Abstract
Chronic low-grade inflammation has been identified as a major contributor in the development of atherosclerosis. Nuclear Factor-κappa B (NF-κB) is a critical transcription factors family of the inflammatory pathway. As a major catalytic subunit of the IKK complex, IκB kinase β (IKKβ) drives canonical activation of NF-κB and is implicated in the link between inflammation and atherosclerosis, making it a promising therapeutic target. Various natural product derivatives, extracts, and synthetic, show anti-atherogenic potential by inhibiting IKKβ-mediated inflammation. This review focuses on the latest knowledge and current research landscape surrounding anti-atherosclerotic drugs that inhibit IKKβ. There will be more opportunities to fully understand the complex functions of IKKβ in atherogenesis and develop new effective therapies in the future.
Similar content being viewed by others
Introduction
Arteries are the conduits that transport blood from the heart to tissues and organs [1]. The artery wall, such as aortic wall, is made up of three layers from inside to outside: the intima, media, and adventitia. The intima consists mainly of a single layer of endothelial cells (ECs) and a thin basal membrane that acts as a barrier to prevent the leakage of blood components into the vessel wall. The media layer regulates the artery elasticity, which is primarily composed of smooth muscle cells (SMCs). The adventitia refers to the connective tissue covering the outer layer [2]. Increased thickness of the vessel wall, stenosis, or occlusion of the lumen may lead to ischemia and dysfunction of tissues and organs. Atherosclerosis is a common cause of ischemic diseases (such as stroke and myocardial ischemia) with high morbidity and mortality worldwide [3]. It is characterized by the formation of atherosclerotic plaques in the intima of large or medium-sized systemic arteries.
It is well established that atherosclerosis is not only a metabolic disorder, but also a chronic low-grade sterile inflammation in the vasculature orchestrated by a network of inflammatory cytokines. ECs, macrophages and migratory SMCs from the media layer are the major cellular components of atherosclerotic lesions [4,5,6]. These cells cooperate to initiate the inflammatory signal and to upregulate the adhesion molecule expression and athermanous plaque is finally formed [7]. Recently, it has demonstrated that nuclear factor-κappa B (NF-κB) is closely related to atherosclerosis-associated inflammation [8, 9]. The researchers found that NF-κB was activated in the key components of atherosclerotic plaques, including ECs, macrophages, and SMCs [10,11,12]. IκB kinase β (IKKβ), the predominant catalytic subunit of the IKK complex [13], is required for the canonical activation of NF-κB, which also known as a critical molecular link between inflammation and atherosclerosis [14]. Fortunately, a variety of natural product-based derivatives, natural extracts, synthetic drugs, as well as peptides et.al other drugs, all display anti-atherogenic potential by inhibiting IKKβ-mediated inflammation, which may be potential therapy medicine for atherosclerosis. As a result, this review provides the current findings on IKK and atherosclerosis, as well as outlines therapeutic interventions used to target IKK for the treatment of atherosclerosis.
Inflammation and atherosclerosis
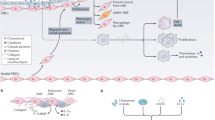
Atherosclerosis, a chronic inflammatory disease of the vessel wall, is characterized by the accumulation of lipid-laden macrophages and fibrous material in the large or medium-sized systemic arteries [15]. A key initiating event is the retention of ApoB-containing lipoprotein particles under the endothelial layer of the arterial wall [16]. There is overwhelming evidence that a transgenic expression of a natural antibody to oxidized phospholipids suppresses lesions in hypercholesterolemic low-density lipoprotein receptor knock out (LDLR−/−) mice, which supports the lipid oxidation hypothesis for atherosclerosis [17]. What's more, as a result of the disturbances in blood flow, the ECs are activated, and the tight junctions between them become "leaky", which facilitates the trans-endothelial transport of plasma LDL and TG-rich lipoproteins or diffusion at cell–cell contact points, and reaches the intima [18]. As a matter of fact, the subsequent activation of ECs is caused by the oxidation of lipoprotein lipids and other mediators of inflammation, which leads to the expression of vascular cell adhesion molecule 1 (VCAM-1), intercellular cell adhesion molecule-1 (ICAM-1), and E-selectin, as well as promotes the adhesion of monocytes and chemokines, and inflammation occurs [19]. This process involves many cells and cytokines, such as ECs, macrophages, lymphocytes (T and B cells), dendritic cells (DCs), interleukin family, adhesion molecules, and TNF-α [20]. Immediately afterwards, monocytes are recruited to the vessel wall and enter the intimal, which are stimulated by a macrophage-stimulating factor (M-CSF) and other cytokines to differentiate into macrophages [21]. In response to local microenvironmental signals, macrophages acquire functionally distinct phenotypes, including the pro-inflammatory M1-like phenotype, and the pro-resolving M2-like phenotype [22]. Macrophages contribute very importantly to lesion progression, in ApoE−/− mice, the number of macrophages in early atherosclerosis is determined by recruitment; in more advanced lesions, however, it was largely dependent on proliferation of local macrophages, rather than monocyte [23, 24]. At the same time, in response to lipid oxidation, LDL transforms into ox-LDL, which is scavenged by monocyte receptors upon infiltration, converting monocytes into lipid-filled macrophage foam cells [25]. With the lesion progresses, SMCs in the media transform from a contractile to a proliferative state, and migrate into the intima [26]. Eventually, the intimal SMCs secrete an extracellular matrix mainly composed of collagen, forming a fibrous cap to protect against plaque rupture. A study of lineage tracing shows that the intimal SMCs can differentiate into macrophage-like and osteochondrogenic descendants [27]. In the presence of lipid, macrophage-like SMCs can produce foam cells, accumulated foam cells undergo apoptosis and inhibited efferocytosis [28]. It is inevitable that some apoptotic foam cells may escape efferocytosis and contribute to the formation of necrotic lipid cores, causing secondary necrosis and inflammation [29] (Fig. 1).
The key inflammatory mechanisms involved in the development of atherosclerosis. Monocytes are first recruited to developing plaques by VCAM-1, ICAM-1, and E-seletin. Then, monocytes differentiate into macrophages, and reactive oxygen species (ROS) from vascular lumen accumulates oxidizes LDL (ox-LDL). Ox-LDL is mostly taken up by macrophage scavenger receptors and becomes foam cells. Macrophages and foam cells secrete inflammatory cytokines, such as IL-6, IL-12 and TNF-α, which in return exacerbates the inflammatory response. Inflammatory cytokines secrete matrix metalloproteinases (MMPs), which degrades the fibrous plaque. This could lead to plaque rupture and thrombosis. In addition, inflammatory cytokines promote the proliferation and migration of smooth muscle cells (SMCs), which contributes to the formation of fibrous cap. Foam cell apoptosis promotes plague rupture
The role of IKKβ/NF‑κB in the development of atherosclerosis
The NF‑κB signaling pathway consists of NF‑κB, the inhibitor of Kinase B (IκB), the IκB kinase (IKK) complex and IKK upstream kinases [30]. There are two main pathways involved in NF-κB activation, namely the canonical (classic) and the non-canonical pathways [31]. The canonical NF-κB pathway is present in most cell types. The most abundant forms of NF-κB activated by the typical pathway are the heterodimers of p50 and p65 [32]. In the resting state, its binding to IκB keeps NF-κB in inactive form in the cytoplasm when nuclear translocalization signals [33, 34]. When cytokines, such as TNF-α, IL-1, and lipopolysaccharide (LPS), attach to their receptors, TNFR, IL-1R, and toll- like receptor (TLR), respectively, IKK is activated (Fig. 2) [35]. Then, IKK induces phosphorylation of IκB on Ser32 and (or) Ser36, and subsequent polyubiquitination. As a result, NF-κB dissociates from the NF-κB/IκB complex, and translocates to nucleus, where it stimulates the transcriptions of cytokines and cell adhesion molecules. IKK consists of two catalytic subunits, IKKα and IKKβ, and an NF-κB essential modifier (NEMO), also known as IKKγ [36]. There is an NEMO-binding domain (NBD) at the C-terminus of IKKα and IKKβ, which mediates the formation of the IKK complex. Although IKKα and IKKβ have similar structural features, they work in different ways. During activation of the canonical pathway, IKKβ is the dominant kinase promoting phosphorylation of IκB on Ser32 and Ser36, instead of IKKα [37]. Once the serine on the activation loop of IKKβ is mutated to alanine, TNF-α, IL-1, and LPS all fail to activate NF-κB [38]. In contrast, the same mutation in IKKα failed to reveal a similar effect [39]. In summary, the IKKβ/NF-κB pathway plays a pivotal role in pro-inflammatory responses, and therefore IKKβ inhibitors may be an effective target in modulating NF-κB activity.
The Activation and regulation of IKKβ/NF-κB pathway. ①The binding of LPS to TLR4 recruits TIRAP. Then MyD88 joins the comlex which is bound by IRAKs and TRAF6 to activate IKKβ. ② MyD88 is recruited upon binds of IL-1 to IL-1RI. Then, IRAK1 comjoins the complex and TRAF6 also assemble to IKK complex. ③ The bingding of TNF with TNFR leads to the binding of TRADD, TRAF2 with the protein kinase RIP1, which forms a platform for the recruitment of TRAF2. When ubiquitinated RIP1 bindings to NEMO, it phosphorylates and activates IKKβ. ④ ROS from vascular lumen interact with various elements of the IKK/NF-κB signaling pathway. On the other hand, the phosphorylation of p65 in which ROS are involved leads to a greater activation of NF-κB. ⑤ The binding of ang II induces endoplasmic reticulum (ER) stress, activates and phosphorylates inositol-requiring 1α (IRE1α). Phosphorylated-IRE1α (p-IRE1α) recruits TRAF2, and then activates IKKβ. In the canonical pathway, IκBα is phosphorylated in an IKKβ- and NEMO(IKKγ)-dependent manner, which results in the nuclear translocation of mostly p65 or p50-containing heterodimers. Then, IκB degradates. The transcriptional p65 and p50 stimulate the production of inflammatory cytokines and finally monocyte adhesion, endothelial dysfunction, inflammatory, SMCs proliferation and migration, apoptosis, and oxidative stress all ensue. TIRAP: Toll/IL-1 receptor adaptor protein; MyD88: myeloid differentiation primary response gene 88; TRAF6: TNF-R-associated factor 6; IRAKs: IL-1 receptor-associated kinases; TRADD: TNF-R-associated death domain; RIP1: receptor-interacting protein 1; TRAF2: TNF-R-associated factor 2
During atherosclerosis, NF-κB activation is dependent on IKKβ rather than IKKα [40]. A small peptide mimicking NBD structure was synthesized and introduced into cells, which significantly inhibited NF-κB activity. In diabetic mice, NBD peptide attenuated NF-κB activation and markedly reduced the size of atherosclerotic plaques by inhibiting IKK complex formation [41]. Similarly, ebselen reduced atherosclerotic lesions in the aorta by inhibiting the phosphorylation of IKKβ, thus abandoned NF-κB activation in diabetic ApoE−/− mice [42]. Furthermore, myeloid-specific IKKβ deficiency alleviated atherosclerosis in LDLR−/− mice [40]. IKKα deficiency did not attenuate atherosclerosis, but only haematopoiesis in ApoE−/− mice [43]. However, some studies treported different conclusion. For instance, IKKβ deficiency did not affect atherosclerotic lesion size, rather it promoted plaque vulnerability and lesional inflammation in obese LDLR−/− mice [44].
Despite some conflicting views, the anti-inflammatory therapy targeting IKKβ is regarded as a effective way in ameliorating atherosclerosis. A variety of drugs, especially natural products-based derivatives, natural extracts, and synthetic drugs, have been shown to inhibit IKKβ, therefore could be candidate drugs to treat atherosclerosis.
The role of IKKβ in key cell types and the influence on atherosclerosis
Endothelial cells IKKβ and atherosclerosis
In atherosclerotic plaques, IKKβ/NF-κB signaling induced by ox-LDL is activated in ECs as well as in vulnerable plaques [45,46,47]. What’s more, the activation of endothelial IKKβ stimulates monocyte infiltration into the arterial intima, thereby exacerbating atherosclerosis [47]. Shear stress [48], TNF-α [49], IL-1β [50], LPS [51], high glucose [52], and insulin resistance [52] are all related to the activation and high expression of IKKs in ECs. ECs are continuously exposed to the shear caused by blood flow, which activates NF-κB that is mediated by integrin/Flk-1, a receptor for VEGF (vascular endothelial growth factor)/IKK pathway [48]. Tripartite motif-containing 14 (TRIM 14) is positively regulated by TNF-α, IL-1β, and LPS, which in turn active NF-κB to form a positive feedback, and drives endothelial activation via the interaction between TRIM14 and NEMO [53]. Notably, TRIM 14 promotes endothelial activation by activating NF-κB to involve in the development of human atherosclerosis [53]. High glucose-induced endothelial dysfunction is accompanied by increased expressions of inflammatory cytokines and adhesion molecules, and adhesion molecules. Endothelial-monocyte adhesion is mediated by the CIKS (connection to IKK and SAPK/JNK), an upstream regulator of NF-κB [54]. Additionally, silver nanoparticles (AgNPs), a potentially hazardous factor for early atherosclerosis, were found to induce HUVECs impairment and dysfunction by activating the IKK/NF-κB pathways [55]. When IKKβ is persistently activated by expressing the dominant interfering mutant, most NF-κB target genes are maximally induced in human microvascular endothelial cell line-1 [56]. The opposite result was observed with dominant negative IKKβ or blocking IKKα/β in response to low shear stress in ECs [57].
Macrophages IKKβ and atherosclerosis
Macrophages are known to play a major role in the development of atherosclerosis, which are not only the major pro-inflammatory cells, but also the essential cellular components of atherosclerotic plaques [58]. Phagocytic macrophages engulf large amounts of ox-LDL and transform into foam cells, which is a hallmark of atherosclerosis [59]. In the plaque microenvironment, there is a vicious cycle between macrophage infiltration and pro-inflammatory factor release [60]. Ox-LDL-activated macrophages upregulated the expression of IKKα and IKKβ, and similar results were found in macrophages induced by LPS in vitro [61, 62]. Clinical studies have shown that obstructive sleep apnea, characterized by intermittent hypoxia, is an independent risk factor for atherosclerosis, especially premature atherosclerosis. It is worth mentioning that its mechanism is closely related to the activation of IKKβ-dependent NF-κB pathway in murine macrophages [63, 64]. Further, excessive nutrition input activated the IKK/NF-κB signaling pathway and inflammation in macrophages, which was strongly attenuated by major vault protein (MVP), an upstream inhibitor of IKK [65].
Vulnerable atherosclerotic plaques are prone to become culprit plaques that cause acute coronary syndromes (ACS), such as acute myocardial infarction, a serious complication of atherosclerosis [66]. Histone deacetylase 9 (Hdac9), a member of the histone deacetylase II family, catalyzes the deacetylation of histone H3K16ac and other non-histone proteins, contributing to atherosclerosis and inflammation [67]. By activating IKK, Hdac9 increases lesional macrophage content and promotes vulnerable plaque formation, whereas hematopoietic Hdac9 knockout promotes the opposite role outcome [68]. Park SH, et al. generated myeloid-specific IKKβ-deficient LDLR−/− mice and found that the lack of IKKβ in macrophage attenuated high-fat diet-induced atherosclerosis in LDLR−/− mice mainly by alleviating inflammatory responses of macrophages [40]. Moreover, chronic uremia promoted atherosclerosis in uremic apoE−/− mice by promoting endoplasmic reticulum (ER) stress-related inflammation, including activating ER stress induced inflammation via activating IKK phosphorylation [69]. Phosphorylated inositol-requiring 1α (p-IRE1α) is an ER stress marker protein expressed mainly in macrophages from atherosclerotic lesions. IRE1α‑siRNA inhibited inflammation and IKK phosphorylation in Ang II-treated RAW264.7 macrophages, thereby suppressing IκB degradation and NF-κB p65 nuclear translocation [69]. Activation of renin-angiotensin system (RAAS) also aggravated atherosclerosis in experimental renal failure apoE−/− mice and upregulated IKK phosphorylation in Ang II-stimulated RAW264.7 macrophages. It suggested that the IKK/NF-κB pathway promotes ER stress-induced inflammation and atherosclerosis [70].
Vascular smooth muscle cells IKKβ and atherosclerosis
During atherogenesis, VSMCs undergo a phenotypic transformation from contractile to synthetic upon the induction of reprogramming transcription factors, such as Krüppel-like factor4 (KLF4) and Octamer-binding transcription factor (OCT4) [27]. Synthetic VSMCs acquire the capacity to proliferate and migrate from the media into intima at the sites of plaques [71]. What’s more, VSMCs synthesize most of the interstitial collagens that stabilize the fibrous caps of plaques [72]. Unlike macrophages, VSMCs transform into a pro-inflammatory phenotype similar to macrophages, acting as both targets and sources of inflammatory factors [26, 73].
Similarly, an activated IKKβ-NF-κB axis has been observed in VSMCs from human atherosclerotic lesions [74]. In vitro, IL-1β-induced proliferation of VSMCs in human saphenous veins via IKKβ activation, which was attenuated by transfection of inactive IKKβ mutants [75]. Oxygen free radicals play a key role in atherogenesis by activating NF-κB in VSMCs, which associated with IKKβ-induced degradation of IκB [76]. Similar results were obtained in VSMCs stimulated by LPS or IL-1β in vitro [77]. IKKβ knockout in VSMCs by the SM22Cre-IKKβ-flox system lead to significant inhibition of vascular inflammation and atherosclerotic plaques in LDLR−/− mice [14, 40]. Furthermore, IKKβ knockout in VSMCs induced by U0126 and SB202190 (inhibitors of p42/p44 MAPK) inhibited cytosolic phospholipase A2 (cPLA2) expression, which exacerbated the atherosclerosis-related vascular inflammation [78].
Anti-inflammatory therapy targeting IKKβ in atherosclerosis
Natural product-based derivatives
Vinpocetine, a derivative of the alkaloid vincamine, is one of the most commonly prescribed medicines for the treatment of cerebrovascular disease and cognitive impairment in many countries [79]. The results of a study revealed that vinpocetine inhibits atherosclerosis in ApoE−/− mice by targeting the Akt/NF-κB receptor dependent pathway [80]. In addition, it has also been shown that vinpocetine is an IKK inhibitor, which inhibits IKK with an IC50 value of approximately 17.17 μM, thereby suppressing the NF-κB-dependent inflammation [81]. A growing body of evidence suggests that vinpocetine is anti-inflammatory in a variety of cell types by directly targeting of IKKβ, including ECs, VSMCs, and monocytes/macrophages [82].
Metformin is a biguanide developed from the guanidine derivative galegine found in Galega officinalis (French lilac), widely used for the treatment of type 2 diabetes mellitus [83]. According to preclinical and clinical studies, metformin has anti-inflammatory properties and performs a protective role in cardiovascular disease, including atherosclerosis [84, 85]. In the atherosclerosis model of rabbits, metformin impeded the atherosclerosis progression, which might be related to inhibiting the adhesion molecules and inflammatory factors by blocking the IKKβ/NF-κB translocation [86]. What's more, there is the conclusive evidence that metformin suppressed the TNF-α–induced phosphorylation of the upstream kinase site p176/17738 on IKKα/β [87]. A study reported that metformin pretreatment (100 ~ 1000 mmol/L) inhibited IKKα/β phosphorylation, IκB degradation, and ultimately IL-6 production in TNF-α-induced HUVECs via the PI3K-dependent AMPK phosphorylation [88].
Naringin is a plant-derived flavonoid, found inmany plants such as grape, citrus species, and fractus aurantii, which has potential for preventing atherosclerosis [89]. In ApoE−/− mice fed a high-fat diet, naringin significantly alleviated atherosclerosis and reduced the serum and liver cholesterol levels by 24.04 and 28.37%, respectively [90]. Interestingly, in TNF-α-stimulated HUVECs, naringin suppressed the activation of NF-κB by inhibiting IKKβ activity [91, 92]. What's more, in a dose-dependent manner, naringin appears to reduce the risk of atherosclerosis by inhibiting the adhesion of THP-1 monocytes to TNF-α-stimulated HUVECs [91, 92].
Emodin is an anthraquinone derivative, naturally occurring in oriental herbs, with diverse biological properties [89]. It has been demonstrated by experimental studies that emodin is capable of attenuating and stabilizing atherosclerotic plaques [93]. Another study found that emodin exhibited inhibitory effects on LIGHT-induced macrophage migration, which was the result of NF-κB activation by NADPH oxidase p47 (phox), suggesting that its anti-atherosclerosis effect was attributed to interventing the IKK [94]. Additionally, emodin inhibited TNF-α-induced activation of NF-κB in rat aortic VSMCs and dose-dependently reduced inflammatory factor gene expression, supporting its anti-atherogenic effects [95].
Green tea polyphenols consist of more than 30 phenolic substances, the main components of which are catechins and their derivatives [96]. Extensive laboratory and epidemiological studies have demonstrated that green tea polyphenols reduce the risk of cardiovascular disease in both animals and humans [97, 98]. As a result of pretreatment with green tea polyphenols, oxLDL-induced proinflammatory cytokine TNF-α and NF-KB activation was reduced by inhibiting the IKK activity in a dose-dependent manner [99]. Stybenpropol A, a resin secreted from the styrax tonkinensis bark, has a protective effect on the vascular endothelium [100]. In vitro, stybenpropol A blocked the monocyte migration, as well as adhesion to TNF-α-induced HUVECs when it inhibited the IKK/NF-κB pathway [100]. Methyl-β-cyclodextrin (MβCD), a cyclodextrins derivative, due to its high affinity for cholesterol, it is one of the most effective agents for removing plasma membrane cholesterol [101]. By downregulating adhesion molecule expression via the LPS/IKK/NF-κB pathway, MβCD may be able to inhibit monocyte endothelial adhesion, which indicates MβCD may have anti-atherosclerosis effects [51].
Natural extracts
Tanshinone IIA is a main lipophilic component derived from the root extract of Salvia miltiorrhiza, which has been widely used in traditional Chinese medicine for the treatment of cardiovascular diseases [102, 103]. According to a study, Tanshinone IIA downregulated the NF-κB activity, and reduced the expression of TNF-α and MCP-1, to stabilize vulnerable atherosclerosis plaque in ApoE−/− mice [104]. What’s more, Cheng-Chieh Chang et al. found that tanshinone IIA (1 ~ 20 µM) inhibited the adhesion of THP-1 monocytes to HUVECs in response to TNF-α stimulation by downregulating IKK/NF-κB mediated VCAM-1, ICAM-1 and fractalkine expression in HUVECs [105]. There is a kind of polyphenol, quercetin, exerts anti-inflammatory effects and contributes to progression of atherosclerosis [106]. There is increasing evidence that both in hypercholesterolemic diet-induced rabbits and high-fat diet fed ApoE−/− mice, quercetin is effective in slowing the progression of atherosclerosis [107, 108]. Similarly, another study demonstrated that in vitro and in vivo, quercetin reduced both VCAM-1 and E-selectin expression, as well as IKK gene expression implicated in local vascular inflammation, with a significant reduction (40%) in the atherosclerotic plaque [109].
Myricetin, also known as alias myricetin, myricetin, is a bark extract from Myrica rubra Sieb. et Zucc, has been found to have vascular protective properties [110]. With the development of medical research, the anti-inflammatory and anti-atherogenic properties of myricetin have been reported successively [111,112,113]. It has been shown that myceritin significantly reduced the plaque area in the aortic root of LDLR−/− mice, as well as improved ox-LDL-induced cholesterol accumulation in macrophages in these mice [114]. Furthermore, according to an early study, myricetin inhibit monocyte adhesion to TNF-α-mediated ECV304 cells (a type of HUVECs) by strongly inhibiting IKK and its downstream signaling NF-κB/IκB [115]. The root of clematis mandshurica is used as anti-inflammatory agent in Chinese pharmacopoeia [116]. Clematichinenoside (a triterpene saponin), extracted from the root of clematis mandshurica, is beneficial in the early stage of atherosclerosis [117]. According to a study, clematichinenoside inhibits VCAM-1 and ICAM-1 expression in TNF-α-treated ECs via the NADPH oxidase-dependent IκB/NF-κB pathway [118].
There is an active bioactive diterpene lactone called andrographolide (AP) ectracted from andrographis paniculata, which has the biological functions, including anti-inflammation, anti-atherosclerosis, and hypoglycemic activities [119]. It is clear from a study that AP is a novel NF-κB Inhibitor, which inhibits the proliferation of VSMCs in atherosclerosis [120]. Another study showed that AP downregulated ICAM-1 expression in TNF-α-treated EA.hy926 cells (HUVECs fusion cell), at least partly by reducing the activation of IKK, indicating a cardioprotective role. Avenanthramide-c, a unique soluble polyphenol, is extracted from oats [121]. As a result of oat bran diets, atheroma lesions are reduced, and high levels of avenanthramides further reduce aortic lesions [122]. An immunofluorescence assay showed that avenanthramide-c reduced the translocation of NF-κB from the cytoplasmic region to the nucleus, and down regulated the expressions of IκB and p-IκB in TNF-α activated human arterial smooth-muscle cells (HASMCs) [123]. Moreover, avenanthramides, a unique polyphenol from oats, decreased the IL-1β-induced proinflammatory cytokines, such as IL-6, IL-8, and MCP-1, in human aortic endothelial cells (HAECs), at least in part by blocking IKK phosphorylation [124].
Cardiac glycoside digitoxins are natural steroid compounds originally exacted from Digitalis sp, there is strong evidence that cardiac glycoside digitoxin is a potent anti-inflammatory agent [125]. Digitoxin inhibits monocyte adhesion to endothelial monolayers, which is associated with inhibiting the IL-1β-induced NF-κB signaling at the level of TAK-1/IKK [126]. Kansuinine A is extracted from Euphorbia kansui L., a well-known medicinal plant in China [127]. There is a study that confirms the anti-atherosclerotic properties of Kansuinine A by inhibiting the IKKβ/IκBα/NF-κB signaling in atherogenic animals and H2O2-stumilated HAECs [42, 128]. Honokiol is a small-molecule polyphenol that is extracted from the Chinese herbal medicine Magnolia officinalis, which has a number of pharmacological properties [129]. There is overwhelming evidence that honokiol suppresses inflammation and oxidative stress in the carotid arteries, inhibiting the formation of atherosclerotic plaque [130]. Surprisingly, in palmitic acid-inducted HUVECs, the expression of NF-KB subunits (p50 and p65), as well as IκB phosphorylation in the IKK/IκB/NF-κB signaling, was significantly inhibited by honokiol [90, 131].
Longxuetongluo Capsule (LTC) is a new drug consisting of the total phenolic extract of Chinese dragon blood. It is believed that Longxuetongluo capsules inhibit monocyte adhesion to the HUVECs through the MAPK/IKK/IκB/NF-κB signaling, thereby reducing atherosclerotic lesions in the aortic sinus of ApoE−/− mice. Pulvones A and C were newly discovered isoflavones from Millettia pulchra, a renowned anti-inflammatory herbal medicine from southeast China [132]. In LPS-stimulated RAW264.7 macrophage cells, pulvones A and C decreased IL-6 and IL-1β expression, reduced the nuclear translocation of NF-κB (p65), and interrupted IκB phosphorylation by directly inhibiting the IKKβ kinase activity (40% inhibition), all of which were validated by docking studies [133].
Acetyl-11-keto-β-boswellic acid (AKBA), the main pharmacological component of Boswellia extract, is considered to be a natural inhibitor of the pro-inflammatory transcription factor NF-κB, exerting powerful anti-inflammatory and antioxidant effects [134]. As a result, AKBA significantly downregulated many NF-κB-dependent genes, including MCP-1, MCP-3, MIP-2, IL-1, VEGF and tissue factor (TF), as well as IKK activity, and resulted in a significant 50% reduction in the size of atherosclerotic lesions in LPS-injected apoE−/− mice; furthermore, similar anti-inflammatory effects were found in LPS-stimulated mouse macrophages and mononuclear cells as well as human macrophages [135]. Ginsenoside Re, a major pharmacological active ingredient of ginseng, has been reported to be a potential therapeutic molecule for atherosclerosis and one of the most promising IKK-β inhibitors [136]. Ginsenoside Re inhibited IKKβ phosphorylation, NF-κB activation, and the expression of proinflammatory cytokines TNF-α and IL-1β in LPS-stimulated peritoneal macrophages, but had no effect on TNF-α-stimulated peritoneal macrophages [137].
Natural pentacyclic triterpenoids (PTs), ursolic acid (UA), and corosolic acid (CA) exhibit a wide range of biological activities, such as anti-inflammatory and cardio-protective effects, which are closely related to particularly the regulation of the NF-κB signaling pathway [138]. According to a hotspot kinase assay and in vitro experiments, UA and CA inhibited IKKβ and down-regulated the proteins expression of IKKβ/NF-κB cascade in LPS-stimulated RAW 264.7 cells, indicating that IKKβ is the main target of PTs-induced NF-κB inhibition [139]. Black pepper (Piper nigrum L.) is commonly used in cooking and traditional medicine in several countries and has been shown to be beneficial in atherosclerosis [140]. Pipernigramides (42–44), a new piperic ester isolated from black pepper EtOH extract, significantly inhibited inducible nitric oxide synthase (iNOS)-mediated release of NO, IL-1β, IL-6, TNF-α, and PGE2 in LPS-stimulated RAW 264.7 cells by targeting IKK-β [141].
Synthetic drugs
Sulforaphane (SFN) is a phytocompound belonging to the isothiocyanate family isothiocyanate derived from cruciferous vegetables, such as broccoli [142]. The aortic histopathologic examination confirmed that SFN significantly reduced the expression of NF-κB in the aortic tissue of fed high cholesterol diet (HCD) rabbits [143]. Due to the inhibition of RhoA/ROCK/NF-κB signaling in human endothelial cells ECV-304, SFN attenuated TNF-α-induced ICAM-1 expression, as well as IKK phosphorylation, suggesting a beneficial role in the atherosclerosis-related inflammation [144,145,146]. Furthermore, SFN also downregulated endothelial lipase expression by inhibiting NF-κB in the same cellular model, which favored HDL cholesterol levels [91]. A hydrophilic vitamin obtained through diet, vitamin C, also known as ascorbic acid, is synthesized by all plants and most animals [147]. Excitingly, according to a clinical study, supplementing with vitamin C can prevent atherosclerosis by improving vascular reactivity and structure in passive smokers [148]. furthermore, vitamin C inhibits NF-κB activation by activating p38 mitogen-activated protein kinases in ECV304 and HUVECs induced by IL-1, PMA, H2O2, TNF, and IFN-γ [149].
As a micronutrient, zinc is essential for human health, which plays a variety of biological roles, such as aiding in growth, metabolism, and immunity [150]. There are evidence that zinc deficiency has a negative role in atherosclerosis in both animal studies and epidemic research [151]. Prasad et.al found that zinc increased A20 and A20–TNF-receptor associated factor-1 complex, decreased inflammatory cytokines by the IKKα/NF-κB signaling pathway, downregelated in HL-60, HUVECs, and SW480 cell lines [152]. 1-deoxynojirimycin, a unique polyhydroxy alkaloid, is the main active component of mulberry (Morus indica L.) leaves and has been found to prevent coronary heart disease (CHD) at least in part by inhibiting the IKK/NF-κB pathway [153]. Similarly, a placebo-controlled, double-blind clinical trial clarifies how 1-deoxynojirimycin does attenuate atherosclerotic lesions in patients with coronary heart disease [153]. Ebselen is a synthetic, organo-selenium radical scavenger compound that functions similarly to glutathione peroxidase [154], which exerts antiatherogenic effects by modulating the transcription factors NF-κB [42].
Polyethylene glycol-superoxide dismutase is an important modifier of SOD that protects ECs [155]. Prostaglandin A1, an anti-inflammatory cyclopentenone prostaglandin, is biosynthesized via dihomo-γ-linolenic acid. Treatment with polyethylene glycol-superoxide dismutase and prostaglandin A1 prevented homocysteine-induced activation of IKK kinase and NF-κB in HUVECs and HAECs [156]. Fatty acid binding protein (FABP) 4/5 is predominantly expressed in macrophages and/or adipocytes and plays essential roles in energy metabolism, inflammation and atherosclerosis [157]. A previous study in patients with angiographically proven coronary artery disease (CAD) showed that FABP 4 plays a critical role in the activation of mononuclear cells and the dysfunction of ECs in atherosclerosis. Interestingly, FABP 4/5 inhibitors, such as compounds A16 and B8, apparently reduced the levels of TNF-α and MCP-1 by inhibiting the IKK/NF-κB pathway, exhibiting anti-inflammatory effects in LPS-stimulated RAW264.7 macrophages [157].
Early reports demonstrated that 8-tosylamino quinolone, a kind of a representative IKK inhibitor (BAY11-7082) analog, has anti-atherogenic effects [158]. Further studies revealed that BAY11-7082 diminished NO, TNF-α, IL-1β, IL-6, and PGE2 production, as well as NF-κB and IKK activation in LPS-activated RAW264.7 cells and peritoneal macrophages in a dose-dependent manner by inhibiting the Akt/IKK/NF-κB pathway [159]. In vivo, losartan, an angiotensin converting enzyme inhibitor, was found to significantly attenuate aortic atherosclerosis, inhibit ER stress, and reduce aortic inflammation in uremic apoE−/− mice; in vitro, losartan inhibited the upregulation of GRP78 in Ang II-stimulated RAW264.7 macrophages and IKK and IκB phosphorylation [70]. It has been suggested that losartan has a protective effect against atherosclerosis in patients with uremic symptoms.
TMP195, a class IIa histone deacetylase inhibitor, reduced the characteristics of plaque vulnerability, thereby enhancing plaque stability in advanced lesions. In addition, transcriptional profiling studies revealed that TMP195 reduced expression of target genes of NF-κB in advanced lesions by inhibiting IKKβ [68]. 9-(2-chlorobenyl)-9H-carbazole-3-carbaldehyde (LCY-2-CHO), an agonist of NRF2, inhibited the inflammatory responses in cultured rat aortic VSMCs. By inhibiting IKK phosphorylation and IκBα degradation, LCY-2-CHO reduced IL-1β-induced inflammatory mediators, such as cyclooxygenase-2 (COX-2) and IL-8 [160]. Based on its anti-inflammatory properties in VSMCs, LCY-2-CHO has therapeutic potential in atherosclerosis [160].
Other drugs
Human ß-defensin 3 (hBD3) is a cardio-protective natural peptide found in mucous membranes, cells of the epithelium, and cells of the endothelium. In ApoE−/− mice, hBD3 inhibited atherosclerosis progression and suppressed P.gingivalis LPS-induced NF-κB activity [161]. What’s more, HBD3 reduces TNF-α-induced inflammation and monocyte adhesion in HUVECs with a dose-dependent effect by decreasing the phosphorylation of IKK-α/β, IκB and p65 subunit [162]. Similarly, glucagon-like peptide 1 (GLP-1) has been shown to be one of the incretin hormones, confers protection against atherosclerosis and myocardial injury [163,164,165].
The Mediterranean dietary is a plant-based, antioxidant-rich, unsaturated fat dietary pattern with lower cardiovascular diseases morbidity and mortality [166]. Whether a mediterranean diet with coenzyme Q (CoQ), 200 mg/day in capsules, contains 15% of energy as protein, 47% of energy as carbohydrate, and 38% of total energy as fat (24% MUFA provided by virgin olive oil, 10% saturated fatty acid, and 4% polyunsaturated fatty acid), affected the inflammatory response genes in elderly individuals was investigated. This dietary pattern reduced postprandial expression of p65 and IKKβ, suggesting anti-inflammatory activity [167].
Inflammatory responses can also be triggered by other stimuli such as TNFα, ox-LDL and Ang II on macrophages. Jianpi Huazhuo Tiaozhi granules (JHTG), a prepared Chinese herbal medicine, including dangshen, poria cocos, tangerine peel, towel gourd, amomum villosum, lotus leaf, atractylodes macrocephala, coix seed, wood fragrance, salvia miltiorrhiza, malt, hawthorn, and fried alisma orientalis, is commonly used clinical practice for the prevention of atherosclerosis [62]. Studies have shown that JHTG attenuates oxidative stress injury induced by ox-LDL in RAW264.7 macrophages, reducing the levels of ROS, the expression of NOX4, IKK-α, IKK-β, and NF-κB by blocking the NOX/ROS-NF-κB pathway [62].
Despite the widespread use of percutaneous coronary intervention (PCI) to treat coronary artery diseases, post-operative arterial restenosis remains a concern [168]. Fufang-Zhenzhu Tiaozhi Capsule (FTZ) is a chinese herbal medicine prescription including rhizoma coptidis, radix salvia miltiorrhiza, radix notoginseng, fructus ligustri lucidi, herba cirsii jeponici, cortex eucommiae, fructus citri sarcodactylis, and radix atractylodes macrocephala. Excitingly, FTZ reduces restenosis by inhibiting NF-κB activity and downregulating inflammatory factor expression in the atherosclerotic lesion of a rabbit restenosis model [76, 169]. It is well known that coronary atherosclerosis is the pathological basis for ischemic heart disease.
Therapeutic potential and future considerations
The compelling evidence has demonstrated the contributory role of IKKβ/NF-κB signaling in the pathogenesis of atherosclerosis. Therefore, the IKKβ is very attractive and promising as a target for the treatment of atherosclerosis. This review expounds on the link between key cellular components of atherosclerosis and IKKβ. It supports the view that targeted inhibition of IKKβ may produce a beneficial effect in preventing atherosclerosis. As a result, inflammation-reducing drugs targeting IKKβ have been developed and applied in several cellular studies and animal models, including natural products-based derivatives, natural extracts, synthetic drugs, as well as peptides et.al other drugs (Table 1). As a matter of fact, we also need to take attention to the potential side effects of these drugs, for example, digestive side effects, such as abdominal pain, nausea, and vomiting, have been observed with vinpocetine [170], metformin [171], andrographolide [172], digitoxin [173], acetyl-11-keto-β-boswellic acid [174], ursolic acid [175], and liraglutide [176]. Additionally, it has been shown in repeated studies that green tea polyphenols [177], acetyl-11-keto-β-boswellic acid [174], and ursolic acid [175] cause liver damage/degeneration, while methyl-β-cyclodextrin [51] (parenteral administration), quercetin [109], and corosolic acid [139] have nephrotoxic potential. Andrographolide [172] and digitoxin [173] cause chest tightness, palpitations, and arrhythmic. Similarly, vinpocetine [170] and andrographolide [172] cause the symptoms including dizziness headache, convulsions, and coma. The side effects of the above mentioned drugs are in fact very difficult to avoid and therefore scientific use of medication is a must.
In summary, as more drugs targeting IKKβ are discovered, there will be more opportunities to fully understand the complex functions of IKKβ in atherogenesis and to develop new effective therapies. Further result should be conducted in the future to enhance the understanding of drugs with potential therapeutic effects to treat atherosclerosis via IKKβ, such as additional validation experiments, comparative efficacy experiments among different drugs, and multicellular targeting experiments and clinical trials, etc. Understanding the pathogenesis of diseases associated with impaired IKKβ activity may provide insight into prevention and treatment of these human diseases.
Availability of data and materials
The declaration is not applicable.
References
Alsharari R, Lip G, Shantsila A. Assessment of Arterial Stiffness in Patients With Resistant Hypertension: Additional Insights Into the Pathophysiology of This Condition? Am J Hypertens. 2020;33(2):107–15.
Francque SM, van der Graaff D, Kwanten WJ. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J Hepatol. 2016;65(2):425–43.
Zhang L, Zhang Y, Wang Y, Zhao Y, Ding H, Li P. Circular RNAs: Functions and Clinical Significance in Cardiovascular Disease. Front Cell Dev Biol. 2020;8:584051. https://doi.org/10.3389/fcell.2020.584051. PMID: 33134301; PMCID: PMC7550538.
Chen CC, Li HY, Leu YL, Chen YJ, Wang CJ, Wang SH. Corylin Inhibits Vascular Cell Inflammation, Proliferation and Migration and Reduces Atherosclerosis in ApoE-Deficient Mice. Antioxidants (Basel). 2020;9(4):275. https://doi.org/10.3390/antiox9040275. PMID: 32218307; PMCID: PMC7222202.
Misra A, et al. Integrin beta3 regulates clonality and fate of smooth muscle-derived atherosclerotic plaque cells. Nat Commun. 2018;9(1):2073.
Parahuleva MS, et al. MicroRNA expression profile of human advanced coronary atherosclerotic plaques. Sci Rep. 2018;8(1):7823.
Cai Y, et al. Rap1 induces cytokine production in pro-inflammatory macrophages through NFκB signaling and is highly expressed in human atherosclerotic lesions. Cell Cycle. 2015;14(22):3580–92.
Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011;13(1):11–22.
Tian S, et al. The miR-378c-Samd1 circuit promotes phenotypic modulation of vascular smooth muscle cells and foam cells formation in atherosclerosis lesions. Sci Rep. 2021;11(1):10548.
Monaco C, et al. Canonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc Natl Acad Sci U S A. 2004;101(15):5634–9.
Hernandez R, Zhou C. Recent Advances in Understanding the Role of IKKβ in Cardiometabolic Diseases. Front Cardiovasc Med. 2021;8:752337. https://doi.org/10.3389/fcvm.2021.752337. PMID: 34957242; PMCID: PMC8692734.
Brand K, et al. Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. J Clin Invest. 1996;97(7):1715–22.
Page A, Navarro M, Suárez-Cabrera C, Bravo A, Ramirez A. Context-Dependent Role of IKKβ in Cancer. Genes (Basel). 2017;8(12):376. https://doi.org/10.3390/genes8120376. PMID: 29292732; PMCID: PMC5748694.
Sui Y, et al. IKKβ links vascular inflammation to obesity and atherosclerosis. J Exp Med. 2014;211(5):869–86.
Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47(8 Suppl):C7-12.
Gofman JW, Lindgren F. The role of lipids and lipoproteins in atherosclerosis. Science. 1950;111(2877):166–71. https://doi.org/10.1126/science.111.2877.166. PMID: 15403115.
Que X, et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature. 2018;558(7709):301–6.
Zhang X, Sessa WC, Fernández-Hernando C. Endothelial Transcytosis of Lipoproteins in Atherosclerosis. Front Cardiovasc Med. 2018;25(5):130. https://doi.org/10.3389/fcvm.2018.00130. PMID: 30320124; PMCID: PMC6167422.
Zhu Y, Xian X, Wang Z, Bi Y, Chen Q, Han X, Tang D, Chen R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules. 2018;8(3):80. https://doi.org/10.3390/biom8030080. PMID: 30142970; PMCID: PMC6163673.
Ranjit N, et al. Psychosocial factors and inflammation in the multi-ethnic study of atherosclerosis. Arch Intern Med. 2007;167(2):174–81.
Tabas I, Bornfeldt KE. Intracellular and Intercellular Aspects of Macrophage Immunometabolism in Atherosclerosis. Circ Res. 2020;126(9):1209–27.
Chen W, et al. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat Rev Cardiol. 2022;19(4):228–49.
Robbins CS, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19(9):1166–72.
Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145(3):341–55.
Maguire EM, Pearce SWA, Xiao Q. Foam cell formation: A new target for fighting atherosclerosis and cardiovascular disease. Vascul Pharmacol. 2019;112:54–71. https://doi.org/10.1016/j.vph.2018.08.002. Epub 2018 Aug 14 PMID: 30115528.
Bennett MR, Sinha S, Owens GK. Vascular Smooth Muscle Cells in Atherosclerosis. Circ Res. 2016;118(4):692–702.
Alencar GF, et al. Stem Cell Pluripotency Genes Klf4 and Oct4 Regulate Complex SMC Phenotypic Changes Critical in Late-Stage Atherosclerotic Lesion Pathogenesis. Circulation. 2020;142(21):2045–59.
Sorokin V, Vickneson K, Kofidis T, Woo CC, Lin XY, Foo R, Shanahan CM. Role of Vascular Smooth Muscle Cell Plasticity and Interactions in Vessel Wall Inflammation. Front Immunol. 2020;11:599415. https://doi.org/10.3389/fimmu.2020.599415. PMID: 33324416; PMCID: PMC7726011.
Bäck M, et al. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol. 2019;16(7):389–406.
Song Q, Ji Q, Li Q. The role and mechanism of β-arrestins in cancer invasion and metastasis (Review). Int J Mol Med. 2018;41(2):631–9.
Noort AR, Tak PP, Tas SW. Non-canonical NF-κB signaling in rheumatoid arthritis: Dr Jekyll and Mr Hyde? Arthritis Res Ther. 2015;17(1):15.
Giridharan S, Srinivasan M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J Inflamm Res. 2018;30(11):407–19. https://doi.org/10.2147/JIR.S140188. PMID: 30464573; PMCID: PMC6217131.
Liu Y, et al. (3R, 7R)-7-Acetoxyl-9-Oxo-de-O-Methyllasiodiplodin, a Secondary Metabolite of Penicillium Sp., Inhibits LPS-Mediated Inflammation in RAW 264.7 Macrophages through Blocking ERK/MAPKs and NF-κB Signaling Pathways. Inflammation. 2019;42(4):1463–73.
Miyauchi T, et al. Up-regulation of FOXO1 and reduced inflammation by β-hydroxybutyric acid are essential diet restriction benefits against liver injury. Proc Natl Acad Sci U S A. 2019;116(27):13533–42.
Yano H, et al. PHD3 regulates glucose metabolism by suppressing stress-induced signalling and optimising gluconeogenesis and insulin signalling in hepatocytes. Sci Rep. 2018;8(1):14290.
Janus P, Szołtysek K, Zając G, Stokowy T, Walaszczyk A, Widłak W, Wojtaś B, Gielniewski B, Iwanaszko M, Braun R, Cockell S, Perkins ND, Kimmel M, Widlak P. Pro-inflammatory cytokine and high doses of ionizing radiation have similar effects on the expression of NF-kappaB-dependent genes. Cell Signal. 2018;46:23–31. https://doi.org/10.1016/j.cellsig.2018.02.011. Epub 2018 Feb 21 PMID: 29476964.
Veerappan K, et al. Inhibition of IKKβ by celastrol and its analogues - an in silico and in vitro approach. Pharm Biol. 2017;55(1):368–73.
Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–63. https://doi.org/10.1146/annurev.immunol.18.1.621. PMID: 10837071.
Hinz M, Scheidereit C. The IκB kinase complex in NF-κB regulation and beyond. EMBO Rep. 2014;15(1):46–61.
Park SH, et al. Myeloid-specific IκB kinase β deficiency decreases atherosclerosis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32(12):2869–76.
Oguiza A, et al. Peptide-based inhibition of IκB kinase/nuclear factor-κB pathway protects against diabetes-associated nephropathy and atherosclerosis in a mouse model of type 1 diabetes. Diabetologia. 2015;58(7):1656–67.
Chew P, et al. Site-specific antiatherogenic effect of the antioxidant ebselen in the diabetic apolipoprotein E-deficient mouse. Arterioscler Thromb Vasc Biol. 2009;29(6):823–30.
Tilstam PV, et al. Bone marrow-specific knock-in of a non-activatable Ikkα kinase mutant influences haematopoiesis but not atherosclerosis in Apoe-deficient mice. PLoS ONE. 2014;9(2):e87452.
Lu W, et al. Deficiency of Adipocyte IKKβ Affects Atherosclerotic Plaque Vulnerability in Obese LDLR Deficient Mice. J Am Heart Assoc. 2019;8(12):e012009.
Zhao W, et al. Danshenol A inhibits TNF-α-induced expression of intercellular adhesion molecule-1 (ICAM-1) mediated by NOX4 in endothelial cells. Sci Rep. 2017;7(1):12953.
Li C, et al. Rhynchophylla total alkaloid rescues autophagy, decreases oxidative stress and improves endothelial vasodilation in spontaneous hypertensive rats. Acta Pharmacol Sin. 2018;39(3):345–56.
Berglund LM, et al. Glucose-Dependent Insulinotropic Polypeptide Stimulates Osteopontin Expression in the Vasculature via Endothelin-1 and CREB. Diabetes. 2016;65(1):239–54.
Wang Y, et al. Shear Stress Regulates the Flk-1/Cbl/PI3K/NF-κB Pathway Via Actin and Tyrosine Kinases. Cell Mol Bioeng. 2009;2(3):341–50.
Hayden MS, Ghosh S. Regulation of NF-κB by TNF family cytokines. Semin Immunol. 2014;26(3):253–66.
Sullivan CB, et al. TNFα and IL-1β influence the differentiation and migration of murine MSCs independently of the NF-κB pathway. Stem Cell Res Ther. 2014;5(4):104.
Chen G, et al. Methyl-β-cyclodextrin suppresses the monocyte-endothelial adhesion triggered by lipopolysaccharide (LPS) or oxidized low-density lipoprotein (oxLDL). Pharm Biol. 2021;59(1):1036–44.
Liu F, Chen Y, Zhao S, Li M, Luo F, Tang CE. Insulin Receptor Substrate p53 Ameliorates High-Glucose-Induced Activation of NF-κB and Impaired Mobility of HUVECs. Biomed Res Int. 2021;6(2021):3210586. https://doi.org/10.1155/2021/3210586. PMID: 33506012; PMCID: PMC7806382.
Huang X, et al. TRIM14 promotes endothelial activation via activating NF-κB signaling pathway. J Mol Cell Biol. 2020;12(3):176–89.
Venkatesan B, et al. CIKS (Act1 or TRAF3IP2) mediates high glucose-induced endothelial dysfunction. Cell Signal. 2013;25(1):359–71.
Shi J, et al. Endothelial cell injury and dysfunction induced by silver nanoparticles through oxidative stress via IKK/NF-κB pathways. Biomaterials. 2014;35(24):6657–66.
Kempe S, et al. NF-kappaB controls the global pro-inflammatory response in endothelial cells: evidence for the regulation of a pro-atherogenic program. Nucleic Acids Res. 2005;33(16):5308–19.
Mohan S, et al. Low shear stress preferentially enhances IKK activity through selective sources of ROS for persistent activation of NF-kappaB in endothelial cells. Am J Physiol Cell Physiol. 2007;292(1):C362–71.
Barrett TJ. Macrophages in Atherosclerosis Regression. Arterioscler Thromb Vasc Biol. 2020;40(1):20–33.
Pirillo A, Norata GD, Catapano AL. LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm. 2013;2013:152786. https://doi.org/10.1155/2013/152786. Epub 2013 Jul 10. PMID: 23935243; PMCID: PMC3723318.
Hoefer IE, Timmers L, Piek JJ. Growth factor therapy in atherosclerotic disease-friend or foe. Curr Pharm Des. 2007;13(17):1803–10.
Liu E, et al. Interacting with α 7 nAChR is a new mechanism for AChE to enhance the inflammatory response in macrophages. Acta Pharm Sin B. 2020;10(10):1926–42.
Liu Y, Liu Z. Jianpi Huazhuo Tiaozhi granules reduce oxidative stress injury in macrophages by inhibiting the nicotinamide adenine dinucleotide phosphate oxidase/reactive oxygen species-nuclear transcription factor kappa B pathway. J Tradit Chin Med. 2020;40(6):922–7.
Imamura T, Poulsen O, Haddad GG. Intermittent hypoxia induces murine macrophage foam cell formation by IKK-β-dependent NF-κB pathway activation. J Appl Physiol (1985). 2016;121(3):670–7.
Lee GS, Lee LA, Wang CY, Chen NH, Fang TJ, Huang CG, Cheng WN, Li HY. The Frequency and Energy of Snoring Sounds Are Associated with Common Carotid Artery Intima-Media Thickness in Obstructive Sleep Apnea Patients. Sci Rep. 2016;29(6):30559. https://doi.org/10.1038/srep30559. PMID: 27469245; PMCID: PMC4965750.
Ben J, et al. Major vault protein suppresses obesity and atherosclerosis through inhibiting IKK-NF-κB signaling mediated inflammation. Nat Commun. 2019;10(1):1801.
Zhou J, Ma W, Wang X, Liu H, Miao Y, Wang J, Du P, Chen Y, Zhang Y, Liu Z. Matrine Suppresses Reactive Oxygen Species (ROS)-Mediated MKKs/p38-Induced Inflammation in Oxidized Low-Density Lipoprotein (ox-LDL)-Stimulated Macrophages. Med Sci Monit. 2019;3(25):4130–6. https://doi.org/10.12659/MSM.917151. PMID: 31156213; PMCID: PMC6561390.
Paul A, Edwards J, Pepper C, Mackay S. Inhibitory-κB Kinase (IKK) α and Nuclear Factor-κB (NFκB)-Inducing Kinase (NIK) as Anti-Cancer Drug Targets. Cells. 2018;7(10):176. https://doi.org/10.3390/cells7100176. PMID: 30347849; PMCID: PMC6210445.
Asare Y, et al. Histone Deacetylase 9 Activates IKK to Regulate Atherosclerotic Plaque Vulnerability. Circ Res. 2020;127(6):811–23.
Holy EW, et al. Carbamylated Low-Density Lipoproteins Induce a Prothrombotic State Via LOX-1: Impact on Arterial Thrombus Formation In Vivo. J Am Coll Cardiol. 2016;68(15):1664–76.
Yang J, et al. Renin-angiotensin system activation accelerates atherosclerosis in experimental renal failure by promoting endoplasmic reticulum stress-related inflammation. Int J Mol Med. 2017;39(3):613–21.
Frismantiene A, Philippova M, Erne P, Resink TJ. Smooth muscle cell-driven vascular diseases and molecular mechanisms of VSMC plasticity. Cell Signal. 2018;52:48–64. https://doi.org/10.1016/j.cellsig.2018.08.019. Epub 2018 Aug 30 PMID: 30172025.
Miano JM, Fisher EA, Majesky MW. Fate and State of Vascular Smooth Muscle Cells in Atherosclerosis. Circulation. 2021;143(21):2110–6.
Rensen SS, Doevendans PA, van Eys GJ. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J. 2007;15(3):100–8.
Zhao G, et al. Activation of nuclear factor-kappa B accelerates vascular calcification by inhibiting ankylosis protein homolog expression. Kidney Int. 2012;82(1):34–44.
Sasu S, Beasley D. Essential roles of IkappaB kinases alpha and beta in serum- and IL-1-induced human VSMC proliferation. Am J Physiol Heart Circ Physiol. 2000;278(6):H1823–31.
Ortego M, et al. HMG-CoA reductase inhibitors reduce I kappa B kinase activity induced by oxidative stress in monocytes and vascular smooth muscle cells. J Cardiovasc Pharmacol. 2005;45(5):468–75.
Yu MH, et al. SAA1 increases NOX4/ROS production to promote LPS-induced inflammation in vascular smooth muscle cells through activating p38MAPK/NF-κB pathway. BMC Mol Cell Biol. 2019;20(1):15.
Wang CC, et al. Involvement of p42/p44 MAPK, p38 MAPK, JNK, and NF-kappaB in IL-1beta-induced VCAM-1 expression in human tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2005;288(2):L227–37.
Lourenco-Gonzalez Y, Fattori V, Domiciano TP, Rossaneis AC, Borghi SM, Zaninelli TH, Bernardy CCF, Alves-Filho JC, Cunha TM, Cunha FQ, Casagrande R, Verri WA Jr. Repurposing of the Nootropic Drug Vinpocetine as an Analgesic and Anti-Inflammatory Agent: Evidence in a Mouse Model of Superoxide Anion-Triggered Inflammation. Mediators Inflamm. 2019;31(2019):6481812. https://doi.org/10.1155/2019/6481812. PMID: 31049025; PMCID: PMC6462340.
Zhuang J, et al. Inhibitory effects of vinpocetine on the progression of atherosclerosis are mediated by Akt/NF-κB dependent mechanisms in apoE-/- mice. PLoS ONE. 2013;8(12):e82509.
Zhang C, Yan C. Updates of Recent Vinpocetine Research in Treating Cardiovascular Diseases. J Cell Immunol. 2020;2(5):211–9.
Jeon KI, et al. Vinpocetine inhibits NF-kappaB-dependent inflammation via an IKK-dependent but PDE-independent mechanism. Proc Natl Acad Sci U S A. 2010;107(21):9795–800.
Graham GG, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50(2):81–98.
Chen H, et al. Effect of metformin on insulin-resistant endothelial cell function. Oncol Lett. 2015;9(3):1149–53.
Wilcox T, et al. Diabetic Agents, From Metformin to SGLT2 Inhibitors and GLP1 Receptor Agonists: JACC Focus Seminar. J Am Coll Cardiol. 2020;75(16):1956–74.
Yang Q, Yuan H, Chen M, Qu J, Wang H, Yu B, Chen J, Sun S, Tang X, Ren W. Metformin ameliorates the progression of atherosclerosis via suppressing macrophage infiltration and inflammatory responses in rabbits. Life Sci. 2018Apr;1(198):56–64. https://doi.org/10.1016/j.lfs.2018.02.017. Epub 2018 Feb 13 PMID: 29452166.
Cameron AR, et al. Anti-Inflammatory Effects of Metformin Irrespective of Diabetes Status. Circ Res. 2016;119(5):652–65.
Huang NL, et al. Metformin inhibits TNF-alpha-induced IkappaB kinase phosphorylation, IkappaB-alpha degradation and IL-6 production in endothelial cells through PI3K-dependent AMPK phosphorylation. Int J Cardiol. 2009;134(2):169–75.
Heidary Moghaddam R, Samimi Z, Moradi SZ, Little PJ, Xu S, Farzaei MH. Naringenin and naringin in cardiovascular disease prevention: A preclinical review. Eur J Pharmacol. 2020;887:173535. https://doi.org/10.1016/j.ejphar.2020.173535. Epub 2020 Sep 8 PMID: 32910944.
Wang F, et al. Naringin Alleviates Atherosclerosis in ApoE(-/-) Mice by Regulating Cholesterol Metabolism Involved in Gut Microbiota Remodeling. J Agric Food Chem. 2020;68(45):12651–60.
Kivelä AM, et al. Sulforaphane inhibits endothelial lipase expression through NF-κB in endothelial cells. Atherosclerosis. 2010;213(1):122–8.
Hsueh TP, Sheen JM, Pang JH, Bi KW, Huang CC, Wu HT, Huang ST. The Anti-Atherosclerotic Effect of Naringin Is Associated with Reduced Expressions of Cell Adhesion Molecules and Chemokines through NF-κB Pathway. Molecules. 2016;21(2):195. https://doi.org/10.3390/molecules21020195. PMID: 26861272; PMCID: PMC6274007.
Luo N, Fang J, Wei L, Sahebkar A, Little PJ, Xu S, Luo C, Li G. Emodin in atherosclerosis prevention: Pharmacological actions and therapeutic potential. Eur J Pharmacol. 2021;890:173617. https://doi.org/10.1016/j.ejphar.2020.173617. Epub 2020 Oct 1 PMID: 33010303.
Heo SK, et al. Emodin and rhein inhibit LIGHT-induced monocytes migration by blocking of ROS production. Vascul Pharmacol. 2010;53(1–2):28–37.
Meng L, et al. Emodin inhibits tumor necrosis factor-α-induced migration and inflammatory responses in rat aortic smooth muscle cells. Int J Mol Med. 2012;29(6):999–1006.
Khan N, Mukhtar H. Tea Polyphenols in Promotion of Human Health. Nutrients. 2018;11(1):39. https://doi.org/10.3390/nu11010039. PMID: 30585192; PMCID: PMC6356332.
Grove KA, Lambert JD. Laboratory, epidemiological, and human intervention studies show that tea (Camellia sinensis) may be useful in the prevention of obesity. J Nutr. 2010;140(3):446–53.
Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43(1):89–143.
Wahyudi S, Sargowo D. Green tea polyphenols inhibit oxidized LDL-induced NF-KB activation in human umbilical vein endothelial cells. Acta Med Indones. 2007;39(2):66–70.
Zhang L, Wang F, Zhang Q, Liang Q, Wang S, Xian M, Wang F. Anti-Inflammatory and Anti-Apoptotic Effects of Stybenpropol A on Human Umbilical Vein Endothelial Cells. Int J Mol Sci. 2019;20(21):5383. https://doi.org/10.3390/ijms20215383. PMID: 31671764; PMCID: PMC6862503.
Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim Biophys Acta. 2007;1768(6):1311–24.
Zhou J, et al. Tanshinone IIA suppresses ovarian cancer growth through inhibiting malignant properties and angiogenesis. Ann Transl Med. 2020;8(20):1295.
Wen J, et al. Tanshinone IIA attenuates atherosclerosis via inhibiting NLRP3 inflammasome activation. Aging (Albany NY). 2020;13(1):910–32.
Chen Z, Gao X, Jiao Y, Qiu Y, Wang A, Yu M, Che F, Li S, Liu J, Li J, Zhang H, Yu C, Li G, Gao Y, Pan L, Sun W, Guo J, Cao B, Zhu Y, Xu H. Tanshinone IIA Exerts Anti-Inflammatory and Immune-Regulating Effects on Vulnerable Atherosclerotic Plaque Partially via the TLR4/MyD88/NF-κB Signal Pathway. Front Pharmacol. 2019;26(10):850. https://doi.org/10.3389/fphar.2019.00850. PMID: 31402870; PMCID: PMC6677033.
Chang CC, et al. The anti-atherosclerotic effect of tanshinone IIA is associated with the inhibition of TNF-α-induced VCAM-1, ICAM-1 and CX3CL1 expression. Phytomedicine. 2014;21(3):207–16.
Jia Q, et al. Quercetin protects against atherosclerosis by regulating the expression of PCSK9, CD36, PPARγ, LXRα and ABCA1. Int J Mol Med. 2019;44(3):893–902.
Bhaskar S, et al. Quercetin alleviates hypercholesterolemic diet induced inflammation during progression and regression of atherosclerosis in rabbits. Nutrition. 2013;29(1):219–29.
Jiang YH, Jiang LY, Wang YC, Ma DF, Li X. Quercetin Attenuates Atherosclerosis via Modulating Oxidized LDL-Induced Endothelial Cellular Senescence. Front Pharmacol. 2020;28(11):512. https://doi.org/10.3389/fphar.2020.00512. Erratumin :Front Pharmacol. 2020 May 29;11:772. PMID: 32410992; PMCID: PMC7198817.
Kleemann R, et al. Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis. 2011;218(1):44–52.
Taheri Y, et al. Myricetin bioactive effects: moving from preclinical evidence to potential clinical applications. BMC Complement Med Ther. 2020;20(1):241.
Afroze N, et al. A review on myricetin as a potential therapeutic candidate for cancer prevention. 3 Biotech. 2020;10(5):211.
Grenier D, et al. Dual Action of Myricetin on Porphyromonas gingivalis and the Inflammatory Response of Host Cells: A Promising Therapeutic Molecule for Periodontal Diseases. PLoS ONE. 2015;10(6):e0131758.
Ciumărnean L, Milaciu MV, Runcan O, Vesa ȘC, Răchișan AL, Negrean V, Perné MG, Donca VI, Alexescu TG, Para I, Dogaru G. The Effects of Flavonoids in Cardiovascular Diseases. Molecules. 2020;25(18):4320. https://doi.org/10.3390/molecules25184320. PMID: 32967119; PMCID: PMC7571023.
Meng Z, Wang M, Xing J, Liu Y, Li H. Myricetin ameliorates atherosclerosis in the low-density-lipoprotein receptor knockout mice by suppression of cholesterol accumulation in macrophage foam cells. Nutr Metab (Lond). 2019;25(16):25. https://doi.org/10.1186/s12986-019-0354-7. PMID: 31049071; PMCID: PMC6482568.
Tsai SH, et al. Suppression of TNFalpha-mediated NFkappaB activity by myricetin and other flavonoids through downregulating the activity of IKK in ECV304 cells. J Cell Biochem. 1999;74(4):606–15.
Dinda B, et al. Naturally occurring triterpenoid saponins. Chem Biodivers. 2010;7(10):2327–580.
Ding H, Han R, Chen X, Fang W, Liu M, Wang X, Wei Q, Kodithuwakku ND, Li Y. Clematichinenoside (AR) Attenuates Hypoxia/Reoxygenation-Induced H9c2 Cardiomyocyte Apoptosis via a Mitochondria-Mediated Signaling Pathway. Molecules. 2016;21(6):683. https://doi.org/10.3390/molecules21060683. PMID: 27248986; PMCID: PMC6273438.
Yan S, Zhang X, Zheng H, Hu D, Zhang Y, Guan Q, Liu L, Ding Q, Li Y. Clematichinenoside inhibits VCAM-1 and ICAM-1 expression in TNF-α-treated endothelial cells via NADPH oxidase-dependent IκB kinase/NF-κB pathway. Free Radic Biol Med. 2015;78:190–201. https://doi.org/10.1016/j.freeradbiomed.2014.11.004. Epub 2014 Nov 13 PMID: 25463279.
Zhang H, Li S, Si Y, Xu H. Andrographolide and its derivatives: Current achievements and future perspectives. Eur J Med Chem. 2021;224:113710. https://doi.org/10.1016/j.ejmech.2021.113710. Epub 2021 Jul 20 PMID: 34315039.
Chen YY, Hsu MJ, Sheu JR, Lee LW, Hsieh CY. Andrographolide, a Novel NF- κ B Inhibitor, Induces Vascular Smooth Muscle Cell Apoptosis via a Ceramide-p47phox-ROS Signaling Cascade. Evid Based Complement Alternat Med. 2013;2013:821813. https://doi.org/10.1155/2013/821813. Epub 2013 Dec 29. PMID: 24489592; PMCID: PMC3893871.
Yu Y, et al. The Progress of Nomenclature, Structure, Metabolism, and Bioactivities of Oat Novel Phytochemical: Avenanthramides. J Agric Food Chem. 2022;70(2):446–57.
Thomas M, et al. High Levels of Avenanthramides in Oat-Based Diet Further Suppress High Fat Diet-Induced Atherosclerosis in Ldlr(-/-) Mice. J Agric Food Chem. 2018;66(2):498–504.
Park J, Choi H, Abekura F, Lim HS, Im JH, Yang WS, Hwang CW, Chang YC, Lee YC, Park NG, Kim CH. Avenanthramide C Suppresses Matrix Metalloproteinase-9 Expression and Migration Through the MAPK/NF- κB Signaling Pathway in TNF-α-Activated HASMC Cells. Front Pharmacol. 2021;12:621854. https://doi.org/10.3389/fphar.2021.621854. PMID: 33841150; PMCID: PMC8027239.
Guo W, et al. Avenanthramides, polyphenols from oats, inhibit IL-1beta-induced NF-kappaB activation in endothelial cells. Free Radic Biol Med. 2008;44(3):415–29.
Yang Q, et al. Gene therapy-emulating small molecule treatments in cystic fibrosis airway epithelial cells and patients. Respir Res. 2019;20(1):290.
Jagielska J, et al. Digitoxin elicits anti-inflammatory and vasoprotective properties in endothelial cells: Therapeutic implications for the treatment of atherosclerosis? Atherosclerosis. 2009;206(2):390–6.
Jiang X, Liu Q, Xue S. LC-MS/MS method for determination of kansuinine a in rat plasma and its application to a rat pharmacokinetic study. Biomed Chromatogr. 2022;36(3):e5282.
Chen CS, Pan BY, Tsai PH, Chen FY, Yang WC, Shen MY. Kansuinine A Ameliorates Atherosclerosis and Human Aortic Endothelial Cell Apoptosis by Inhibiting Reactive Oxygen Species Production and Suppressing IKKβ/IκBα/NF-κB Signaling. Int J Mol Sci. 2021Sep 24;22(19):10309. https://doi.org/10.3390/ijms221910309. PMID: 34638650; PMCID: PMC8508741.
Rauf A, Olatunde A, Imran M, Alhumaydhi FA, Aljohani ASM, Khan SA, Uddin MS, Mitra S, Emran TB, Khayrullin M, Rebezov M, Kamal MA, Shariati MA. Corrigendum to “Honokiol: A review of its pharmacological potential and therapeutic insights” [Phytomedicine, 153647]. Phytomedicine. 2021;92:153769. https://doi.org/10.1016/j.phymed.2021.153769. Epub 2021 Sep 28. Erratum for: Phytomedicine. 2021 Sep;90:153647. PMID: 34597906.
Liu Y, Cheng P, Wu AH. Honokiol inhibits carotid artery atherosclerotic plaque formation by suppressing inflammation and oxidative stress. Aging (Albany NY). 2020;12(9):8016–28.
Qiu L, et al. Honokiol ameliorates endothelial dysfunction through suppression of PTX3 expression, a key mediator of IKK/IκB/NF-κB, in atherosclerotic cell model. Exp Mol Med. 2015;47(7):e171.
Nguyen V, Huang J, Doan V, Lin X, Tang X, Huang Y, Tang A, Yang X, Huang R. Hepatoprotective effects of Yulangsan polysaccharide against nimesulide-induced liver injury in mice. J Ethnopharmacol. 2015;22(172):273–80. https://doi.org/10.1016/j.jep.2015.06.048. Epub 2015 Jul 2 PMID: 26144697.
Xue LL, Wu WS, Ma X, Pei HY, Tang MH, Kuang S, Cai XY, Wang L, Li Y, Zhang RJ, Hong F, Peng AH, Ye HY, Chen LJ. Modulation of LPS-induced inflammation in RAW264.7 murine cells by novel isoflavonoids from Millettia pulchra. Bioorg Chem. 2020;97:103693. https://doi.org/10.1016/j.bioorg.2020.103693. Epub 2020 Feb 22 PMID: 32120079.
Raja AF, Ali F, Khan IA, Shawl AS, Arora DS. Acetyl-11-keto-β-boswellic acid (AKBA); targeting oral cavity pathogens. BMC Res Notes. 2011Oct;13(4):406. https://doi.org/10.1186/1756-0500-4-406. PMID: 21992439; PMCID: PMC3201914.
Cuaz-Pérolin C, et al. Antiinflammatory and antiatherogenic effects of the NF-kappaB inhibitor acetyl-11-keto-beta-boswellic acid in LPS-challenged ApoE-/- mice. Arterioscler Thromb Vasc Biol. 2008;28(2):272–7.
Hua F, Shi L, Zhou P. Phytochemicals as potential IKK-β inhibitor for the treatment of cardiovascular diseases in plant preservation: terpenoids, alkaloids, and quinones. Inflammopharmacology. 2020;28(1):83–93.
Lee IA, et al. Ginsenoside Re ameliorates inflammation by inhibiting the binding of lipopolysaccharide to TLR4 on macrophages. J Agric Food Chem. 2012;60(38):9595–602.
Khlebnicova TS, Piven YA, Baranovsky AV, Lakhvich FA, Sorokina IV, Tolstikova TG. Fluorine-containing lupane triterpenoid acid derivatives: Design, synthesis and biological evaluation as potential anti-inflammatory agents. Steroids. 2019;147:62–9. https://doi.org/10.1016/j.steroids.2018.10.001. Epub 2018 Oct 5 PMID: 30296549.
Patil KR, et al. Pentacyclic Triterpenoids Inhibit IKKβ Mediated Activation of NF-κB Pathway: In Silico and In Vitro Evidences. PLoS One. 2015;10(5):e0125709.
Kattupalli D, Srinivasan A, Soniya EV. A Genome-Wide Analysis of Pathogenesis-Related Protein-1 (PR-1) Genes from Piper nigrum Reveals Its Critical Role during Phytophthora capsici Infection. Genes (Basel). 2021;12(7):1007. https://doi.org/10.3390/genes12071007. PMID: 34208836; PMCID: PMC8303604.
Pei H, et al. Alkaloids from Black Pepper (Piper nigrum L.) Exhibit Anti-Inflammatory Activity in Murine Macrophages by Inhibiting Activation of NF-κB Pathway. J Agric Food Chem. 2020;68(8):2406–17.
Liu H, et al. Biomarker Exploration in Human Peripheral Blood Mononuclear Cells for Monitoring Sulforaphane Treatment Responses in Autism Spectrum Disorder. Sci Rep. 2020;10(1):5822.
Shehatou GS, Suddek GM. Sulforaphane attenuates the development of atherosclerosis and improves endothelial dysfunction in hypercholesterolemic rabbits. Exp Biol Med (Maywood). 2016;241(4):426–36.
Haodang L, Lianmei Q, Ranhui L, Liesong C, Jun H, Yihua Z, Cuiming Z, Yimou W, Xiaoxing Y. HO-1 mediates the anti-inflammatory actions of Sulforaphane in monocytes stimulated with a mycoplasmal lipopeptide. Chem Biol Interact. 2019;1(306):10–8. https://doi.org/10.1016/j.cbi.2019.04.007. Epub 2019 Apr 6 PMID: 30965051.
Barthelemy J, Sanchez K, Miller MR, Khreis H. New Opportunities to Mitigate the Burden of Disease Caused by Traffic Related Air Pollution: Antioxidant-Rich Diets and Supplements. Int J Environ Res Public Health. 2020;17(2):630. https://doi.org/10.3390/ijerph17020630. PMID: 31963738; PMCID: PMC7014349.
Hung CN, et al. Sulforaphane inhibits TNF-α-induced adhesion molecule expression through the Rho A/ROCK/NF-κB signaling pathway. J Med Food. 2014;17(10):1095–102.
Padayatty SJ, Levine M. Vitamin C: the known and the unknown and Goldilocks. Oral Dis. 2016;22(6):463–93.
Woo KS, et al. Vitamins B-12 and C Supplementation Improves Arterial Reactivity and Structure in Passive Smokers: Implication in Prevention of Smoking-Related Atherosclerosis. J Nutr Health Aging. 2021;25(2):248–54.
Bowie AG, O’Neill LA. Vitamin C inhibits NF-kappa B activation by TNF via the activation of p38 mitogen-activated protein kinase. J Immunol. 2000;165(12):7180–8.
Tamura Y. The Role of Zinc Homeostasis in the Prevention of Diabetes Mellitus and Cardiovascular Diseases. J Atheroscler Thromb. 2021;28(11):1109–22.
Shen T, Zhao Q, Luo Y, Wang T. Investigating the Role of Zinc in Atherosclerosis: A Review. Biomolecules. 2022;12(10):1358. https://doi.org/10.3390/biom12101358. PMID: 36291566; PMCID: PMC9599385.
Prasad AS, et al. Zinc-suppressed inflammatory cytokines by induction of A20-mediated inhibition of nuclear factor-κB. Nutrition. 2011;27(7–8):816–23.
Ma Y, et al. 1-Deoxynojirimycin in Mulberry (Morus indica L.) Leaves Ameliorates Stable Angina Pectoris in Patients With Coronary Heart Disease by Improving Antioxidant and Anti-inflammatory Capacities. Front Pharmacol. 2019;10:569.
Azad GK, Tomar RS. Ebselen, a promising antioxidant drug: mechanisms of action and targets of biological pathways. Mol Biol Rep. 2014;41(8):4865–79.
Xiao Y, et al. Inhibition of S-Adenosylhomocysteine Hydrolase Induces Endothelial Dysfunction via Epigenetic Regulation of p66shc-Mediated Oxidative Stress Pathway. Circulation. 2019;139(19):2260–77.
Au-Yeung KK, et al. Hyperhomocysteinemia activates nuclear factor-kappaB in endothelial cells via oxidative stress. Circ Res. 2004;94(1):28–36.
Maeda K, et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 2005;1(2):107–19.
Evans CE, et al. The role of hydrostatic pressure in foam cell formation upon exposure of macrophages to LDL and oxidized LDL. Atherosclerosis. 2008;197(2):596–601.
Jung Y, et al. 8-(Tosylamino)quinoline inhibits macrophage-mediated inflammation by suppressing NF-κB signaling. Acta Pharmacol Sin. 2012;33(8):1037–46.
Ho FM, et al. The anti-inflammatory actions of LCY-2-CHO, a carbazole analogue, in vascular smooth muscle cells. Biochem Pharmacol. 2007;74(2):298–308.
Bian T, Li L, Lyu J, Cui D, Lei L, Yan F. Human β-defensin 3 suppresses Porphyromonas gingivalis lipopolysaccharide-induced inflammation in RAW 264.7 cells and aortas of ApoE-deficient mice. Peptides. 2016;82:92–100. https://doi.org/10.1016/j.peptides.2016.06.002. Epub 2016 Jun 11 PMID: 27298203.
Bian T, et al. Human β-Defensin 3 Reduces TNF-α-Induced Inflammation and Monocyte Adhesion in Human Umbilical Vein Endothelial Cells. Mediators Inflamm. 2017;2017:8529542.
Jonik S, Marchel M, Grabowski M, Opolski G, Mazurek T. Gastrointestinal Incretins-Glucose-Dependent Insulinotropic Polypeptide (GIP) and Glucagon-like Peptide-1 (GLP-1) beyond Pleiotropic Physiological Effects Are Involved in Pathophysiology of Atherosclerosis and Coronary Artery Disease-State of the Art. Biology (Basel). 2022;11(2):288. https://doi.org/10.3390/biology11020288. PMID: 35205155; PMCID: PMC8869592.
Liu Y, et al. Protective effect of glucagon-like peptide-1 mediated by ultrasound microbubbles on myocardial injury in rats with diabetic cardiomyopathy. Bioengineered. 2022;13(2):3251–61.
Shiraki A, et al. The glucagon-like peptide 1 analog liraglutide reduces TNF-α-induced oxidative stress and inflammation in endothelial cells. Atherosclerosis. 2012;221(2):375–82.
Stewart RA, et al. Dietary patterns and the risk of major adverse cardiovascular events in a global study of high-risk patients with stable coronary heart disease. Eur Heart J. 2016;37(25):1993–2001.
Lopez-Moreno J, et al. Mediterranean Diet Supplemented With Coenzyme Q10 Modulates the Postprandial Metabolism of Advanced Glycation End Products in Elderly Men and Women. J Gerontol A Biol Sci Med Sci. 2018;73(3):340–6.
Zhang R, et al. Fufang-Zhenzhu-Tiaozhi Capsule reduces restenosis via the downregulation of NF-kappaB and inflammatory factors in rabbits. Lipids Health Dis. 2018;17(1):272.
Bailey CJ. Metformin: historical overview. Diabetologia. 2017;60(9):1566–76.
Smith TW, et al. Treatment of life-threatening digitalis intoxication with digoxin-specific Fab antibody fragments: experience in 26 cases. N Engl J Med. 1982;307(22):1357–62.
Efferth T, Oesch F. Anti-inflammatory and anti-cancer activities of frankincense: Targets, treatments and toxicities. Semin Cancer Biol. 2022;80:39–57. https://doi.org/10.1016/j.semcancer.2020.01.015. Epub 2020 Feb 4 PMID: 32027979.
SSun Q, He M, Zhang M, Zeng S, Chen L, Zhou L, Xu H. Ursolic acid: A systematic review of its pharmacology, toxicity and rethink on its pharmacokinetics based on PK-PD model. Fitoterapia. 2020;147:104735. https://doi.org/10.1016/j.fitote.2020.104735. Epub 2020 Sep 30 PMID: 33010369.
Drucker DJ. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018;27(4):740–56.
Younes M, et al. Scientific opinion on the safety of green tea catechins. EFSA J. 2018;16(4):e05239.
Rahimi M, et al. The effect of methyl-beta-cyclodextrin on DNA absorption and quality of posttransfected sperm. Poult Sci. 2021;100(5):101058.
Andres S, Pevny S, Ziegenhagen R, Bakhiya N, Schäfer B, Hirsch-Ernst KI, Lampen A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol Nutr Food Res. 2018;62(1):1700447. https://doi.org/10.1002/mnfr.201700447. Epub 2017 Dec 19. PMID: 29127724.
Zheng JQ, Zheng CM, Lu KC. Corosolic acid-induced acute kidney injury and lactic acidosis in a patient with impaired kidney function. Am J Kidney Dis. 2010;56(2):419–20.
He Y, et al. Identification of new dual FABP4/5 inhibitors based on a naphthalene-1-sulfonamide FABP4 inhibitor. Bioorg Med Chem. 2019;27(19):115015.
Yubero-Serrano EM, et al. Mediterranean diet supplemented with coenzyme Q10 modifies the expression of proinflammatory and endoplasmic reticulum stress-related genes in elderly men and women. J Gerontol A Biol Sci Med Sci. 2012;67(1):3–10.
Patyar S, et al. Role of vinpocetine in cerebrovascular diseases. Pharmacol Rep. 2011;63(3):618–28.
Flory J, Lipska K. Metformin in 2019. JAMA. 2019;321(19):1926–7.
Shang YX, Shen C, Stub T, Zhu SJ, Qiao SY, Li YQ, Wang RT, Li J, Liu JP. Front Pharmacol. 2022;13:773282. https://doi.org/10.3389/fphar.2022.773282. PMID: 35153776; PMCID: PMC8831758.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 82074211), the Natural Science Foundation of Tianjin City, China (No. 21JCQNJC01170), 2020 Annual Graduate Students Innovation Fund (School of Integrative Medicine, Tianjin University of Traditional Chinese Medicine,Tianjin, China; No. ZXYCXLX202014).
Funding
National Natural Science Foundation of China, Grant/Award Numbers: 82074211; Natural Science Foundation of Tianjin City, China, Grant/Award Numbers: 21JCQNJC01170; 2020 Annual Graduate Students Innovation Fund, Grant/Award Number: ZXYCXLX202014.
Author information
Authors and Affiliations
Contributions
Jiali Gan and Lin Guo drafted the manuscript. Xijuan Jiang and Maojuan Guo designed and supervise manuscript. Xiaolu Zhang verified the contents and revised the manuscript. Qun Yu, Qiuyue Yang, Yilin Zhang, Wenyun Zeng critically revised the manuscript. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that the paper was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gan, J., Guo, L., Zhang, X. et al. Anti-inflammatory therapy of atherosclerosis: focusing on IKKβ. J Inflamm 20, 8 (2023). https://doi.org/10.1186/s12950-023-00330-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12950-023-00330-5