colorectal cancer, and intestinal ischemia and reperfusion injury via the regulation of intestinal immune homeostasis, restoring gut microbiota composition and promote the intestinal mucous production, by alleviating oxidative stress, cell apoptosis and inflammation, as well as reducing the ROS and lysosome accumulation.
Milk-derived exosomes have been confirmed to exert no adverse immune and inflammatory responses, nontoxicity, high biocompatibility and bioavailability and has the potential of mass production for clinical therapy for various targeted diseases. However, further studies are required to establish and promote the standardization production platform of exosomes in milk to improve the utilization and obtain higher concentration and purity and more complete exosomes obtained from milk. In addition, several clinical in vivo studies should be carried out to explore the pharmacological effects and the pharmacokinetics of the milk-derived exosome-based drug delivery carriers for the therapy of targeted diseases, thereby to establish milk-derived exosomes as a mature drug delivery system and promote its widely use in the treatment of various diseases. Taken together, the use of milk-derived exosomes is useful in preventing and treating diseases in both humans and animals. Studies on the dietary supplementation of milk-derived exosomes could alleviate piglet diarrhea post-weaning and proliferative enteropathy in pigs require further exploration.
Data availability
Not applicable.
Abbreviations
- AMPK:
-
adenosine monophosphate-activated protein kinase
- ASC:
-
apoptosis-associated speck-like protein
- ATF6:
-
activating transcription factor 6
- Axin2:
-
axis inhibition protein 2
- C13:0:
-
tridecanoic acid
- C15:1:
-
methyl cis-10-pentadecenoate
- C20:1:
-
cis-11-eicosenoic acid
- C20:2:
-
eicosadienoic acid
- C20:5:
-
eicosapentaenoic acid
- C22:6:
-
docosahexaenoic acid
- CCL3/4/11:
-
CC chemokine ligand 3/4/11
- CHOP:
-
CCAAT-enhancer-binding homologous protein
- CLDN1:
-
claudins 1
- COX2:
-
cyclooxygenase-2
- CXCL2/3/5:
-
C-X-C motif chemokine ligand
- DNMT1:
-
DNA methyltransferase1
- EBI3:
-
epstein-barr virus-induced gene 3
- EGF:
-
epidermal growth factor
- ER:
-
endoplasmic reticulum
- GRP94:
-
glucose-regulated protein 94
- GSK3β:
-
glycogen synthase kinase-3beta
- Iba1:
-
ionized calcium binding adaptor molecule 1
- IFN-γ:
-
interferon-gamma
- IL-6:
-
interleukin 6
- iNOS:
-
inducible nitric oxide synthase
- IRE1:
-
inositol-requiring enzyme 1
- JNK:
-
c-Jun N-terminal kinase
- Lgr5:
-
Leucine-rich repeat-containing G-protein coupled receptor 5
- MDEs:
-
milk-derived exosomes
- MBP:
-
myelin basic protein
- MMP9:
-
matrix metalloproteinase 9
- MPO:
-
myeloperoxidase
- MUC2:
-
mucin 2
- NFκB:
-
nuclear factor kappaB
- NLRP3:
-
nod-like receptor family pyrin domain containing 3
- NOS2:
-
nitric oxide synthase
- OCLN:
-
occludin
- PCNA:
-
proliferating cell nuclear antigen
- PERK:
-
protein kinase RNA-like ER kinase
- PTEN:
-
phosphatase and tensin homolog
- PTGS2:
-
prostaglandin-endoperoxide synthase 2 (also known as COX-2)
- ROS:
-
reactive oxygen species
- SERPINE1:
-
serine protease inhibitor clade E member 1
- SIRT1:
-
sirtuin 1
- TFF3:
-
trefoil factor 3
- TGF-β:
-
transforming growth factor β
- TLR4:
-
Toll-like receptor 4
- TNFAIP3:
-
TNF-alpha-induced protein 3
- TNF-α:
-
tumour necrosis factor alpha
- XBP1:
-
X-box binding protein 1
- Zo-1:
-
zona occludens 1
References
Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, Zhou K, Liu X, Ren X, Wang F, et al. Cancer-derived exosomal mir-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. 2018;9:5395.
Zhang Q, Deng T, Zhang H, Zuo D, Zhu Q, Bai M, Liu R, Ning T, Zhang L, Yu Z, et al. Adipocyte-derived exosomal MTTP suppresses ferroptosis and promotes Chemoresistance in Colorectal Cancer. Adv Sci (Weinh). 2022;9:e2203357.
Filip R. An update on the role of Extracellular vesicles in the pathogenesis of necrotizing enterocolitis and Inflammatory Bowel Diseases. Cells. 2021;10:3202.
Shen Q, Huang Z, Yao J, Jin Y. Extracellular vesicles-mediated interaction within intestinal microenvironment in inflammatory bowel Disease. J Adv Res. 2022;37:221–33.
Schorey JS, Bhatnagar S. Exosome function: from Tumor immunology to pathogen biology. Traffic. 2008;9:871–81.
Sancho-Albero M, Navascués N, Mendoza G, Sebastián V, Arruebo M, Martín-Duque P, Santamaría J. Exosome origin determines cell targeting and the transfer of therapeutic nanoparticles towards target cells. J Nanobiotechnol. 2019;17:16.
Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–79.
Alzhrani GN, Alanazi ST, Alsharif SY, Albalawi AM, Alsharif AA, Abdel-Maksoud MS, Elsherbiny N. Exosomes: isolation, characterization, and biomedical applications. Cell Biol Int. 2021;45:1807–31.
Lane RE, Korbie D, Anderson W, Vaidyanathan R, Trau M. Analysis of exosome purification methods using a model liposome system and tunable-resistive pulse sensing. Sci Rep. 2015;5:7639.
Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteom. 2010;73:1907–20.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 2020;367:eaau6977.
Yue B, Yang H, Wang J, Ru W, Wu J, Huang Y, Lan X, Lei C, Chen H. Exosome biogenesis, secretion and function of exosomal miRNAs in skeletal muscle myogenesis. Cell Prolif. 2020;53:e12857.
Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93.
Pathan M, Fonseka P, Chitti SV, Kang T, Sanwlani R, Van Deun J, Hendrix A, Mathivanan S. Vesiclepedia 2019: a compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019;47:D516–d519.
van Balkom BW, Eisele AS, Pegtel DM, Bervoets S, Verhaar MC. Quantitative and qualitative analysis of small RNAs in human endothelial cells and exosomes provides insights into localized RNA processing, degradation and sorting. J Extracell Vesicles. 2015;4:26760.
Zhou Q, Li M, Wang X, Li Q, Wang T, Zhu Q, Zhou X, Wang X, Gao X, Li X. Immune-related microRNAs are abundant in breast milk exosomes. Int J Biol Sci. 2012;8:118–23.
Arntz OJ, Pieters BC, Oliveira MC, Broeren MG, Bennink MB, de Vries M, van Lent PL, Koenders MI, van den Berg WB, van der Kraan PM, van de Loo FA. Oral administration of bovine milk derived extracellular vesicles attenuates arthritis in two mouse models. Mol Nutr Food Res. 2015;59:1701–12.
Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113:1–11.
Trams EG, Lauter CJ, Salem N Jr., Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645:63–70.
Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunol Lett. 2006;107:102–8.
Zhang T, Ma S, Lv J, Wang X, Afewerky HK, Li H, Lu Y. The emerging role of exosomes in Alzheimer’s Disease. Ageing Res Rev. 2021;68:101321.
Su L, Li R, Zhang Z, Liu J, Du J, Wei H. Identification of altered exosomal microRNAs and mRNAs in Alzheimer’s Disease. Ageing Res Rev. 2022;73:101497.
Guo M, Wang J, Zhao Y, Feng Y, Han S, Dong Q, Cui M, Tieu K. Microglial exosomes facilitate α-synuclein transmission in Parkinson’s Disease. Brain. 2020;143:1476–97.
Castaño C, Kalko S, Novials A, Párrizas M. Obesity-associated exosomal miRNAs modulate glucose and lipid metabolism in mice. Proc Natl Acad Sci U S A. 2018;115:12158–63.
Zhao H, Shang Q, Pan Z, Bai Y, Li Z, Zhang H, Zhang Q, Guo C, Zhang L, Wang Q. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in White Adipose tissue. Diabetes. 2018;67:235–47.
Ying W, Gao H, Dos Reis FCG, Bandyopadhyay G, Ofrecio JM, Luo Z, Ji Y, Jin Z, Ly C, Olefsky JM. MiR-690, an exosomal-derived miRNA from M2-polarized macrophages, improves insulin sensitivity in obese mice. Cell Metab. 2021;33:781–790e785.
Phu TA, Ng M, Vu NK, Bouchareychas L, Raffai RL. IL-4 polarized human macrophage exosomes control cardiometabolic inflammation and Diabetes in obesity. Mol Ther. 2022;30:2274–97.
Patwardhan S, Mahadik P, Shetty O, Sen S. ECM stiffness-tuned exosomes drive Breast cancer motility through thrombospondin-1. Biomaterials. 2021;279:121185.
Zhang X, Sai B, Wang F, Wang L, Wang Y, Zheng L, Li G, Tang J, Xiang J. Hypoxic BMSC-derived exosomal miRNAs promote Metastasis of Lung cancer cells via STAT3-induced EMT. Mol Cancer. 2019;18:40.
Xu G, Zhang B, Ye J, Cao S, Shi J, Zhao Y, Wang Y, Sang J, Yao Y, Guan W, et al. Exosomal miRNA-139 in cancer-associated fibroblasts inhibits gastric cancer progression by repressing MMP11 expression. Int J Biol Sci. 2019;15:2320–9.
Zhu L, Li J, Gong Y, Wu Q, Tan S, Sun D, Xu X, Zuo Y, Zhao Y, Wei YQ, et al. Exosomal tRNA-derived small RNA as a promising biomarker for cancer diagnosis. Mol Cancer. 2019;18:74.
Jiang C, Hopfner F, Berg D, Hu MT, Pilotto A, Borroni B, Davis JJ, Tofaris GK. Validation of α-Synuclein in L1CAM-Immunocaptured exosomes as a biomarker for the stratification of parkinsonian syndromes. Mov Disord. 2021;36:2663–9.
Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV. Exosomes as drug delivery vehicles for Parkinson’s Disease therapy. J Control Release. 2015;207:18–30.
Gao ZS, Zhang CJ, Xia N, Tian H, Li DY, Lin JQ, Mei XF, Wu C. Berberine-loaded M2 macrophage-derived exosomes for spinal cord injury therapy. Acta Biomater. 2021;126:211–23.
Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18:1606–14.
Zhao X, Xue X, Cui Z, Kwame Amevor F, Wan Y, Fu K, Wang C, Peng C, Li Y. microRNAs-based diagnostic and therapeutic applications in liver fibrosis. Wiley Interdiscip Rev RNA 2022:e1773.
Wijenayake S, Eisha S, Tawhidi Z, Pitino MA, Steele MA, Fleming AS, McGowan PO. Comparison of methods for pre-processing, exosome isolation, and RNA extraction in unpasteurized bovine and human milk. PLoS ONE. 2021;16:e0257633.
Li X, Su L, Zhang X, Chen Q, Wang Y, Shen Z, Zhong T, Wang L, Xiao Y, Feng X, Yu X. Recent advances on the function and purification of milk exosomes: a review. Front Nutr. 2022;9:871346.
Rashidi M, Bijari S, Khazaei AH, Shojaei-Ghahrizjani F, Rezakhani L. The role of milk-derived exosomes in the treatment of Diseases. Front Genet. 2022;13:1009338.
Momen-Heravi F, Bala S. Extracellular vesicles in oral squamous carcinoma carry oncogenic miRNA profile and reprogram monocytes via NF-κB pathway. Oncotarget. 2018;9:34838–54.
Yamauchi M, Shimizu K, Rahman M, Ishikawa H, Takase H, Ugawa S, Okada A, Inoshima Y. Efficient method for isolation of exosomes from raw bovine milk. Drug Dev Ind Pharm. 2019;45:359–64.
Ferreira RF, Blees T, Shakeri F, Buness A, Sylvester M, Savoini G, Agazzi A, Mrljak V, Sauerwein H. Comparative proteome profiling in exosomes derived from porcine colostrum versus mature milk reveals distinct functional proteomes. J Proteom. 2021;249:104338.
Blans K, Hansen MS, Sørensen LV, Hvam ML, Howard KA, Möller A, Wiking L, Larsen LB, Rasmussen JT. Pellet-free isolation of human and bovine milk extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles. 2017;6:1294340.
Vaswani K, Koh YQ, Almughlliq FB, Peiris HN, Mitchell MD. A method for the isolation and enrichment of purified bovine milk exosomes. Reprod Biol. 2017;17:341–8.
Vaswani K, Mitchell MD, Holland OJ, Qin Koh Y, Hill RJ, Harb T, Davies PSW, Peiris H. A Method for the Isolation of Exosomes from Human and Bovine Milk. J Nutr Metab 2019, 2019:5764740.
Merchant ML, Rood IM, Deegens JKJ, Klein JB. Isolation and characterization of urinary extracellular vesicles: implications for biomarker discovery. Nat Rev Nephrol. 2017;13:731–49.
Livshits MA, Khomyakova E, Evtushenko EG, Lazarev VN, Kulemin NA, Semina SE, Generozov EV, Govorun VM. Isolation of exosomes by differential centrifugation: theoretical analysis of a commonly used protocol. Sci Rep. 2015;5:17319.
Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, Nolte-‘t Hoen EN, Piper MG, Sivaraman S, Skog J et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2013, 2.
Mathivanan S, Simpson RJ. ExoCarta: a compendium of exosomal proteins and RNA. Proteomics. 2009;9:4997–5000.
Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM, Simpson RJ. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon Cancer cell line LIM1863-derived exosomes. Methods. 2012;56:293–304.
Zarovni N, Corrado A, Guazzi P, Zocco D, Lari E, Radano G, Muhhina J, Fondelli C, Gavrilova J, Chiesi A. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods. 2015;87:46–58.
He M, Zeng Y. Microfluidic exosome analysis toward Liquid Biopsy for Cancer. J Lab Autom. 2016;21:599–608.
Ukkola J, Pratiwi FW, Kankaanpää S, Abdorahimzadeh S, KarzarJeddi M, Singh P, Zhyvolozhnyi A, Makieieva O, Viitala S, Samoylenko A, et al. Enrichment of bovine milk-derived extracellular vesicles using surface-functionalized cellulose nanofibers. Carbohydr Polym. 2022;297:120069.
Sánchez-Abarca TLR, Muntión LI, Preciado S, Puig S, López-Ruano N, Hernández-Hernández G, Redondo Á, Ortega A, Rodríguez R. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun Signal. 2016;14:2.
Li H, Ding Y, Huang J, Zhao Y, Chen W, Tang Q, An Y, Chen R, Hu C. Angiopep-2 modified exosomes load rifampicin with potential for treating Central Nervous System Tuberculosis. Int J Nanomedicine. 2023;18:489–503.
Dong B, Wang C, Zhang J, Zhang J, Gu Y, Guo X, Zuo X, Pan H, Hsu AC, Wang G, Wang F. Exosomes from human umbilical cord mesenchymal stem cells attenuate the inflammation of severe steroid-resistant Asthma by reshaping macrophage polarization. Stem Cell Res Ther. 2021;12:204.
Siwaponanan P, Kaewkumdee P, Phromawan W, Udompunturak S, Chomanee N, Udol K, Pattanapanyasat K, Krittayaphong R. Increased expression of six-large extracellular vesicle-derived miRNAs signature for nonvalvular atrial fibrillation. J Transl Med. 2022;20:4.
Lozano-Andrés E, Libregts SF, Toribio V, Royo F, Morales S, López-Martín S, Valés-Gómez M, Reyburn HT, Falcón-Pérez JM, Wauben MH, et al. Tetraspanin-decorated extracellular vesicle-mimetics as a novel adaptable reference material. J Extracell Vesicles. 2019;8:1573052.
Dehghani M, Gaborski TR. Fluorescent labeling of extracellular vesicles. Methods Enzymol. 2020;645:15–42.
Kamei N, Nishimura H, Matsumoto A, Asano R, Muranaka K, Fujita M, Takeda M, Hashimoto H, Takeda-Morishita M. Comparative study of commercial protocols for high recovery of high-purity mesenchymal stem cell-derived extracellular vesicle isolation and their efficient labeling with fluorescent dyes. Nanomedicine. 2021;35:102396.
Kapustin AN, Chatrou ML, Drozdov I, Zheng Y, Davidson SM, Soong D, Furmanik M, Sanchis P, De Rosales RT, Alvarez-Hernandez D, et al. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ Res. 2015;116:1312–23.
Ter-Ovanesyan D, Norman M, Lazarovits R, Trieu W, Lee JH, Church GM, et al. Framework for rapid comparison of extracellular vesicle isolation methods. Elife. 2021;10:e70725.
Macías M, Rebmann V, Mateos B, Varo N, Perez-Gracia JL, Alegre E, González Á. Comparison of six commercial serum exosome isolation methods suitable for clinical laboratories. Effect in cytokine analysis. Clin Chem Lab Med. 2019;57:1539–45.
Shojaati G, Khandaker I, Funderburgh ML, Mann MM, Basu R, Stolz DB, Geary ML, Dos Santos A, Deng SX, Funderburgh JL. Mesenchymal stem cells reduce corneal fibrosis and inflammation via Extracellular vesicle-mediated delivery of miRNA. Stem Cells Transl Med. 2019;8:1192–201.
Karimi N, Dalirfardouei R, Dias T, Lötvall J, Lässer C. Tetraspanins distinguish separate extracellular vesicle subpopulations in human serum and plasma - contributions of platelet extracellular vesicles in plasma samples. J Extracell Vesicles. 2022;11:e12213.
Wen Z, Mai Z, Zhu X, Wu T, Chen Y, Geng D, Wang J. Mesenchymal stem cell-derived exosomes ameliorate cardiomyocyte apoptosis in hypoxic conditions through microRNA144 by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11:36.
Huang Y, Zhu L, Li H, Ye J, Lin N, Chen M, Pan D, Chen Z. Endometriosis derived exosomal miR-301a-3p mediates macrophage polarization via regulating PTEN-PI3K axis. Biomed Pharmacother. 2022;147:112680.
Lin X, Li S, Wang YJ, Wang Y, Zhong JY, He JY, Cui XJ, Zhan JK, Liu YS. Exosomal Notch3 from high glucose-stimulated endothelial cells regulates vascular smooth muscle cells calcification/aging. Life Sci. 2019;232:116582.
Lichá K, Pastorek M, Repiská G, Celec P, Konečná B. Investigation of the presence of DNA in human blood plasma small extracellular vesicles. Int J Mol Sci. 2023;24:5915.
Xiao B, Chai Y, Lv S, Ye M, Wu M, Xie L, Fan Y, Zhu X, Gao Z. Endothelial cell-derived exosomes protect SH-SY5Y nerve cells against ischemia/reperfusion injury. Int J Mol Med. 2017;40:1201–9.
Zhang M, Xie Y, Li S, Ye X, Jiang Y, Tang L, Wang J. Proteomics analysis of Exosomes from patients with active Tuberculosis reveals Infection profiles and potential biomarkers. Front Microbiol. 2021;12:800807.
Gao HN, Guo HY, Zhang H, Xie XL, Wen PC, Ren FZ. Yak-milk-derived exosomes promote proliferation of intestinal epithelial cells in an hypoxic environment. J Dairy Sci. 2019;102:985–96.
Hoshino A, Kim HS, Bojmar L, Gyan KE, Cioffi M, Hernandez J, Zambirinis CP, Rodrigues G, Molina H, Heissel S, et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell. 2020;182:1044–1061e1018.
Grey M, Dunning CJ, Gaspar R, Grey C, Brundin P, Sparr E, Linse S. Acceleration of α-synuclein aggregation by exosomes. J Biol Chem. 2015;290:2969–82.
Biagini V, Busi F, Anelli V, Kerschbamer E, Baghini M, Gurrieri E, et al. Zebrafish melanoma-derived interstitial EVs are carriers of ncRNAs that induce inflammation. Int J Mol Sci. 2022;23:5510.
Chen J, Chen J, Cheng Y, Fu Y, Zhao H, Tang M, Zhao H, Lin N, Shi X, Lei Y, et al. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem Cell Res Ther. 2020;11:97.
Yang X, Shi G, Guo J, Wang C, He Y. Exosome-encapsulated antibiotic against intracellular Infections of methicillin-resistant Staphylococcus aureus. Int J Nanomedicine. 2018;13:8095–104.
Ibáñez F, Montesinos J, Ureña-Peralta JR, Guerri C, Pascual M. TLR4 participates in the transmission of ethanol-induced neuroinflammation via astrocyte-derived extracellular vesicles. J Neuroinflammation. 2019;16:136.
Peng Q, Zhang J, Zhou G. Circulating exosomes regulate T-cell-mediated inflammatory response in oral lichen planus. J Oral Pathol Med. 2019;48:143–50.
Ross M, Atalla H, Karrow N, Mallard BA. The bioactivity of colostrum and milk exosomes of high, average, and low immune responder cows on human intestinal epithelial cells. J Dairy Sci. 2021;104:2499–510.
Takov K, Yellon DM, Davidson SM. Confounding factors in vesicle uptake studies using fluorescent lipophilic membrane dyes. J Extracell Vesicles. 2017;6:1388731.
Lässer C, Alikhani VS, Ekström K, Eldh M, Paredes PT, Bossios A, Sjöstrand M, Gabrielsson S, Lötvall J, Valadi H. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med. 2011;9:9.
Ocansey DKW, Zhang L, Wang Y, Yan Y, Qian H, Zhang X, Xu W, Mao F. Exosome-mediated effects and applications in inflammatory bowel Disease. Biol Rev Camb Philos Soc. 2020;95:1287–307.
Heidari N, Abbasi-Kenarsari H, Namaki S, Baghaei K, Zali MR, Ghaffari Khaligh S, Hashemi SM. Adipose-derived mesenchymal stem cell-secreted exosome alleviates dextran sulfate sodium-induced acute Colitis by Treg cell induction and inflammatory cytokine reduction. J Cell Physiol. 2021;236:5906–20.
Yu H, Yang X, Xiao X, Xu M, Yang Y, Xue C, Li X, Wang S, Zhao RC. Human adipose mesenchymal stem cell-derived exosomes protect mice from DSS-Induced Inflammatory Bowel Disease by promoting intestinal-stem-cell and epithelial regeneration. Aging Dis. 2021;12:1423–37.
Yang S, Liang X, Song J, Li C, Liu A, Luo Y, Ma H, Tan Y, Zhang X. A novel therapeutic approach for inflammatory bowel Disease by exosomes derived from human umbilical cord mesenchymal stem cells to repair intestinal barrier via TSG-6. Stem Cell Res Ther. 2021;12:315.
Ju S, Mu J, Dokland T, Zhuang X, Wang Q, Jiang H, Xiang X, Deng ZB, Wang B, Zhang L, et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced Colitis. Mol Ther. 2013;21:1345–57.
Sriwastva MK, Deng ZB, Wang B, Teng Y, Kumar A, Sundaram K, Mu J, Lei C, Dryden GW, Xu F, et al. Exosome-like nanoparticles from Mulberry bark prevent DSS-induced Colitis via the AhR/COPS8 pathway. EMBO Rep. 2022;23:e53365.
Cao Y, Wang Z, Yan Y, Ji L, He J, Xuan B, et al. Enterotoxigenic bacteroidesfragilis promotes intestinal inflammation and malignancy by inhibiting exosome-packaged miR-149-3p. Gastroenterology. 2021;161:1552–66.e12.
Liu T, Zhang X, Du L, Wang Y, Liu X, Tian H, Wang L, Li P, Zhao Y, Duan W, et al. Exosome-transmitted mir-128-3p increase chemosensitivity of oxaliplatin-resistant Colorectal cancer. Mol Cancer. 2019;18:43.
Zhang Z, Xing T, Chen Y, Xiao J. Exosome-mediated miR-200b promotes Colorectal cancer proliferation upon TGF-β1 exposure. Biomed Pharmacother. 2018;106:1135–43.
Shang A, Gu C, Wang W, Wang X, Sun J, Zeng B, Chen C, Chang W, Ping Y, Ji P, et al. Exosomal circPACRGL promotes progression of Colorectal cancer via the miR-142-3p/miR-506-3p- TGF-β1 axis. Mol Cancer. 2020;19:117.
Zheng R, Zhang K, Tan S, Gao F, Zhang Y, Xu W, Wang H, Gu D, Zhu L, Li S, et al. Exosomal circLPAR1 functions in Colorectal cancer diagnosis and tumorigenesis through suppressing BRD4 via METTL3-eIF3h interaction. Mol Cancer. 2022;21:49.
Guo MM, Zhang K, Zhang JH. Human breast milk-derived exosomal miR-148a-3p protects against necrotizing enterocolitis by regulating p53 and sirtuin 1. Inflammation. 2022;45:1254–68.
Yan X, Liu L, Yao S, Chen Y, Yu Q, Jiang C, Chen W, Chen X, Han S. LncRNA and mRNA profiles of human milk-derived exosomes and their possible roles in protecting against necrotizing enterocolitis. Food Funct. 2022;13:12953–65.
Hu X, Zhang R, Liang H, An J, Yang Y, Huo J, et al. Comparison and investigation of exosomes from human amniotic fluid stem cells and human breast milk in alleviating neonatal necrotizing enterocolitis. Stem Cell Rev Rep. 2022;19:754–66.
Pisano C, Besner GE. Potential role of stem cells in Disease prevention based on a murine model of experimental necrotizing enterocolitis. J Pediatr Surg. 2019;54:413–6.
Zhang G, Wan Z, Liu Z, Liu D, Zhao Z, Leng Y. Exosomes Derived from BMSCs ameliorate Intestinal Ischemia-Reperfusion Injury by regulating mir-144-3p-Mediated oxidative stress. Dig Dis Sci. 2022;67:5090–106.
Yang J, Zheng XG, Wu YL, Wang AP, Wang CH, Chen WX, Zhong S, Yang H. Intestinal epithelial cell-derived exosomes package microRNA-23a-3p alleviate gut damage after ischemia/reperfusion via targeting MAP4K4. Biomed Pharmacother. 2022;149:112810.
Li YJ, Xu QW, Xu CH, Li WM. MSC promotes the secretion of Exosomal miR-34a-5p and improve intestinal barrier function through METTL3-Mediated Pre-miR-34A m(6)a modification. Mol Neurobiol. 2022;59:5222–35.
Zempleni J, Sukreet S, Zhou F, Wu D, Mutai E. Milk-derived exosomes and metabolic regulation. Annu Rev Anim Biosci. 2019;7:245–62.
Ayyar KK, Moss AC. Exosomes in intestinal inflammation. Front Pharmacol. 2021;12:658505.
Miyake H, Lee C, Chusilp S, Bhalla M, Li B, Pitino M, Seo S, O’Connor DL, Pierro A. Human breast milk exosomes attenuate intestinal damage. Pediatr Surg Int. 2020;36:155–63.
Aarts J, Boleij A, Pieters BCH, Feitsma AL, van Neerven RJJ, Ten Klooster JP, M’Rabet L, Arntz OJ, Koenders MI, van de Loo FAJ. Flood Control: how milk-derived extracellular vesicles can help to improve the intestinal barrier function and Break the Gut-Joint Axis in Rheumatoid Arthritis. Front Immunol. 2021;12:703277.
Garcia C, Duan RD, Brévaut-Malaty V, Gire C, Millet V, Simeoni U, Bernard M, Armand M. Bioactive compounds in human milk and intestinal health and maturity in preterm newborn: an overview. Cell Mol Biol (Noisy-le-grand). 2013;59:108–31.
Kosaka N, Izumi H, Sekine K, Ochiya T. microRNA as a new immune-regulatory agent in breast milk. Silence. 2010;1:7.
Pieters BC, Arntz OJ, Bennink MB, Broeren MG, van Caam AP, Koenders MI, van Lent PL, van den Berg WB, de Vries M, van der Kraan PM, van de Loo FA. Commercial cow milk contains physically stable extracellular vesicles expressing immunoregulatory TGF-β. PLoS ONE. 2015;10:e0121123.
Zhou Y, Yu Z, Wang X, Chen W, Liu Y, Zhang Y, Yin J, Han S. Exosomal circRNAs contribute to intestinal development via the VEGF signalling pathway in human term and preterm colostrum. Aging. 2021;13:11218–33.
Chen T, Xie MY, Sun JJ, Ye RS, Cheng X, Sun RP, Wei LM, Li M, Lin DL, Jiang QY, et al. Porcine milk-derived exosomes promote proliferation of intestinal epithelial cells. Sci Rep. 2016;6:33862.
Silberg DG, Swain GP, Suh ER, Traber PG. Cdx1 and cdx2 expression during intestinal development. Gastroenterology. 2000;119:961–71.
Zeng B, Wang H, Luo J, Xie M, Zhao Z, Chen X, et al. Porcine milk-derived small extracellular vesicles promote intestinal immunoglobulin production through pIgR. Animals (Basel). 2021;11:1522.
Xie MY, Chen T, Xi QY, Hou LJ, Luo JY, Zeng B, Li M, Sun JJ, Zhang YL. Porcine milk exosome miRNAs protect intestinal epithelial cells against deoxynivalenol-induced damage. Biochem Pharmacol. 2020;175:113898.
Xie MY, Hou LJ, Sun JJ, Zeng B, Xi QY, Luo JY, Chen T, Zhang YL. Porcine milk exosome MiRNAs attenuate LPS-Induced apoptosis through inhibiting TLR4/NF-κB and p53 pathways in intestinal epithelial cells. J Agric Food Chem. 2019;67:9477–91.
Zhou F, Paz HA, Sadri M, Cui J, Kachman SD, Fernando SC, Zempleni J. Dietary bovine milk exosomes elicit changes in bacterial communities in C57BL/6 mice. Am J Physiol Gastrointest Liver Physiol. 2019;317:G618–g624.
Maghraby MK, Li B, Chi L, Ling C, Benmoussa A, Provost P, Postmus AC, Abdi A, Pierro A, Bourdon C, Bandsma RHJ. Extracellular vesicles isolated from milk can improve gut barrier dysfunction induced by Malnutrition. Sci Rep. 2021;11:7635.
Shome S, Jernigan RL, Beitz DC, Clark S, Testroet ED. Non-coding RNA in raw and commercially processed milk and putative targets related to growth and immune-response. BMC Genomics. 2021;22:749.
Samuel M, Fonseka P, Sanwlani R, Gangoda L, Chee SH, Keerthikumar S, Spurling A, Chitti SV, Zanker D, Ang CS, et al. Oral administration of bovine milk-derived extracellular vesicles induces senescence in the primary Tumor but accelerates cancer Metastasis. Nat Commun. 2021;12:3950.
Santos-Coquillat A, González MI, Clemente-Moragón A, González-Arjona M, Albaladejo-García V, Peinado H, Muñoz J, Ximénez Embún P, Ibañez B, Oliver E, et al. Goat milk exosomes as natural nanoparticles for detecting inflammatory processes by Optical Imaging. Small. 2022;18:e2105421.
Hock A, Miyake H, Li B, Lee C, Ermini L, Koike Y, Chen Y, Määttänen P, Zani A, Pierro A. Breast milk-derived exosomes promote intestinal epithelial cell growth. J Pediatr Surg. 2017;52:755–9.
Ma J, Wang C, Long K, Zhang H, Zhang J, Jin L, Tang Q, Jiang A, Wang X, Tian S, et al. Exosomal microRNAs in giant panda (Ailuropoda melanoleuca) breast milk: potential maternal regulators for the development of newborn cubs. Sci Rep. 2017;7:3507.
Zhang W, Zhou B, Yang X, Zhao J, Hu J, Ding Y, Zhan S, Yang Y, Chen J, Zhang F, et al. Exosomal circEZH2_005, an intestinal injury biomarker, alleviates intestinal ischemia/reperfusion injury by mediating Gprc5a signaling. Nat Commun. 2023;14:5437.
Console L, Scalise M, Indiveri C. Exosomes in inflammation and role as biomarkers. Clin Chim Acta. 2019;488:165–71.
Larabi A, Barnich N, Nguyen HTT. Emerging role of exosomes in diagnosis and treatment of infectious and inflammatory bowel diseases. Cells. 2020;9:1111.
Hodson R. Inflammatory bowel Disease. Nature. 2016;540:97.
Höie O, Wolters F, Riis L, Aamodt G, Solberg C, Bernklev T, Odes S, Mouzas IA, Beltrami M, Langholz E, et al. Ulcerative Colitis: patient characteristics may predict 10-yr Disease recurrence in a european-wide population-based cohort. Am J Gastroenterol. 2007;102:1692–701.
Baumgart DC, Carding SR. Inflammatory bowel Disease: cause and immunobiology. Lancet. 2007;369:1627–40.
Sinopoulou V, Gordon M, Akobeng AK, Gasparetto M, Sammaan M, Vasiliou J, Dovey TM. Interventions for the management of abdominal pain in Crohn’s Disease and inflammatory bowel Disease. Cochrane Database Syst Rev. 2021;11:Cd013531.
Gecse KB, Vermeire S. Differential diagnosis of inflammatory bowel Disease: imitations and Complications. Lancet Gastroenterol Hepatol. 2018;3:644–53.
Ponder A, Long MD. A clinical review of recent findings in the epidemiology of inflammatory bowel Disease. Clin Epidemiol. 2013;5:237–47.
Zhang YZ, Li YY. Inflammatory bowel Disease: pathogenesis. World J Gastroenterol. 2014;20:91–9.
Danese S, Fiocchi C. Etiopathogenesis of inflammatory bowel Diseases. World J Gastroenterol. 2006;12:4807–12.
Podolsky DK. Inflammatory bowel Disease. N Engl J Med. 2002;347:417–29.
Melnik BC, Stremmel W, Weiskirchen R, John SM, Schmitz G. Exosome-derived MicroRNAs of human milk and their effects on infant health and development. Biomolecules. 2021;11:851.
Li DF, Yang MF, Xu J, Xu HM, Zhu MZ, Liang YJ, Zhang Y, Tian CM, Nie YQ, Shi RY, et al. Extracellular vesicles: the Next Generation Theranostic Nanomedicine for Inflammatory Bowel Disease. Int J Nanomedicine. 2022;17:3893–911.
Zhong J, Xia B, Shan S, Zheng A, Zhang S, Chen J, Liang XJ. High-quality milk exosomes as oral drug delivery system. Biomaterials. 2021;277:121126.
Tong L, Hao H, Zhang Z, Lv Y, Liang X, Liu Q, Liu T, Gong P, Zhang L, Cao F, et al. Milk-derived extracellular vesicles alleviate ulcerative Colitis by regulating the gut immunity and reshaping the gut microbiota. Theranostics. 2021;11:8570–86.
Benmoussa A, Diallo I, Salem M, Michel S, Gilbert C, Sévigny J, Provost P. Concentrates of two subsets of extracellular vesicles from cow’s milk modulate symptoms and inflammation in experimental Colitis. Sci Rep. 2019;9:14661.
Reif S, Elbaum-Shiff Y, Koroukhov N, Shilo I, Musseri M, Golan-Gerstl R. Cow and human milk-derived exosomes ameliorate colitis in DSS murine model. Nutrients. 2020;12:2589.
Du C, Zhao Y, Wang K, Nan X, Chen R, Xiong B. Effects of milk-derived extracellular vesicles on the colonic transcriptome and proteome in murine model. Nutrients. 2022;14:3057.
Du C, Wang K, Zhao Y, Nan X, Chen R, Quan S, et al. Supplementation with milk-derived extracellular vesicles shapes the gut microbiota and regulates the transcriptomic landscape in experimental colitis. Nutrients. 2022;14:1808.
Du C, Quan S, Zhao Y, Nan X, Chen R, Tang X, Xiong B. Bovine milk-derived extracellular vesicles prevent gut inflammation by regulating lipid and amino acid metabolism. Food Funct. 2023;14:2212–22.
Mecocci S, De Paolis L, Fruscione F, Pietrucci D, De Ciucis CG, Giudici SD, Franzoni G, Chillemi G, Cappelli K, Razzuoli E. In vitro evaluation of immunomodulatory activities of goat milk extracellular vesicles (mEVs) in a model of gut inflammation. Res Vet Sci. 2022;152:546–56.
Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364:255–64.
Henry MC, Moss RL. Necrotizing enterocolitis. Annu Rev Med. 2009;60:111–24.
Galley JD, Besner GE. The therapeutic potential of breast milk-derived extracellular vesicles. Nutrients. 2020;12:745.
Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336:1519–23.
Quigley M, Embleton ND, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2018;6:Cd002971.
Egan CE, Sodhi CP, Good M, Lin J, Jia H, Yamaguchi Y, Lu P, Ma C, Branca MF, Weyandt S, et al. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Invest. 2016;126:495–508.
Yazji I, Sodhi CP, Lee EK, Good M, Egan CE, Afrazi A, Neal MD, Jia H, Lin J, Ma C, et al. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proc Natl Acad Sci U S A. 2013;110:9451–6.
Good M, Sodhi CP, Egan CE, Afrazi A, Jia H, Yamaguchi Y, Lu P, Branca MF, Ma C, Prindle T Jr., et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol. 2015;8:1166–79.
Madden JW. Human breast milk exosomes may protect against necrotizing enterocolitis in preterm infants. Pediatr Res. 2021;90:244–5.
Wang X, Yan X, Zhang L, Cai J, Zhou Y, Liu H, Hu Y, Chen W, Xu S, Liu P, et al. Identification and peptidomic profiling of exosomes in Preterm Human milk: insights into necrotizing enterocolitis Prevention. Mol Nutr Food Res. 2019;63:e1801247.
Dong P, Zhang Y, Yan DY, Wang Y, Xu X, Zhao YC, Xiao TT. Protective effects of Human milk-derived exosomes on intestinal stem cells damaged by oxidative stress. Cell Transpl. 2020;29:963689720912690.
Pisano C, Galley J, Elbahrawy M, Wang Y, Farrell A, Brigstock D, Besner GE. Human breast milk-derived extracellular vesicles in the Protection Against Experimental Necrotizing enterocolitis. J Pediatr Surg. 2020;55:54–8.
Martin C, Patel M, Williams S, Arora H, Brawner K, Sims B. Human breast milk-derived exosomes attenuate cell death in intestinal epithelial cells. Innate Immun. 2018;24:278–84.
Chen W, Chen X, Qian Y, Wang X, Zhou Y, Yan X, Yu B, Yao S, Yu Z, Zhu J, Han S. Lipidomic profiling of human milk derived exosomes and their emerging roles in the Prevention of Necrotizing enterocolitis. Mol Nutr Food Res. 2021;65:e2000845.
He S, Liu G, Zhu X. Human breast milk-derived exosomes may help maintain intestinal epithelial barrier integrity. Pediatr Res. 2021;90:366–72.
Gao R, Zhang R, Qian T, Peng X, He W, Zheng S, Cao Y, Pierro A, Shen C. A comparison of exosomes derived from different periods breast milk on protecting against intestinal organoid injury. Pediatr Surg Int. 2019;35:1363–8.
Li B, Hock A, Wu RY, Minich A, Botts SR, Lee C, Antounians L, Miyake H, Koike Y, Chen Y, et al. Bovine milk-derived exosomes enhance goblet cell activity and prevent the development of experimental necrotizing enterocolitis. PLoS ONE. 2019;14:e0211431.
Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–502.
Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019;11:164.
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467–80.
Brody H. Colorectal cancer. Nature. 2015;521:1.
Hu G, Drescher KM, Chen XM. Exosomal miRNAs: Biological properties and therapeutic potential. Front Genet. 2012;3:56.
Glass SE, Coffey RJ. Recent advances in the study of Extracellular vesicles in Colorectal Cancer. Gastroenterology. 2022;163:1188–97.
Hon KW, Abu N, Ab Mutalib NS, Jamal R. Exosomes as potential biomarkers and targeted therapy in Colorectal Cancer: a Mini-review. Front Pharmacol. 2017;8:583.
Wang X, Zhang H, Yang H, Bai M, Ning T, Deng T, Liu R, Fan Q, Zhu K, Li J, et al. Exosome-delivered circRNA promotes glycolysis to induce chemoresistance through the mir-122-PKM2 axis in Colorectal cancer. Mol Oncol. 2020;14:539–55.
Guo G, Tan Z, Liu Y, Shi F, She J. The therapeutic potential of stem cell-derived exosomes in the ulcerative Colitis and Colorectal cancer. Stem Cell Res Ther. 2022;13:138.
Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu Y, Zhang Z, Cai S, Xu Y, Li X, et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver Metastasis of Colorectal cancer. J Hematol Oncol. 2020;13:156.
Munagala R, Aqil F, Jeyabalan J, Agrawal AK, Mudd AM, Kyakulaga AH, Singh IP, Vadhanam MV, Gupta RC. Exosomal formulation of anthocyanidins against multiple cancer types. Cancer Lett. 2017;393:94–102.
Golan-Gerstl R, Elbaum Shiff Y, Moshayoff V, Schecter D, Leshkowitz D, Reif S. Characterization and biological function of milk-derived miRNAs. Mol Nutr Food Res 2017, 61.
Melnik BC, Schmitz G. MicroRNAs: milk’s epigenetic regulators. Best Pract Res Clin Endocrinol Metab. 2017;31:427–42.
Reif S, Elbaum Shiff Y, Golan-Gerstl R. Milk-derived exosomes (MDEs) have a different biological effect on normal fetal colon epithelial cells compared to colon Tumor cells in a miRNA-dependent manner. J Transl Med. 2019;17:325.
Jing B, Gao Y, Guo F, Jiang D, Guo R, Wang J, Li Y, Xie Y, Chen Y, Li H, et al. Engineering goat milk-derived extracellular vesicles for multiple bioimaging-guided and photothermal-enhanced therapy of colon Cancer. Biomater Sci. 2023;11:1408–21.
Martino E, Balestrieri A, Mele L, Sardu C, Marfella R, D’Onofrio N, et al. Milk exosomal miR-27b worsen endoplasmic reticulum stress mediated colorectal cancer cell death. Nutrients. 2022;14:5081.
Wang Z, Sun R, Wang G, Chen Z, Li Y, Zhao Y, Liu D, Zhao H, Zhang F, Yao J, Tian X. SIRT3-mediated deacetylation of PRDX3 alleviates mitochondrial oxidative damage and apoptosis induced by intestinal ischemia/reperfusion injury. Redox Biol. 2020;28:101343.
Zhang Q, Liu XM, Hu Q, Liu ZR, Liu ZY, Zhang HG, Huang YL, Chen QH, Wang WX, Zhang XK. Dexmedetomidine inhibits mitochondria damage and apoptosis of enteric glial cells in experimental intestinal ischemia/reperfusion injury via SIRT3-dependent PINK1/HDAC3/p53 pathway. J Transl Med. 2021;19:463.
Cai J, Chen X, Liu X, Li Z, Shi A, Tang X, Xia P, Zhang J, Yu P. AMPK: the key to ischemia-reperfusion injury. J Cell Physiol. 2022;237:4079–96.
Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian D, Liu D, Zhang F, Ning S, Yao J, Tian X. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019;26:2284–99.
Li S, Zhou Y, Gu X, Zhang X, Jia Z. NLRX1/FUNDC1/NIPSNAP1-2 axis regulates mitophagy and alleviates intestinal ischaemia/reperfusion injury. Cell Prolif. 2021;54:e12986.
Wen J, Xu B, Sun Y, Lian M, Li Y, Lin Y, Chen D, Diao Y, Almoiliqy M, Wang L. Paeoniflorin protects against intestinal ischemia/reperfusion by activating LKB1/AMPK and promoting autophagy. Pharmacol Res. 2019;146:104308.
Zhang X, Wu J, Liu Q, Li X, Li S, Chen J, Hong Z, Wu X, Zhao Y, Ren J. mtDNA-STING pathway promotes necroptosis-dependent enterocyte injury in intestinal ischemia reperfusion. Cell Death Dis. 2020;11:1050.
Sheng X, Zhao J, Li M, Xu Y, Zhou Y, Xu J, He R, Lu H, Wu T, Duan C, et al. Bone marrow mesenchymal stem cell-derived exosomes accelerate functional recovery after spinal cord Injury by promoting the phagocytosis of macrophages to clean myelin debris. Front Cell Dev Biol. 2021;9:772205.
Liu J, Chen T, Lei P, Tang X, Huang P. Exosomes released by bone marrow mesenchymal stem cells attenuate Lung Injury Induced by Intestinal Ischemia Reperfusion via the TLR4/NF-κB pathway. Int J Med Sci. 2019;16:1238–44.
Zhao J, Chen XD, Yan ZZ, Huang WF, Liu KX, Li C. Gut-derived exosomes induce Liver Injury after intestinal Ischemia/Reperfusion by promoting hepatic macrophage polarization. Inflammation. 2022;45:2325–38.
Chen XD, Zhao J, Yang X, Zhou BW, Yan Z, Liu WF, Li C, Liu KX. Gut-derived Exosomes Mediate Memory Impairment after intestinal Ischemia/Reperfusion via activating Microglia. Mol Neurobiol. 2021;58:4828–41.
Shiou SR, Yu Y, Guo Y, Westerhoff M, Lu L, Petrof EO, Sun J, Claud EC. Oral administration of transforming growth factor-β1 (TGF-β1) protects the immature gut from injury via Smad protein-dependent suppression of epithelial nuclear factor κB (NF-κB) signaling and proinflammatory cytokine production. J Biol Chem. 2013;288:34757–66.
Liao W, Du Y, Zhang C, Pan F, Yao Y, Zhang T, Peng Q. Exosomes: the next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater. 2019;86:1–14.
Somiya M, Yoshioka Y, Ochiya T. Biocompatibility of highly purified bovine milk-derived extracellular vesicles. J Extracell Vesicles. 2018;7:1440132.
Wang L, Gao R, Li B, Alganabi M, He W, Shen C, Zhu H, Pierro A. Human breast milk-derived exosomes protect against intestinal ischemia and reperfusion injury in neonatal rats. J Pediatr Surg. 2022;57:1264–8.
Li S, Tang Y, Dou Y. The potential of milk-derived exosomes for Drug Delivery. Curr Drug Deliv. 2021;18:688–99.
Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371:48–61.
Wu L, Wang L, Liu X, Bai Y, Wu R, Li X, Mao Y, Zhang L, Zheng Y, Gong T, et al. Milk-derived exosomes exhibit versatile effects for improved oral drug delivery. Acta Pharm Sin B. 2022;12:2029–42.
González-Sarrías A, Iglesias-Aguirre CE, Cortés-Martín A, Vallejo F, Cattivelli A, Del Pozo-Acebo L, et al. Milk-derived exosomes as nanocarriers to deliver curcumin and resveratrol in breast tissue and enhance their anticancer activity. Int J Mol Sci. 2022;23:2860.
Carobolante G, Mantaj J, Ferrari E, Vllasaliu D. Cow milk and intestinal epithelial cell-derived extracellular vesicles as systems for enhancing oral drug delivery. Pharmaceutics. 2020;12:226.
Sedykh S, Kuleshova A, Nevinsky G. Milk exosomes: perspective agents for anticancer drug delivery. Int J Mol Sci. 2020;21:6646.
Qu S, Han Y, Liu Y, Zhu J, Acaroz U, Shen J, Zhu K. Milk exosomes facilitate oral delivery of Drugs against intestinal bacterial Infections. J Agric Food Chem. 2022;70:16069–79.
Vashisht M, Rani P, Onteru SK, Singh D. Curcumin encapsulated in milk exosomes resists human digestion and possesses enhanced intestinal permeability in Vitro. Appl Biochem Biotechnol. 2017;183:993–1007.
Agrawal AK, Aqil F, Jeyabalan J, Spencer WA, Beck J, Gachuki BW, Alhakeem SS, Oben K, Munagala R, Bondada S, Gupta RC. Milk-derived exosomes for oral delivery of paclitaxel. Nanomedicine. 2017;13:1627–36.
Wallen M, Aqil F, Spencer W, Gupta RC. Milk/colostrum exosomes: a nanoplatform advancing delivery of cancer therapeutics. Cancer Lett. 2023;561:216141.
Yan C, Chen J, Wang C, Yuan M, Kang Y, Wu Z, Li W, Zhang G, Machens HG, Rinkevich Y, et al. Milk exosomes-mediated mir-31-5p delivery accelerates diabetic wound healing through promoting angiogenesis. Drug Deliv. 2022;29:214–28.
Del Pozo-Acebo L, Hazas MLL, Tomé-Carneiro J, Gil-Cabrerizo P, San-Cristobal R, Busto R, et al. Bovine milk-derived exosomes as a drug delivery vehicle for miRNA-based therapy. Int J Mol Sci. 2021;22:1105.
Warren MR, Zhang C, Vedadghavami A, Bokvist K, Dhal PK, Bajpayee AG. Milk exosomes with enhanced mucus penetrability for oral delivery of siRNA. Biomater Sci. 2021;9:4260–77.
Shandilya S, Rani P, Onteru SK, Singh D. Small interfering RNA in milk exosomes is resistant to digestion and crosses the Intestinal Barrier in Vitro. J Agric Food Chem. 2017;65:9506–13.
Aqil F, Munagala R, Jeyabalan J, Agrawal AK, Kyakulaga AH, Wilcher SA, Gupta RC. Milk exosomes - natural nanoparticles for siRNA delivery. Cancer Lett. 2019;449:186–95.
Xiang X, Chen J, Jiang T, Yan C, Kang Y, Zhang M, et al. Milk-derived exosomes carrying siRNA-KEAP1 promote diabetic wound healing by improving oxidative stress. Drug Deliv Transl Res. 2023;13:2286–96.
Ngu A, Wang S, Wang H, Khanam A, Zempleni J. Milk exosomes in nutrition and drug delivery. Am J Physiol Cell Physiol. 2022;322:C865–c874.
Tian MY, Hao DX, Liu Y, He J, Zhao ZH, Guo TY, Li X, Zhang Y. Milk exosomes: an oral drug delivery system with great application potential. Food Funct. 2023;14:1320–37.
Pullan J, Dailey K, Bhallamudi S, Feng L, Alhalhooly L, Froberg J, Osborn J, Sarkar K, Molden T, Sathish V, et al. Modified bovine milk exosomes for Doxorubicin Delivery to Triple-negative Breast Cancer cells. ACS Appl Bio Mater. 2022;5:2163–75.
Li D, Gong L, Lin H, Yao S, Yin Y, Zhou Z, et al. Hyaluronic acid-coated bovine milk exosomes for achieving tumor-specific intracellular delivery of miRNA-204. Cells. 2022;11:3065.
Li D, Yao S, Zhou Z, Shi J, Huang Z, Wu Z. Hyaluronan decoration of milk exosomes directs tumor-specific delivery of doxorubicin. Carbohydr Res. 2020;493:108032.
Zhang Q, Xiao Q, Yin H, Xia C, Pu Y, He Z, Hu Q, Wang J, Wang Y. Milk-exosome based pH/light sensitive drug system to enhance anticancer activity against oral squamous cell carcinoma. RSC Adv. 2020;10:28314–23.
Li Y, Xing L, Wang L, Liu X, Wu L, Ni M, Zhou Z, Li L, Liu X, Huang Y. Milk-derived exosomes as a promising vehicle for oral delivery of hydrophilic biomacromolecule Drugs. Asian J Pharm Sci. 2023;18:100797.
Gong L, Zhou H, Zhang S, Wang C, Fu K, Ma C, Zhang Y, Peng C, Li Y. CD44-Targeting Drug Delivery System of exosomes Loading Forsythiaside A combats Liver Fibrosis via regulating NLRP3-Mediated pyroptosis. Adv Healthc Mater 2023:e2202228.
Funding
This study was supported by the Natural Science Foundation of Chongqing (2023NSCQ-MSX1184), the Fundamental Research Funds for the Central Universities (SWU-KQ22080), and the National Natural Science Foundation of China (Grant No. 32350410427).
Author information
Authors and Affiliations
Contributions
Author Contributions StatementConceptualization, ZC and FKA; Literature review, ZC, FKA, XZ; Writing–original draft, ZC; Figures preparation, ZC, XL, CM, and AL; Table preparation, ZC, JP, XP, and LL; Writing–review & editing, XL, and LL. All authors have seen and approved the final version of the submitted manuscript.
Corresponding authors
Ethics declarations
Competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cui, Z., Amevor, F.K., Zhao, X. et al. Potential therapeutic effects of milk-derived exosomes on intestinal diseases. J Nanobiotechnol 21, 496 (2023). https://doi.org/10.1186/s12951-023-02176-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-023-02176-8