Abstract
Refractory diabetic wounds can cause persistent inflammation and delayed healing due to hypoxia. Currently, no optimal solution is available. Exosomes of adipose stem cells (ADSCs-exo) may promote skin wound healing, however, molecular mechanisms remains mysterious. We found significantly enhanced survival and proliferation of adipose stem cells after hypoxia induction compared to normoxia. Here, we aimed to investigate if hypoxic adipose stem cells exosomes (HypADSCs-exo) participate in hypoxia adaptability and accelerate diabetic wound healing. Based on high-throughput sequencing, 215 microRNAs (miRNAs) were upregulated and 369 miRNAs downregulated in HypADSCs-exo compared to ADSCs-exo. Up-regulated miR-21-3p, miR-126-5p, miR-31-5p whereas down-regulated gene miR-99b and miR-146-a correlated with wound healing. According to Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG), miRNAs might regulate cell metabolism, differentiation and Transforming growth factor-β (TGF-β) function. Consistently, HpyADSCs-exo could promote diabetic wounds healing and inhibit inflammation through PI3K/AKT signaling pathway. Collectively, HpyADSCs-exo can promote diabetic wound healing as an alternative strategy to improve wound healing.

Similar content being viewed by others
Introduction
For diabetes, molecular mechanisms underlying wound healing are complex and involve dysfunction of multiple signaling pathways and processes. In particular, diabetic wound is susceptible to infection and scarring, while non-union or slow healing becomes a major culprit that seriously affects patients’ quality of life [1,2,3,4]. At initial stage of wound, cellular adaptability to hypoxia was decreased, resulting in persistent inflammation and substantially delayed healing [5]. Traditional treatment of diabetic trauma mainly focuses on late dressing, negative pressure, electrical stimulation, hyperbaric oxygen and skin grafting, but the treatment effect is not satisfactory [6]. At present, many new materials have been applied, bringing more possibilities for the complete cure of diabetic wounds, among which the application of exosome therapy shows a strong potential [7,8,9,10,11].
Adipose stem cells (ADSCs) hold a good prospect in wound repair [12]. Exosomes, extracellular vesicles with a diameter of 30–150 nm, mediate remote communication between cells. Exosomes can transferred to recipient cells, delivering proteins, RNAs and DNAs [13, 14]. Compared with cell therapy, exosomes may minimize immune mediated rejection and malignant transformation [15]. Hypoxia activates hypoxia inducible factor (HIF-1α), which regulates angiogenesis [11, 16, 17]. Interestingly, ADSCs-exo are involved in a wide range of biological processes by affecting tissue responses to injury, infection and diseases [18,19,20,21,22,23]. ADSCs secrete more exosomes under hypoxic environment, while HypADSCs-exo can improve blood perfusion and survival of transplanted tissues and reduce inflammatory filtration in adipose [24,25,26,27]. A variety of miRNAs in exosomes especially can promote diabetic wound healing [28, 29]. Although evidence on high-quality skin wound healing is still lacking at this stage, these results provide a new perspective for HypADSCs-exo in soft tissue repair.
Our study show that ADSCs-exo and HypADSCs-exo to identify differentially expressed miRNAs with performed high-throughput sequencing. Notably, HypADSCs-exo induced proliferation, collagen metabolism and migration through PI3K/AKT signaling pathway in human skin fibroblasts. Furthermore, we established a diabetic wound model in nude mice to analyze speed and quality of wound tissue healing, and to explore molecular mechanism underlying beneficial effects of HypADSCs-exo. Our study provides a promising strategy for cell-free therapy.
Materials and methods
Cell culture
Subcutaneous adipose and skin tissue samples were harvested from the abdomen of women (aged 20–50 years) who received liposuction from January to May 2020 at the Second Affiliated Hospital of Harbin Medical University. Informed consent was obtained from each subject. Acquisition of human subcutaneous fat aimed to establish primary culture of adipose stem cells. The cell pellet was resuspended in Dulbecco’s Modified Eagle Medium F12 (DMEM/F12; Corning, New York, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, Rockville, MD, USA) and 100 IU penicillin/100 mg/mL streptomycin (Solarbio, Beijing, China), and cultured in a humidified 5% CO2 atmosphere at 37 °C. Similarly, we conducted primary cultures of human fibroblasts (HF).
Isolation and analysis of exosomes
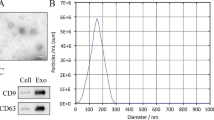
Human ADSCs (hADSCs) at 70–80% confluence were washed with PBS and cultured in microvascular endothelial cell growth medium‐2 media deprived of FBS with supplement of 1× serum replacement solution (PeproTech) for 24 h. Similarly, the HADSCs were subjected to hypoxia conditions (1% O2/5% CO2/94% N2) for 24 h to obtain the cell supernatant and isolate the HypADSCs-exo. Then, the cell supernatant was then collected and centrifuged at 300g for 10 min, followed by centrifugation at 2000×g for 10 min at 4 °C. Then, the cell supernatant was filtered with a 0.22 μm sterile filter (sterile micropores, Burlington). We follow the instructions to add the ExoQuick‐TC reagent. After centrifugation at 1500g for 30 min, discarding the cell supernatant and resuspend the exosome‐containing pellet in PBS. Exosomes were either applied immediately for experiments or stored at − 80 °C. Dimensions of purified exosomes were determined with NanoSight LM10 (Malvern Instruments) nanoparticle tracking system. Levels of HSP70 and CD9 proteins were detected by Western blotting (abcam, USA). ADSCs-exos were labeled using PKH26 Red Fluorescent Cell Linker Kits according to the manufacturer’s instructions (Sigma, USA). Specific concentrations of proteins in exosomes were assessed with bicinchoninic acid assay kits (Beyotime, China). Ultrastructure of extracellular vesicles was analyzed by Transmission electron microscopy (TEM) with Libra 120 instrument (Zeiss).
MiRNAs high-throughput sequence
Exosomas miRNAs was isolated using a commercially available total exosomes RNA isolation kit (Qiagen’s exoRNeasy Serum Plasma Kit). RNA integrity was verifified on a Bioanalyzer 2100 using an Agilent RNA 6000 Nano Assay. QPCR’s KAPA Biological System Library Quantization Kit for quantitative sequencing applications (Kapa Biosystems, Inc., Woburn, Mass). Each library was diluted to a final concentration of 10 nM and pooled equimolar before clustering. Also, to meet the sample compliance requirements, we performed paired end sequencing and analysis of gene coverage and thermal graph. Quality analysis of gene coverage graph and thermal graph. GO and KEGG analyses for differentially expressed miRNAs identified downstream genes regulated by miRNAs (P < 0.05, |log2 (fold change)|> 1).
RNA extraction and real-time PCR
Total RNA was isolated from hADSCs after treatments using RNAiso Plus (TaKaRa Biotechnology, Shiga, Japan) according to the manufacturer’s instructions. Reverse the separation RNA to cDNA. Next, mRNAs expression was measured via real-time PCR in an ABI Prism 7500 Sequence Detection System (ABI, CA, USA) using SYBR Premix ExTaq (TaKaRa Biotechnology) (Additional file 2: Table S1).
Human fibroblasts (HF) treatment
HF(5 × 106 cell) treated with ADSCs-exo, HypADSCs-exo (100 μg/mL) or PBS (control). Next, we treat the cells with the PI3K/AKT inhibitor LY294002 (50 nM, boss from MedChem Express, Monmouth Junction, NJ, USA). Cells were harvested 30 min after treatment for western blotting and after 48 h or for Edu assays.
Cell proliferation assay
Cell proliferation was detected using the Edu colorimetric immunoassay kit (Cell Proliferation ELISA, Roche Diagnostics, Germany). Cell proliferation was expressed as the mean percentage of the control values (set at 100%).
Western blot analysis
Total separated protein components, control, ADSCs-exo, HypADSCs-exo (100 μg/mL) or PBS induced HF 48 h and cell lysis buffer treated 15 min. The lysate centrifuged at 12,000 rpm at 4 °C for 15 min. Then, 20 μg was separated by 15% SDS/PAGE and transferred onto polyvinylidene diflfluoride membranes (Millipore, Mississauga, Canada). The membranes were blocked in milk for 1 h and were incubated overnight at 4 °C with primary antibodies GAPDH (ab181602, Abcam), Type I collagen (COL I) (ab34710, Abcam), Type III collagen (COL III) (ab184993, Abcam), P-AKT (13038, Cell Signaling Technology), AKT (9272, Cell Signaling Technology) in blocking solution at a compatible dilution. Membranes were treatment with anti-rabbit/anti-mouse secondary antibodies (Abcam) in blocking solution at a dilution of 1:8000 and incubated for 1 h at RT. The bands were detected using an ECL kit (Beyotime BioTech, Shanghai, China) according to the manufacturer’s protocol. The film signals were digitally scanned with ImageQuant LAS 4000 mini machine (GE).
Cell migration
6-well plate plated into 2 × 105 human fibroblasts (HF) and scrape smooth edges with the 200 μl fluid head. The serum-free F-12 medium (500 μl) with exosomes (50 μg/ml ADSCs-exo, HypADSCs-exo) or PBS was added to the well. Shooting with a Lecka inverted microscope after 0, 12, 24 h, Migration area is calculated after 0, 12, 24 h (Leica Microscopy for camera).
Similarly, the the bottom of the transwell plates add to exosomes. HF were plated in serum-free medium in the upper chamber of a Transwell. After 24 h, remove upper membrane cells with cotton swab and rinse. We used 0.1% crystal violet staining and Lecka inverted microscope for photos.
Diabetic wound healing evaluation
BALB/c nude mice (4 weeks old) were obtained from the Second Affiliated Hospital of Harbin Medical University and approved by IACUC and fed with 45% high fat diet for 5 weeks. After fasting for 12 h without food and water, the mice were intraperitoneally injected with streptozotocin (35 mg/kg in 0.1 M citrate-buffered saline, pH 4.5) to induce Diabetes Mellitus (DM). Glucose was measured with blood sugar test paper (Roche, Basel, Switzerland). Glucose level > 16.7 mmol/L indicated DM. To establish a stable animal model of DM, diabetic nude mice were re-fed with high-fat diet for 4 week and blood glucose levels were reconfirmed before wound formation. Anesthesia was performed with intraperitoneal injection of 10% chloral hydrate solution (250 μl/100 g). A square full thick skin injury (0.8 cm × 0.8 cm) was produced on the back of each nude mice. Then diabetic nude mice were randomly divided into three groups, respectively, in which ADSCs-exo (DW + exo), HypADSCs-exo (DW + hexo) (2 mg in 100 μL PBS) or 100 μL PBS (DW) were subcutaneously injected into four mid-points of the wound edge. Normal nude mice (Ctrl) with normal diet and water received the same wound operation. The wound was photographed on days 0, 3, 7 and 14, respectively after surgery to observe the healing process. Image J software was used to analyze wound size on days 0, 3, 7 and 14, respectively.
Histology
Tissue specimens were fixed with 4% paraformaldehyde solution and then embedded in paraffin. Sections were sectioned and cultured overnight with primary antibodies against β-actin, CD31, COLIII, IL-6, or TGF-β overnight at 4 °C and with secondary antibodies (Abcam) for 1 h at 37 °C. The sections were stained with 3,3-diaminobenzidine and counter stained with hematoxylin. Immunofluorescence staining on COLI (1:100; ab34710) was performed. Hematoxylin–eosin (H&E) and Masson's trichrome staining were conducted. The expression levels of COLI, TGF-β, PDGF and VEGF mRNA in tissues of nude mice were detected by qRT-PCR. Get images with a Lecka microscope and randomly select five different fields.
Statistical analysis
All data were used of GraphPad Prism13.0 software and Image J analyses and expressed as mean ± standard deviation (SD). One-way ANOVA and t-test was used for comparison of two or more groups. P < 0.05 was considered to be statistically significant.
Results
Characterization of ADSCs-exo
Under transmission electron microscopy, ADSCs-exo were round vesiculas (Fig. 1A), expressing specific markers HSP70 and CD9 (Fig. 1B), with size of 110 nm (Fig. 1C). We observed red PKH26-labeled exosomes around the nucleus, indicating that ADSCs-exo were internalized by fibroblasts (Fig. 1D).
Expression profiles of miRNAs in ADSCs-exo and HypADSCs-exo
Differential expression profiles of miRNAs were described in Fig. 2. As shown in Fig. 2A, B, clustered heat map and volcano plot of differentially expressed miRNAs depicted up- and down-regulated miRNAs in HypADSCs-exo, compared to ADSCs-exo. 369 miRNAs were downregulated whereas 215 upregulated as presented by Volcano Plot filtering. Downstream genes of differentially expressed miRNAs were analyzed with GO and KEGG. GO enrichment was shown in Fig. 3A, B. The most common biological process was regulation of macromolecule metabolism (GO:0060255). The most enriched cellular component was perinuclear region of cytoplasm (GO:0048471). The most enriched molecular function was binding (GO:0005488). As shown in Fig. 3C, KEGG pathway analysis revealed thyroid hormone signaling pathway as the most enrichment factor.
High-throughput sequencing analysis ADSCs-exo and HypADSCs-exo miRNA differential expression. A Clustered heat map of differentially expressed miRNAs depicting up and down regulated miRNAs. B Volcano plot of differentially expressed miRNAs depicting up and down regulated miRNAs. C, D Schematic representation of the predicted target genes and corresponding cellular functions of the miRNAs (C up-regulated miRNAs and D down-regulated miRNA) senriched in exosomes
HypADSCs-exo promotes fibroblast proliferation and migration in vitro
Based on scratch assay, we found that the migration of HypADSCs-exo component fibroblasts increased significantly (Fig. 4A). Similar result was demonstrated by transwell assay (Fig. 4B). HypADSCs-exo significantly promoted fibroblast proliferation (Fig. 4C).
HypADSCs-exo significantly promote the proliferation and migration of HF. A, B The migration ability of HF treated with HypADSCs-exo, measured by scratch and Transwell test assays. C The proliferation of cells by Edu assays. Data are represented as mean ± SD. n = 3. *P < 0.01, **P < 0.001, ***P < 0.0001. Scale bars, 100 μm.
HypADSCs-exo regulate fibroblast chemokines and extracellular matrix formation
Expression of HypADSCs-exo proteins in fibroblasts was significantly increased (Fig. 5A). ADSCs-exo gene expression of COLI, TGF-β, EGF and bFGF was significantly increased in fibroblasts (Fig. 5B). As a result, HypADSCs-exo regulated the production of extracellular proteins and chemokines in fibroblasts and exerted potential effects on angiogenesis.
HypADSCs-exo promote the expression and secretion of extracellular matrix and growth factors in HF. A Analysis of protein expression levels in treated HF. B QRT-PCR analysis of mRNA levels, including bFGF, PDGF, COLI, EGF. Data are represented as mean ± SD. *, vs ADSCs-exo; #, vs Ctrl. C Western-blot analysis of protein levels of P-AKT induced by different concentrations of control, ADSCs-exo and HypADSCs-exo. Ly294002 inhibit the activation of PI3K/ AKT induced by control, ADSCs-exo and HypADSCs-exo, respectively. D Edu assay showed that HypADSCs-exo-mediated HF proliferation was suppressed by inhibitors Ly294002, compared with ADSCs-exo. Data are represented as mean ± SD. *P < 0.01, **P < 0.001, ***P < 0.0001. Scale bars, 100 μm
Regulation of PI3K/AKT signaling in fibroblast
PI3K/AKT signaling pathway promotes cell proliferation, migration and wound healing. To investigate if PI3K/AKT could regulate behaviors of fibroblasts, we pretreated fibroblasts with Ly294002 (PI3K/AKT inhibitor). Accordingly, AKT phosphorylation induced by HypADSCs-exo was significantly inhibited (Fig. 5C). Upon inhibiting PI3K/AKT signaling, HypADSCs-exo mediated proliferation of fibroblast was significantly attenuated (Fig. 5D). Thus, proliferation and migration of fibroblasts regulated by HypADSCs-exo might be dependent on PI3K/AKT signaling.
HypADSCs-exo accelerate skin wound healing in diabetic mice
We established a diabetic wound model with nude mice. From the images taken, the wound healing of DW-hexo group was significantly better than that of DW-hexo, DW or Ctrl (Fig. 6A). In DW-hexo group, wounds were almost completely closed on day 14. As for wound closure rate, HypADSCs-exo treated wounds contracted much faster than ADSCs-exo treated wounds on days 7 and 14. Additionally, HypADSCs-exo treated skin wounds had complete re-epithelialization and cuticle covering on the epidermis as demonstrated by blue skin fibers (Fig. 6B). By contrast, skin wounds treated with ADSCs-exo exhibited less re-epithelialization.
Observation of wound healing quality and velocity after operation. A After HypADSCs-exo treatment 0, 3, 7 and 14 days were taken. B HE staining of tissue wounds 14 days after operation. Masson staining of tissue wounds 14 days after operation. Data are represented as mean ± SD. *P < 0.01, **P < 0.001, ***P < 0.0001. Scale bars, 100 μm
HypADSCs-exo regulate inflammatory factors, chemokines and extracellular matrix formation in diabetic mice
In wound tissue of DW-hexo group, upregulated expression of collagens and growth factors (TGF-β, PDGF, COLI and VEGF) in skin tissue cells could accelerated wound healing (Fig. 7A). Potential effects of DW-hexo on stromal-related factors CD31, TGF-β, COLI, COLIII as well as inflammatory factor IL-6 in diabetic wound were investigated. Interestingly, expression of CD31, TGF-β, COLI and COLIII was upregulated while IL-6 was downregulated in DW-hexo treatment group at week 2 (Fig. 7B), in comparison with DW-exo, DW or Ctrl (Additional file 1: Figure S1).
Genetic and histological analysis of wound healing in diabetes A QRT-PCR analysis of wound tissue mRNA level 14 days after operation, including COLI, TGF-β, VEGF, PDGF. B, C Expression of CD31, COLIII, IL-6, TGF-β, COLI in wound tissue was observed 21 days after operation. Data are represented as mean ± SD. *P < 0.01, **P < 0.001, ***P < 0.0001. Scale bars, 100 μm
Discussion
Incidence and prevalence of diabetes keep rising globally [30]. Because of slow healing and even non-healing, diabetic wound has brought a great challenge to therapy [31]. Diabetic patients with foot ulcers are 2.5 times more likely to die in 5 years [32]. Therefore, it is necessary to explore molecular mechanisms underlying diabetic wound healing and to identify effective treatment of diabetic wound. Importantly, ADSCs together with conditioned medium may promote skin wound healing and regeneration [33, 34]. Compared with adipose stem cell transplantation, ADSCs-exo has greater potential, and ADSCs-exo under hypoxia has advantages in promoting angiogenesis and bone healing compared with that under normorxia [35, 36]. In this study, we have demonstrated that HypADSCs-exo promotes fibroblast proliferation and migration by activating PI3K/AKT pathway, which can accelerate healing of diabetic wounds.
In the early stage of diabetic healing, cells adapt to hypoxia; while in the late stage, inflammatory response and growth factors directly affect the process of wound healing [37,38,39]. Therefore, we analyzed differential expression of miRNAs and related pathways, cell functions and target proteins between hypoxic exosomes and normoxic exosomes with high-throughput sequencing. 215 miRNAs were upregulated whereas 369 downregulated in hypoxic exosomes compared with normoxic exosomes. Upregulated miR-21-3p/miR-126-5p/miR-31-5p whereas down-regulated miR-99b/miR-146-a might play a role in promoting fibroblast proliferation and migration, as well as in regulating immune response by activating targeted signaling pathways [40, 41]. GO and KEGG indicate regulatory effects of adipose stem cell exosomes on cell metabolism, differentiation and TGF-β secretion under hypoxia, partially through activating PI3K/AKT and MARK pathways. Thus, we propose HypADSCs-exo can accelerate diabetic wound healing. Consistently, HypADSCs-exo can regulate inflammation and extracellular matrix secretion, partially through PI3K/AKT signal pathway, to accelerate wound healing in diabetes. This finding provides a new solution for refractory diabetic wounds.
Fibroblasts are the important effector cells in skin wounds [3]. Skin fibroblasts interact with keratinocytes, adipocytes, mast cells and extracellular matrix (including collagen) [42, 43]. Accordingly, HypADSCs-exo induced proliferation and migration of fibroblasts. HypADSCs-exo also increases production of extracellular matrix and growth factors. PI3K/AKT signaling pathway is involved in regulating cell proliferation and migration, while exosomes can activate PI3K/AKT for survival [44, 45]. Notably, HypADSCs-exo induce AKT fast channels, which can be weakened by Ly294002, suggesting that survival-promoting signals in fibroblasts can weaken HypADSCs-exo fast channels to improve cell viability. ADSCs-hexo induced fibroblast proliferation and migration partially depends on PI3K/AKT pathway. Besides, TGF-β stimulates fibroblast proliferation in coordination with bFGF [46]. EGF as a chemokine promotes fibroblast proliferation and migration [47]. In our study, TGF-β, EGF, COLI, bFGF induced by HypADSCs-exo were increased significantly in fibroblasts. HypADSCs-exo may regulate the expression of multiple growth factors, to promote the proliferation and migration of fibroblasts, as well as angiogenesis.
A diabetic wound model using nude mouse was developed and treated with HypADSCs-exo in vivo. It’s noteworthy that HypADSCs-exo can accelerate high-quality healing of diabetic wounds compared with ADSCs-exo. It’s well-known that extracellular matrix plays a supporting and elastic role in wound healing and regeneration, depending on the secretion of collagen I and III. HypADSCs-exo may regulate extracellular matrix formation, thereby accelerating wound healing in diabetes. The expression of IL-6 was decreased in wound tissue of HypADSCs-exo treated diabetic nude mice, while the expression of VEGF was increased. Our study suggests that HypADSCs-exo may modulate the remodeling of external matrix in diabetic wound healing and thus improve scar fibrosis. These results suggest that HypADSCs-exo may play a key role in extracellular matrix remodeling during wound healing and thus partially improving scar fibrosis. We detected a significant increase in TGF-β, COLI, PDGF and VEGF expression in HypADSCs-exo treated diabetic mice. In this regard, VEGF has the potential to initiate angiogenesis and promote wound healing [48]. PDGF released by platelets binds to fibroblast surface receptors during wound repair [49]. HypADSCs-exo may promote skin regeneration by regulating the secretion and expression of growth factors. Meanwhile, the expression of CD31 was up-regulated, indicating increased angiogenesis. Taken together, HypADSCs-exo may accelerate high-quality healing of diabetic wound, which provides the possibility for clinical application of HypADSCs-exo.
Nevertheless, our study has some limitations. For example, potential effects of specific miRNAs derived from HypADSCs-exo on fibroblasts need further investigation. Moreover, PI3K/AKT signaling may not be the only pathway in HypADSCs-exo that affects fibroblasts, which requires illustration. Furthermore, clinical application of HypADSCs-exo requires more accurate injection dose and time to achieve an optimal therapeutic efficacy.
Conclusion
Collectively, HypADSCs-exo may accelerate the rate of diabetic wound healing, improve the quality of wound healing whereas inhibit inflammation. HypADSCs-exo promotes the proliferation and migration of fibroblasts by activating the PI3K/Akt pathway, and thus enhancing the secretion of vascular growth factors and extracellular matrix. These results provide a new treatment strategy by applying HypADSCs-exo in diabetic wound repair.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- ADSCs-exo:
-
Adipose stem cells exosomes
- HypADSCs-exo:
-
Hypoxic adipose stem cells exosomes
- bFGF:
-
Basic fibroblast growth factor
- COLI:
-
Collegen I
- COLIII:
-
Collegen III
- Ctrl:
-
Control
- DW:
-
Diabetic wound
- DW + exo:
-
Diabetic wound exosomes
- DW + hexo:
-
Diabetic wound hypoxic exosomes
- DMEM/F12:
-
Dulbecco’s Modified Eagle Medium F12
- FBS:
-
Fetal bovine serum
- GO:
-
Gene ontology
- HIF-1α:
-
Hypoxia inducible factor-1α
- HF:
-
Human fibroblast
- H&E:
-
Hematoxylin and eosin
- IRB:
-
Institutional review board
- IL-6:
-
Interleukin-6
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- SDF-1:
-
Stromal cell-derived factor-1
- TGF-β:
-
Transforming growth factor-β
- TEM:
-
Transmission electron microscopy
- P-AKT:
-
Phosphate-AKT
- PMSF:
-
Phenylmethanesulfonyl fluoride
- PDGF:
-
Platelet derived growth factor
- qRT-PCR:
-
Quantitative reverse transcription-polymerase chain reaction
- SD:
-
Standard deviation
- VEGF:
-
Vascular endothelial growth factor
References
Francia P, Anichini R, Seghieri G, De Bellis A, Gulisano M. History, prevalence and assessment of limited joint mobility, from stiff hand syndrome to diabetic foot ulcer prevention: a narrative review of the literature. Curr Diabetes Rev. 2018;14(5):411–26.
Hamed S, Ullmann Y, Masoud M, Hellou E, Khamaysi Z, Teot L. Topical erythropoietin promotes wound repair in diabetic rats. J Invest Dermatol. 2010;130(1):287–94.
Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–46.
Lan CC, Wu CS, Huang SM, Kuo HY, Wu IH, Liang CW, Chen GS. High-glucose environment reduces human β-defensin-2 expression in human keratinocytes: implications for poor diabetic wound healing. Br J Dermatol. 2012;166(6):1221–9.
Pankajakshan D, Agrawal DK. Mesenchymal stem cell paracrine factors in vascular repair and regeneration. J Biomed Technol Res. 2014;1(1):10.
Boateng J, Catanzano O. Advanced therapeutic dressings for effective wound healing—a review. J Pharm Sci. 2015;104(11):3653–80.
Ouyang J, Ji X, Zhang X, Feng C, Tang Z, Kong N, et al. In situ sprayed NIR-responsive, analgesic black phosphorus-based gel for diabetic ulcer treatment. Proc Natl Acad Sci USA. 2020;117(46):28667–77.
Xiao Y, Sun H, Du J. Sugar-breathing glycopolymersomes for regulating glucose level. J Am Chem Soc. 2017;139(22):7640–7.
Ji X, Ge L, Liu C, Tang Z, Xiao Y, Chen W, et al. Capturing functional two-dimensional nanosheets from sandwich-structure vermiculite for cancer theranostics. Nat Commun. 2021;12(1):1124.
Lv X, Walton JH, Druga E, Wang F, Aguilar A, McKnelly T, et al. Background-free dual-mode optical and 13C magnetic resonance imaging in diamond particles. Proc Natl Acad Sci USA. 2021;118(21): e2023579118.
An Y, Lin S, Tan X, Zhu S, Nie F, Zhen Y, et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021;54(3): e12993.
Hu ZC, Chen D, Guo D, Liang YY, Zhang J, Zhu JY, et al. Randomized clinical trial of autologous skin cell suspension combined with skin grafting for chronic wounds. Br J Surg. 2015;102(2):e117-23.
Park BS, Jang KA, Sung JH, Park JS, Kwon YH, Kim KJ, et al. Adipose-derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatol Surg. 2008;34(10):1323–6.
Vu NB, Nguyen HT, Palumbo R, Pellicano R, Fagoonee S, Pham PV. Stem cell-derived exosomes for wound healing: current status and promising directions. Minerva Med. 2021;112(3):384–400.
Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89.
Horvathova L, Padova A, Tillinger A, Osacka J, Bizik J, Mravec B. Sympathectomy reduces tumor weight and affects expression of tumor-related genes in melanoma tissue in the mouse. Stress. 2016;19(5):528–34.
Ding J, Wang X, Chen B, Zhang J, Xu J. Exosomes derived from human bone marrow mesenchymal stem cells stimulated by deferoxamine accelerate cutaneous wound healing by promoting angiogenesis. Biomed Res Int. 2019. https://doi.org/10.1155/2019/9742765.
Yu M, Liu W, Li J, Lu J, Lu H, Jia W, et al. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther. 2020;11(1):350.
Lee Y, El Andaloussi S, Wood MJ. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012;21(R1):R125-34.
Wu J, Wang Y, Li L. Functional significance of exosomes applied in sepsis: a novel approach to therapy. Biochim Biophys Acta Mol Basis Dis. 2017;1863(1):292–7.
Szatanek R, Baj-Krzyworzeka M, Zimoch J, Lekka M, Siedlar M, Baran J. The methods of choice for extracellular vesicles (EVs) characterization. Int J Mol Sci. 2017;18(6):1153.
Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35(4):851–8.
Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820(7):940–8.
Han YD, Bai Y, Yan XL, Ren J, Zeng Q, Li XD, et al. Co-transplantation of exosomes derived from hypoxia-preconditioned adipose mesenchymal stem cells promotes neovascularization and graft survival in fat grafting. Biochem Biophys Res Commun. 2018;497(1):305–12.
Xue C, Shen Y, Li X, Li B, Zhao S, Gu J, et al. Exosomes derived from hypoxia-treated human adipose mesenchymal stem cells enhance angiogenesis through the PKA signaling pathway. Stem Cells Dev. 2018;27(7):456–65.
Liu W, Yu M, Xie D, Wang L, Ye C, Zhu Q, et al. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11(1):259.
Geiger A, Walker A, Nissen E. Human fibrocyte-derived exosomes accelerate wound healing in genetically diabetic mice. Biochem Biophys Res Commun. 2015;467(2):303–9.
Huang J, Yu M, Yin W, Liang B, Li A, Li J, et al. Development of a novel RNAi therapy: engineered miR-31 exosomes promoted the healing of diabetic wounds. Bioact Mater. 2021;6(9):2841–53.
Lv Q, Deng J, Chen Y, Wang Y, Liu B, Liu J. Engineered human adipose stem-cell-derived exosomes loaded with miR-21-5p to promote diabetic cutaneous wound healing. Mol Pharm. 2020;17(5):1723–33.
Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389(10085):2239–51.
Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719–24.
Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet Med. 2016;33(11):1493–8.
Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res. 2010;316(14):2213–9.
Yang JA, Chung HM, Won CH, Sung JH. Potential application of adipose-derived stem cells and their secretory factors to skin: discussion from both clinical and industrial viewpoints. Expert Opin Biol Ther. 2010;10(4):495–503.
Han Y, Ren J, Bai Y, Pei X, Han Y. Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF-R. Int J Biochem Cell Biol. 2019;109:59–68.
Zhang Y, Hao Z, Wang P, Xia Y, Wu J, Xia D, et al. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019;52(2): e12570.
Noor S, Zubair M, Ahmad J. Diabetic foot ulcer—a review on pathophysiology, classification and microbial etiology. Diabetes Metab Syndr. 2015;9(3):192–9.
Braun L, Kim PJ, Margolis D, Peters EJ, Lavery LA, Wound Healing Society. What’s new in the literature: an update of new research since the original WHS diabetic foot ulcer guidelines in 2006. Wound Repair Regen. 2014;22(5):594–604.
Markuson M, Hanson D, Anderson J, Langemo D, Hunter S, Thompson P, et al. The relationship between hemoglobin A(1c) values and healing time for lower extremity ulcers in individuals with diabetes. Adv Skin Wound Care. 2009;22(8):365–72.
Casado-Díaz A, Quesada-Gómez JM, Dorado G. Extracellular vesicles derived from mesenchymal stem cells (MSC) in regenerative medicine: applications in skin wound healing. Front Bioeng Biotechnol. 2020;8:146.
Zhang D, Xuan J, Zheng BB, Zhou YL, Lin Y, Wu YS, et al. Metformin improves functional recovery after spinal cord injury via autophagy flux stimulation. Mol Neurobiol. 2017;54(5):3327–41.
Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48(1):15–24.
Olczyk P, Mencner Ł, Komosinska-Vassev K. The role of the extracellular matrix components in cutaneous wound healing. Biomed Res Int. 2014. https://doi.org/10.1155/2014/747584.
Vrijsen KR, Maring JA, Chamuleau SA, Verhage V, Mol EA, Deddens JC, et al. Exosomes from cardiomyocyte progenitor cells and mesenchymal stem cells stimulate angiogenesis via EMMPRIN. Adv Healthc Mater. 2016;5(19):2555–65.
Kim S, Lee SK, Kim H, Kim TM. Exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. Int J Mol Sci. 2018;19(10):3119.
Piazuelo E, Lanas A, Jimenez P, García-Gonzalez A, Esteva F. In vitro wound repair by human gastric fibroblasts: implications for ulcer healing. Dig Dis Sci. 1998;43(6):1230–40.
Clark RA, McCoy GA, Folkvord JM, McPherson JM. TGF-beta 1 stimulates cultured human fibroblasts to proliferate and produce tissue-like fibroplasia: a fibronectin matrix-dependent event. J Cell Physiol. 1997;170(1):69–80.
Narayanan R, Huang CC, Ravindran S. Hijacking the cellular mail: exosome mediated differentiation of mesenchymal stem cells. Stem Cells Int. 2016. https://doi.org/10.1155/2016/3808674.
Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835–70.
Acknowledgements
The authors thank the scientific research center at the Second Affiliated Hospital of Harbin Medical University for technical support. They also extend a special thanks to the Experimental Animal Center of the Second Affiliated Hospital of Harbin Medical University for helping with our animal experiments.
Funding
This work was sponsored by the National Natural Science Foundation of China (Grant No. 81471796) and Excellent Youth Foundation of Heilongjiang Province of China (Grant JC2017019).
Author information
Authors and Affiliations
Contributions
ZX, JW and HW designed and performed experiments. JW and HW contributed to data compilation and paper preparation. ZX, JW, HW, YP, YQ, YZ provided critical feedback on the study and contributed to the preparation of the paper. ZX and JW devised the study and oversaw the research program. All authors listed reviewed the paper and provided feedback. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the Second Hospital of Harbin Medical University and conducted in accordance with the 2000 Helsinki Declaration.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
(A) ROS generation was evaluated by fluorescence microscopy, and a stronger red fluorescence intensity indicates a higher production of ROS. (B) HIF-1α protein levels increased after HypADSCs-exo treatment of HF. Data are represented as mean ± SD. n = 3. *P < 0.05, **P < 0.01. Scale bars 100 μm.
Additional file 2: Table S1.
Primer sequences of the study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, J., Wu, H., Peng, Y. et al. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J Nanobiotechnol 19, 202 (2021). https://doi.org/10.1186/s12951-021-00942-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-021-00942-0